Abstract
Because the six major known resistance genes to Asian soybean rust (ASR) are pathotype-specific, they rarely offer durable resistance to all ASR pathogens. Thus, other types of resistance such as field resistance or tolerance that confer broad-spectrum resistance to ASR pathogens are required for soybean breeding. The Chinese soybean variety Lu Pi Dou has a green cotyledon color (CC) and a no leaf yellowing (LY) phenotype was previously identified during ASR infection of this strain. This phenotype may reduce yield losses of soybean cultivars infected with ASR infections if it is independent of pathotype-specific major resistance genes and undesirable phenotypes. Thus, to identify associations of LY and CC and other color traits and loci that are linked to the six known ASR resistance genes, recombinant inbred lines (RILs) and backcross (BC) BC2F5 lines were derived from Lu Pi Dou and BRS184 plant strains. Comparisons of LY, CC, and genotypes of simple sequence repeat (SSR) markers revealed that the absence of LY is tightly associated with green CC in accordance with the expression of the genes d1 and d2. However, although variations of LY in RILs with yellow CC predominantly reflected the infection index (IFI), no associations were observed between LY and pubescence color, pod color, seed coat color, or flanking SSR markers for six known ASR resistance loci. An association of LY with CC and IFI was also shown in data from BC populations. Therefore, prevention of LY during ASR infection of Lu Pi Dou was primarily dependent on the double-recessive genotype d1d1d2d2 for CC and secondarily on low IFI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fungus Phakopsora pachyrhizi is the causal agent of Asian soybean rust (ASR) and is one of the most serious economic threats for soybean growers (Goellner et al. 2010). It has caused severe losses to soybean production in South America (Yorinori 2008). Foliar disease is characterized by rapid leaf yellowing (LY) and premature defoliation, which result in yield reductions from both fewer and smaller seeds. Hence, the development of soybean varieties that are resistant to ASR is considered the most economical management strategy for this disease (Hartman et al. 2005; Ribeiro et al. 2007; Arias et al. 2008). However, the six known major P. p achyrhizi ASR resistance genes Rpp 1–6 (Hyten et al. 2007, 2009; Garcia et al. 2008; Silva et al. 2008; Li et al. 2012) are pathotype-specific and are not always durable (Oliveira et al. 2005). Moreover, some of these genes are not effective in South America because of the presence of highly virulent and divergent ASR populations (Yamanaka et al. 2010, 2011; Akamatsu et al. 2013). Therefore, provision of multiple effective ASR resistance genes (Rpp1–6) in a single soybean cultivar and/or use of low specificity resistance modalities such as field resistance or tolerance to ASR are considered ideal strategies for providing widespread resistance against the diverse P. pachyrhizi populations in South America (Lemos et al. 2011; Yamanaka et al. 2013b).
In a previous study (Yamanaka et al. 2011), ASR infection promoted LY in both resistant and susceptible varieties during severe infection with the highly virulent ASR population, BRP-2. However, the two Chinese soybean varieties Lu Pi Dou and Hei Dou showed LY preventative phenotypes, despite being highly susceptible to ASR infection. These two varieties also showed a minor characteristic of a green cotyledon color (CC) of seeds. Hence, this LY resistant phenotype may reduce yield losses of soybean cultivars and maximize seed productivity under conditions of severe infection with ASR if it is independent of the major pathotype-specific resistance genes and does not produce undesirable phenotypes.
