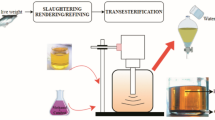
Abstract
In the present study, we present a holistic approach to emphasize the importance of process conditions of In-situ transesterification to evaluate madhuca biodiesel sustainability. The current study proposes the utilization of Plackett–Burman design followed by central composite design to maximize the biodiesel yield, exegy analysis and kinetics of biodiesel. The LCA analysis and energy spent on different techniques used in biodiesel synthesis were also studied. Screening of variables using Plackett–Burman design was carried out to identify maximum oil yield following central composite design and exergy analysis. Plackett–Burman screening design revealed seed weight, hexane volume, sulfuric acid, and temperature were the important variables (P < 0.05) influencing biodiesel yield. Gas chromatography analysis showed the dominance of oleic acid 36.95%, stearic acid 26.115%, linoleic acid 20.05%, and strong methylene peaks attributing to fatty acid methyl esters followed by FT-IR analysis. In addition, kinetic model with varying temperature on biodiesel production fitted first order equation at an R2 value of 98% with an activation energy of 19.16 kJ mol−1. Thus, to compare influence of process variables on biodiesel yield following In-situ transesterification, based on experimental yield and material consumption, central composite design (CCD) and exergy analysis were used. The results from the analysis showed that both CCD and exergy analysis revealed 92–95% biodiesel yield with significant change in process variables. The ANOVA results showed that all the variables in CCD model were significant with R2 97.37%, R-squared adjusted 94.55%, R-squared predicted value as 82.82%. Energy spent in biodiesel synthesis from seed to biodiesel for mechanical extraction (55.656 kJ), solvent extraction (48.312 kJ) and 19.8 kJ for in-situ process (19.8 kJ).The less energy spent for in-situ transesterification due to direct synthesis of biodiesel from seeds. Finally, life cycle assessment (LCA) was performed for these variables from CCD and exergy of madhuca biodiesel and compared with mechanical and solvent extraction presenting a holistic approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biodiesel is obtained by converting oil from various biomass enriched with lipids into fatty acid methyl ester (Thakkar et al., 2018). Due to the inherent properties such as lower vapour pressure, higher cetane number and higher flash point, several studies have demonstrated that emissions are halved compared to fossil fuels in CI engines (Kavitha & Murugavelh, 2019; Subramaniam et al., 2013). These evidence supports biodiesel as a sustainable energy source that which can minimize the impact of human health and environment (Acharya et al., 2016; Venugopal et al., 2023). Most countries, specifically the USA, European countries, Malaysia, and the Philippines are depending on the edible oil source for biodiesel production which includes soybean, rapeseed, sunflower, palm, and coconut (Mo et al., 2013). Besides, nonedible oil sources and waste cooking oil was mostly preferred for biodiesel production instead of edible oil sources to suppress food demand (Corral-Bobadilla et al., 2022; Kanitkar et al., 2011; Stamenkovi et al., 2012). In India, Madhuca indica is a non-edible oil source can be used in the biofuel industry. Madhuca indica is a medium-sized tree seen in central and southern parts of India and comes under the sapotaceae family. The tree grows around 20 m in height, and based on the tree size, the seed yield varies from 20 to 200 kg per year. It is usually grown in an arid region and has a maximum oil content of 50% (Jena et al., 2010; Kumar & Sharma, 2011). It can grow in different soil conditions and generally seen in the forest area. It is a potential feedstock for biodiesel production since the availability of the seed is around 60 million tons per year. The conventional transesterification for biodiesel production is in practice and widely adopted process, but often limited with longer reaction time and the yield is dependent on the homogenous catalyst or heterogeneous catalyst and feedstock used (Aghbashlo et al., 2017; Hosseinpour et al., 2016). Specifically for the synthesis of biodiesel from non-edible seeds, conventional transesterification is less considered due to the presence of high free fatty acid and moisture content (Charoenchaitrakool & Thienmethangkoon, 2011; Lim & Lee, 2013). Further, the practice of conventional transesterification using base catalyst for production of biodiesel from non-edible seeds like Jatropha seeds can account for low yield due to limitation of saponification reactions (Hincapié et al., 2011; Santori et al., 2012). Alternatively, two-stage transesterification, pre-treatment methods were considered as promising methods overcome the limitations of conventional transesterification and yet is limited to too many steps leading to significant capital cost (Dubey et al., 2014; Haas et al., 2006).
In-situ transesterification of biomass into biodiesel eliminates a few processes like oil extraction, oil purification, and degumming to produce esters in a single step thus saving energy limits the use of solvent in the process (Georgogianni et al., 2007; Kasim & Harvey, 2011). Evidently, several studies have tested and modified In-situ transesterification methods for the production of biodiesel particularly from non-edible seeds (Hailegiorgis et al., 2013; Harrington et al., 1985; Martínez et al., 2019). Biodiesel from In-situ transesterification of castor seed produced 97% of methyl ester through RSM (Rani et al., 2017). Notably, this In-situ transesterification success criteria on biodiesel yield from non-edible seeds is dependent on the diffusion of solvents and various parameters, which aids in swift cell wall rupture (Sitepu et al., 2020). Sustainability is an emerging concept to drive and foster green energy covering three key concepts focussing on resource efficiency, environment friendly and economic feasibility (Praveen et al., 2022). Despite, the promising application of biodiesel from non-edible seeds, it is also very evident that identifying best conditions is imminent for sustainability (Khounani et al., 2019; Sitepu et al., 2020). The use of central composite design of response surface methodology is overwhelming reported for identifying process variables to enhance biodiesel yield (Rajendran et al., 2022). However, the results from synthesis of acetin through continuous esterification using glycerol in acetic acid highlighted that optimizing conditions based on yield could be misleading due to thermodynamic irreversibility (Aghbashlo et al., 2018). Thus, exergy based narrowing of process variables on influencing biodiesel yield is another important analysis that delineate the process with respect to material and energy (Demirel, 2013; Ofori-Boateng et al., 2012). For instance, studies have reported that employing exergy in tranesterification process has contributed to remove waste and optimize the process based on energy and material usuage (Sakthivel et al., 2013). The application of exergy in biodiesel production focuses on the simultaneous production of biodiesel and the decrease of exergy destruction (Hoang et al., 2023). In addition, life cycle analysis helps to understand the environmental impact for any process based on the computable assessment of energy flow, material usage and its impact on the environment (Gnansounou et al., 2009; Kim & Dale, 2009). LCA is an influential methodology for evaluating the environmental problems related to biofuel production and involves recognising the materials and energy spent in the biofuel production and allows to identify the improvement in the environment (Lostado-Lorza et al., 2023; SETAC 1993). TRACI software is used for life cycle assessment, pollution prevention, design of process and sustainability. This software needs data which helps to study the impact on current and future generations (Bare et al., 2012).
