Abstract
Cowpea (Vigna unguiculata (L.) Walp) is a legume of economic importance world-wide, especially in Western Africa, where it is an important part of the population’s diet. The rapidly increasing population growth in Africa requires substantial increase in cowpea production, which can be achieved by expanding land areas for agricultural purposes. In addition, prevalence of soil acidity in Africa constrains such an alternative since phosphorus availability, a key element for plant growth and development, is limited, thus resulting in poor cowpea production. The objectives of this study were to conduct an association analysis for adaptation to low phosphorus conditions and rock phosphate response in cowpea, and to identify SNP markers associated with these two traits. A total of 357 cowpea accessions, collected worldwide, was evaluated for phosphorus stress and response to addition of rock phosphate. Association analysis was conducted using 1018 SNPs obtained using genotyping-by-sequencing (GBS). TASSEL 5 and R were used for association mapping studies based on six different models. The results indicated that: (1) substantial variability in adaptation to low phosphorus conditions and rock phosphate response exists in the USDA cowpea accession panel; (2) ten SNP markers, C35006753_110, C35028233_482, C35072764_1384, C35084634_455, Scaffold21750_4938, Scaffold26894_5408, Scaffold41885_14420, Scaffold45170_4650, Scaffold50732_679; and Scaffold88448_741 were found to be associated with tolerance to low phosphorus conditions in cowpea, and (3) eight SNP markers, C35028233_482, C35058535_121, Scaffold26894_5408, Scaffold45170_4650, Scaffold51609_507, Scaffold53730_7339, Scaffold74389_5733, and Scaffold87916_4921 were highly associated with rock phosphate response. These SNP markers can be used in a marker-assisted breeding (MAS) program to improve cowpea tolerance to phosphorus stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cowpea (Vigna unguiculata (L.) Walp) is a diploid self-pollinated legume species (2n = 2x = 22). It belongs to the family Fabaceae (Verdcourt 1970). Molecular analysis revealed a discrepancy in cowpea domestication events occurring in East and West Africa (Badiane et al. 2014). Cowpea nomenclature depends upon regions. It is known as southern pea or blackeye pea in the US, ‘niebe’, ‘wake’ or ‘ewa’ in Africa, and ‘caupi’ in Brazil (Agbicodo et al. 2010). Cowpea is primarily grown for human consumption, and provides a cheap source of protein (Caygill et al. 1981). Worldwide cowpea production is estimated at 5.4 million tons of dry seed (Olufajo 2012). Cowpea can be grown in temperature of 18–28 °C, with an optimum of 28 °C (Hall et al. 2003).
Phosphorus is a key element in plant growth and development. In addition to being a critical component of nucleic acids, phytins, phospholipids, nucleotides coenzymes and enzymes (Thakur et al. 2014), phosphorus is involved in different cellular metabolism and plays an important role in the transformation of energy within cells (Mc Vickar et al. 1963). Early root development, growth, and plant establishment require phosphorus (Hayman 1975). Deficiency in phosphorus unfavorably affects plants, resulting in a drop in tissue phosphorus concentration, which leads to plant death (Veneklaas et al. 2012).
Increased cowpea production can be achieved by expanding lands allocated to cowpea cultivation. However, soil acidity, prevalent in developing countries (Wang et al. 2012), limits phosphorus availability since phosphorus is a very reactive element (Jones et al. 2015). The use of inorganic phosphate fertilizers to tackle phosphorus deficiency is expensive. Low phosphorus condition as a major production constraint is an important problem for sub-Saharan Africa, where fertilization is still a main challenge in cowpea cultivation. Low phosphorus also affect the effectiveness of nitrogen fixation of cowpea, which is one of the main advantages of grow cowpea in the cropping system.
Understanding the genetic mechanism leading to plant tolerance to low soil phosphorus conditions is crucial to rapidly develop low soil phosphorus-tolerant plants. Studies reported relatively high heritability of low phosphorus-related parameters in crops. Coltman and Gabelman (1982) estimated tomato (Solanum lycopersicum L.) broad-sense heritability for phosphorus uptake (mg P/plant) and phosphorus utilization ration (mg SDW/mg P) ranging from 61 to 67%. In addition, investigations demonstrated dominant and epistatic genetic effect controlling tolerance to low soil phosphorus conditions in common bean (Phaseolus vulgaris L.) (Fawole et al. 1982) and cucumbers (Cucumis sativus L.) (Ghaderi and Lower 1979). However, other reports stated that crop tolerance to low soil phosphorus conditions were likely to be controlled by genes with additive effects (Araújo et al. 2005; Parentoni et al. 2010).
