Abstract
Seven clones of colchicine-induced tetraploid purple coneflower (Echinacea purpurea L.) plants were grown on a farm for comparison with their corresponding original diploid plants. In the first year of cultivation, the plant height, number of capitula, and inflorescence (branched flower stalk) were significantly reduced in all of the tetraploid plants, while the length-to-width leaf ratio, pollen size, and root thickness were significantly increased. Although the tetraploid plants had larger seeds, most of the seeds of tetraploid plants were not fully filled and the naked seeds (i.e., seeds observed without the outer seed coat) were noticeably smaller than those of the diploid plants. In the second year of cultivation, the phenological stages were markedly delayed in the tetraploid plants; the number of capitula increased much more in tetraploid plants than in diploid plants. Among the seven tetraploid clones, two yielded significantly higher plant biomass and higher cichoric acid content per gram dry weight as well as per plant. The results of the present experiments indicate that some effects of tetraploidization of diploid E. purpurea are genotype-dependent, suggesting that the tetraploidization of more genotypes of the diploid is needed to breed better tetraploid varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Purple coneflower (Echinacea purpurea L.) is one of the most popular medicinal plants worldwide, and it is commonly used for treating respiratory and urinary diseases (Barrett 2003). Artificial polyploidization is an effective strategy for modifying various traits in various crops such as leaf, flower, and plant body size, chlorophyll content index, heat tolerance, and secondary metabolite accumulation (Luckett 1989; Dhawan and Lavania 1996; Majdi et al. 2010; Zhang et al. 2010; Lavania et al. 2012).
One year after our first report in 2009 on tetraploid E. purpurea induced by treating in vitro cultured petiole explants with colchicine (Nilanthi et al. 2009), Koul et al. (2010) also obtained tetraploid E. purpurea plants by treating seeds with colchicine. Very recently, Abdoli et al. (2013) obtained tetraploid E. purpurea plants by treating seedling root tips with colchicine.
Subsequently, two comparative studies on diploid and tetraploid E. purpurea plants have been reported (Abdoli et al. 2013; Xu et al. 2014) using materials originating from different seeds within a seed lot. Because E. purpurea is an entomophilous plant and, therefore, heterozygous (Sejdler and Dabrowska 1998; Stephens 2008), plants originating from different seeds are of different genotypes and might perform very differently (Chen et al. 2013a, b; Li et al. 2013). To more precisely evaluate the effects of duplication of the chromosome number in E. purpurea without the influence of the different genotypes, we cultivated seven diploid E. purpurea genotypes with their corresponding colchicine-induced tetraploids and compared several important traits among them. The details of the results are described in this paper.
Materials and methods
Diploid plant cloning
Seeds of purple coneflower (Echinacea purpurea L.) were surface-sterilized by immersion in 70 % ethanol for 1 min and soaking in 1 % sodium hypochlorite solution containing one drop of Tween-20 per 50 mL for 10 min. The surface-sterilized seeds were germinated on 0.5 % agar-gelled MS medium (Murashige and Skoog 1962) containing 2 % sucrose in a culture room with a temperature range of 25–27 °C under darkness for 1 week and then under fluorescent light (approximately 40 μmoL m−2 s−1) with a 12-h photoperiod. One month later, the seedlings were transferred to fresh MS medium containing 3 % sucrose and 0.3 mg/L benzyladenine (BA) to stimulate axillary bud proliferation. Genotypes that did not grow well on the medium or had evident symptoms of hyperhydricity were discarded. Shoots produced from the axillary buds were isolated and subcultured again in fresh MS medium. When enough shoots grew from one genotype, the shoots were transferred to plain MS medium with only 3 % sucrose for rooting.
Doubling chromosome number of the diploid plants and cloning the resulting tetraploid plants
Petioles were isolated from in vitro plantlets of each diploid clone and then cut into small segments (0.7–10 mm) and used as explants. These explants were cultured on agar-gelled MS medium containing 0.3 mg/L BA and 120 mg/L colchicine to induce chromosome doubling. All of the methods used to prepare the explants, double the chromosomes, and identify ploidy status were as described previously (Nilanthi et al. 2009). After treatment, the tetraploid shoots were isolated and cultured on agar-gelled MS medium containing 3 % sucrose, 0.01 mg/L NAA, and 0.5 mg/L BA (Chen et al. 2012) where they developed axillary shoots. These axillary shoots could be isolated and cultured repeatedly to yield more axillary shoots. When enough shoots were achieved, the shoots were rooted on agar-gelled MS medium containing 3 % sucrose and 0.01 mg/L NAA to become intact plantlets. After the reconfirmation of the tetraploid status, the cloned tetraploid plantlets were used in the present experiments for comparison with their corresponding original diploids.