Based on our perusal of literature using Web of Science there are ~ 3500 scientific reports identified using the keyword “biodiesel from seeds”. Further screening with keywords “response surface methodology” only less than < 10% of studies; “exergy” and “Sustainability” < 1% were identified. This clearly shows the significant knowledge gap on non-edible biodiesel studies particularly focussing on identifying process variable for enhancing biodiesel yield. Previously (Baroi & Dalai, 2015) evaluated process sustainability homogeneous and heterogeneous acid catalyzed biodiesel from green seed canola based on process economics, process safety, energy efficiency and environmental impact. In this study, we present a holistic approach in process sustainability of non-edible seed (madhuca) biodiesel synthesis and its life cycle assessment. Thus, several process parameters were initially screened using Plackett–Burman design to identify significant process variables. Further process sustainability of biodiesel yield of was carried out using central composite design and exergy analysis. In addition, and life cycle assessment following TRACI Method was performed to unravel the environmental impact as shown in Fig. 1.
1.1 Objective of the study
The literature study reveals many researchers have worked on exergy analysis of biodiesel production. However, a gap is identified in the exergy efficiency analysis of in-situ transesterification. Limited reports were available on the in-situ transesterification and optimization of madhuca for biodiesel production. This study reports on the in-situ transesterification of madhuca seeds and its optimization using RSM. Plackett–Burmann design eliminates the insignificant factor and identifies the significant factor. Significant factors identified by the PB design are applied to CCD, thereby optimising the process factors to maximize the yield. The importance of the variables in the process and their impact on the transesterification is well-known by applying PB and CCD, since it maximises the yield. Evaluating exergy efficiency for biodiesel production will impact the economy and thus improve production on a commercial scale and help minimise the waste generated by the process. The exergy efficiency is calculated for biodiesel production using CCD to reveal the resources utilization efficiency.
Thermodynamic analysis of biodiesel synthesis generates reliability and increases the data accuracy. Thus current work analysed the kinetic of biodiesel synthesis. The energy spent on biodiesel production through the conventional method (mechanical and solvent extraction of oil from madhuca seeds) and in-situ transesterification were studied. The energy utilized in these processes were also compared and the LCA of the each process was analysed to evaluate its impact on the environment and human health.
2 Materials and methods
Madhuca indica seeds were bought from the local seed distributor in Vellore. Subsequently, the seeds were sun-dried, to separate kernels consisting of lipids, which were then used for biodiesel production. Methanol (96% purity), n- hexane (99% purity), sulphuric acid (98% purity) was purchased from Merck chemicals, India.
2.1 In-situ transesterification (Screening of process variables)
Screening of important process variables influencing In-situ transesterification of madhuca seeds was performed based on Plackett–Burman design generated using Minitab (V.16). The coded variables and its quantity considered are shown in Table 1. All the experiments were executed in a screw-capped bottle as per the experimental design as shown in Table 2 incubated in a temperature-controlled water bath. After completion of the process, the obtained biodiesel cooled to room temperature and the filtrate was subjected to evaporation to separate biodiesel and stored for further analysis. All the experiments were performed thrice, and the mean value was reported. The yield of biodiesel from madhuca seed was calculated through Eq. 1.
2.1.1 Characterisation of biodiesel
The fatty acid composition of madhuca biodiesel was analysed using an Agilent 6890 gas chromatography fitted with flame Ionization Detector (FID) using a cyano silicone column (DB- 225, 30 m × 0.25 mm × 0.2 µm). The initial oven temperature was 160 °C which was slowly raised to 230 °C and finally stabilized to 250 °C for sample injection. A detailed GCMS specification employed in this study is provided in Table 3. In addition, the biodiesel obtained was also determined using an FT-IR spectroscopy through Shimadzu IR Affinity-1 working in mid-IR energy range (4000–400 cm−1) in ATR mode. A total of 16 scans obtained for each sample were co-averaged to improve signal-to-noise ratio at a resolution of 8 cm−1 using air-cooled DTGS detector following the literature (Abinandan et al., 2019).
2.1.2 Kinetic studies
Kinetic studies were conducted in this study to determine the effect of time and temperature on biodiesel yield. An excess of methanol concentration causes methyl ester formation, so the reaction is a pseudo first-order reaction (Sambasivam & Murugavelh, 2019). The product concentration and time were plotted to obtain the rate constant. The kinetics is expressed as
where B is the yield of madhuca methyl esters, t is the reaction time in minutes, and k is the rate constant of the reaction in min−1. The following equation is obtained on integrating both sides.
It is arranged in the form y = mx + C
The plot between ln [B] and ln [dB]/ [dt] was determined to be linear, and the slope indicates the rate constant of the reaction. The reaction rate was found to increase with an increase in temperature. The activation energy required for this reaction can be calculated using the Arrhenius equation. The relation between the reaction rate constant and temperature can be expressed as
where k is the reaction rate constant in min−1, A is the Arrhenius constant, Ea is the activation energy in J/mol, R is the universal gas constant, and T is the absolute temperature in kelvin (K). In the plot between the ln(k) and 1/T, the slope gives activation energy (− Ea/R), and the intercept gives Arrhenius constant, lnA. The activation thermodynamic parameters were estimated using the transition state theory.
Here N is the Avogadro’s constant, h represents Planck’s constant, ΔS++ is the activation entropy, ΔH++ is the activation enthalpy, and ΔG++ is Gibbs free energy.
Here Yi is equilibrium constant, Bt is the biodiesel yield concerning the temperature, Bu is unconverted oil into biodiesel, ΔS is entropy change, ΔH is enthalpy change, and ΔG is Gibb’s free energy. In the plot between ln Bt versus 1/T, the slope gives the enthalpy change ΔH for biodiesel production.
2.2 Evaluation of the process sustainability to identify significant variables influencing biodiesel synthesis
Following screening of process variables, further optimization of variables using central composite design was carried out under the response surface methodology (RSM) was employed to illustrate the nature of the response surface in the experimental design and to elucidate the optimal conditions of the most significant independent variables. However, the experimental results for the yield based on the esterification conditions can be ambiguous and thus an exergy analysis was also performed consequentially.
2.2.1 Central composite design
The full factorial CCD design matrix of top four independent variables from screening trails and their coded and uncoded values are presented in Table 4. These independent variables were varied over two level relative to the centre following second order polynomial model (Eq. 11) was designed using Minitab V 16.0. Further, the goodness of fit is performed through co-efficient determination and analysis of variance. The second-order polynomial equation, including both the linear and interaction effects of the process variable, is shown in Eq. (11)
where Y is the dependent variable (biodiesel yield), A, B, C and D are the independent variable, β0 is the intercept, β1, β2, β3 and β4 represents the linear coefficient and β11, β22, β33 and β44 denotes the squared coefficient and β12 β13 β23 β44, β11 β22 β33 β44 indicates the interaction effect of process variables. The generated model was assessed by the values of regression coefficient, P, F and ANOVA. Based on the R-Squared values the fitness of the model was assessed. The statistical software Minitab 16 was employed to predict the optimum experimental conditions for biodiesel synthesis using Plackett–Burmann and CCD design.