Efforts to perform linkage and QTL mapping studies for tolerance to low soil phosphorus conditions have been carried out in different crops of economic value. Wissuwa et al. (2002) conducted a study to map the Pup1 locus for phosphorus-deficient soil conditions in rice (Oryza sativa L.). Linkage mapping was performed using a population consisting of 160 F3 individuals. Their results indicated that Pup1 was flanked by markers S14025 and S13126, with a 3-cM interval. Co-segregation between Pup1 and S13126 was observed in substitution mapping. The Pup1 locus contributes to increasing root growth, thus resulting in rice tolerance to low phosphorus conditions (Wissuwa et al. 2002). Hammond et al. (2009) evaluated 90 Brassica oleracea L. double haploids for shoot phosphorus and phosphorus use efficiency. Results showed a significant correlation between root development and phosphorus use efficiency. Significant QTLs for shoot phosphorus and phosphorus use efficiency were reported on chromosomes 3 and 7 in Brassica oleracea L. In common bean (Phaseouls vulgaris), QTL analysis for adventitious root formation under contrasting phosphorus availability revealed two major QTLs located on linkage groups B2 and B8 (Ochoa et al. 2006). Regarding soybean (Glycine max L. Merr.), QTLs associated with phosphorus deficiency tolerance (Li et al. 2005) and phosphorus efficiency-related traits such as root growth (Liang et al. 2010) have been reported. Transcriptomic profiling based on four QTL regions in two transgressive soybean RILs, genotype B20 (low phosphorus-tolerant) and genotype B18 (low phosphorus-sensitive) revealed 22 up-regulated phosphatase family genes, and overexpression of acid phosphatase gene, GmACP2, triggered phosphorus efficiency in soybean (Zhang et al. 2017). In cowpea, Rothe (2014) conducted a QTL analysis in an F6 recombinant inbred population (RIL) consisting of 125 individuals, derived from a cross between IT98K-476-8 (positive for low phosphorus conditions) and Aloka (negative for low phosphorus conditions). SSR markers, CLM0721, MA135, 181/182, 221/22, CLM0269, EX41, CLM0089, and CLM0298, located on linkage group 3 were reported to be associated with tolerance to low phosphorus in cowpea.
To date, single nucleotide polymorphism (SNP) has been shown to be the fundamental molecular marker of choice (Varshney et al. 2009). To our knowledge, a SNP marker associated with soil phosphorus stress in cowpea has not been reported. The use of SNP markers has proven to be cost-effective in molecular assisted selection (MAS). Molecular markers for cowpea tolerance to low soil phosphorus conditions remain limited, and the genetic mechanism underlying phosphorus stress tolerance in cowpea is poorly understood. Therefore, this study aimed to conduct an association analysis for adaptation to low phosphorus conditions and response to rock phosphate in cowpea, and to identify SNP markers associated with these two traits.
Materials and methods
Plant materials
The cowpea panel consisted of 357 United States Department of Agriculture (USDA) Germplasm Resources Information Network (GRIN) cowpea germplasm accessions (Table S1). Cowpea accessions originated from 53 countries (Table S1). Cowpea seeds were obtained from the USDA Plant Genetic Resources Conservation Unit at Griffin, GA. Seeds were increased at the Research and Extension Station of the University of Arkansas at Fayetteville, AR in the summers 2014 and 2015.
Phenotyping for phosphorus stress and rock phosphate response
Greenhouse evaluation of cowpea genotypes for phosphate stress and response to the addition of rock phosphate was conducted by Mahamane et al. (2008) at the Texas AgriLife Research Center at Bushland, Texas. Average greenhouse day/night temperatures were 32/16 °C, and relative humidity was 60%. Since phosphorus deficiency is prevalent in West Africa (Payne et al. 1990), soil medium used for the screening consisted of Betis sand surface horizon (sandy, siliceous, thermic Psammentic Paleustafl), whose physical and chemical properties are similar to those of the coarse, sandy soils found in much of West Africa. Soil pH was 4.7, with a 1:1 water to soil mixture ratio. The Melich III method revealed 3 mg kg−1 of available phosphorus in Betis sand. Soil medium was air dried, 2 mm sieved, and was completely mixed to ensure soil uniformity. Deep containers of 20 cm in length (Ray Leach Conetainers®, Portland, Oregon) were filled with 200 g of soil medium. A paper filter previously placed in each container prevented the soil medium from leaking.
Non-addition of any P-related materials to Betis sand induced phosphorus stress. Tahoua rock phosphate (RP) from Niger was used as phosphate source for the evaluation of rock phosphate response in cowpea. Addition of 400 mg crused Tahoua RP per container provided 300 mg kg−1 of phosphorus. Tahoua RP consisted of 15% P (34.5% P2O5) and 8.3% of neutral ammonium-citrate soluble P (Mahamane et al. 2008).
The experimental design was a completely randomized design (CRD). The treatments were cowpea accessions and phosphorus application. Three replications per treatment combination were used. Each container contained one plant per cowpea accession and was irrigated with water containing insignificant phosphorus content during the germination process. Approximately 25–35 ml of r water was applied once every two days upon plant establishment.
