Abstract
The impact of the ploidy level on biomass accumulation and the production of high-value secondary metabolites was studied in Echinacea purpurea (L.) Moench. Tetraploid E. purpurea was obtained by treating diploid explants with colchicine. The morphology, biomass yield, the contents of caffeic acid derivatives and alkamides, and the activities of phenylalanine ammonia-lyase (PAL) and cinnamate 4-hydroxylase (C4H) were compared between diploid plants and tetraploid plants of E. purpurea. The total fresh root weight and total dry root weight of the tetraploid plants were 39.32 and 40.48 % higher than those of the diploid plants, respectively. The chemical profiles of the diploid and tetraploid E. purpurea plants were similar, as determined through a comparison of their FTIR spectra and second derivative spectra. The caffeic acid derivatives and alkamides in the diploid and tetraploid plants were determined by HPLC. The tetraploid plants had higher contents of both of these types of molecules. In addition, the tetraploid plants had higher PAL and C4H activities compared with the diploid plants. The enhancement in the PAL and C4H activities was accompanied with an increase in the cichoric acid content, which indicates that the induction of polyploidy in E. purpurea resulted in higher PAL and C4H expression and promoted the biosynthesis of cichoric acid. Therefore, the induction of polyploidy may be a valid strategy to achieve a higher yield of biomass and bioactive compounds in E. purpurea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyploidy is frequently accompanied by conspicuous changes in morphology and metabolite production (Dhawan and Lavania 1996; Kaensaksiri et al. 2011; Lavania 2005; Nilanthi et al. 2009; Sun et al. 2011; Van Laere et al. 2011). In particular, polyploidy may increase the content of bioactive secondary metabolites in medicinal plants (Berkov and Philipov 2002; Dehghan et al. 2012; Dhawan and Lavania 1996; Gao et al. 1996; Lavania et al.2012). Similarly, the impact of polyploidy on biomass is generally positive, but sometimes this may be negative depending upon the metabolic cost of metabolite being produced i.e. longer the metabolic pathaway larger is the negative effect of polyploidy on biomass (Lavania et al. 2012). Ploidy level manipulation has been suggested as an interesting approach for increasing both plant biomass accumulation and the production of high-value plant secondary metabolites (Lavania 2005). Thus, the induction of polyploidy is a strategy to achieve a higher yield of bioactive chemicals.
Echinacea purpurea (L.) Moench, which is an herb native to North America, is well known for its effects on immune modulation and is widely used as a dietary supplement, functional food ingredient, and food additive (Lindenmuth and Lindenmuth 2000; Barrett 2003; Hall 2003). Caffeic acid derivatives and alkamides are considered the major bioactive compounds of E. purpurea (Barnes et al. 2005). Phenolic compounds are derived from phenylalanine through the core phenylpropanoid pathway by the action of phenylalanine ammonia-lyase (PAL) and cinnamate-4-hydroxylase (C4H), which are responsible for the hydroxylation of cinnamic acid to p-coumaric acid (Pina et al. 2012). The PAL and C4H activities are positively correlated to phenylpropanoid product accumulation (Bate et al. 1994; Bi et al. 2007; Schilmiller et al. 2009). Tetraploid E. purpurea has been obtained by treating diploid petiole explants with colchicine (Nilanthi et al. 2009). However, the impact of the ploidy level on the biosynthesis and accumulation of caffeic acid derivatives and alkamides has not yet been studied.
In this work, ploidy manipulation was used to obtain tetraploid E. purpurea. The caffeic acid derivatives and alkamides contents of diploid (2n = 22) and induced tetraploid (4n = 44) E. purpurea were compared. In addition, the impact of the ploidy level on caffeic acid derivatives biosynthesis was studied through the determination of the activities of phenylalanine ammonia-lyase (PAL) and cinnamate 4-hydroxylase (C4H).
Materials and methods
Plant source
Primary E. purpurea seeds were supplied by the Company of Plantation Products (Norton, MA, USA) and were cultivated in the Garden of Chinese Medicinal Plants on the campus of South China Agricultural University. The offspring seeds were used in this study.