2.2.2 Exergy analysis
In the madhuca transesterification process, the interactions between work and heat were analysed using the four balance equations in steady-state. Equation (12) represents the balance between mass input and mass output. The first law of thermodynamics is applied to balance the energy input and energy output through Eq. (13). An increase in the entropy and part of energy destruction can be calculated using Eqs. (14) and (15). The total Exergy component has been divided into four segments: chemical, physical, potential and kinetic energy shown in Eq. (16).
Exkin and Expot represent the kinetic and potential energy that is negligible in the process since the variation with speed and elevation is minimal. The other two physical and chemical energy factors are calculated using Eqs. (17) and (18).
Physical energy is based on temperature, enthalpy and entropy. In contrast, chemical exergy was calculated from Eq. (18), in which ΔG represents the gibbs free energy, nelem is the number of atoms in each element, and ech is the chemical exergy of each element (Demirel, 2013). Thus chemical exergy of each combination is calculated from Eq. (19). The heat and work flow exergy calculations were calculated from Eqs. (20) & (21).
2.3 LCA for biodiesel production
LCA methodology is categorized into four phases as per ISO14040 as (i) goal and scope definition (ii) inventory analysis (iii) impact assessment, and (iv) interpretation (Finkbeiner et al., 2006; Sabando-Fraile et al., 2023). This current study explores LCA methodology for biodiesel production from madhuca seed following TRACI 2.1 method (Bare et al., 2012). This work compares the energy spent on biodiesel synthesis from madhuca seed through in-situ transesterification results from CCD, exergy analysis and also compares the biodiesel production and oil extraction from madhuca seed by mechanical and solvent extraction methods. The goal and scope of the current work and also reveals the amount of energy and mass flow for this process and includes its impact on the environment. LCA helps to show the connection between the biofuel production system and its environmental impact.
2.3.1 Goal and scope definition
The initial phase of LCA is to define the goal and scope. In this work, the process of biofuel production from in-situ and conventional methods was compared. This study compares the materials involved in the process and the energy utilized for biofuel production. LCA was analysed for biodiesel production through in-situ, mechanical and solvent extraction from madhuca seeds. The study comprises of the amount acid usage for degumming, esterification and transesterification and also solvent used for oil extraction and biofuel production were compared with different process methods. This allows us to compare the eco toxicity potential, human health toxicity, acidification air, smog air and eutrophication potential.
2.3.2 The functional unit (FU)
The current study utilises 30 g of madhuca seeds in each process for biodiesel production. The biofuel synthesis is produced through in-situ, oil extraction by mechanical means and solvent extraction.
2.3.3 System boundary
Biodiesel synthesis from 30 g of madhuca seeds includes drying of seeds, oil extraction, degumming of oil, esterification, transesterification and recovery of solvents. The inventory analysis explained the materials involved and energy consumed in these processes.
2.4 Inventory analysis
2.4.1 Drying of seeds
Seeds from madhuca tree are cleaned and dried to remove excess moisture, and thereby the seeds were acceptable for further biodiesel synthesis. Higher moisture content of seed hinders the DT process. Thus, proper drying of seeds enhances both oil extraction and biodiesel synthesis.
2.4.2 Oil extraction
Seeds after drying were subjected to the oil extraction process through the mechanical method by passing the seeds through a screw press, or seeds were powdered into fine particles and mixed with optimum solvent concentration to extract the oil from solvent extraction. The extracted oil is further separated from solids by filtration in mechanical means, whereas in solvent extraction, the solvent is removed from the oil and stored for further analysis.
2.4.3 Degumming of oil
Extracted oil from either mechanical or solvent method has impurities to be removed to reduce the free fatty acids. Hence the phosphoric acid is employed for the purification of oil. As a result, phosphatides, waxes and other impurities and FFA content were removed to a few extents.
2.4.4 Esterification and transesterification
Esterification is required before the transesterification process to reduce the free fatty acid content in the oil. Madhuca oil has high free fatty acid content. Thus esterification is necessary before the transesterification process. Oil with low FFA content is used directly in the transesterification process and avoids the esterification or pre-treatment of oil. Transesterification is a process that converts oil into methyl ester in the presence of a catalyst. Biodiesel synthesis produces biodiesel and glycerol as by-product.
2.4.5 Recovery of solvents
Solvents were recovered during oil extraction and biodiesel production, and thus the solvents can be reused. The solvent and oil will be in a mixture during oil extraction using a solvent. Simple Distillation was used to recover the oil from the solvent. The unreacted methanol solvent can be recovered during biodiesel production and thus used in biodiesel synthesis.
2.4.6 Life cycle inventory analysis
The process includes case 1 (Biodiesel synthesis through in-situ method from madhuca seeds), case 2 (Biodiesel synthesis through conventional method- Mechanical means of oil extraction), case 3 (Biodiesel synthesis through conventional method- oil extraction using solvent). The materials used in these biodiesel syntheses and the energy required for these processes were also studied. TRACI was used to perform the LCA in all these methods.
2.4.7 Life cycle impact assessment
Comparative studies of biodiesel synthesis through conventional and in-situ transesterification were studied. The volume of solvents and acids used in biodiesel synthesis was also studied. The following midpoint was characterized from oil extraction to biodiesel synthesis. The midpoints were Acidification (kg SO2 eq/kg), Eutrophication (kg N eq/kg), Smog air (kg O3 eq/kg), Ecotoxicity (CTUeco/kg) and Human health (CTUcancer/kg). The methodologies utilized in the TRACI is based on the usage of chemicals or resource which emits the impact in the media (Air, water, urban air, nonurban air, freshwater, seawater, natural soil and agricultural soil) and also based on the calculated effectiveness of the stressor. Based on the experimental data, the impact category varies and for few impact categories site does not decide the fate, transport of the potency and thus CF (characterization factor) represents global utilization in the case of global climate change and stratospheric depletion. In these situations, a general Eq. (22) without considering location would be;
Here Ii—represents the potential impact of chemicals for a particular impact, CFxm—CF of the chemical released in the media, M—mass of chemical discharged to media.
2.4.8 Data interpretation
The impact analysis of the resource used in the process will be evaluated based on the impact category. The comparison of the three cases (Biodiesel through in-situ process, mechanical oil extraction and oil extraction using solvent) were studied. The analysis has been done to analyse which method has more influence on the environment. Thus this study reveals which process has worst impact to the environment.