Evaluation of tolerance to low phosphorus condition and rock phosphate response was conducted 8 weeks after planting. Tolerance to low phosphorus conditions and rock phosphate response was scored on a 1–5 scale (Adaptation to low phosphorus conditions: 1 = most efficient, 2 = efficient, 3 = moderately efficient, 4 = inefficient, and 5 = most inefficient; rock phosphate response: 1 = highly responsive, 2 = responsive, 3 = moderately responsive, 4 = poorly responsive, and 5 = least responsive). Data on phosphorus stress and rock phosphate response scores were published and obtained from the USDA GRIN database by choosing the VIGNA option (https://npgsweb.ars-grin.gov/gringlobal/descriptors.aspx). Descriptive statistics were performed using “Tabulate” in JMP Genomics 7 (SAS Institute, Cary, North Carolina). Bar charts and correlation analysis were generated using Excel.
Genotyping
DNA extraction and sequencing
Genomic DNA was extracted from freeze-dried cowpea young leaves using the CTAB (hexadecyltrimethyl ammonium bromide) protocol as described by Kisha et al. (1997). DNA extraction buffer was added to each ground cowpea leave sample, and centrifuged at 13,000 rpm for 10 min. Proteins were denatured by adding 1 ml of cholorform-isoamyl alcohol (24:1) to each sample. Addition of 1 ml of isopropanol made the DNA precipitate. Samples were stored over night at −20 °C, and DNA pellets were washed with 70 and 90% ethanol, and dried for 30 min. A total of 200 µl of 0.1X TE and 3 µl of RNAse were supplied to each sample afterwards. DNA quantification was conducted using a NanoDrop 200c spectrophotometer (Thermo SCIENTIFIC, Wilmington, DE), and DNA quality was checked on a 1%-agarose gel with ethidium bromide stain.
Genotyping-by-sequencing libraries were constructed following the ApeKI protocol (Elshire et al. 2011). DNA sequencing was done using the GBS method (Bastien et al. 2014; Elshire et al. 2011) by HiSeq series in Beijing Genome Institute (BGI). Genotyping-by-sequencing libraries were quality checked. DNA was digested by the restriction enzyme ApeKI. Adaptors were ligated to the restriction products, and in situ PCR was performed using specific primer pairs (GBS protocol of BGI).
SNP assembly, mapping, discovery, and filtering
SNP assembly, mapping, discovery, and filtering were carried out by BGI using SOAP family software (http://soap.genomics.org.cn/). Short-reads were aligned to a cowpea genome reference (cowpea_Genome_0.03.fa) by SOAPalinger/soap2 (http://soap.genomics.org.cn/). Initial SNP calls were performed using SOAPsnp v 1.05 (Li et al. 2009). Dr. Timothy J. Close from the University of California, Riveside, CA, provided the cowpea_Genome_0.03.fa (644,126 scaffolds or contigs).
Cowpea accessions with more than 30% missing SNP data were not considered for analysis. For each SNP, if its heterozygous calls were greater than 30%, the SNP was removed from the analysis; the minor allele frequency threshold was set at 0.05 for SNP callings. SNPs containing more than 30% missing data were also removed from the analysis. A total of 1018 SNPs postulated from GBS were used for this association analysis after SNP filtering.
Population structure analysis
Population structure was inferred using STRUCTURE 2.3.4 (Pritchard et al. 2000). An admixture model with a correlated allele frequency model, independent for each run, was used to assess the population structure (K). The use of such models has been proven to provide consistent results in cowpea (Shi et al. 2016). A total of ten runs were performed for each estimated K value. Markov Chain Monte Carlo (MCMC) length of the burn-in period was 30,000. After the burn-in period, the number of MCMC iterations was adjusted to 50,000. In order to screen appropriate K-values, values of delta K and optimal K were computed using STRUCTURE Harvester (Earl and VonHoldt 2011; http://taylor0.biology.ucla.edu/structureHarvester/) using the formula developed by Evanno et al. (2005). After defining K optimal, Q-matrix with the K vectors were generated and used for association analysis using TASSEL 5. Each cowpea genotype was allocated to each cluster (Q). The cut-off probability for assigning an accession to a Q cluster was 0.55. Structure among the cowpea panel was visualized by bar plots using STRUCTURE PLOT with the option “Sort by Q” and based on K optimal were designed (Ramasamy et al. 2014).
Genetic diversity
The analysis of genetic diversity and the drawing of phylogenetic trees were performed using MEGA 7 (Kumar et al. 2016). The Maximum Likelihood tree was adopted as statistical method and the parameters were set in MEGA 7 (Shi et al. 2016): Analysis: Phylogeny Reconstruction; Statistical method: Maximum Likelihood; Test of phylogeny: None; Substitutions type: Nucleotide; Model/Method: Tamura-Nei Model; Rates among sites: Gamma distributed with Invariant sites (G+I); No of Discrete Gamma Categories: 5; Gaps/Missing Data treatment; ML Heuristic Method: Nearest-Neighbor-Interchange (NNI); Initial Tree for ML: Make initial tree automatically (Default—NJ/BioNJ); Branch Swap Filter: Moderate; Number of threads: 1; Test of Phylogeny: None; No. of Bootstrap Replications: 500; Model/Method: General Time Reversible Model; Rates among Sites: Gamma distributed with invariant sites (G+1); Number of discrete gamma categories: 5; Gaps/Missing data treatment: use of all sites.