Cultivation of cloned diploid and tetraploid plants
At the end of January 2012, plantlets of seven clones of diploids and their corresponding tetraploids grown in vitro were removed from the culture bottles. After hardening, these plantlets were randomly cultivated in early March at a farm situated in a field on a gentle slope (24°28′N, 108°21′E; ~120 m above sea level; 20.1 °C average temperature). For each clone, at least 18 healthy plantlets were cultivated. Throughout the experimental period, all of the plants were tended evenly using conventional methods.
Observation of pollen grains
Pollen grains were collected from blooming flowers on glass slides with a small amount of pure water and covered with a glass cover slip before being observed under a microscope.
Determination of phenological states
Phenological states of both the diploid and the corresponding tetraploid were determined using clone 4 as a representative. Observations were conducted every 3 or 4 days. In the second year, 20 diploid and tetraploid plants each were observed during the cultivation period. A “resuming growth state” was recorded when at least two plants in the group resumed growth after winter dormancy. A “squaring state” was noted when at least two plants developed flower buds. An “early flowering state” was recorded when at least two plants bloomed. A “blooming state” was noted when at least 10 plants bloomed. A “fruiting state” was recorded when ray flowers on at least two plants dropped from the capitula. A “fruit maturing state” was noted when the capitula on at least two plants became dehydrated. A “dormant state” was noted when the above ground parts of at least 10 plants became dry.
Data collection and analysis
Leaf data were recorded in the middle of June in the first year, while data for the ray flowers of the capitula were recorded in the middle of August in the first and second years. For each plant, the length and width of the three largest leaves were measured; the number of ray flowers were counted on three blooming capitula, and the length and width of the ray flowers were measured. Plant height, number of capitula, and inflorescences (branched flower stalks) per plant were recorded when the plants were harvested to obtain above and below ground dry weight (DW). The diameter of each infructescence was measured upon maturity. Root data were recorded soon after harvest. Seed data were obtained when infructescence became dry. The cichoric acid concentration (mg g−1, DW) was determined on whole plants as described by Xu et al. (2014). All of the data were analyzed statistically using the Student Newman-Keuls means separation test (SAS software, SAS Institute, Cary, NC, USA; 1995). Significant differences among means were determined using Duncan’s multiple range tests. Values of P ≤ 0.05 were considered significant.
Results
Comparison of seven diploid plant clones with their corresponding tetraploid plants in the first year of cultivation
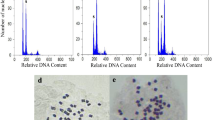
Clear differences in the general appearance of the plants were observed among the clones of the same ploidy level and between each pair of corresponding diploid and tetraploid clones (Fig. 1), and significant differences in plant height and root thickness between each pair of diploid and tetraploid plants were calculated for all seven pairs of clones with an average decrease in 33.2 % in plant height and an average increase of 78.1 % in root thickness for the tetraploid plants (Table 1). Leaves were longer in diploid plants than in tetraploid plants, but differences in leaf length were not statistically significant. Leaves were wider in more tetraploid plants than diploid plants, but the difference was not as large as that for leaf length. Significant differences among diploid and tetraploid plants from the same clone were also observed in the generative parts. All diploid clones had a greater number of capitula than their corresponding tetraploid plants. The tetraploid plants had 34.9 % as many capitula, with the most significant decrease in clone 6, which had only 18.8 % as many capitula as its diploid complement (Table 2). For ray flowers, although not all of the plants of each diploid clone had more ray flowers per capitulum, all of the diploid clones had longer ray flowers than their corresponding tetraploids. The diameter of the infructescence was smaller in the tetraploid clones (Table 3). In addition, intact seeds were larger in the tetraploid clones (Fig. 2), whereas naked seeds (without outer seed coats) were significantly smaller in the tetraploid clones (Table 3; Fig. 3). Furthermore, pollen grains from the tetraploid plants appeared morphologically normal under a microscope; however, they were obviously larger and had rougher surfaces than their diploid counterparts (Fig. 4). Differences in biomass yield and cichoric acid content per gram of DW between diploids and their corresponding tetraploids were not always statistically significant. The biomass yield from the above ground part of the plants tended to be higher in diploid plants, whereas the biomass yield from the below ground part was higher in tetraploid plants, as was the cichoric acid content per gram DW, although the difference was not large (Table 4). Notably, tetraploid clone 5 had the highest yield (33.9 % higher than average) from the below ground part of the plant and the highest cichoric acid content per gram DW (60.3 % higher than average).