Green or yellow CC is reportedly associated with the unlinked loci d1 and d2 in soybean (Woodworth 1921), and the double-recessive genotype (d1d1d2d2) preserves most leaf thylakoid proteins during senescence and leads to maintenance of green color (Guiamét et al. 1991; Guiamét and Giannibelli 1996). These loci were mapped (Ott et al. 2013) on the linkage groups (LG) D1a (Chromosome 1) and B1 (Chromosome 11). In addition, the soybean orthologous STAY-GREEN (SRG) genes GmSGR1 and GmSGR2, which encode chloroplast proteins, were recently identified to be responsible for the green-cotyledon/stay-green phenotype in d1d1d2d2 soybean mutants (Nakano et al. 2014). Moreover, several candidate pleiotropic effects of d1d1d2d2 with the gene G, which preserves chlorophyll in the mature seed coat, were associated with agronomic and physiological characters such as seed yield and stomata conductance (Guiamét et al. 1990; Luquez and Guiamét 2001). However, the d1d1d2d2 genotype was more susceptible to water stress than wild type (Luquez and Guiamét 2002). In addition, Young and Ross (1978) demonstrated co-inheritance of the green CC phenotype and non-chlorotic responses to brown spots (Septoria glycines) in disease resistant soybean plants.
Because most cultivated soybeans have yellow CC, disease resistance may be associated with genes that are independent of the green CC, and these could be used to breed soybeans with yellow CC. Thus, in the present study we clarified the associations of ASR-associated LY with CC and various other traits and genes using an independent population of recombinant inbred lines (RILs) and backcrossed lines that were derived from the cross between Lu Pi Dou and the ASR susceptible LY variety BRS184.
Materials and methods
Plant materials
Two experiments were conducted in this study. For the first experiment, 70 recombinant inbred lines (RILs, F6) were developed using F2-derived single seed descent (SSD) from the cross of Lu Pi Dou × BRS184. Lu Pi Dou is susceptible to ASR but has no LY phenotype during ASR infection, whereas BRS184 is a susceptible variety that shows LY during ASR infection (Yamanaka et al. 2011). A total of 70 F6 lines (one plant each of 70 RILs) were evaluated for their CC (green/yellow), pubescence color (brown/white), and pod color (dark/light colored), and DNA was then extracted from samples for analyses of simple sequence repeat (SSR) markers. F7 seeds were harvested from each F6 plant and were evaluated for CC and seed coat color (green/yellow). Degrees of black pigmentations on seed coats were not evaluated, and three F7 plants from each of 70 RILs were sown to evaluate degrees of LY and to calculate the infection index (IFI).
In the second experiment, a total of 58 BC2F5 plants were generated from a cross of the single F3 plant with the lowest degree of LY (1.0, see below), which was derived from the same cross used for the first experiment. BRS184 was used as the recurrent and pollen-producing parent. During backcrossing and selfing, plants showing no or low LY (1.0 ≤ LY < 1.5) were selected and used to produce the following generation. All 58 BC2F5 plants were evaluated for their CC, degree of LY, and IFI and were then sampled for DNA extraction and SSR marker analysis.
Evaluation of leaf yellowing and infection index
The Japanese ASR isolate T1-2 (Akamatsu et al. 2013; Yamanaka et al. 2013b; Hossain et al. 2015) was used in LY and IFI experiments. A spore suspension of T1-2 was prepared at the concentration of 115,000 spores/mL and was used to inoculate plants grown in a greenhouse according to Yamanaka et al. (2013a). Three weeks after inoculation, degrees of LY and IFI scores on three trifoliate leaves from each plant were averaged using the scale and formula for IFI described by Yamanaka et al. (2013a). Mean values from three F7 plants from each RIL and those from a single BC2F5 plant were included in subsequent statistical analyses.
Simple sequence repeat (SSR) markers
To analyze the CC-related loci d1 and d2, the SSR markers Sat_160 and Sat_272, which were approximately 1 cM from the d1 locus of the linkage group (LG) D1a (Chromosome 1) and approximately 14 cM from the d2 locus in the LG B1 (Chromosome 11), respectively, were chosen based on the linkage map GmComposite2003 in SoyBase (Grant et al. 2010). The six SSR markers Sat_117, Satt366, Sat_263, AF162283, Sat_280, and Satt324 were used to detect Rpps 1–6, respectively (Hyten et al. 2007, 2009; Garcia et al. 2008; Silva et al. 2008; Li et al. 2012). The SSR markers Satt408 and BE806308 (Grant et al. 2010) were polymorphic between the parents and were expected to be linked with d1 and d2, respectively, and were used in addition to Sat_160 and Sat_272. PCR and band detection were performed as described by Yamanaka et al. (2013a).