Maximum likelihood heuristic method: Subtree-Pruning-Regrafting-Extensive (SPR level 5); Initial tree for ML: Make initial tree automatically (Neighbor Joining); and Branch swap filter: Moderate.
The population structure and Q clusters were imported to MEGA 7 for combined analysis of genetic diversity while drawing the phylogenetic trees. For the sub-trees for each cluster (Q), the shape of “Node/Subtree Marker” and the “Branch Line” were colored similarly as the bar plots displayed by STUCTURE PLOTS.
Association analysis
Association analysis was conducted using TASSEL 5 (Bradbury et al. 2007) and R package (Liu et al. 2016). Six types of models were used. These models involved a single marker regression (SMR) for all SNPs without structure and kinship, a general linear model (GLM) using structure outputs (GLM_Q), and PCA (GLM_PCA), a mixed linear model (MLM) involving structure and Kinship (MLM_Q + K), PCA and Kinship (MLM_PCA + K), and a fixed and random model Circulating Probability Unification (FarmCPU) using R. PCA and Kinship were obtained from TASSEL 5, The LOD threshold for significant SNPs was between 2 and 3 (Lander and Botstein 1989).
Favorable allele, SNP selection accuracy, and SNP selection efficiency
For each significant SNP, number of genotype AA, CC, GG, TT, AC, AG, AT, CG, CT, and GT were calculated using VB code developed by Dr. Shi (University of Arkansas, Fayetteville) (unpublished). Percentage of homozygote genotype AA, CC, GG, TT, major allele frequency, minor allele frequency, heterozygosity percentage, and missing data were generated using VB code developed by Dr. Shi as well. Selection accuracy and selection efficiency SNP were calculated using the method described by Shi et al. (2016). Briefly, these methods were computed using the following formulas.
-
Selection accuracy (%) = 100 × (number of phosphorus-deficient tolerant or highly rock phosphate responsive cowpea accessions in the benefit SNP allele/(number of phosphorus-deficient tolerant or highly rock phosphate responsive cowpea accessions in the benefit SNP allele + number of phosphorus-deficient tolerant or highly rock phosphate responsive cowpea accession genotypes in the benefit SNP allele),
-
Selection efficiency (%) = 100 × (number of phosphorus-deficient tolerant or highly rock phosphate responsive cowpea accessions in the benefit SNP allele/total genotype number).
Cowpea accessions were divided into two groups depending upon the phosphorus stress and rock phosphate response scores. Accessions having a score equal to 1 for either trait were considered as favorable parents; whereas those with a score of 5 were considered as accessions contributing to negative alleles.
Results
Phenotyping for adaptation to low phosphorus conditions and rock phosphate response in cowpea
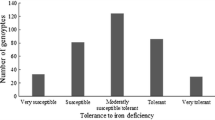
Cowpea response to phosphorus stress and addition of rock phosphate was assessed using a 1–5 scale. Adaptation to low phosphorus conditions scores varied from 1.0 to 5.0, with a mean of 3.5 and a standard deviation of 1.20. Distribution of adaptation to low phosphorus condition scores was skewed to 1 (Fig. 1). Seventeen cowpea accessions, PI148681, PI163448, PI175962, PI279845, PI293505, PI293573, PI307558, PI307561, PI339565, PI582343, PI582465, PI582469, PI582471, PI582670, PI582736, PI582923, and PI583209 had the lowest phosphorus stress score, indicating that the 17 accessions performed best under a soil phosphorus-deficient condition, thus highly tolerant to phosphorus stress. In addition, 19.9% of the accessions were scored 2, 23.8% with a score of 3, and 26.3% belonged to 4. The highest phosphorus stress score was recorded for over 25.0% of the cowpea genotypes evaluated for adaptation to low phosphorus condition (Table S1), suggesting that these genotypes were highly susceptible to phosphorus stress.
Rock phosphate response scores ranged between 1.0 and 5.0, with a mean of 3.4 and a standard deviation of 1.21. Distribution of rock phosphate response score was skewed to 1, which was similar to that of a tolerance to low phosphorus score (Fig. 1). Nineteen cowpea accessions, PI148681, PI293505, PI293573, PI307558, PI307561, PI339565, PI582343, PI582469, PI582923, PI583209, PI152196, PI170869, PI205141, PI292871, PI292891, PI307556, PI353289, PI582707, and PI583182 had the lower rock phosphate score (Table S1), suggesting that these 19 accessions were highly responsive to the addition of rock phosphate to the soil medium. Moreover, 19.3% of the accessions were responsive to the phosphorus application, 26.1% were moderately responsive, and 24.4% were poorly responsive. The results suggested that 24.9% of the cowpea accessions were scored 5, indicating that these accessions were least responsive to the addition of rock phosphate.