3 Results and discussion
3.1 Screening of parameters using Plackett Burman design
Plackett Burmann design was used to identify the significant factors involved in in-situ transesterification and maximise the yield of madhuca biodiesel. According to Pareto chart (Fig. 2), the influence of process parameters was in the order of hexane > temperature > seed weight > sulphuric acid. Amongst, the higher concentration of hexane (4 mL) alone yielded 93.68% which is 1.62 fold greater than the lower concentration of hexane. Because, hexane has higher affinity towards the fatty acid present in the oil (Sambasivam & Murugavelh, 2019). Similarly, the greater biodiesel yield of madhuca oil was also favoured with high temperature, which shows improved lipid solubility (Hidalgo et al., 2013; Meurah et al., 2021). Because the amount of biodiesel produced is proportional to the amount of oil contained in the seed, the quantity of seed that is used in the in-situ transesterification process has a significant impact on the amount of biodiesel produced. In the present study, when the sample volume was around 0.6 g, the biodiesel yield was 90%, but when 1.0 g of seed was used, the yield increased to 99.68%. As shown in Table 2, variables such as temperature, hexane volume, and sulphuric acid all significantly affect the biodiesel yield in addition to the number of seeds used. In general, sulfuric acid is a suitable catalyst for in-situ transesterification when the feedstock free fatty acid content is higher than 3% (Park et al., 2016). The lesser volume of sulphuric acid produced more biodiesel yield than the high volume of sulphuric acid. Thus, the dominant variables involved in the direct transesterification of madhuca oil were found out from the PB design with R2 95.85%, R-squared adjusted 76.11%, R-squared predicted values 90.88% (Table 5), which can be used for further optimization using CCD design to determine the exact optimized condition for the improved yield.
3.1.1 Characterisation of biodiesel through in-situ transesterification
The fatty acid composition of Madhuca indica biodiesel was analysed using a GC–MS showed dominance of palmitic, stearic, oleic, linoleic and arachidic acid, as shown in Table 6. The major fatty acid of madhuca includes oleic acid 36.95%, followed by stearic acid 26.115%, linoleic acid 20.05% and palmitic acid 15.082%. Arachidic acid was found to be in a minor concentration of 1.440%. FT-IR analysis of biodiesel obtained from madhuca seeds synthesized from in-situ transesterification was analysed for the confirmation of biodiesel conversion and shown in Fig. 3. The presence of methyl and methylene of lipids was confirmed by 340 triplet bands, which is seen in the wavelength of 2980–2800 cm−1. Biodiesel synthesis was confirmed from madhuca seeds by the presence of the ester band at 1710 cm−1. Thespesia populnea seed oil is transformed into biodiesel and confirmed by FT-IR analysis by detecting spectral bands near 1710 cm−1 and 2800 cm−1 (Rashid et al., 2011). Biodiesel synthesis from sunflower oil showed the wavelength at 1746 cm−1 represented biodiesel production (Guzatto et al., 2012). According to the literature study, the bands from 1438–1462 to 1244–1377 cm−1 denotes the synthesis of biodiesel. The existence of cis olefins was predictable concerning the band at 721 cm−1 (Saloua et al., 2020). The greater content of fatty acid revealed by the presence of peak at 720 cm−1 (Prasad et al., 2017).
3.1.2 Kinetics for Madhuca biodiesel synthesis
Biodiesel synthesis from madhuca oil was studied with varying temperature of 45, 55, 65 and 75 °C and at different reaction rates. The data obtained from biodiesel synthesis fits first-order kinetics, and the best conditions required from the transesterification process was determined. The calculated rate constant, entropy and Gibbs free energy for biodiesel yield is shown in Table 7. The relationship between ln k versus 1/T, and the slope obtained from this graph provides activation energy, which is depicted in Fig. 4a. The activation energy calculated for the production of madhuca biodiesel was 19.16 kJ mol−1. The obtained activation energy was lower than the 22.306 kJ/mol activation energy for madhuca biodiesel that was previously reported (Muthukumaran et al., 2017). This shows the in-situ processs requires less heat energy. The enthalpy change was obtained from the graph between Yt versus 1/T shown in Fig. 4b. The slope of the graph was 4.45 kJ mol−1, which represents the enthalpy change. The value obtained is positive which indicates endothermic reaction and thus requires energy for the process (Sambasivam & Murugavelh, 2019).
3.2 Process sustainability analysis
3.2.1 Central composite design
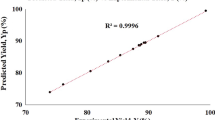
The process variables used in the CCD process comprises Seed weight, hexane volume, Sulfuric acid and temperature (Table 8). One way ANOVA results showed that all the variables used in the CCD model were significant with R2 97.37%, R-squared adjusted 94.55%, R-squared predicted values 82.82% (Table 8). The maximum biodiesel yield was seen with the interaction between variables of seed weight along with sulphuric acid, hexane volume, and temperature which were shown in Fig. 5. In addition, the maximum biodiesel yield were seen with respect to the interaction between sulfuric acid with hexane volume and temperature elucidating maximum biodiesel yield 90% through in-situ transesterification in Fig. 5. Sulphuric acid improves the transesterification rate and simultaneously degrade the cell wall to release the lipid molecule (Ms, 2019). The acid catalyst usage like sulphuric, hydrochloric acid is essential for the oil with high FFA content. Madhuca is a nonedible oil with high FFA content, is a suitable source that has to treat with sulphuric acid (Aranda et al., 2008). The optimum usage of acid catalyst around 0.2 mL is required for the enhanced yield of 94.9%, whereas the higher amount of acid catalyst showed a decrease in biodiesel yield to 14.8%. Thus, acid catalyst usage beyond the optimal value may lead to the side reaction, formation of catalyst lumps in the reaction mixture, thereby restricting mass transfer and interaction between reactants (Koutsouki et al., 2015).
Higher biodiesel yield (94%) was obtained with 3 mL of hexane, 0.2 mL of sulphuric acid at a temperature of 75 °C. However, when the temperature increased to 105 °C there was ~ 40% reduction of biodiesel yield (Fig. 5). Temperature is an essential parameter that has a significant contribution to the success of IT of madhuca seeds. The diffusivity of solvents and seed wall lysis is based on the reaction temperature. The blending of lipids from the feedstock with solvent is successful at a particular temperature (Leung et al., 2010). Increased temperature has a positive impact on the yield of biodiesel by enhancing the viscosity of the fluid. In contrast, in a few cases, it may lead to a secondary reaction. Thus, there is a decline in the biodiesel yield of madhuca oil. The biodiesel product is around 96% at 75 °C and reduced to 53% at 105 °C, which is due to higher temperature than required for the reaction, it will lead to secondary reactions, thus minimizing the yield. The possibility of secondary products at high temperature and solubility of lipid components may be the reason for the decline in the biodiesel yield (Rashid et al., 2011). Hexane as a co-solvent increases biodiesel yield in the presence of sulphuric acid, whereas the use of base catalyst lowers the lipid extraction in in-situ transesterification (Guzatto et al., 2012). A higher amount of hexane is required for the maximum yield owing to the solubility FAME content of madhuca oil. Almost 96% of biodiesel was produced using 4 mL of hexane in the reaction mixture and the 0.1 mL of sulphuric acid at 90 °C. The hexane volume and sulphuric acid play a significant role in biodiesel production from the result.