Comparison of general appearance between each pair of clones. First row from left to right Diploid clones 1, 2, 3, and 4. Second row from left to right Tetraploid clones 1, 2, 3, and 4. Third row from left to right Diploid clones 5, 6, and 7. Fourth row from left to right tetraploid clones 5, 6, and 7
Comparison of seven clones of diploid plants with their corresponding tetraploid plants in the second year of cultivation
In the second year, the plant heights for both diploid and tetraploid clones increased and the diploid plants were still significantly taller than their tetraploid counterparts in most of the clones (except for clone 5, which was taller but not significantly). Most of the tetraploid plants (not clones 6 and 7) had significantly more basal shoots than the corresponding diploid plants; the average increase in the number of basal shoots was 95.1 %. Clone 5 exhibited the largest increase (276 %; Table 5). The number of inflorescences per plant was much higher in most of the tetraploid clones in the second year with an average increase of 135.9 %. Only four tetraploid clones had a higher number of capitula than their corresponding diploid clones (Table 6), although the tetraploid plants tended to have more capitula. In the second year of cultivation, the biomass yield from the above ground part of the plants increased by approximately threefold for both the diploid and tetraploid plants, yet most of the diploid clones had higher biomass yields than the tetraploids (Table 7). Compared with the increase in biomass yield from the above ground part of the plants, the increase in the biomass yield from the below ground part of the plants for both the diploid and tetraploid clones was smaller. For cichoric acid concentration, an increase of ~ 50 % was observed for both the diploid and tetraploid clones on average; the tetraploid plants had slightly higher cichoric acid content per gram DW, with the tetraploid clone 4 having the highest cichoric acid content per gram DW (13.6 mg/g).
Comparison of phenology and plant styling between a diploid clone and its counterpart tetraploid clone
Obvious differences in phenological phenomena were observed between diploid and tetraploid clone plants, with the diploid clones reaching all measured growth and development stages much earlier (Table 8; Fig. 5). Plain differences in plant styling were also observed, with the tetraploid plants being more compact and steady, especially at the blooming stage (Fig. 6).
Discussion and conclusion
Although quite a number of polyploid plants have been produced with advantages over diploid plants such as increases in the yield and improvement of fiber quality in cotton (Luckett 1989), enhanced heat tolerance in Dioscorea (Zhang et al. 2010), and the accumulation of more secondary metabolites in a range of plant species (Dhawan and Lavania 1996; Majdi et al. 2010; Lavania et al. 2012) including E. purpurea (Abdoli et al. 2013; Xu et al. 2014), polyploidization may for many reasons bring also disadvantages to plants as reviewed by Comai (2005) and Yoo et al. (2014a, b). In investigating diploid-autotetraploid paired sets of eight diverse clones of six species of aromatic grasses, Lavania et al. (2012) found that polyploidization differentially influences body size in plants, some species become larger but some become smaller. Similarly, the results of the present study demonstrate that the effects of tetraploidization are highly genotype dependent, which has the advantages of certain traits such as biomass yield and cichoric acid content for only some of the genotypes. These facts indicate that the validity of evaluation of the effects of polydization should be carefully restricted to only the genotypes investigated, and in order to precisely evaluate the effects of tetraploidization of the diploid E. purpurea, the comparison should be limited to the induced tetraploid and its original diploid genotype.