Correlation analysis was conducted between tolerance to low phosphorus conditions and rock phosphate response. The two traits were highly correlated (r = 0.75). Ten accessions, PI148681, PI293505, PI293573, PI307558, PI307561, PI339565, PI582343, PI582469, PI582923, and PI583209 were not only phosphorus-deficient tolerant but also highly responsive to phosphorus application; whereas, 62 cowpea accessions were highly susceptible to phosphorus deficiency and were least responsive to phosphate application (Table S1). Tolerance to low phosphorus conditions and rock phosphate response scores by country were calculated (Table 1) and the differences in tolerance to low phosphorus condition and rock phosphate response were also observed.
Genetic diversity and population structure
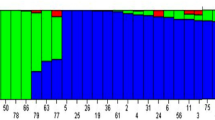
Population structure was inferred using STRUCTURE 2.3.4; optimal delta K was captured using Structure harvester. Including population structure into association analysis models reduces false positive. Delta K was optimal at K equal to 2, suggesting that there were two subpopulations in the cowpea accession panel (Fig. 2a). Subpopulation 1 (Q1) is shown in red whereas subpopulation 2 (Q2) is represented in green (Fig. 2b). Q1 made up 45.4% of the cowpea accessions tested for phosphorus stress and addition of rock phosphate. Q2 consisted of 43.4% of the cowpea population. Admixture, denoted as Q1Q2, was observed and made up 11.2% of total cowpea accessions.
Model-based populations of 357 cowpea accessions evaluated for adaptation to low phosphorus condition and rock phosphate response. a Delta K values obtained from Structure harvester for different numbers of populations assumed (K) in STRUCTURE analysis. b Classification of the 1357 cowpea accessions into two populations using STRUCTURE 2.3.4. The subgroup membership is presented on the y axis, and each accession on the x axis. Distribution of the 357 cowpea accessions is revealed by color coding (Q1 or cluster1: red, and Q2 or cluster2: green). c Phylogenetic tree of the 357 cowpea accessions drawn using MEGA 7 and using maximum likelihood (ML) as a statistical method. Q1 and Q2 are consistent between b and c. Admixture Q1Q2 is represented in blue. (Color figure online)
A phylogenetic tree involving 357 cowpea accessions was drawn using MEGA 7. The red circles show accessions belonging to Q1, the green squares represent Q2, and the blue triangles are the admixture Q1Q2 (Fig. 2c). Accessions belonging to either subpopulation were clustered in the phylogenetic tree. Such results indicated that the structure outcomes were consistent with that of MEGA 7 and confirmed there two well-clustered subpopulation existed in this association set.
Association mapping
Association mapping was conducted using TASSEL 5 and R package. We used six different models, SMR, GLM (Q), GLM (PCA), MLM (Q+K), MLM (PCA+K), and FarmCPU for association analysis study. LOD values associated with SNPs usually decreased from SMR to FarmCPU (Table 2). Ten SNP markers, C35006753_110, C35028233_482, C35072764_1384, C35084634_455, Scaffold21750_4938, Scaffold26894_5408, Scaffold41885_14420, Scaffold45170_4650, Scaffold50732_679, and Scaffold88448_741 were found to be highly associated with tolerance to low phosphorus conditions in cowpea. Adaptation to low phosphorus conditions SNP-associated LOD values varied from 2.63 to 6.89, 2.48 to 6.92, 1.56 to 6.19, 1.28 to 3.82, 1.30 to 3.64, and 1.14 to 5.44 using SMR, GLM (Q), GLM (PCA), MLM (Q+K), MLM (PCA+K), and FarmCPU, respectively (Table 2). R-square values ranged between 3.50–8.86%, 3.28–8.73%, 2.02–7.53%, 1.77–5.10%, and 1.81–4.88% using SMR, GLM (Q), GLM (PCA), MLM (Q + K), and MLM (PCA+K), respectively. Scaffold26894_5408 and Scaffold41885_14420 had an LOD value greater than 2.60 under the six models used in the current analysis (Table 2). These SNPs were consistent regardless of the models. Distribution of SNPs within the QQ plot confirmed the robustness of the SNPs (Fig. 3).
Eight SNP markers, C35028233_482, C35058535_121, Scaffold26894_5408, Scaffold45170_4650, Scaffold51609_507, Scaffold53730_7339, Scaffold74389_5733, and Scaffold87916_4921 were highly associated with rock phosphate response in cowpea. LOD values ranged between 2.17–5.60%, 1.58–3.55%, 1.88–4.40%, 2.23–5.52%, 1.25–3.27%, and 1.32–5.99% under SMR, GLM (Q), GLM (PCA), MLM (Q+K), MLM (PCA+K), and FarmCPU, respectively (Table 2). Overall, LOD values decreased from SMR to FarmCPU, which was similar to that found in tolerance to low phosphorus condition. R square values varied from 3.00 to 7.12, 2.12 to 4.86, 2.36 to 5.41, 3.10 to 7.04, and 1.68 to 4.52% using SMR, GLM (Q), GLM (PCA), MLM (Q+K), and MLM (PCA+K), respectively (Table 2). Scaffold74389_5733 had an LOD greater than 2.99, irrespective of the models involved in the association analysis study. Distribution patterns of the SNP markers used for rock phosphate response within the QQ plot were almost similar to that of adaptation to low phosphorus condition (Fig. 3).