Statistical analysis
Comparisons of observed and expected segregation ratios of SSR marker genotypes, differences in SSR marker determined haplotypes (d loci), and frequencies of CC in RILs were identified using Chi square (χ2) analyses. One-way analysis of variance (ANOVA) was performed to identify significant relationships between LY and pubescence color, pod color, seed coat color, and CC-related genes d1, d2, and Rpps 1–6 in RILs and between LY and CC frequencies in BC2F5 plants. Numbers of green, segregating, and yellow CCs in RILs and BC2F5 plants were recorded as 2, 1, and 0, respectively, and regression analyses were performed to estimate associations between LY and CC.
Results
Leaf yellowing and cotyledon color in recombinant inbred lines
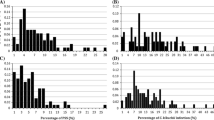
In the first experiment, no or low LY (1.0 ≤ LY < 1.5) due to infection after the Japanese ASR isolate T1-2 was observed in the parental variety Lu Pi Dou and in approximately 24 % of RILs (Fig. 1). However, infection with T1-2 resulted in widespread LY in 76 % of the RIL population, which included the parental variety BRS184. Degrees of LY among RILs were distributed from 1.0 to 5.6, and only two RILs showed higher levels of LY than BRS184 plants (Fig. 1). The CC of single F6 plants was identical to their F7 progeny (data not shown), except for five RILs. Specifically, single F6 plants had yellow CC, whereas these five F7 progeny RILs showed segregation of yellow and green CC in each line (shown as “segregating” CC in Fig. 1 and subsequent Tables). All the 13 RILs with green CC showed no (LY = 1.0 in 12 lines) or almost no yellowing (LY = 1.1 in one line; Fig. 2; Table 1). In contrast, LY was observed in BRS184 and RILs with yellow or segregating CC (Fig. 1; Table 1). Average LY values in segregating and yellow-fixed RILs were 3.2 (range 2.0–4.4) and 2.9 (range 1.0–5.6), respectively, and traces to 10 % of leaflet area showed yellowing. Significantly differing degrees of LY were observed between the three groups of RILs (green, yellow, and segregating; P < 0.001, Table 1). Among RILs, CC explained 34.9 % of variance in LY (Table 1), but four RILs had yellow CC but no LY.
Numbers of recombinant inbred lines (RILs) with differing degrees of leaf yellowing (LY). Color separation in the graph is based on phenotypes of cotyledon colors among F7 plants. Values for parents and the LY scale (Yamanaka et al. 2011) are shown on the top and the bottom of the figure, respectively. (Color figure online)
Cotyledon color and d loci in recombinant inbred lines
Haplotypes of RILs were determined using the two SSR markers Sat_160 and Sat_272, which are linked to d1 and d2, respectively, and the corresponding segregations of CC within each haplotype are shown in Table 2. The ratio of green to segregating to yellow CC in 70 RILs was 13:5:52 and corresponded with the theoretical ratio of 16.4:2.2:51.4. Moreover, green CC reflected the two independent double recessive loci (P = 0.1435, Table 2). The number of RILs with nine classes of marker haplotypes fitted to the number of RILs with two unlinked loci (P = 0.7461). Twelve of 13 RILs with green CC had the AA haplotype (both alleles from Lu Pi Dou), whereas only one RIL with green CC had the BA haplotype (BRS184 allele at Sat_160 and Lu Pi Dou allele at Sat_272), reflecting potential recombination between Sat_160 and d1. In contrast, 52 RILs with yellow cotyledons had AA, AB, BA, HB, BH, or BB haplotypes (Table 2). The HH (H, heterozygous) haplotype was not identified among the present RILs.
Leaf yellowing and infection index in recombinant inbred lines
No significant associations were found between the degree of LY and any of the three color characters, the eight SSR markers that were linked to the two d loci, or the six Rpp loci (Table 3). However, regression analysis indicated a significant (P < 0.001) positive correlation (r = 0.5899) between IFI and LY among RILs with yellow CC (Fig. 2).