Induction of tetraploids and determination of ploidy level
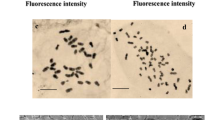
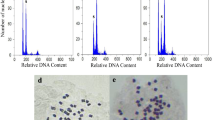
Tetraploid E. purpurea was obtained by treating diploid petiole explants with colchicine. The petiole explants were precultured on shoot regeneration medium for 1 week to heal the cutting wound and initiate cell division and then transferred to shoot regeneration medium with different concentrations (0, 30, 60, 120, or 240 mg/L) of colchicine for 30 days or with 120 mg/L colchicine for different time periods (7, 14, 21, or 28 days). Eight explants were cultured in one bottle. After colchicine treatment, the treated explants were transferred to shoot regeneration medium and cultured for 40 days. The regenerated shoots were cut from the mother tissues and cultured on rooting medium for the initiation of roots and further growth of the intact regenerated plantlets. The determination of the ploidy level was based on the observation of the chromosome number. The detailed procedures are described in our previous study (Nilanthi et al. 2009).
Cultivation of diploid and tetraploid
Diploid seedlings germinated from seeds and tetraploid plantlets obtained from the tissue culture with similar size and developmental stage, as determined by their morphological features, were cultivated in the Teaching and Research Base of South China Agricultural University in April 2011. The 2-year-old plants were harvested for observation and analysis in April 2013.
Observation of the morphological features of the root system
Three healthy diploid plants and three healthy tetraploid plants were randomly selected, and their aerial parts were removed. Their root systems with earth were soaked in water for 12 h. The earth was slowly rinsed off by flowing water. The roots that fell off were carefully collected by a sieve. The root system was scanned using a WinRhizo Pro LA2400 instrument (Regent Instruments, Quebec, Canada). The morphological features of the root axis as an organ, including the root length, root surface area, root volume, and root mean diameter, were evaluated using a computer image analysis software (WinRHIZO-2004a, Regent Instruments, Quebec, Canada). After morphological analysis, the fresh weight of the root system was measured. It was then heated at 105 °C in an oven until a constant weight was obtained, and the dry weight was recorded.
Chemical profile analysis using FTIR
The flowers, stems, leaves, roots, and rhizomes of the diploid and tetraploid plants were dried with hot air at 40 °C for 48 h and pulverized. The powder was sieved using a 200 mesh sieve. Accurately weighed 1 mg of dry sample powder was well mixed into 200 mg of dry KBr powder in an agate mortar. The mixture was ground to a fine powder, transferred into a pellet-forming die, and pressed to form a transparent pellet. The pellet was measured at 25 °C in an atmosphere with 30 % relative humidity using a VERTEX70 FTIR spectrometer (Bruker Optics, Ettlingen, Germany) over a region from 4,000 to 400 cm−1 with a resolution of 4 cm−1. The background was determined from a pellet of KBr that contained no sample and subtracted from the sample spectra. Five replicate pellets were prepared for each, and each sample was measured four times. The mean of the 20 spectra obtained for each sample was used for analysis and comparison.
Determination of caffeic acid derivatives and alkamides
Accurately weighed 0.1 g of the sample powder (described in Chemical profile analysis using FTIR) was extracted with 20 ml of 70 % ethanol for 20 min assisted by ultrasonic treatment (40 kHz). The extract solution was centrifuged for 10 min at a speed of 3,500 rpm (Eppendorf 5804R, Germany), and the supernatant was collected. The precipitate was extracted again, and the supernatants were combined. The solvent of the supernatant was evaporated under vacuum. The residue was reconstituted with 10 ml of 70 % ethanol. The solution was filtered through a 0.45-μm microporous membrane, and the filtrate was used as the sample solution for the determination of caffeic acid derivatives. The sample solution for the determination of alkamides was prepared using the same procedure as above with the exception that the 70 % ethanol was replaced by methanol.
Cichoric acid, caffeic acid, and chlorogenic acid were dissolved in appropriate volume of 70 % ethanol and diluted to obtain a solution of 0.0400 mg/ml.