SNP markers C35028233_482, Scaffold26894_5408, and Scaffold45170_4650 were overlapping both tolerance to low phosphorus conditions and rock phosphate response, which is consistent with the fact that these two traits were highly correlated (r = 0.75). These SNP markers showed consistent LOD values across the six models. However, these aforementioned SNP markers contributed to only 2.29–8.31% of the variation in the phenotypes of interest (Table 2).
Major allele frequency, minor allele frequency, selection accuracy, and selection efficiency were calculated for significant SNPs associated with phosphorus-deficient stress and application with rock phosphate in cowpea. For all SNPs, major allele frequency varied from 53.3 to 90.5%; minor allele frequency ranged between 3.3 and 39.9%. Regarding the SNP markers associated with low phosphorus efficiency in cowpea, selection accuracy varied from 47.1 to 88.2%, with a mean of 64.7% and a standard deviation of 17.8%; selection efficiency ranged between 12.6 and 91.6%, with a mean of 65.4% and a standard deviation of 24.71% (Table 3). With respect to the SNP markers found associated with rock phosphate response, selection accuracy ranged between 52.9 and 100%, with a mean of 68.4% and a standard deviation of 19.1%; selection efficiency ranged between 67.8 and 88.5%, with a mean of 80.0% and a standard deviation of 6.11% (Table 3).
Discussion
Large genetic variability in a germplasm collection offers plant breeders valuable alternatives to address both biotic and abiotic-related issues. In regard to soil phosphorus stress in cowpea, important variability in adaptation to low phosphorus condition and rock phosphate response was found among the 357 cowpea accessions involved in this study (Mahamane et al. 2008). Abaidoo et al. (2017) reported significant differences in phosphorus utilization index in a total of 175 cowpea genotypes as well. Fita et al. (2011) reported substantial variability in phosphorus uptake and use between forty Cucumis melo L. accessions.
We reported ten SNP markers, C35006753_110, C35028233_482, C35072764_1384, C35084634_455, Scaffold21750_4938, Scaffold26894_5408, Scaffold41885_14420, Scaffold45170_4650, Scaffold50732_679, and Scaffold88448_741 to be associated with tolerance to low phosphorus condition in cowpea. In addition, eight SNP markers C35028233_482, C35058535_121, Scaffold26894_5408, Scaffold45170_4650, Scaffold51609_507, Scaffold53730_7339, Scaffold74389_5733, and Scaffold87916_4921 were found to be associated with rock phosphate response in cowpea. Overlapping SNPs were found for both tolerance to low phosphorus condition and rock phosphate response, which was confirmed by the high correlation between the two traits. SNP markers with minor effects from this study suggested there were QTLs controlling low phosphorus tolerance in cowpea, which were consistent with the findings from the report by Rothe (2014), where four QTLs, QAB.tam-3Chr.1, QAB.tam-3Chr2, QLP.tam-3Chr, and QPSI.tam-3chr were identified to be associated with low phosphorus conditions in cowpea and the R-square values associated with these aforementioned QTLs varied from 6.4 to 14.5%. Yan et al. (2004) reported two QTLs, Pup4.1, and Pup10.1 in common bean associated with phosphorus uptake in a low phosphorus field. The two QTLs accounted for 14% of the variation in phosphorus uptake in common bean. Beebe et al. (2006) reported a total of 26 QTLs associated with phosphorus acquisition in a common bean population involving 86 F5:7 recombinant inbred lines, which were derived from a cross between G19833 and DOR 364.
Marker-assisted selection has proven to be effective across cultivar improvement and breeding programs (Moose and Mumm 2008; Collard and Mackill 2008; Xu and Crouch 2008). The use of SNP markers showed promising results in studies pertaining to traits of agronomic interest in cowpea (Shi et al. 2016; Qin et al. 2016; Xiong et al. 2016). This current study provides potential SNP markers which can be used in a breeding program aiming at improving cowpea tolerance to soil phosphorus deficiency. These SNP markers will permit rapid screening of cowpea genotypes which are tolerant to phosphorus stress through marker-assisted selection. In addition, the outcomes from this investigation will provide a substantial contribution to the genetic architecture underlying cowpea tolerance to a low phosphorus environment, since studies in regard to the genetics of phosphorus stress in legumes remain scarce.