3.3 Estimating material and energy loss: an exergy analysis
The chemical exergy for biodiesel synthesis were classified into inputs, outputs and wastes for in-situ transesterification of madhuca seeds. The seeds of 30 g were used for the in-situ transesterification and the parameters were chosen from PB design. The selected parameters were utilised in CCD design, and the optimum yield was obtained. The optimum biodiesel yield of 94.94% was obtained at a temperature of 75 °C, sample weight of 0.8 g, solvent volume of 3 mL (hexane), with a sulfuric acid concentration of 0.2 mL as shown in Table 9. Based on the exergy output (total) and exergy input (total) of the biodiesel synthesis, the internal energy destruction of the process was calculated. The total internal exergy destruction can be calculated by subtracting exergy output (total) from the exergy input (total). The addition of exergy of the wastes, which include unreacted methanol, oil, solvents and glycerides, will help to calculate the external energy destruction of the process. Hence, the mass balance of the materials and exergy balance are shown in Table 10. The main inputs of in-situ transesterification include madhuca seed, sulfuric acid, hexane, methanol and the output comprises biodiesel, unreacted madhuca oil, recovered methanol, and hexane. Exergy incorporated for biodiesel is 42,547.02 kJ/kg, which is higher than the exergy of madhuca oil 39,928.64 kJ/kg in the present study. Exergy of material involved in the process and its output and by-products were calculated and it helps us to minimize the usage of all the chemicals and raw materials involved (Saloua et al., 2020).
The maximum yield obtained from the in-situ transesterification from madhuca seed is around 94.94% yield, and its exergy efficiency is 82.26%. This might be due to the presence of water in the reactor or due to the simultaneous oil extraction and biodiesel synthesis in the same reactor. The 30 g of madhuca seed was used in the in-situ transesterification for which 9.2 g of sulphuric acid, 138.5 g of methanol, 132 g of hexane was utilized. The biodiesel synthesis of 14.25 g was obtained from the 30 g of madhuca seed which is around 94% yield. The hexane and methanol utilized in the in-situ transesterification was recovered after biodiesel synthesis. Recovered methanol and hexane from the in-situ transesterification were 119 and 82.5 g respectively. Solvent recovery can be even increased by recovering the solvent from the sludge of the madhuca seed. The solvent usage will be high in DT compared to conventional biodiesel production process, whereas many steps in the production process and energy involved in the biodiesel synthesis are reduced. Exergy efficiency of 80% was achieved for biodiesel production using lipase enzyme (Karimi, 2016), whereas current work reports an efficiency of 82%. The exergy efficiency of 82% for in-situ transesterification is satisfactory since it eliminates few processes and reduces the energy required for biofuel synthesis.
3.4 LCA analysis
In the present study, LCA was performed for several process of biodiesel synthesis such as in-situ transesterification based on CCD, exergy, and conventional method. In conventional method, the oil extraction is assumed to use screw press and soxhlet apparatus. The impact category of these processes include acidification, eutrophication, smog formation, freshwater eco toxicity and human health toxicity. The impact category was analysed based on the mass of chemical/solvent used in each process.
The impact percentage of acidification, smog formation and ecotoxicity potential with respect to the biodiesel synthesis through in-situ CCD, in-situ exergy mechanical, solvent oil extraction (Fig. 6a). Acidification potentials for air emissions of in-situ transesterification is high due to the utilization of sulphuric acid and shown in Fig. 6a. The higher concentration of sulphuric acid is utilized in in-situ transesterification compared to mechanical and solvent process. The impact percentage was exhibited around 28% (in-situ CCD) and 50% for in-situ exergy, whereas 6% for mechanical and 16% for solvent process. The impact percentage for in-situ exergy was higher compared to in-situ CCD, which exhibits the higher concentration of sulphuric acid. However, the sulphuric acid concentration utilized in mechanical and solvent were less compared to in situ exergy and in-situ CCD. The enhanced utilization of sulphuric acid in in-situ transesterification than the conventional biodiesel production, because the acid not only involve in esterification but also participate in lysis of cell wall of seed to release the oil (Ms, 2019). Irrespective of the extraction method, sulphuric acid is utilized for esterification and transesterification process of biodiesel synthesis for the feedstock with high FFA content (Veljković et al., 2006). Better oil yield was obtained through solvent extraction compared to mechanical process. Thus a slight high concentration of sulphuric acid is utilized in biodiesel production through solvent extraction which exhibits 16% of impact percentage compared to mechanical extraction based biodiesel synthesis which is around 6%. Figure 6a shows the smog emissions in air due to the utilization of volatile solvents in biodiesel synthesis. The increased emission of smog was shown in solvent process around 68% of impact compared to other process, due to solvent usage for both oil extraction and biodiesel generation. in-situ process also utilizes a considerable amount of solvent for biodiesel synthesis. However the impact percentage for in-situ exergy and in-situ ccd were 12% and 19% irrespective of the solvent recovery was around 85% for methanol and 62.5% (hexane). Similarly, even after recovering of 70% solvent from the in-situ process it exhibited significant impact to the environment (Chopra et al., 2020). Comparing to biodiesel synthesis through solvent extraction and in-situ transesterification, mechanical extraction of oil followed by biodiesel synthesis showed a negligible impact around 1%. Overall, it is concluded that the incorporating exergy in in-situ process is beneficial for the efficient utilization of chemicals in biodiesel synthesis.
The eutrophication impact on air and water of biodiesel synthesis were around 30% and 70% for both mechanical and solvent Process as shown in Fig. 6a. This implies the usage of phosphoric acid in both the process for oil degumming to reduce the free fatty acid content. The solvent technique has high impact than mechanical is due to the increased oil yield. However, the in-situ process eliminates the usage of phosphoric acid, since simultaneous biofuel production and oil extraction occur in a single reactor. Comparing to other process for biodiesel production in-situ has no impact to the eutrophication emission to air and water.
The ecotoxicity discharge potential of air, freshwater and soil of various process involved in biodiesel production (Fig. 6b). The impact percentage of ecotoxicity air emission rural, urban, natural and agricultural soil potential for in-situ exergy was high around 62% compared to in-situ ccd 34%, mechanical (2%) and solvent (2%) as shown in Fig. 6b. This clearly illustrates the higher sulphuric acid concentration and solvents utilisation in in-situ process has more impact to the environment compared to the conventional biodiesel production. However, higher impact is created by the solvent method to the freshwater ecotoxicity around 38% compared to other process. It clearly indicates the lesser amount of solvent used in mechanical and solvent process has least impact compared to in-situ transesterification process.
The pollutant was categorized into human health indicators based on carcinogenic and non-carcinogenic and shown in Fig. 6c. Human health non-cancer and cancer potentials for air, water and soil were analysed for these process. Solvent method exhibits the impact percentage around 76%, while in-situ CCD and exergy were 14% and 10%. Mechanical means of biodiesel synthesis has negligible impact compared to solvent and in-situ process. This is due to the low concentration of solvent were utilised in this technique. Oil extraction using solvent followed by biodiesel synthesis will have higher impact to human health toxicity potential compared to other process. The in-situ process has less impact in all the emission categories listed above than the biodiesel production through solvent oil extraction.
