Abstract
Catalysts play a major role in the transesterification process. In recent years, heterogeneous catalysts have gathered attention due to the advantage of reusability and easy separation. The use of renewable sources for catalyst preparation has advanced the use of heterogeneous catalysts. The biomass-derived catalysts are important in decreasing the cost of production and promoting the commercialization of biodiesel. Various renewable sources such as sea sand, shells, fish bones, large-scale industrial wastes can be used for catalyst preparation. Catalysts prepared from these wastes can make the transesterification reaction more sustainable and cost-effective. Thus, this work comprises a review of the advancements of various catalyst technologies used in biodiesel production, including the use of waste biomass for catalyst preparation. The paper also discussed the Bimetallic and trimetallic heterogeneous catalysts for several applications in energy and biodiesel generation from microalgal lipids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The growth of the world and its well-being is directly reliant on energy growth. Rapid urbanization and industrial evolution have increased the high demand for energy worldwide [1, 2]. Hence, energy plays an important role in supporting our economic and social growth [3, 4]; however, the growing energy requirements and limited reserves make it difficult. Thus, it is a serious concern to develop alternative energy sources to meet the rising energy demand. The world energy consumption from 2008 to 2018 is shown in Table 1.
As shown in Table 1, it can be observed that there is a continuous increase in energy consumption per year which is making world energy security a tough task. Figure 1 shows our over-dependency on fossil fuels. This over-dependency is causing the depletion of fossil fuels. This depletion can lead us to fail to meet the energy demand in upcoming years. Besides energy security, the burning of fossil fuels also causes high harmful gaseous emissions that affect the environment and human beings [6, 7, 8].
Primary energy sources (MT oil equivalent) [5]
Thus, there is a high need to work for alternative energy sources so as to meet the energy demand and control the pollution in upcoming years [9, 10]. Considering these problems, various researchers, industrialists, and world leaders are efficiently promoting energy sustainability. The only way is to maximize the use of alternative sources of energy.
This research aims to examine the technical and economic aspects of the various types of catalysts used in biodiesel production. It includes a thorough examination of the latest developments in various biodiesel catalyst technologies, including waste biomass for catalyst manufacturing. In addition, Bimetallic and trimetallic heterogeneous catalysts for energy and biodiesel synthesis from microalgal lipids were also studied in the article.
1.1 Biofuel production
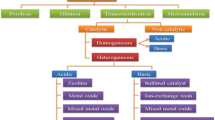
Fuels derived from biomass have been promising alternatives due to their renewable nature and less exhaust emissions [11, 12]. Therefore, among different biofuels, biodiesel has gained attention all over the world. Various processes produce biodiesel, but transesterification is the most commonly used [13, 14]. Figure 2 shows the procedure of biodiesel production from two methods using different catalysts.
Worldwide biodiesel production by various feedstock [17]
In Fig. 3, an increasing trend in biodiesel production can be observed worldwide. This is because the transport sector has been proved the most energy-consuming and emission-producing sector globally. Hence, biodiesel has been promoted as an alternative to petroleum due to its biodegradability [18].
Biodiesel is a fatty acid alkyl ester produced from vegetable oils and animal fats. There are four common methods to produce biodiesel like micro-emulsification, blending, thermal cracking, and transesterification. Transesterification is the method given more attention to producing biodiesel by various researchers as it produces biodiesel with good fuel properties [2, 19]. In transesterification reaction, triglycerides react with alcohol in the presence of a catalyst to produce alkyl ester and glycerol as a by-product [20, 21, 22]. The process undergoes three consecutive reversible reactions, where triglyceride is sequentially converted into diglyceride, monoglyceride, and glycerol. As a result, an alkyl ester is produced at each stage.
2 Parameters affecting the reaction
Four different parameters take part in the transesterification reaction. These are catalyst concentration, methanol to oil ratio, reaction time, and reaction temperature. The catalyst is used to speed up the reaction for converting triglycerides into fatty acid alkyl ester. Initially, a lower amount of catalyst is used to get enough time to complete the reaction. On further increasing the catalyst amount, a decrease in biodiesel yield has been observed due to a decrease in the diffusion rate of the reactants [23]. Excessive use of catalyst is found to form emulsions reflecting on higher viscosity, thereby making biodiesel recovery difficult. In the case of high FFA, the esterification process is performed, which produces water as a byproduct. The water content in the reaction can decrease the catalyst’s reactivity, thus affecting the biodiesel yield.