Conclusion
A cowpea panel consisting of 357 accessions was evaluated for tolerance to low phosphorus conditions and rock phosphate response in this study. Substantial variabilities in both traits was found and will be of interest in breeding programs. Ten SNP markers, C35006753_110, C35028233_482, C35072764_1384, C35084634_455, Scaffold21750_4938, Scaffold26894_5408, Scaffold41885_14420, Scaffold45170_4650, Scaffold50732_679, and Scaffold88448_741 were found to be associated with adaptation to low phosphorus conditions in cowpea. In addition, eight SNP markers, C35028233_482, C35058535_121, Scaffold26894_5408, Scaffold45170_4650, Scaffold51609_507, Scaffold53730_7339, Scaffold74389_5733, and Scaffold87916_4921 were highly associated with rock phosphate response. These SNP markers can be used as a tool in a breeding program to enhance tolerance of cowpea to soil phosphorus deficiency. To our knowledge, this current study is one of the first investigations reporting SNP markers associated with low phosphate stress tolerance in cowpea.
References
Abaidoo R, Dare MO, Killani S (2017) Evaluation of early maturing cowpea (Vigna unguiculata) germplasm for variation in phosphorus use efficiency and biological nitrogen fixation potential. J Agric Sci 155(1):102–116
Agbicodo EM, Fatokun CA, Bandyopadhyay R, Wydra K, Diop NN, Muchero W, Ehlers JD, Roberts PA, Close TJ, Visser RGF, Van der Linden (2010) Identification of markers associated with bacterial blight resistance loci in cowpea [Vign aunguiculata (L.) Walp]. Euphytica 175:215–226
Araújo AP, Antunes IF, Teixeira MG (2005) Inheritance of root traits and phosphorus uptake in common bean (Phaseolus vulgaris L.) under limited soil phosphorus supply. Euphytica 145(1–2):33–40
Badiane FA, Diouf M, Diouf D (2014) Cowpea in broadening the genetic base of grain legumes. Springer, India, pp 95–114
Bastien M, Sonah H, Belzile F (2014) Genome wide association mapping of resistance in soybean with a genotyping-by-sequencing approach. Plant Genome. doi:10.3835/plantgenome2013.10.0030
Beebe SE, Rojas-Pierce M, Yan X, Matthew WB, Pedraza F, Muñoz F, Tohme J, Jonathan PL (2006) Quantitative trait loci for root architecture traits correlated with phosphorus acquisition in common bean. Crop Sci 46(1):413–423
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23(19):2633–2635
Caygill JC, Janis AJ, Carol EMF (1981) Imitation milks from Cicer arietinum (L.), Vigna unguiculata (L.) Walpers and Vigna radiata (L.) Wilczek and other legumes. J Sci Food Agric 32(6):601–607
Collard BCY, Mackill DJ (2008) Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Biol Sci 363(1491):557–572
Coltman RGG, Gabelman W (1982) Intraspecific variation in growth, phosphorus acquisition and phosphorus utilization in tomatoes under phosphorus-deficiency stress. In: Proceedings of the Ninth International Plant Nutrition Colloquium, Warwick University, England
Earl DA, VonHoldt BM (2011) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4(2):359–361
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6(5):e19379
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14(8):2611–2620
Fawole I, Gabelman WH, Gerloff GC (1982) Heritability of efficiency in phosphorus utilization in beans (Phaseolus vulgaris L.) grown under phosphorus stress. J Am Soc Hortic Sci 107:94–97
Fita A, Nuez F, Picó B (2011) Diversity in root architecture and response to P deficiency in seedlings of Cucumis melo L. Euphytica 181(3):323–339
Ghaderi A, Lower RL (1979) Gene effects of some vegetative characters of cucumber. J Am Soc Hortic Sci 104:141–144
Hall AE, Cisse N, Thiaw S, Elawad HO, Ehlers JD, Ismail AM, Fery RL, Roberts PA, Kitch LW, Murdock LL, Boukar O (2003) Development of cowpea cultivars and germplasm by the bean/cowpea CRSP. Field Crops Res 82(2):103–134
Hammond JP, Broadley MR, White PJ, King GJ, Bowen HC, Hayden R, Meacham MC, Mead A, Overs T, Spracklen WP, Greenwood DJ (2009) Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. J Exp Bot 60(7):1953–1968
Hayman DS (1975) Phosphorus cycling by soil microorganisms and plant roots. In: Walker N (ed) Soil microbiology. Butterworths, London, pp 67–92
Jones C, Hatier JH, Cao M, Fraser K, Rasmussen S (2015) Metabolomics of plant phosphorus-starvation response. Annu Plant Rev 48:215–236
Kisha TJ, Sneller CH, Diers BW (1997) Relationship between genetic distance among parents and genetic variance in populations of soybean. Crop Sci 37(4):1317–1325
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Lander ES, Botstein D (1989) Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121(1):185–199
Li YD, Wang YJ, Tong YP, Gao JG, Zhang JS, Chen SY (2005) QTL mapping of phosphorus deficiency tolerance in soybean (Glycine max L. Merr.). Euphytica 142(1–2):137–142
Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J (2009) SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics 25(15):1966–1967
Liang Q, Cheng X, Mei M, Yan X, Liao H (2010) QTL analysis of root traits as related to phosphorus efficiency in soybean. Ann Bot 106(1):223–234
Liu X, Huang M, Fan B, Buckler ES, Zhang Z (2016) Iterative usage of fixed and random effect models for powerful and efficient genome-wide association studies. PLoS Genet 12(2):e1005767
Mahamane S, Payne WA, Loeppert RH, Miller JC Jr, Reed DW (2008) Evaluation of cowpea genotypes for adaptation to low soil P conditions and rock phosphate application. HortScience 43(3):618–619
Mc Vickar MH, Bridger GL, Nelson LB (1963) Advances in phosphate fertilizers. Soil Sci Soc Am 17:155–187
Moose SP, Mumm RH (2008) Molecular plant breeding as the foundation for 21st century crop improvement. Plant Physiol 147(3):969–977
Ochoa IE, Blair MW, Lynch JP (2006) QTL analysis of adventitious root formation in common bean under contrasting phosphorus availability. Crop Sci 46(4):1609–1621
Olufajo OO (2012) Agronomic performance of improved cowpea varieties under natural infestation with Alectra vogelii (Benth.) in the northern Guinea savannah of Nigeria. Agric Trop Subtrop 45(2):66–71
Parentoni SN, de Souza Jr CL, de Carvalho Alves VM, Gama EE, Coelho AM, De Oliveira AC, Guimarães PE, Guimarães CT, Vasconcelos MJ, Patto Pacheco CA, Meirelles WF (2010) Inheritance and breeding strategies for phosphorous efficiency in tropical maize (Zea mays L.). Maydica 55(1):1–15
Payne WA, Wendt CW, Lascano RJ (1990) Root zone water balances of three low-input millet fields in Niger. West Afr Agron J 82(4):813–819
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155(2):945–959
Qin J, Shi A, Xiong H, Mou B, Motes D, Lu W, Miller JC Jr, Scheuring DC, Nzaramba MN, Weng Y, Yang W (2016) Population structure analysis and association mapping of seed antioxidant content in USDA Cowpea (Vigna unguiculata L. Walp.) core collection using SNPs. Can J Plant Sci 96(6):1026–1036
Ramasamy RK, Ramasamy S, Bindroo BB, Naik VG (2014) STRUCTURE PLOT: a program for drawing elegant STRUCTURE bar plots in user friendly interface. SpringerPlus 3(1):431
Rothe JC (2014) Breeding for tolerance of cowpea to low phosphorus soil conditions through physiological and genetic studies. Ph.D. Dissertation, Texas A&M University
Shi A, Buckley B, Mou B, Motes D, Morris JB, Ma J, Xiong H, Qin J, Yang W, Chitwood J, Weng Y (2016) Association analysis of cowpea bacterial blight resistance in USDA cowpea germplasm. Euphytica 208(1):143–155
Thakur D, Kaushal R, Shyam V (2014) Phosphate solubilising microorganisms: role in phosphorus nutrition of crop plants-A review. Agric Rev 35(3):159–171
Varshney RK, Nayak SN, May GD, Jackson SA (2009) Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol 27(9):522–530
Veneklaas EJ, Lambers H, Bragg J, Finnegan PM, Lovelock CE, Plaxton WC, Price CA, Scheible WR, Shane MW, White PJ, Raven JA (2012) Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol 195(2):306–320
Verdcourt B (1970) Studies in the Leguminosae-Papilionoïdeae for the flora of tropical East Africa. Kew Bull 24(3):507–569
Wang L, Butterly CR, Yang XL, Wang Y, Herath HM, Jiang X (2012) Use of crop residues with alkaline slag to ameliorate soil acidity in an ultisol. Soil Use Manag 28(2):148–156
Wissuwa M, Wegner J, Ae N, Yano M (2002) Substitution mapping of Pup1: a major QTL increasing phosphorus uptake of rice from a phosphorus-deficient soil. Theor Appl Genet 105(6–7):890–897
Xiong H, Shi A, Mou B, Qin J, Motes D, Lu W, Ma J, Weng Y, Yang W, Wu D (2016) Genetic diversity and population structure of cowpea (Vigna Unguiculata L. Walp). PLoS ONE 11(8):e0160941
Xu Y, Crouch JH (2008) Marker-assisted selection in plant breeding: from publications to practice. Crop Sci 48(2):391–407
Yan X, Liao H, Beebe SE, Blair MW, Lynch JP (2004) QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant Soil 265(1–2):17–29
Zhang D, Zhang H, Chu S, Li H, Chi Y, Triebwasser-Freese D, Lv H, Yu D (2017) Integrating QTL mapping and transcriptomics identifies candidate genes underlying QTLs associated with soybean tolerance to low-phosphorus stress. Plant Mol Biol 93(1–2):137–150
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ravelombola, W., Qin, J., Shi, A. et al. Association mapping revealed SNP markers for adaptation to low phosphorus conditions and rock phosphate response in USDA cowpea (Vigna unguiculata (L.) Walp.) germplasm. Euphytica 213, 183 (2017). https://doi.org/10.1007/s10681-017-1971-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-017-1971-8