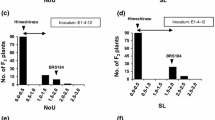
Leaf yellowing, cotyledon color, and infection index in the BC2F5 population
Minimum LY values (LY = 1.0) were observed in BC2F5 plants with green CC. In contrast, plants with yellow CC showed various degrees of LY and an average value of 3.7. Significant differences in degrees of LY were observed between BC2F5 plants with green and yellow CC (P < 0.001, Table 1). In this population, the observed segregation for CC was 28 green to 30 yellow (Table 1). In the analyses of segregation of the two d2 locus-linked SSR markers BE806308 and Sat_272 linked, 27 of 28 plants with green CC were homozygous for the Lu Pi Dou allele at Sat_272, whereas seven of 28 plants were heterozygous or homozygous for the BRS184 allele at BE806308. The other one BC2F5 plant with green CC was homozygous for the Lu Pi Dou allele at the BE806308 locus and heterozygous at the Sat_272 locus. These segregations indicate that d2 is located between BE806308 and Sat_272, although the linkage to Sat_272 appears stronger. In contrast, all BC2F5 plants with Sat_160 and Satt408 were homozygous for BRS184 and Lu Pi Dou, respectively, indicating that Satt408 is more tightly linked with d1 than Sat_160 and that all BC2F5 plants had Lu Pi Dou alleles and were homozygous for d1.
CC explained 61.3 % of the variation in LY among BC2F5 plants (Table 1), and IFI and LY were highly correlated (r = 0.8023, P < 0.001) in BC2F5 plants with yellow CC (Fig. 3), indicating that the degree of LY in plants with the yellow cotyledon genotype was largely determined by IFI.
Discussion
In this study of unique Lu Pi Dou phenotypes, no LY was observed during infection of the Japanese ASR isolate T1-2. A similar phenotype was observed in our previous study after the same variety was infected with the Brazilian ASR population: BRP-2 (Yamanaka et al. 2011), although these two ASR pathogens have differing virulence in resistant varieties (Yamanaka et al. 2013b). The distribution of LY phenotypes in 70 RILs was continuous (Fig. 1). In addition, LY values in most of the RILs ranged between those of Lu Pi Dou and BRS184 (Fig. 2), indicating that Lu Pi Dou and BRS184 carry factors for preventing and promoting LY, respectively and that LY may be controlled by multiple factors or environmental variables. Most RILs with no or almost no LY had green CC, and all 13 RILs with green CC belonged to this class (Fig. 1). In addition, the degree of LY differed significantly between RILs with green, segregating, and yellow CC (Table 1), indicating that the loci for CC predominantly determines the degree of LY. This conclusion was also supported in subsequent experiments with BC2F5 plants, which showed greater differences in LY between CC groups and greater explanatory power of CC (Table 1). Our preliminary test using 45 F5 lines also revealed significant differences in LY between CC classes (data not shown). Moreover, analyses of the SSR markers that are linked to the known double recessive loci d1 and d2 (Woodworth 1921) indicated that green CC in Lu Pi Dou is controlled by these loci, although some recombination was observed between markers and CC phenotypes in RILs and BC2F5 plants (Table 2). In conclusion, LY prevention during ASR infection in Lu Pi Dou primarily reflects green CC controlled by double recessive loci for CC (d1 and d2). Young and Ross (1978) also observed co-inheritance of green CC controlled by double recessive genes and non-chlorotic responses to brown spot caused by Septoria glycines. In addition, they observed non-chlorotic responses to S. glycines in plantlets of only 8 of 626 plant introduction (PI) lines, including the two ASR resistant lines PI 230970 (Rpp2) and PI 339866 (Hartwig and Bromfield 1983; Laperuta et al. 2008). The PI 79609 had green CC and black lesion brown spot disease symptoms without showing any LY. Moreover, the green CC variety Lu Pi Dou produced dark-colored (but highly sporulating) ASR lesions and showed no LY phenotype. Brogin (2005) also demonstrated the presence of a gene on LG C2 of the soybean variety FT-2 that conferred resistance against ASR and brown spot, potentially reflecting similar mechanisms of resistance.