Colchicine is one of the most commonly used chemicals for inducing chromosome doubling in plants (Hansen and Andersen 1996; Ade and Rai 2010). To our knowledge, colchicine is the only chemical used in the induction of chromosome doubling leading to the formation of tetraploid plants (Nilanthi et al. 2009; Koul et al. 2010; Abdoli et al. 2013) in E. purpurea. We were the first to report successful induction of chromosome doubling in E. purpurea (Nilanthi et al. 2009) using in vitro culturing and petioles dissected from in vitro grown plantlets as an explant source. In the experiments, the best result of 23.5 % tetraploid among all the regenerated shoots was obtained when the explants were treated with 120 mg/L colchicine for 28 days; a lower concentration and/or shorter treating duration resulted in the yield of more ploidy chimera (mixoploid). The other two cases reported by different research groups in E. purpurea chromosome doubling were achieved by conventional in vivo methods. Koul et al. (2010) treated 1200 E. purpurea seeds with 0.01 % colchicine for 24 h and obtained one tetraploid and 17 mixoploid among 210 seedlings recovered from the treated seeds. Abdoli et al. (2013) treated the root tips of two true leaf seedlings with 0.25 % colchicine for 24, 48, and 72 h and obtained a 4–5 % tetraploid induction rate in the experiments. From the results above, it is clear that treating the root tips of two true leaf seedlings in vivo was relatively simple and most efficient.
Cloned tetraploid plants used in the present experiments were obtained following the method described in our previous paper (Nilanthi et al. 2009). Although it is not difficult to induce chromosome doubling in E. purpurea in vitro or in vivo, all cases of E. purpurea treated with colchicine resulted in only the formation of autotetraploid because of their autodiploid origin. Unlike allotetraploids, which can set seeds quite normally, autotetraploids with four sets of chromosomes originating from the same species generally have difficulty mating with sister chromosomes during meiosis by the base complementarity method. Because of this, autotetraploids tend to form aneuploid gametes (Doyle 1986; Joppa and Williams 1988). In the present experiments, the lower seed-setting rate for the tetraploid plants might be well explained by this autopolyploid method of producing aneuploid gametes.
It was observed that in the first year of cultivation, the numbers of capitula and inflorescences (Table 2) were reduced significantly in tetraploid plants of most of the clones, while in the second year of cultivation, tetraploid plants had greater numbers of basal shoots (Table 5) and inflorescences (Table 6) per tetraploid plant and largely delayed flowering time (Table 8). These obvious differences between diploids and tetraploids might be accounted for by the sophisticated non-additive modes of gene expression or regulation (Kim and Chen 2011; Yoo et al. 2014a, b), novel gene expression (Osborn et al. 2003; Adams and Wendel 2005), and greater gene expression (Udall and Wendel 2006; Chen and Ni 2006) in the tetraploid plants. However, the carryover effects of plant growth regulators (BA and NAA) that accumulated in the plants during the cloning procedure might not be completely excluded.
Although significant variations of quite a few traits were detected in our present experiments among diploid and tetraploid clones, some common features of tetraploids were confirmed, such as thicker roots (Xu et al. 2014), larger seeds, and pollen grains (Abdoli et al. 2013). After removing the outer seed coats, we found that the naked seeds were smaller for the tetraploid clones (Fig. 3), while by comparing the pollen grain sizes, we found that the difference between diploid and tetraploid was reasonable, i.e. about twice as large for the tetraploid pollen grains (Fig. 4), not very large as the case reported by Abdoli et al. (2013), who showed a tetraploid pollen grain almost 10 times as large as a diploid pollen grain (Fig. 3 in Abdoli et al. 2013). Furthermore, we found that the infructescence size was smaller in mature tetraploids (Table 2), which may be attributable to the much lower seed-setting rate in the tetraploid plants (Table 3).
Biomass yield and cichoric acid concentration are two most important parameters for purple coneflower production. Although tetraploidization can significantly increase the below ground plant part biomass yield in both the first and the second year, it did not seem to noticeably change the cichoric acid concentration but largely decreased the above ground plant biomass yield (Tables 4, 7). These results, together with the significant variation in most of the traits tested among diploid clones and tetraploid clones shown in Tables 1 to 6, clearly demonstrated that tetraploidization might not be a valid strategy for improving the purple coneflower production as suggested by Xu et al. (2014) and Abdoli et al. (2013) if the genotypes for tetraploidization were not properly chosen.