Energy spent for biodiesel extraction through conventional method (mechanical and solvent extraction) and in-situ processes are shown in Table 11. Drying of seeds was done in all three processes and utilized 19.8 kJ of energy. Oil extraction is done through mechanical and solvent extraction techniques, energy used for mechanical pressing 21.6 kJ and 28.512 kJ for solvent extraction. Extracted oil requires purification and consumes around 14.256 kJ energy for both mechanical and solvent processes. Thus energy spent from seed to oil was around 55.656 kJ for mechanical, 48.312 kJ for solvent and 19.8 kJ of energy utilized for in-situ process. Energy used for mechanical, solvent-based biodiesel production is around 42.768 kJ for each operation, including solvent recovery, whereas in-situ transesterification is around 28.512 kJ, including solvent recovery after biodiesel separation.
The results of our study on madhuca biodiesel production processes through in-situ transesterification following CCD and exergy analysis aimed to enhance its sustainability. GC and FTIR analysis confirmed the rich fatty acids are abundant in madhuca biodiesel showing the quality that could be exploited as substitute for conventional fuels. Thus, screening of variables through Placket Burman design identified seed amount, methanol volume, hexane and sulphuric acid influenced biodiesel yield. Further optimization following CCD and exergy analysis showed the process conditions varied rather than biodiesel yield which remained constant. In addition, the LCA analysis for these process variables showed less incorporating exergy in In-situ process is beneficial for the efficient utilization of chemicals, which in turn showed less impact to the environment. However, under the impact category of ecotoxicity potential, the acid catalysed transesterification requires attention to enhance madhuca biodiesel sustainability.
4 Conclusion
The optimization of biodiesel production processes through in-situ transesterification is necessary to enhance the biodiesel yield and scaling up the industrial-scale process. Stearic acid was found to be in higher concentration in mahua seeds from GC–MS analysis. PB design was used in this current study to identify variables involved in the in-situ transesterification. Six variables were used in the PB design which were seed amount (1.0 g), methanol volume (6 mL), hexane volume (4 mL), sulphuric acid (0.15 mL), temperature (90 °C) and time (90 min) and yields around 93.68%. The critical parameters were identified and further optimized using CCD design. The maximum yield of 95.56% was obtained from the experimental investigations of the optimization process through CCD design. The optimization process were vial for the maximum yield and to analyse the impact of sulphuric concentration for the degradation of seed cell wall. The activation energy for the optimum yield of biodiesel was 19.16 kJ mol−1 and the enthalphy value was 4.45 kJ mol−1 indicating the endothermic reaction. The FT-IR analysis of biodiesel was performed, and the presence of a peak at a wavelength of 1710 cm−1 indicates the existence of ester in the biodiesel sample. This proves that mahua oil was converted into biodiesel through in-situ transesterification. Energy used in biodiesel synthesis through mechanical and solvent were high due to the pre-processing steps in biodiesel production like drying, degumming, and extraction of oil from seeds which were eliminated in in-situ process. Acidification potentials for air emissions, eco-toxicity for in-situ transesterification is high compared to solvent and mechanical. Smog emission, eutrophication and human health (carcinogenic and non-carcinogenic potential) were high in solvent extraction compared to mechanical and solvent. The utilization of sulphuric acid and solvents were the major demerits in in-situ transesterification from the point of sustainability whereas it saves time and economical by less energy utilization. The future research needs to focus on the elimination of solvents and acid for eco-friendly biodiesel synthesis.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
- IT:
-
In-situ transesterification
- PBD:
-
Plackett–Burman design
- CCD:
-
Central composite design
- FAME:
-
Fatty acid methyl ester
- GC-MS:
-
Gas chromatography-mass spectrometry
- FID:
-
Flame ionization detector
- FFA:
-
Free fatty acid
- TRACI:
-
Tool for the reduction and assessment of chemical and other environmental impacts
- LCA:
-
Life cycle analysis
- CTU:
-
Comparative toxic units
- FT-IR:
-
Fourier-transform infrared spectroscopy
- ANOVA:
-
Analysis of variance
- R-Sq:
-
R-squared
- R-Sq (Adj):
-
R-squared adjacent
- R-Sq(Pred):
-
R-squared prediction
References
Abinandan, S., Subashchandrabose, S. R., Cole, N., Dharmarajan, R., Venkateswarlu, K., & Megharaj, M. (2019). Sustainable production of biomass and biodiesel by acclimation of non-acidophilic microalgae to acidic conditions. Bioresource Technology, 271, 316–324.
Acharya, N., Nanda, P., Panda, S., & Acharya, S. (2016). Analysis of properties and estimation of optimum blending ratio of blended mahua biodiesel. Engineering Science and Technology, an International Journal. https://doi.org/10.1016/j.jestch.2016.12.005
Aghbashlo, M., Tabatabaei, M., Hosseinpour, S., Khounani, Z., & Hosseini, S. S. (2017). Exergy-based sustainability analysis of a low power, high frequency piezo-based ultrasound reactor for rapid biodiesel production. Energy Conversion and Management, 148, 759–769.
Aghbashlo, M., Tabatabaei, M., Rastegari, H., & Ghaziaskar, H. S. (2018). Exergy-based sustainability analysis of acetins synthesis through continuous esterification of glycerol in acetic acid using Amberlyst® 36 as catalyst. Journal of Cleaner Production, 183, 1265–1275.
Aranda, D. A., Santos, R. T., Tapanes, N. C., Ramos, A. L., & Antunes, O. A. (2008). Acid-catalyzed homogeneous esterification reaction for biodiesel production from palm fatty acids. Catalysis Letters. https://doi.org/10.1007/s10562-007-9318-z
Bare, J., Young, D., Qam, S., Hopton, M., & Chief, S. (2012). Tool for the reduction and assessment of chemical and other environmental impacts (TRACI). US Environmental Protection Agency.
Baroi, C., & Dalai, A. K. (2015). Process sustainability of biodiesel production process from green seed canola oil using homogeneous and heterogeneous acid catalysts. Fuel Processing Technology, 133, 105–119.
Charoenchaitrakool, M., & Thienmethangkoon, J. (2011). Statistical optimization for biodiesel production from waste frying oil through two-step catalyzed process. Fuel Processing Technology, 92(1), 112–118. https://doi.org/10.1016/j.fuproc.2010.09.012
Chopra, J., Tiwari, B. R., Dubey, B. K., & Sen, R. (2020). Environmental impact analysis of oleaginous yeast based biodiesel and bio-crude production by life cycle assessment. Journal of Cleaner Production, 271, 122349.
Corral-Bobadilla, M., Lostado-Lorza, R., Somovilla-Gómez, F., & Íñiguez-Macedo, S. (2022). Life cycle assessment multi-objective optimization for eco-efficient biodiesel production using waste cooking oil. Journal of Cleaner Production, 359, 132113.
Demirel, Y. (2013). Thermodynamic analysis. Arabian Journal for Science and Engineering, 38(2), 221–249.