The alcohol to oil molar ratio has a significant effect on the biodiesel yield. In case of an insufficient amount of methanol, a reverse reaction occurs due to which a decrease in yield takes place. On the contrary, in the case of using excess alcohol, a higher conversion percentage takes place, which increases the yield. However, this excess methanol requires higher energy to recover the unreacted methanol after the reaction, increasing production costs [24]. Furthermore, methanol with polar hydroxyl group results in the emulsification of glycerol and the biodiesel formed during the reaction. This aids to the backward reaction, i.e., recombination of glycerol and esters, thus decreasing the biodiesel yield. Therefore, it must be noted that the transesterification reaction is reversible, and therefore a large amount of alcohol is required to keep the reaction in the forward direction.
Biodiesel yield is generally observed to increase with an increase in the reaction time. However, excess reaction time also negatively affects the biodiesel yield, resulting in a decrease in yield. This excess time also increases the energy cost. On the other hand, an increase in temperature is found to speed up the reaction, and more yield is achieved, which may be due to the reduction of oil’s viscosity on increasing the temperature, resulting in better mixing of oil with alcohol and faster separation glycerol from biodiesel. The reaction time and temperature majorly depend upon the type of catalyst and the amount of catalyst used in the reaction [25, 26]. Chavan et al. [27] have reported the maximum yield for the varying amount of catalyst and reaction temperature with different feedstock; for catalyst (3 wt%) with Pongamia oil, 90% yield obtained at the reaction temperature 65 ℃, whereas the reaction temperature decreases to 50 ℃ yield decreases to below 80%.
It was observed that the reaction parameters mainly vary according to the catalyst. Thus, this work focuses on studying different catalysts used in biodiesel production and discusses various advantages and disadvantages of different types of catalysts.
3 Homogeneous catalyst
Homogeneous catalysts are the commonly used catalysts in biodiesel production. These catalysts are also used in commercialized biodiesel production as they possess high catalytic activity; however, mainly solid base catalysts are used in the reaction. Nevertheless, using the base catalyst in case of high free fatty acids in the feedstock oil, saponification occurs to prevent this; acid pretreatment is performed over the oil to reduce its FFA. This process of treating biodiesel with acid is known as esterification [28, 29, 30]. Mostly used base catalysts are potassium hydroxide (KOH) and sodium hydroxide (NaOH) [31] and acid catalysts used are sulfuric acid (H2SO4) and phosphoric acid (H3PO4) [32] (Table 2).
The use of homogeneous catalysts increases the production cost of biodiesel as they are non-recyclable. These catalysts also face problems in separating homogeneous phase products and increasing the amount of wastewater produced during fuel purification [46]. Furthermore, from a technical point of view, the homogeneous catalysts consume two step-process due to the presence of FFA in oils which is a time-consuming and cost-increasing factor. Therefore, there is a need to introduce recyclable catalysts that can treat the problem of high FFA, reducing the steps in the process.
4 Heterogeneous catalysts
4.1 Solid acid catalyst
In concern of various disadvantages of using a homogeneous catalyst, heterogeneous catalysts were developed. These catalysts possess various advantages over homogeneous catalysts. These catalysts are prepared from the naturally aspirated metals and their derivatives. These catalysts are reusable, recyclable, and not affected by the amount of FFA present in triglycerides [47]. Thus, these can improve the overall energy consumption and reduce the time of pretreatment. Table 5 depicts various studies which show the potential use of heterogeneous acid catalysts with the process parameters in biodiesel production. The application of these catalysts obtains a high yield (Table 3).
Besides the advantages of homogeneous acid catalysts, there are certain disadvantages of using these catalysts, which must be considered when considering the catalyst. First, some catalysts possess low reactivity, which directly affects biodiesel yield. Second, they require high reaction temperature and poor reusability [63]. Finally, some catalysts of these categories have complicated preparation processes. Thus, there is a need to develop other catalysts that can tackle these disadvantages of homogeneous acid catalysts mentioned earlier.
4.2 Solid base catalyst
CaO is the most widely used alkaline metal catalyst in transesterification reactions for biodiesel production. The catalyst’s reactivity depends on the calcination temperature of the catalyst, whereas the reusability depends upon the efficiency of extraction of the catalyst from pure biodiesel during the cleaning process. Therefore, these catalysts have been found to have very effective reusable properties [64, 65]. The reactivity of these catalysts is very high thus, resulting in high biodiesel yield. In addition, these catalysts possess high catalytic life and are non-corrosive in nature. Table 4 depicts various studies performed by researchers using the solid base catalyst for biodiesel production from different feedstocks and their impact on other process parameters in Table 6.