The HPLC determination was conducted on a LC 20A system equipped with a LC-20A-series solvent delivery pump, SPD-M20A PDA detector, SIL-20A autosampler, and an LCSolution workstation (Shimadzu, Japan). A Gemini C18 reversed-phase column (4.6 mm × 250 mm, 5 μm, Phenomenex, USA) was used. Water containing 0.1 % formic acid (A) and acetonitrile (B) was used as the mobile phase. A gradient elution at a flow rate of 1.0 ml/min was programmed as follows: 0–9 min, 10–18.5 % B; 9–9.5 min, 18.5–45 % B; 9.50–39.50 min, 45–80 % B; 39.5–42.0 min, 80–100 % B; 42.0–45.0 min, 100–10 % B. The UV spectra were recorded in the range from 200 to 400 nm, and 330 and 254 nm were used for the quantification of caffeic acid derivatives and alkamides, respectively (Luo et al. 2003). The HPLC determination of each sample was conducted in triplicate.
The main peaks of alkamides on the HPLC chromatograms were identified by comparing the UV spectra and ESI/MS/MS data with literature data. The ESI/MS/MS data of a peak was acquired through the off-line coupling of HPLC to ESI–MS as follows: The eluate of the peak was collected from the HPLC outlet during the corresponding elution time and was introduced into a Finnigan TSQ Quantum triple-quadrupole mass spectrometer (Thermo Electron Corporation, USA) equipped with an ESI source through a syringe pump at a flow rate of 10 μl/min. The alkamides were determined in the positive ion mode. The automatic compound optimization procedure of the Xcalibur 2.0 workstation (Thermo Electron Corporation, USA) was used to fine-tune and optimize the mass spectrometer for the compound in the ESI/MS/MS mode. The ESI/MS/MS data were then acquired using the Tune Master Procedure.
Determination of PAL and C4H activities
Accurately weighed 1 g of fresh Echinacea leaves or roots and rhizomes was homogenized in 2 ml of potassium phosphate buffer (200 mM, pH 7.5, containing 2 mM 2-mercaptoethanol). After filtration and centrifugation (15 min at 10,000×g), the supernatant was diluted 20-fold and used for enzymatic analysis.
The PAL activity was determined spectrophotometrically using the method described by Edwars and Kessmann (1992), which is based on the rate of the conversion of l-phenylalanine into trans-cinnamic acid measured by the absorbance at 290 nm. The absorbance of 0.5 ml of enzyme extract, 2 ml of 50 mM Tris–H2SO4 buffer (pH 8.8), and 1 ml of 20 mM l-phenylalanine was measured before and after the incubation of the mixture in water at 40 °C for 15 min. The enzyme activity was stopped by the addition of 0.1 ml of 6 M HCl. One unit of activity was defined as the amount of extract that catalyzes the production of cinnamic acid and increases the absorbance by 0.01 per hour, and PAL activity was expressed as units per gram per hour.
The C4H activity was assayed using the method described by Lamb and Rubery (1975) with a slight modification. The extract (0.2 ml) was added to 2 ml of reaction buffer (50 mM phosphate buffer containing 2 mM 2-mercaptoethanol, 2 mM trans-cinnamic acid, and 0.5 mM NADPH). The solution was incubated for 1 h at 37 °C. The reaction was stopped through the addition of 0.1 ml of 6 M HCl and readjusted to pH 11 with 6 M NaOH. The absorbance at 340 nm was measured before and after the incubation of the mixture. One unit of activity was defined as the amount of extract that catalyzes the production of p-hydroxyl-cinnamic acid and increases the absorbance by 0.01 per h. The C4H activity was expressed as units per gram per hour.
Statistical analysis
The statistical analysis of the morphological and chemical results was performed using the SPSS 21 software (IBM, USA). The measured values are expressed as the mean ± standard deviation (SD) from three independent experiments (n = 3). The unpaired Student’s t test at a probability of 5 % was used to compare the obtained data to determine the differences between the diploid and tetraploid plants.