The smaller size of the naked seeds revealed the poor embryonic development after fertilization in these plants and might partially be accounted since the autotetraploid zygotes were formed between aneuploid gametes or one of them was aneuploid. E. purpurea has been confirmed to be self-sterile and entomophilous (Sejdler and Dabrowska 1998; Stephens 2008), whereas in the cultivation experiments, we did not pollinate the flowers of either diploid or tetraploid plants, plainly seeds obtained in the present experiments were set after random pollination by insects. In a somewhat similar study of watermelon by Muhammad et al. (2005), larger seeds with and without the outer seed coat were observed in tetraploid watermelon fruits, demonstrating the smooth embryonic development. Obviously, this result was different for E. purpurea, suggesting that the mating of sister chromosomes in tetraploid watermelon was regulated by genes such as those in polyploid sugarcane (Jannoo et al. 2004) and many other plant species (Cifuentes et al. 2010) rather than complementary base pairing. In addition, in watermelon, the seed coat of the tetraploid seeds was thicker, which might have been the main factor causing the lower germination rate in tetraploid seeds (Grange et al. 2003).
Lower germination rates for seeds collected from tetraploid plants were observed in our recent experiments as well (data not shown), but we doubt that the thicker seed coat was the main factor. A poor-quality or disordered genome resulting from the combination of aneuploid gametes in the cells of the embryos may be a more likely cause. Although a thicker seed coat and smaller seed size could underscore the poor seed quality, seeds collected from tetraploid plants might not have exactly three sets of chromosomes when pollinated with monoploid pollens from diploid plants, or they might not have exactly four sets of chromosomes when pollinated with diploid pollens from tetraploid plants. Abnormal chromosomal behavior has been detected during the meiosis of the pollen mother cells in tetraploid plants, and the segregation of these chromosomes might be uneven (Chen et al. 2013a, b).
With the exceptions mentioned above, we also found that the phenological states were delayed largely in the second year in tetraploid plants, which may have allowed the tetraploid plants to grow for a longer period of time during the year (Table 8), contributing to the increase of the total biomass yield and the accumulation of functional secondary metabolites. In addition, more compact plant styling for tetraploid plants (Fig. 6) could have horticultural value when E. purpurea is cultivated as an ornamental plant (Ault 2007).
In conclusion, although two closely related reports have compared diploid and tetraploid clones of E. purpurea (Xu et al. 2014; Abdoli et al. 2013), this is the first report of results from 2 years of cultivation and multiple genotypes. Because the effects of tetraploidization are highly genotype-dependent for E. purpurea, the production of more tetraploid genotypes is necessary for the selection and breeding of better varieties for large-scale cultivation.
References
Abdoli M, Moieni A, Badi HN (2013) Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol Plant 35:2075–2083
Adams KL, Wendel JF (2005) Novel patterns of gene expression in polyploid plants. Trends Genet 21:539–543
Ade R, Rai MK (2010) Review: colchicine, current advances and future prospects. Bioscience 2:90–96
Ault JR (2007) Coneflower, Echinacea species. In: Anderson NO (ed) Flower breeding and genetics: issues, challenges and opportunities for the 21 century. Springer, Dordrecht, pp 801–824
Barrett B (2003) Medicinal properties of Echinacea: a critical review. Phytomedicine 10:66–86
Chen ZJ, Ni Z (2006) Mechanisms of genomic rearrangements and gene expression changes in plant polyploids. BioEssays 28:240–252
Chen R, Chen XL, Li QL, Yang YS, Wu H (2012) Micropropagation by repeatedly inducing axillary bud formation of different gene dosage purple coneflower plants. In: Proceedings of the international conference on biomedical engineering and biotechnology, Macao, pp 1056–1059. May 28–30 2012
Chen R, Jin YH, Li QL, Chen XL, Yang YS, Wu H (2013) Some effective methods for dealing with the problem of hyperhydricity in cloning purple coneflower. In: 2013 International conference on biological, medical and chemical engineering (BMCE2013), Dec. 1-2, 2013, Hong Kong, pp 319–325