Dubey, S. M., Gole, V. L., & Gogate, P. R. (2014). Cavitation assisted synthesis of fatty acid methyl esters from sustainable feedstock in presence of heterogeneous catalyst using two step process. Ultrasonics Sonochemistry. https://doi.org/10.1016/j.ultsonch.2014.08.019
Finkbeiner, M., Inaba, A., Tan, R., Christiansen, K., & Klüppel, H.-J. (2006). The new international standards for life cycle assessment: ISO 14040 and ISO 14044. The International Journal of Life Cycle Assessment, 11(2), 80–85.
Georgogianni, K. G., Kontominas, M. G., Pomonis, P. J., Avlonitis, D., & Gergis, V. (2007). Conventional and in situ transesterification of sunflower seed oil for the production of biodiesel. Fuel Processing Technology. https://doi.org/10.1016/j.fuproc.2007.10.004
Gnansounou, E., Dauriat, A., Villegas, J., & Panichelli, L. (2009). Life cycle assessment of biofuels: Energy and greenhouse gas balances. Bioresource Technology, 100(21), 4919–4930.
Guzatto, R., Defferrari, D., Reiznautt, Q. B., Cadore, Í. R., & Samios, D. (2012). Transesterification double step process modification for ethyl ester biodiesel production from vegetable and waste oils. Fuel, 92(1), 197–203. https://doi.org/10.1016/j.fuel.2011.08.010
Haas, M. J., Mcaloon, A. J., Yee, W. C., & Foglia, T. A. (2006). A process model to estimate biodiesel production costs. Bioresource Technology, 97, 671–678. https://doi.org/10.1016/j.biortech.2005.03.039
Hailegiorgis, S. M., Mahadzir, S., & Subbarao, D. (2013). Parametric study and optimization of in situ transesterification of Jatropha curcas L. assisted by benzyltrimethylammonium hydroxide as a phase transfer catalyst via response surface methodology. Biomass and Bioenergy, 49, 63–73. https://doi.org/10.1016/j.biombioe.2012.12.003
Harrington, K. J., Arcy-evans, C. D., & Division, C. (1985). Comparison of conventional and in situ methods of transesterification of seed oil from a series of sunflower cultivars. Journal of the American Oil Chemists Society, 62(6), 1009–1013.
Hidalgo, P., Toro, C., Ciudad, G., & Navia, R. (2013). Advances in direct transesterification of microalgal biomass for biodiesel production. Reviews in Environmental Science and Bio/technology. https://doi.org/10.1007/s11157-013-9308-0
Hincapié, G., Mondragón, F., & López, D. (2011). Conventional and in situ transesterification of castor seed oil for biodiesel production. Fuel, 90, 1618–1623. https://doi.org/10.1016/j.fuel.2011.01.027
Hoang, A. T., Balasubramanian, D., Venugopal, I. P., Rajendran, V., Nguyen, D. T., Lawrence, K. R., et al. (2023). A feasible and promising approach for diesel engine fuelled with a blend of biodiesel and low-viscosity Cinnamon oil: A comprehensive analysis of performance, combustion, and exergy. Journal of Cleaner Production, 401, 136682.
Hosseinpour, S., Aghbashlo, M., Tabatabaei, M., & Khalife, E. (2016). Exact estimation of biodiesel cetane number (CN) from its fatty acid methyl esters (FAMEs) profile using partial least square (PLS) adapted by artificial neural network (ANN). Energy Conversion and Management, 124, 389–398.
Jena, P. C., Raheman, H., Kumar, G. V. P., & Machavaram, R. (2010). Biodiesel production from mixture of mahua and simarouba oils with high free fatty acids. Biomass and Bioenergy, 34(8), 1108–1116.
Kanitkar, A., Balasubramanian, S., Lima, M., & Boldor, D. (2011). Bioresource technology CZbean and rice bran oil in the presence of microwaves. Bioresource Technology, 102(17), 7896–7902. https://doi.org/10.1016/j.biortech.2011.05.091
Karimi, M. (2016). Immobilization of lipase onto mesoporous magnetic nanoparticles for enzymatic synthesis of biodiesel. Biocatalysis and Agricultural Biotechnology, 8, 182–188.
Kasim, F. H., & Harvey, A. P. (2011). Influence of various parameters on reactive extraction of Jatropha curcas L. for biodiesel production. Chemical Engineering Journal, 171(3), 1373–1378. https://doi.org/10.1016/j.cej.2011.05.050
Kavitha, M. S., & Murugavelh, S. (2019). Optimization and transesterification of sterculia oil: Assessment of engine performance, emission and combustion analysis. Journal of Cleaner Production. https://doi.org/10.1016/j.jclepro.2019.06.240
Khounani, Z., Nazemi, F., Shafiei, M., Aghbashlo, M., & Tabatabaei, M. (2019). Techno-economic aspects of a safflower-based biorefinery plant co-producing bioethanol and biodiesel. Energy Conversion and Management, 201, 112184.
Kim, S., & Dale, B. E. (2009). Regional variations in greenhouse gas emissions of biobased products in the United States—corn-based ethanol and soybean oil. The International Journal of Life Cycle Assessment, 14(6), 540–546.
Koutsouki, A. A., Tegou, E., Kontakos, S., Kontominas, M. G., Pomonis, P. J., & Manos, G. (2015). In situ transesterification of Cynara cardunculus L. seed oil via direct ultrasonication for the production of biodiesel. Fuel Processing Technology. https://doi.org/10.1016/j.fuproc.2015.01.024
Kumar, A., & Sharma, S. (2011). Potential non-edible oil resources as biodiesel feedstock: An Indian perspective. Renewable and Sustainable Energy Reviews, 15(4), 1791–1800. https://doi.org/10.1016/j.rser.2010.11.020
Leung, D. Y. C., Wu, X., & Leung, M. K. H. (2010). A review on biodiesel production using catalyzed transesterification. Applied Energy, 87(4), 1083–1095. https://doi.org/10.1016/j.apenergy.2009.10.006
Lim, S., & Lee, K. T. (2013). Process intensification for biodiesel production from Jatropha curcas L. seeds: Supercritical reactive extraction process parameters study. Applied Energy, 103, 712–720.
Lostado-Lorza, R., Corral-Bobadilla, M., Íñiguez-Macedo, S., & Somovilla-Gómez, F. (2023). Characterization, LCA and FEA for an efficient ecodesign of novel stainless steel woven wire mesh reinforced recycled aluminum alloy matrix composite. Journal of Cleaner Production, 411, 137380.
Martínez, A., Mijangos, G. E., Romero-ibarra, I. C., Hernández-altamirano, R., & Mena-cervantes, V. Y. (2019). In-situ transesterification of Jatropha curcas L. seeds using homogeneous and heterogeneous basic catalysts. Fuel, 235(1), 277–287. https://doi.org/10.1016/j.fuel.2018.07.082
Meurah, T., Riayatsyah, I., Thaib, R., Silitonga, A. S., Milano, J., Shamsuddin, A. H., et al. (2021). Biodiesel production from Reutealis trisperma oil using conventional and ultrasonication through esterification and transesterification. Sustainability, 13(6), 3350.