Results
Comparison of morphological features of diploid and tetraploid plants
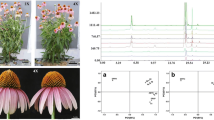
Tetraploid plants were more “stocky” than diploid plants. The tetraploid plants were shorter than the diploid plants, the stem diameter of the tetraploid plants was larger than that of the diploid plants, and the tetraploid plants were found to have shorter but broader leaves compared with the diploid plants (Table 1; Fig. 1). Compared with the diploid plants, the tetraploid plants had a higher number of thick roots, a shorter total root length, and fewer lateral roots (Fig. 2). Through a root system analysis (Fig. 3), the total root length of tetraploid plants was found to be 77.27 % of that of diploid plants; however, the total root surface area, total root volume, root mean diameter, total fresh root weight, and total dry root weight were 9.47, 55.17, 40.79, 39.32, and 40.48 % higher than those of the diploid plants, respectively. According to the distribution diagram of the root length and root diameter (Fig. 3), the length of the roots of tetraploid plants with a diameter greater than 1.4 mm was markedly longer than that of diploid plants, and the length of the roots of tetraploid plants with a diameter greater 2.4 mm was significantly longer than that of the diploid plants. This result indicates that the root system of tetraploid plants was more developed.
Chemical profile analysis using FTIR
The FTIR spectra and second-derivative spectra of the flowers, stems, leaves, and roots and rhizomes of 2-year-old diploid plants and tetraploid plants are shown in Figs. 4 and 5. The peak at 3,390 cm−1 displays the characteristic absorptions of the O–H stretching vibrations of polysaccharides, glycosides, and polyphenols. The C–H stretching vibrations at 2,930 cm−1 and the C–H bending vibration at 1,420 cm−1 indicate the presence of unsaturated alkyls in the sample. The peak at 1,630 cm−1 displays the characteristic absorptions of the O–H bending vibration of water, the COO− asymmetrical stretching vibration of organic acids and amino acid residues, and the stretching vibration of the benzene ring of aromatic compounds, which was confirmed by the high contents of polyphenols and alkamides in E. purpurea. The peak at 1,250 cm−1 displays the characteristic absorptions of the C=C, C=O–C, and P=O stretching vibrations of polysaccharides, glycosides, and phospholipids. The peak at 1,050 cm−1 displays the characteristic absorptions of the C–OH bending vibrations of polysaccharides and glycosides.
The spectra of different parts of the diploid plants and tetraploid plants shared similar peaks, which indicates that their chemical profiles were similar. Of these, the spectra of the flowers were more similar to those of the leaves, and the spectra of the stems were more similar to those of the roots and rhizomes.
Because herbal medicine is a mixture of chemicals and the FTIR spectrum consists of the overlapped spectra of many chemicals, some features will be unnoticeable on the original FTIR spectrum. Therefore, a second derivative spectrum of the fingerprint region, namely from 1,800 to 400 cm−1, was used to further compare the chemical profile of the different samples. As shown in Fig. 5, the spectra of different samples exhibit a similar profile. However, these spectra showed more differences than the original spectra. The region from 1,600 to 1,450 cm−1 displays the stretching vibration of the benzene ring of aromatic compounds. In this region, the spectra of different parts of the plants showed an obvious difference. The spectra of the flowers and leaves showed a stronger absorption, which indicates that these parts have higher contents of aromatic compounds.
Determination of caffeic acid derivatives and alkamides
Caffeic acid derivatives are the main bioactive compounds in E. purpurea and are conventionally used as marker compounds for quality control. Therefore, their contents in diploid plants and tetraploid plants were compared. Fig. 6 shows the HPLC chromatograms of caffeic acid derivatives of different samples. Different parts of the diploid and tetraploid plants shared similar peaks, which indicates that these samples contained similar caffeic acid derivatives. Little chlorogenic acid and no caffeic acid were detected in all of the samples. Cichoric acid accounted for most of the contents of caffeic acid derivatives. Therefore, the cichoric acid content was used as a marker of caffeic acid derivatives. Its contents in the different samples are listed in Table 2. The results show that the cichoric acid contents of the flowers, leaves, stems, and roots and rhizomes of tetraploid plants were 125.2, 121.1, 122.4, and 127.8 % of those of the diploid plants, respectively. It has been reported that the phenylpropanoid content is generally significantly higher in tetraploids compared with diploid S. commersonii (Caruso et al. 2011). Therefore, it appears that the induction of polyploidy generally increases the phenylpropanoid content in plants. The underlying mechanisms are worthy of further study.