Although LY prevention in Lu Pi Dou was shown to primarily reflect the green CC by the known double recessive loci d1 and d2, large variations in LY were observed among plants with yellow CC in the two populations. In the present study, IFI, pubescence color, pod color, and seed coat color, as well as eight SSR markers that are linked to six known resistance loci (Rpp1–6) and two d loci were evaluated as additional indicators of the association between LY and yellow CC. However, pubescence color, pod color, seed coat color, and the six known ASR resistance genes were not associated with LY, and no association was found between LY and the two d loci markers, suggesting that each of d loci is not exclusive determinates of LY solely (Table 3). In contrast, positive associations of IFI and degrees of LY were significant in both of the present experiments (Figs. 1, 2), indicating that IFI could be considered a determinate of LY in the progeny of Lu Pi Dou × BRS184 plants. In particular, initial experiments identified four RILs with yellow CC and similarly low levels of LY (mean 1.2; range 1.0–1.3) to those of 13 RILs with green CC (Fig. 1). Moreover, the corresponding IFI values were the lowest (mean 0.45; range 0.28–0.69) among the 52 RILs with yellow CC (Fig. 2). These observations further indicate that IFI is a significant determinate of LY. Accordingly, among genotypes with yellow CC, IFI explained 64 % of the variance in LY in BC2F5 plants but only 34.8 % in RILs (34.8 %), potentially reflecting relative uniformity of the genetic background among BC2F5 plants and fewer minor genetic contributors to LY. Taken together, these data indicate that in addition to d loci, IFI and other genetic factor(s) influence LY.
Both of the experiments in this study indicated that the absence of the LY character in Lu Pi Dou is associated with green CC and is regulated by known double recessive loci (d1d1d2d2). Thus, introduction of LY prevention phenotypes of the green CC variety Lu Pi Dou into soybean breeding programs of the widely grown yellow CC varieties may not be possible. However, low IFI reduced LY in genotypes with yellow CC. Thus, low IFI is genetically determined and may be exploited to reduce LY due to ASR infection in soybean breeding programs.
References
Akamatsu H, Yamanaka N, Yamaoka Y, Soares RM, Morel W, Ivancovich AJG, Bogado AN, Kato M, Yorinori JT, Suenaga K (2013) Pathogenic diversity of soybean rust in Argentina, Brazil, and Paraguay. J Gen Plant Pathol 79:28–40. doi:10.1007/s10327-012-0421-7
Arias CAA, Toledo JFF, Almeida LA, Pipolo AE, Carneiro GES, Abdelnoor RV, Rachid BF, Ribeiro AS (2008) Asian rust in Brazil: Varietal resistance. In: Kudo H, Suenaga K, Soares RM, Toledo A (eds) JIRCAS Working Report No. 58: Facing the challenge of soybean rust in South America. JIRCAS, Tsukuba, pp 29–30
Brogin RL (2005) Mapeamento de genes de resistência à ferrugem e de QTLs envolvidos na resistência à septoriose em soja. Dissertation, Universidade de São Paulo
Garcia A, Calvo ES, Kiihl RAS, Harada A, Hiromoto DM, Vieira LGE (2008) Molecular mapping of soybean rust (Phakopsora pachyrhizi) resistance genes: discovery of a novel locus and alleles. Theor Appl Genet 117:545–553. doi:10.1007/s00122-008-0798-z
Goellner K, Loehrer M, Langenbach C, Conrath U, Koch E, Schaffrath U (2010) Phakopsora pachyrhizi, the causal agent of Asian soybean rust. Mol Plant Pathol 11:169–177. doi:10.1111/J.1364-3703.2009.00589.X