Mo, M., Atabani, A. E., Masjuki, H. H., Kalam, M. A., & Masum, B. M. (2013). A study on the effects of promising edible and non-edible biodiesel feedstocks on engine performance and emissions production: A comparative evaluation. Renewable and Sustainable Energy Reviews, 23, 391–404. https://doi.org/10.1016/j.rser.2013.03.009
Ms, K. (2019). In situ acid catalysed transesterification of biodiesel production from Sterculia foetida oil and seed. International Journal of Green Energy. https://doi.org/10.1080/15435075.2019.1671418
Muthukumaran, C., Praniesh, R., Navamani, P., Swathi, R., Sharmila, G., & Kumar, N. M. (2017). Process optimization and kinetic modeling of biodiesel production using non-edible Madhuca indica oil. Fuel, 195, 217–225.
Ofori-Boateng, C., Keat, T. L., & JitKang, L. (2012). Feasibility study of microalgal and jatropha biodiesel production plants: Exergy analysis approach. Applied Thermal Engineering, 36, 141–151.
Park, J., Kim, B., & Lee, J. W. (2016). Bioresource technology In-situ transesterification of wet spent coffee grounds for sustainable biodiesel production. Bioresource Technology, 221, 55–60. https://doi.org/10.1016/j.biortech.2016.09.001
Prasad, Y., Vongsvivut, J., Adhikari, R., & Adhikari, B. (2017). Physicochemical and thermal characteristics of Australian chia seed oil. Food Chemistry, 228, 394–402. https://doi.org/10.1016/j.foodchem.2017.02.021
Praveen, K., Abinandan, S., Venkateswarlu, K., & Megharaj, M. (2022). Sustainability evaluation of immobilized acid-adapted microalgal technology in acid mine drainage remediation following emergy and carbon footprint analysis. Molecules, 27(3), 1015.
Rajendran, N., Kang, D., Han, J., & Gurunathan, B. (2022). Process optimization, economic and environmental analysis of biodiesel production from food waste using a citrus fruit peel biochar catalyst. Journal of Cleaner Production, 365, 132712.
Rani, S., Jagannath, A., Goud, V. V., Sahoo, N., & Kulkarni, V. N. (2017). In-situ alkaline transesterification of castor seeds: Optimization and engine performance, combustion and emission characteristics of blends. Energy Conversion and Management, 142, 200–214. https://doi.org/10.1016/j.enconman.2017.03.044
Rashid, U., Anwar, F., & Knothe, G. (2011). Biodiesel from Milo (Thespesia populnea L.) seed oil. Biomass and Bioenergy, 35(9), 4034–4039. https://doi.org/10.1016/j.biombioe.2011.06.043
Sabando-Fraile, C., Corral-Bobadilla, M., Lostado-Lorza, R., & Somovilla-Gomez, F. (2023). Multiresponse performance evaluation and life cycle assessment for the optimal elimination of Pb (II) from industrial wastewater by adsorption using vine shoot activated carbon. Sustainability, 15(14), 11007.
Sakthivel, G., Nagarajan, G., Ilangkumaran, M., Kumar, S. S., & Suresh, K. S. (2013). A hybrid multi–criteria decision support system for selection of optimum fuel blend. International Journal of Exergy, 12(4), 463–490.
Saloua, F., Saber, C., & Hedi, Z. (2020). Bioresource technology methyl ester of [Maclura pomifera (Rafin.) Schneider] seed oil: Biodiesel production and characterization. Bioresource Technology, 101(9), 3091–3096. https://doi.org/10.1016/j.biortech.2009.11.100
Sambasivam, K. M., & Murugavelh, S. (2019). Optimisation, experimental validation and thermodynamic study of the sequential oil extraction and biodiesel production processes from seeds of Sterculia foetida. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-019-06214-7
Santori, G., Di Nicola, G., Moglie, M., & Polonara, F. (2012). A review analyzing the industrial biodiesel production practice starting from vegetable oil refining. Applied Energy, 92, 109–132.
Setac, B. (1993). Guidelines for life-cycle assessment: A code of practice. In Consoli, Allen, Boustead, Fava, Franklin, Jense de Oude, Parrish, Perriman, Postelthwaite, Quay, Sequin, and Vigon.
Sitepu, E. K., Heimann, K., Raston, C. L., & Zhang, W. (2020). Critical evaluation of process parameters for direct biodiesel production from diverse feedstock. Renewable and Sustainable Energy Reviews, 123(March 2019), 109762. https://doi.org/10.1016/j.rser.2020.109762
Stamenkovi, O. S., Veljkovi, V. B., & Bankovi, I. B. (2012). Biodiesel production from non-edible plant oils. Renewable and Sustainable Energy Reviews, 16, 3621–3647. https://doi.org/10.1016/j.rser.2012.03.002
Subramaniam, D., Murugesan, A., Avinash, A., & Kumaravel, A. (2013). Bio-diesel production and its engine characteristics—An expatiate view. Renewable and Sustainable Energy Reviews, 22, 361–370. https://doi.org/10.1016/j.rser.2013.02.002
Thakkar, K., Shah, K., Kodgire, P., & Kachhwaha, S. S. (2018). In-situ reactive extraction of castor seeds for biodiesel production using the coordinated ultrasound–microwave irradiation: Process optimization and kinetic modeling. Ultrasonics - Sonochemistry. https://doi.org/10.1016/j.ultsonch.2018.08.007
Veljković, V. B., Lakićević, S. H., Stamenković, O. S., Todorović, Z. B., & Lazić, M. L. (2006). Biodiesel production from tobacco (Nicotiana tabacum L.) seed oil with a high content of free fatty acids. Fuel, 85(17–18), 2671–2675.
Venugopal, I. P., Balasubramanian, D., Rajarajan, A., & Suresh, K. (2023). Quantification of φ-operating range with impact of exhaust gas recirculation under low-temperature combustion mode with polyoxymethylene dimethyl ether: A perspective study. Journal of Cleaner Production, 411, 137298.
Xue, J., Grift, T. E., & Hansen, A. C. (2011). Effect of biodiesel on engine performances and emissions. Renewable and Sustainable Energy Reviews, 15(2), 1098–1116. https://doi.org/10.1016/j.rser.2010.11.016
Acknowledgements
The authors thank VIT for providing ‘VIT SEED GRANT’ (FY202021) for carrying out this research work.
Author information
Authors and Affiliations
Contributions
K.M-S.: Original draft, data curation, formal analysis, methodology. C.D.: Data curation. R.G.S, R.S.: Data curation, formal analysis. K.P.: Review & editing. S.A.: Resources, Conceptualization. S.A, KM-S.: Review & editing. S.A, KM-S.: Supervision, Conceptualization, Resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sambasivam, K.M., Devarajulu, C., ShenaviGhode, R.G. et al. Comprehensive analysis of in-situ transesterification for madhuca biodiesel: from synthesis to life cycle assessment. Environ Dev Sustain (2024). https://doi.org/10.1007/s10668-024-04600-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10668-024-04600-x