Alkamides are the other class of bioactive compounds in E. purpurea. There are many alkamide compounds in E. purpurea plants, and their contents are very low. Their high-purity reference standards for quantitative determination are not easily available. Therefore, the peaks of alkamides on the HPLC chromatograms were putatively identified by comparing the UV spectra and ESI/MS/MS data with literature data (Luo et al. 2003), and the alkamide contents in different parts of the diploid and tetraploid plants were compared based on the total peak area of the identified peaks per gram. Three main alkamides at the retention times (Rts) of 26.84, 31.62, and 41.80 min were putatively identified (Table 3; Fig. 7).
Figure 8 shows the HPLC chromatograms of the alkamides present in the different samples. The different parts of the diploid plants and tetraploid plants shared similar peaks, which indicates that these parts contain similar alkamides. The alkamide contents of the flowers, leaves, stems, and roots and rhizomes of tetraploid plants were 119.57, 117.64, 121.24, and 123.57 % of those of diploid plants, respectively (Table 4).
PAL and C4H activities of diploid and tetraploid plants
Caffeic acid derivatives are more commonly known as phenylpropanoids, which are synthesized from the amino acid phenylalanine (Dixon and Pavia 1995). Phenylalanine is ammonolyzed to cinnamic acid by the action of PAL, and cinnamic acid is hydroxylated to p-coumaric acid by the action of C4H and further hydroxylated to caffeic acid (Hahlbrock and Scheel 1989). Therefore, the impact of the ploidy level on the caffeic acid derivative biosynthesis was studied through the determination of the activities of PAL and C4H. The results are listed in Table 5.
The activities of PAL and C4H in tetraploid plants were higher than those in diploid plants, which indicates that the tetraploid plants exhibit a higher degree of secondary metabolite biosynthesis at the proteomic level.
Discussion
The FTIR spectra and the second derivative spectra of the flowers, stems, leaves, and roots and rhizomes of 2-year-old diploid and tetraploid plants showed that the chemical profiles of diploid and tetraploid plants are similar. The HPLC determination of caffeic acid derivatives and alkamides in the diploid and tetraploid plants showed that the tetraploid plants have higher contents of these compounds. Moreover, the total fresh root weight and total dry root weight of tetraploid plants were found to be 39.32 and 40.48 % higher than those of diploid plants, respectively. Therefore, the induction of polyploidy may be a valid strategy to achieve a higher yield of biomass and bioactive compounds in E. purpurea. Tetraploid E. purpurea would thus exhibit higher medicinal value and has a good application prospect.
Polyploidy is frequently accompanied by conspicuous changes in morphology and metabolite production (Lavania 2005; Kaensaksiri et al. 2011; Sun et al. 2011; Van Laere et al. 2011; Dehghan et al. 2012). Similarly, our observations also revealed that tetraploid plants have higher contents of caffeic acid derivatives and alkamides than diploid E purpurea plants. However, the molecular mechanisms through which polyploidy affects secondary metabolites remain obscure. It is known that polyploidy is not simply a chromosome doubling and is always accompanied by changes in the genome structure and gene expression model (Comai 2000; Wendel 2000; Kashkush et al. 2002; Adams et al. 2004; Adams and Wendel 2005; Chen and Ni 2006). Furthermore, genomic plasticity has downstream effects on the transcriptome, proteome, and metabolome that can generate phenotypic variation in polyploids that exceed that found in the parents (Leitch and Leitch 2008).