Chen XL, Chen R, Li QL, Yang YS, Wu H (2013) Cytological comparison of diploid, triploid, tetraploid and hexaploid in purple coneflower (Echinacea purpurea L.). 2013 International conference on biological, medical and chemical engineering (BMCE2013), Dec. 1-2, 2013, Hong Kong, pp 212–220
Cifuentes M, Grandont L, Moore G, Chèvre AM, Jenczewski E (2010) Genetic regulation of meiosis in polyploid species: new insights into an old question. New Phyto 186:29–36
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev 6:836–846
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87:81–89
Doyle GG (1986) Aneuploidy and inbreeding depression in random mating and self-fertilizing autotetraploid populations. Theor Appl Genet 72:799–806
Grange S, Leskovar DI, Pike LM, Cobb BG (2003) Seed coat structure and oxygen-enhanced environments affect germination of triploid watermelon. J Am Soc Hortic Sci 128:253–259
Hansen NJP, Andersen SB (1996) In vitro chromosome doubling potential of colchicine, oryzalin, trifluralin, and APM in Brassica napus microspore culture. Euphytica 88:159–164
Jannoo N, Grivet L, David J, D’Hont A, Glaszmann JC (2004) Differential chromosome pairing affinities at meiosis in polyploid sugarcane revealed by molecular markers. Heredity (Edinb) 93:460–467
Joppa LR, Williams ND (1988) Langdon durum disomic substitution lines and aneuploid analysis in tetraploid wheat. Genome 30:222–228
Kim ED, Chen ZJ (2011) Unstable transcripts in Arabidopsis allotetraploids are associated with nonadditive gene expression in response to abiotic and biotic stresses. PLoS One 6(8):e24251
Koul S, Sambyal M, Kitchlu SK, Bakshi SK, Kaul MK (2010) Development, micropropagation and characterization of colchiploid of Echinacea purpurea (L.) Moench. Indian J Biotechnol 9:221–224
Lavania UC, Srivastava S, Lavania S, Basu S, Misra NK, Mukai Y (2012) Autopolyploidy differentially influences body size in plants, but facilitates enhanced accumulation of secondary metabolites, causing increased cytosine methylation. Plant J 71:539–549
Li QL, Chen R, Chen XL, Yang YS, Wu H (2013) Estimation of the cloning potential in six selected genotypes of purple coneflower (Echinacea purpurea L.). Biotechnol Biotech Equip 27:3911–3917
Luckett DJ (1989) Colchicine mutagenesis is associated with substantial heritable variation in cotton. Euphytica 42:177–182
Majdi M, Karimzadeh G, Malboobi MA, Omidbaigi R, Mirzaghaderi G (2010) Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): morphological, physiological, cytological, and phytochemical changes. HortScience 45:16–21
Muhammad J, Jaskani S, Kwon W, Kim DH (2005) Comparative study on vegetative, reproductive and qualitative traits of seven diploid and tetraploid watermelon lines. Euphytica 145:259–268
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nilanthi D, Chen XL, Zhao FC, Yang YS, Wu H (2009) Induction of tetraploids from petiole explants through colchicine treatments in Echinacea purpurea L. J Biomed Biotech. doi:10.1155/2009/343485
Osborn TC, Pires JC, Birchler JA, Auger DL, Chen ZJ, Lee HS, Comai L, Madlung A, Doerge RW, Colot V, Martienssen RA (2003) Understanding mechanisms of novel gene expression in polyploids. Trends Genet 19:141–147
Sejdler LK, Dabrowska J (1998) Studies on the biology of flowering and fruiting of purple coneflower (Echinacea purpurea Moench). Part 1. Biology of flowering and fruiting. Herba Polonica 42:83–87
Stephens LC (2008) Self-incompatibility in Echinacea purpurea. HortScience 43:1350–1354
Udall JA, Wendel JF (2006) Polyploidy and crop improvement. Crop Sci 46:3–14
Xu CG, Tang TX, Chen R, Liang CH, Liu XY, Wu CL, Yang YS, Yang DP, Wu H (2014) A comparative study of bioactive secondary metabolite production in diploid and tetraploid Echinacea purpurea (L.) Moench. Plant Cell Tissue Organ Cult 116:323–332. doi:10.1007/s11240-013-0406-z
Yoo MJ, Liu X, Pires CJ, Soltis PS, Soltis DE (2014a) Nonadditive gene expression in polyploids. Ann Rev Genet 48:485–517
Yoo MJ, Liu X, Pires JC, Soltis PS, Soltis DE (2014b) Nonadditive gene expression in polyploids. Annu Rev Genet 48:485–517
Zhang XY, Hu CG, Yao JL (2010) Tetraploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J Plant Physiol 167:88–94
Acknowledgments
This research was funded by a grant from Science and Technology Planning Project of Guangdong Province, China (2011B031700026).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, R., Jiang, Wz., Li, Ql. et al. Comparison of seven colchicine-induced tetraploid clones with their original diploid clones in purple coneflower (Echinacea purpurea L.). Euphytica 207, 387–399 (2016). https://doi.org/10.1007/s10681-015-1556-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1556-3