Unlike heterogeneous acid catalysts, these catalysts are highly sensitive to the amount of water and FFA present in the feedstock oil. FFA requirement is less than 1%. The have a slow reaction rate compared to homogeneous catalysts, and in some cases, saponification occurs, resulting in degradation in the quality of biodiesel produced. The preparation method is also complex and expensive [85].
Besides the advantage of catalyst reusability, the high cost of biodiesel production is still a serious concern. The naturally aspirated metals present in the catalyst increase the cost of the catalyst as well as the process. Thus, there is a need to focus on eco-friendly methods to prepare the catalysts from renewable sources to be economical and less energy-consuming.
4.3 Heterogeneous catalyst derived from waste biomass
On considering problems with natural heterogeneous-based catalysts, researchers worldwide have worked for alternate sources for catalysts preparation. The new method of catalysts was developed by using lignocellulosic waste biomass and converting it into value-added products, i.e., heterogeneous catalysts. These catalysts are prepared from various types of biomass. Tables 5 and 6 depict various catalysts prepared from different types of feedstocks for transesterification reaction. This can help in reducing the cost of biodiesel production and can promote the commercialization of biodiesel.
4.3.1 Solid base catalyst from waste biomass
These catalysts are mainly prepared by direct calcination of biomass and integrating biomass with existing CaO catalysts. CaO was found one of the most widely used and promising heterogeneous base catalysts for biodiesel production [86]. Table 7 depicts various heterogeneous base catalysts developed from different feedstocks of biomass. These catalysts possess low surface reactivity, thus are integrated with other compounds to increase the reactivity and improve the biodiesel yield. As mentioned in above section, these base catalysts require low FFA oil for the transesterification reaction. Otherwise, there might be the formation of soap during the process. That means a pretreatment of oil is required in the process.
For further improvement in the performance of biomass CaO catalysts, researchers developed another method of integrating biomass with CaO. Researchers have limited the use of available CaO catalysts. Instead, more emphasis is provided on the CaO production from calcination of waste biomass [25]. Thus, various studies have been proposed o biomass-derived CaO and biomass as supporting materials for CaO.
4.3.2 Solid acid catalyst from waste biomass
The most common method for preparing acid catalysts is the sulfonation method comprising direct sulfonation by thermal treatment of carbon material concentrated with H2SO4. These carbon materials are produced from waste biomass. These catalysts are ineffective of high FFA present in triglycerides of oils. This sulfonation process helps in high biodiesel yield at the low reaction temperature. However, the corrosive behavior of H2SO4 has limited the use of solid acid catalysts [97].
4.4 Bimetallic and tri metallic catalyst
Nowadays, bimetallic and trimetallic heterogeneous catalysts have been examined for a variety of applications in energy and Biodiesel production and be used for environmental remediation. These catalysts have tunable properties that is controlled by their metallic compositions, morpho structure, and preparation method [107]. The performance of some bimetallic catalysts such as Au–Ag, Ca/Fe, Al–MCM-41, Ni–W, Mo–Zn, Mo–Mn, Mo–Sn supported on mixed metal oxide, W–Zr/CaO have been evaluated by different authors [82, 108, 109, 110] for the efficient production of biodiesel. However, few trimetallic heterogenous catalyst offered better catalytic properties over the mono and bimetallic catalyst. Abdulkareem et al. [111] experimentally reported the catalytic performance of Fe–Co–Ni predominates over Fe–Ni, Fe–Co, Ni–Co or their monometallic form. In addition to mono, bi and tri metallic catalyst, some ternary and quaternary metal compound such as Cu/Zn/Ca/Al2O3 and Cu/Ni/Ca/Al2O3 are also competent to produce methyl esters with greater yield and quality [112].
4.5 Catalysts used for biodiesel synthesis from microalgae
As the demand for oil crops increases for human consumption, it is not desirable to use oil crops for biofuel production. Hence, the researchers and scientists focused on alternative oil source lipids that contain aqua biomass in terms of microalgae or macroalgae. The application of microalgae over macroalgae toward biodiesel production has several advantages such as higher growth rate (doubling in 24 h); higher yield (15–300 times) as compared to the conventional oil crops in area wise; harvesting is also possible multi times in a year; they are highly biodegradable and nontoxic. Biodiesel can be made from the esterification and transesterification of a variety of microalgal/macroalgal lipids (such as Chlorella protothecoides, Oedigonium and Spirogyra) and an alcohol with the help of a catalyst [113]. Several homogenous catalysts such as sulfuric acid or sodium hydroxide used with methanol due to their high reaction at low temperature and atmospheric pressure. In addition to the homogenous catalysts, various heterogenous catalysts such as alumina supported calcium oxide and magnesium oxide, H-Beta zeolite, calcium methoxide, chromium–aluminum mixed oxide [114] are also used to produce methyl ester biodiesel from Nannochloropsis oculate, N. gaditana, Scenedesmus obliquus and Spirulina sp. microalgal lipids, respectively [113].