Grant D, Nelson RT, Cannon SB, Shoemaker RC (2010) SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Res 38:843–846. doi:10.1093/nar/gkp798
Guiamét JJ, Giannibelli MC (1996) Nuclear and cytoplasmic `stay-green’ mutations of soybean alter the loss of leaf soluble proteins during senescence. Physiol Plant 96:655–661. doi:10.1111/j.1399-3054.1996.tb00239.x
Guiamét JJ, Teeri JA, Noodén LD (1990) Effects of nuclear and cytoplasmic genes altering chlorophyll loss on gas exchange during monocarpic senescence in soybean. Plant Cell Physiol 31:1123–1130
Guiamét JJ, Schwartz E, Pichersky E, Noodén LD (1991) Characterization of cytoplasmic and nuclear mutations affecting chlorophyll and chlorophyll-binding proteins during senescence in soybean. Plant Physiol 96:227–231. doi:10.1104/pp.96.1.227
Hartman GL, Miles MR, Frederick RD (2005) Breeding for resistance to soybean rust. Plant Dis 89:664–666. doi:10.1094/PD-89-0664
Hartwig EE, Bromfield KR (1983) Relationships among three genes conferring specific resistance to rust in soybeans. Crop Sci 23:237–239. doi:10.2135/cropsci1983.0011183X002300020012x
Hossain MM, Akamatsu H, Morishita M, Mori T, Yamaoka Y, Suenaga K, Soares RM, Bogado AN, Ivancovich AJG, Yamanaka N (2015) Molecular mapping of Asian soybean rust resistance in soybean landraces PI 594767A, PI 587905 and PI 416764. Plant Pathol 64:147–156. doi:10.1111/ppa.12226
Hyten DL, Hartman GL, Nelson RL, Frederick RD, Concibido VC, Narvel JM, Gregan PB (2007) Map location of the Rpp1 locus that confers resistance to soybean rust in soybean. Crop Sci 47:835–838. doi:10.2135/cropsci2006.07.0484
Hyten DL, Smith JR, Frederick RD, Tucker ML, Song Q, Cregan PB (2009) Bulked segregant analysis using the GoldenGate assay to locate the Rpp3 locus that confers resistance to soybean rust in soybean. Crop Sci 49:265–271. doi:10.2135/cropsci2008.08.0511
Laperuta LDC, Arias CAA, Ribeiro AS, Rachid BF, Pierozzi PHB, Toledo JFFD, Pípolo AE, Carneiro GEDS (2008) New genes conferring resistance to Asian soybean rust: allelic testing for the Rpp2 and Rpp4 loci. Pesqui Agropecu Bras 43:1741–1747. doi:10.1590/S0100-204X2008001200014
Lemos NG, Braccini AL, Abdelnoor RV, Oliveira MCN, Suenaga K, Yamanaka N (2011) Characterization of genes Rpp2, Rpp4, and Rpp5 for resistance to soybean rust. Euphytica 182:53–64. doi:10.1007/s10681-011-0465-3
Li S, Smith JR, Ray JD, Frederick RD (2012) Identification of a new soybean rust resistance gene in PI 567102B. Theor Appl Genet 125:133–142. doi:10.1007/s00122-012-1821-y
Luquez VM, Guiamét JJ (2001) Effects of the ‘Stay Green’ genotype GGd1d1d2d2 on leaf gas exchange, dry matter accumulation and seed yield in soybean (Glycine max L. Merr.). Ann Bot 87:313–318. doi:10.1006/anbo.2000.1324
Luquez VM, Guiamét JJ (2002) The stay green mutations d1 and d2 increase water stress susceptibility in soybeans. J Exp Bot 53:1421–1428. doi:10.1093/jexbot/53.373.1421
Nakano M, Yamada T, Masuda Y, Sato Y, Kobayashi H, Ueda H, Morita R, Nishimura M, Kitamura K, Kusaba M (2014) A green-cotyledon/stay-green mutant exemplifies the ancient whole-genome duplications in soybean. Plant Cell Physiol 55:1763–1771. doi:10.1093/pcp/pcu107
Oliveira ACB, Godoy CV, Martins MC (2005) Avaliação da tolerância de cultivares de soja à ferrugem asiática no Oeste da Bahia. Fitopatol Bras 30:658–662