Current evidence reveals that increased PAL activity is accompanied with increased concentrations of cichoric acid, caftaric acid, and chlorogenic acid in E. purpurea hairy roots (Abashi et al. 2012). We found that the PAL and C4H activities in tetraploid plants were stronger than those in diploid plants. The enhancement of PAL and C4H activities was accompanied with an increase in the cichoric acid content, which indicates that the induction of polyploidy in E. purpurea resulted in higher PAL and C4H expression and promoted that biosynthesis of cichoric acid. The correlation between the ploidy level and a certain metabolite is not clear at present (Buggs et al. 2009). However, after chromosome doubling, an increased number of genes may lead to an increased concentration and activity of some enzymes (Caruso et al. 2011). The enhancement of PAL and C4H activities and cichoric acid content might be caused by the upregulation of the corresponding genes. In addition, the genes that regulate the biosynthesis of cichoric acid in tetraploid plants may be dose-dependent (Hegarty and Hiscock 2008). In the future, the study of the variation in the mRNA levels of the genes encoding PAL and C4H in E. purpurea varieties with different ploidy levels will provide additional evidence to elucidate the regulatory mechanism through which the ploidy level impacts the contents of metabolites in E. purpurea.
References
Abashi BH, Stiles AR, Saxena PK, Liu CZ (2012) Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl Biochem Biotechnol 168:2057–2066
Adams K, Wendel J (2005) Polyploidy and genome evolution in plants. Curr Opin Plant Biol 8:135–141
Adams K, Precifield R, Wendel J (2004) Organ-specific silencing of duplicated genes in a newly synthesized cotton allotetraploid. Genetics 168:2217–2226
Barnes J, Anderson LA, Gibbons S, Phillipson JD (2005) Echinacea species (Echinacea Angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology and clinical properties. J Pharm Pharmcol 57:929–954
Barrett B (2003) Medicinal properties of Echinacea: a critical review. Phytomedicine 10:66–86
Bate NJ, Orr J, Ni W, Meroni A, Nadler-Hassar T, Doerner PW, Dixon RA, Lamb CJ, Elkind Y (1994) Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate determining step in natural product synthesis. Proc Natl Acad Sci USA 91:7608–7612
Berkov S, Philipov S (2002) Alkaloid production in diploid and autotetraploid plants of Mature stramonium. Pharm Biol 40:617–621
Bi HH, Zeng RS, Su LM, An M, Luo SM (2007) Rice allelopathy induced by methyl jasmonate and methyl salicylate. J Chem Ecol 33:1089–1103
Buggs RJA, Doust AN, Tate JA, Kon J, Soltis K, Feltus FA, Paterson AH, Soltis PS, Soltis DE (2009) Gene loss and silencing in Tragopogon miscellus (Asteraceae): comparison of natural and synthetic allotetraploids. Heredity 103:73–81
Caruso I, Lepore L, De Tommasi N, Piaz FD, Frusciante L, Aversano R, Garramone R, Carputo D (2011) Secondary metabolite profile in induced tetraploids of wild Solanum commersonii Dun. Chem Biodivers 8:2226–2237
Chen ZJ, Ni ZF (2006) Mechanisms of genomic rearrangements and gene expression changes in plant polyploids. BioEssays 28:240–252
Comai L (2000) Genetic and epigenetic interactions in allopolyploid plants. Plant Mol Biol 43:387–399
Dehghan E, Hakkinen S, Oksman-Caldentey KM, Ahmadi FS (2012) Production of tropane alkaloids in diploid and tetraploid plants and in vitro hairy root cultures of Egyptian henbane (Hyoscyamus muticus L.). Plant Cell Tissue Organ Cult 110:35–44
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87:81–89
Dixon R, Pavia N (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Edwars R, Kessmann H (1992) Isoflavonoid phytoalexins and their biosynthetic enzymes. In: Gurr SJ, McPherson MJ, Bowles DJ (eds) Molecular plant pathology: a practical approach. IRL Press, Oxford, pp 45–62