5 Economics of biodiesel
Several important factors drive the economics of biodiesel production and its feasibility in a practical world. First, government policies play a critical role in the biodiesel economy. Over the last decade, biodiesel production and usage have increased substantially with the involvement of governments and non-profit organizations in the form of biofuel mandates, subsidies to user groups and production companies, tax advantages, and compulsory targets. In the initial stages, vegetable oil feedstock was used for biodiesel production due to its principal usage for food and high cost. Hence the focus has been shifted to produce biodiesel from waste stream feedstock such as non-edible oils, micro-algae, waste plastics, and waste tyres. Thus, the major factors that influence the economics of biodiesel are crude oil prices, the production cost of feedstock, market requirements, and government contribution in the form of tax subsidies. Biodiesel made from microalgae has become a viable alternative to traditional feedstock in terms of industrial production and commercialization. Microalgae have a high rate of growth and carbon sequestration and can be easily grown in fresh and/or marine water without the use of arable soil. The cost of producing microalgae biodiesel is 0.38 USD per litre [115], which is commercially viable over a 10-year period when considering the economic value of residual biomass and glycerol by-products.
6 Major findings
The significant findings based on the current study are appended as follows:
-
Potassium hydroxide (KOH) and sodium hydroxide (NaOH) are the most commonly used base catalysts, while sulfuric acid (H2SO4) and phosphoric acid are the most commonly used acid catalysts (H3PO4).
-
In the transesterification reaction for biodiesel production, CaO is the most extensively utilized alkaline metal catalyst among KOH (32%)/ZrO2-5, Nd2O3–KOH, Sr/MgO, BaCeO3, and K2Al2O4.
-
Alternative catalyst preparation sources have been sought by researchers all around the world. The new catalytic approach was created by turning waste lignocellulosic biomass into value-added. Despite the benefit of catalyst reusability, the high cost of biodiesel manufacturing remains a major concern. In addition, the presence of naturally aspirated metals in the catalyst raises the cost of both the catalyst and the process. As a result, eco-friendly approaches for preparing catalysts from renewable sources must be prioritized in order for the process to be cost-effective and energy efficient.
-
Bimetallic and trimetallic heterogeneous catalysts are being investigated for several applications in energy and biodiesel generation and environmental cleanup. Trimetallic Fe–Co–Ni outperforms bimetallic Fe–Ni, Fe–Co, Ni–Co or its monometallic form.
-
Various heterogeneous catalysts are used to produce methyl ester biodiesel from Nannochloropsis oculate, N. gaditana, Scenedesmus obliquus, and Spirulina sp. microalgal lipids, including alumina supported calcium oxide and magnesium oxide, H-Beta zeolite, calcium methoxide, and chromium–aluminum mixed oxide.
7 Conclusions
For commercial biodiesel production, most firms employ homogenous catalysts, which causes separation and waste neutralization issues. In addition, during the purification of biodiesel, these catalysts produce a substantial volume of wastewater. Advances in heterogeneous catalysts have helped to alleviate the issues associated with homogeneous catalysts while also lowering the cost of producing biodiesel. They have a non-corrosive nature and can be reused. The use of biomass for catalyst manufacture has also advanced the preparation of heterogeneous catalysts. As a result, the transesterification reaction is processed in a green manner. These catalysts lower the transesterification process’ activation energy, reducing energy consumption and reaction time. The use of biomass-derived catalysts lowers the cost of manufacturing biodiesel while maintaining a high output.