Ott A, Yang Y, Bhattacharyya M, Horner HT, Palmer RG, Sandhu D (2013) Molecular mapping of D1, D2 and ms5 revealed linkage between the cotyledon color locus D2 and the male-sterile locus ms5 in soybean. Plants 2:441–454. doi:10.3390/plants2030441
Ribeiro AS, Moreira JUV, Pierozzi PHB, Rachid BF, De Toledo JFF, Arias CAA, Soares RM, Godoy CV (2007) Genetic control of Asian rust in soybean. Euphytica 15:15–25. doi:10.1007/s10681-007-9404-8
Silva DCG, Yamanaka N, Brogin RL, Arias CAA, Nepomuceno AL, Di Mauro AO, Pereira SS, Nogueira LM, Passianotto ALL, Abdelnoor RV (2008) Molecular mapping of two loci that confer resistance to Asian rust in soybean. Theor Appl Genet 117:57–63. doi:10.1007/s00122-008-0752-0
Woodworth CM (1921) Inheritance of cotyledon, seed-coat, hilum and pubescence colors in soy-beans. Genetics 6:487–553
Yamanaka N, Yamaoka Y, Kato M, Lemos NG, Passianotto ALL, Santos JVM, Benitez ER, Abdelnoor RV, Soares RM, Suenaga K (2010) Development of classification criteria for resistance to soybean rust and differences in virulence among Japanese and Brazilian rust populations. Trop Plant Pathol 35:153–162. doi:10.1590/S1982-56762010000300003
Yamanaka N, Lemos NG, Akamatsu H, Yamaoka Y, Silva DCG, Passianotto ALL, Abdelnoor RV, Soares RM, Suenaga K (2011) Soybean breeding materials useful for resistance to soybean rust in Brazil. JARQ 45:385–395
Yamanaka N, Akamatsu H, Yamaoka (2013a) Laboratory manual for studies on soybean rust resistance. JIRCAS website. http://www.jircas.affrc.go.jp/english/manual/soybean_rust/soybean_rust.html. Accessed 1 December 2013
Yamanaka N, Lemos NG, Uno M, Akamatsu H, Yamaoka Y, Abdelnoor RV, Braccini AL, Suenaga K (2013b) Resistance to Asian soybean rust in soybean lines with the pyramided three Rpp genes. Crop Breed Appl Biotechnol 13:75–82. doi:10.1590/S1984-70332013000100009
Yorinori JT (2008) Soybean germplasms with resistance and tolerance to Asian rust and screening methods. In: Kudo H, Suenaga K, Soares RM, Toledo A (eds) JIRCAS Working Report No. 58: Facing the challenge of soybean rust in South America. JIRCAS, Tsukuba, pp 70–87
Young LD, Ross JP (1978) Resistance evaluation and inheritance of a nonchlorotic response to brown spot of soybean. Crop Sci 18:1075–1077. doi:10.2135/cropsci1978.0011183X001800060043x
Acknowledgments
We thank Mio Morishita, Tomomi Mori, Akiko Takahashi, Sachiko Nishimura, Yukie Muraki, and Iwao Ohyama (JIRCAS) for their technical assistance, advice, and encouragement. We are grateful to the Jilin Academy of Agricultural Sciences (JAAS) in China and the Brazilian Agricultural Research Corporation (Embrapa) for providing seeds of the parental varieties. This study was financially supported and conducted by the JIRCAS research project “Development of Breeding Technologies toward Improved Production and Stable Supply of Upland Crops.” The authors N. G. L. and M. M. H. were financially supported by the JIRCAS Visiting Research Fellowship Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamanaka, N., Lemos, N.G., Jara, R.C. et al. Prevention of leaf yellowing in Asian soybean rust infected plants is associated with green cotyledon color and the infection index. Euphytica 205, 475–482 (2015). https://doi.org/10.1007/s10681-015-1414-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1414-3