Gao SL, Zhu DN, Cai ZH, Xu DR (1996) Autotetraploid plants from colchicine treated bud culture of Salvia miltiorrhiza Bge. Plant Cell Tissue Organ Cult 47:73–77
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol 40:347–369
Hall C III (2003) Echinacea as a functional food ingredient. Adv Food Nutr Res 47:113–173
Hegarty MJ, Hiscock SJ (2008) Genomic clues to the evolutionary success of review polyploid plants. Curr Biol 18:4352–4441
Kaensaksiri T, Soontornchainaksaeng P, Soonthornchareonnon N, Prathanturarug S (2011) In vitro induction of polyploidy in Centella asiatica (L.) Urban. Plant Cell Tissue Organ Cult 107:187–194
Kashkush K, Feldman M, Levy A (2002) Gene loss, silencing and activation in a newly synthesized wheat allotetraploid. Genetics 160:1651–1659
Lamb C, Rubery PH (1975) A spectrophotometric assay for trans-cinnamic acid 4-hydroxylase activity. Anal Biochem 68:554–561
Lavania UC (2005) Genomic and ploidy manipulation for enhanced production of phyto-pharmaceuticals. Plant Genet Resour 3:170–177
Lavania UC, Srivastava S, Lavania S, Basu S, Misra NK, Mukai Y (2012) Autopolyploidy differentially influences body size in plants, but facilitates enhanced accumulation of secondary metabolites, causing increased cytosine methylation. Plant J 71:539–549
Leitch AR, Leitch IJ (2008) Genomic plasticity and the diversity of polyploid plants. Science 320(25):481–483
Lindenmuth GF, Lindenmuth EB (2000) The efficacy of Echinacea compound herbal tea preparation on the severity and duration of upper respiratory and flu symptoms: a randomized, double-blind placebo-controlled study. J Altern Complement Med 6:327–334
Luo XB, Chen B, Yao SZ, Zeng JG (2003) Simultaneous analysis of caffeic acid derivatives and alkamides in roots and extracts of Echinacea purpurea by high-performance liquid chromatography–photodiode array detection-electrospray mass spectrometry. J Chromatogr A 986:73–81
Nilanthi D, Chen XL, Zhao FC, Yang YS, Wu H (2009) Induction of tetraploids from petiole explants through colchicine treatments in Echinacea purpurea L. J Biomed Biotech. doi:10.1155//343485
Pina A, Zhebentyayeva T, Errea P (2012) Isolation and molecular characterization of cinnamate 4-hydroxylase from apricot and plum. Biol Plant 56(3):441–450
Schilmiller AL, Stout J, Weng JK, Humphreys J, Ruegger MO, Chapple C (2009) Mutations in the cinnamate 4-hydroxylase gene impact metabolism, growth and development in Arabidopsis. Plant J 60:771–782
Sun Q, Sun H, Bell RL, Li H, Xin L (2011) Variation of phenotype, ploidy level, and organogenic potential of in vitro regenerated polyploids of Pyrus communis. Plant Cell Tissue Organ Cult 107:131–140
Van Laere K, Franca SC, Vansteenkiste H, Van Huylenbroeck J, Steppe K, Van Labeke MC (2011) Influence of ploidy level on morphology, growth and drought susceptibility in Spathiphyllum wallisii. Acta Physiol Plant 33:1149–1156
Wendel J (2000) Genome evolution in polyploids. Plant Mol Biol 42:225–249
Acknowledgements
This research study was funded by grants from a special program for Enterprise, University, and Research Institute Cooperation of Guangdong Province and the Ministry of Education of China (No. 2008B090500250) and from the Science and Technology Planning Project of Guangdong Province, China (No. 2011B031700026).
Author information
Authors and Affiliations
Corresponding author
Additional information
Chuan-gui Xu and Tie-xin Tang are co-first authors.
Rights and permissions
About this article
Cite this article
Xu, Cg., Tang, Tx., Chen, R. et al. A comparative study of bioactive secondary metabolite production in diploid and tetraploid Echinacea purpurea (L.) Moench. Plant Cell Tiss Organ Cult 116, 323–332 (2014). https://doi.org/10.1007/s11240-013-0406-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-013-0406-z