References
Jasrotia, A., Shukla, A. K., & Kumar, N. (2020). Impact of nanoparticles on the performance and emissions of diesel engine using mahua biodiesel. In: Yadav, S., Singh, D. B., Arora, P. K., Kumar, H. (Eds.), Proceedings of international conference in mechanical and energy technology. Springer, Singapore (pp.49–59). https://doi.org/10.1007/978-981-15-2647-3_5
Dwivedi, G., & Sharma, M. P. (2013). Performance evaluation of diesel engine using biodiesel from pongamia oil. International Journal of Renewable Energy Research, 3, 325–330. https://doi.org/10.20508/ijrer.99993
Dwivedi, G., Pillai, S., & Shukla, A. K. (2019). Study of performance and emissions of engines fueled by biofuels and its blends. In A. K. Agarwal, A. Gautam, N. Sharma, & A. P. Singh (Eds.), Methanol and the alternate fuel economy (pp. 77–106). Singapore: Springer . https://doi.org/10.1007/978-981-13-3287-6_5
Dwivedi, G., & Sharma, M. P. (2014). Potential and limitation of straight vegetable oils as engine fuel—An Indian perspective. Renewable and Sustainable Energy Reviews, 33, 316–322. https://doi.org/10.1016/j.rser.2014.02.004
BPSTATS (2019). BP statistical review of world energy statistical review of world, 68th edition. The Editor BP Statistical review of world energy (pp. 1–69).
Kumar Shukla, A., Ahmad, Z., Sharma, M., Dwivedi, G., Nath Verma, T., Jain, S., & Zare, A. (2020). Advances of carbon capture and storage in coal-based power generating units in an indian context. Energies, 13, 4124.
Verma, T. N., Shrivastava, P., Rajak, U., Dwivedi, G., Jain, S., Zare, A., & Verma, P. (2021). A comprehensive review of the influence of physicochemical properties of biodiesel on combustion characteristics, engine performance and emissions. Journal of Traffic and Transportation Engineering (English Edition), 8, 510–533. https://doi.org/10.1016/j.jtte.2021.04.006
Sharma, A., Shukla, A. K., Singh, O., & Sharma, M. (2021). Recent advances in gas/steam power cycles for concentrating solar power. International Journal of Ambient Energy. https://doi.org/10.1080/01430750.2021.1919552
Verma, P., Sharma, M. P., & Dwivedi, G. (2016). Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Reports, 2, 8–13. https://doi.org/10.1016/j.egyr.2015.12.001
Verma, P., Sharma, M. P., & Dwivedi, G. (2016). Potential use of eucalyptus biodiesel in compressed ignition engine. Egyptian Journal of Petroleum, 25, 91–95. https://doi.org/10.1016/j.ejpe.2015.03.008
Dwivedi, G., & Sharma, M. P. (2013). Performance evaluation of diesel engine using biodiesel from pongamia oil.
Verma, S., Sharma, B., Ahmad, J., Dwivedi, G., & Nandan, G. (2018). Impact assessment of ethanol as fuel for engine operation. Materials Today: Proceedings, 5, 6115–6120. https://doi.org/10.1016/j.matpr.2017.12.217
Madhu, D., Arora, R., Sahani, S., Singh, V., & Sharma, Y. C. (2017). Synthesis of high-quality biodiesel using feedstock and catalyst derived from fish wastes. Journal of Agricultural and Food Chemistry, 65, 2100–2109.
Madhu, D., Chavan, S. B., Singh, V., Singh, B., & Sharma, Y. C. (2016). An economically viable synthesis of biodiesel from a crude Millettia pinnata oil of Jharkhand, India as feedstock and crab shell derived catalyst. Bioresource Technology, 214, 210–217.
Chhabra, M., Dwivedi, G., Baredar, P., Shukla, A. K., Garg, A., & Jain, S. (2020). Production & optimization of biodiesel from rubber oil using BBD technique. Materials Today: Proceedings.
Rizwanul Fattah, I. M., Ong, H. C., Mahlia, T. M. I., Mofijur, M., Silitonga, A. S., Rahman, S. M. A., & Ahmad, A. (2020). State of the art of catalysts for biodiesel production. Frontiers of Energy Research, 8, 101.
11.4 Economics of Biodiesel Production (accessed September 3, 2020). Including Economics of Algae | EGEE 439: Alternative Fuels from Biomass Sources, (n.d.). https://doi.org/https://www.e-education.psu.edu/egee439/node/722
Jain, S., & Sharma, M. P. (2010). Biodiesel production from Jatropha oil Biodiesel production from Jatropha curcas oil. Renewable and Sustainable Energy Reviews. https://doi.org/10.1016/j.rser.2010.07.047
Sharma, Y. C., Kumar, A., Prasad, R., & Upadhyay, S. N. (2017). Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renewable and Sustainable Energy Reviews, 74, 89–103.
Jain, S., & Sharma, M. P. (2010). Prospects of biodiesel from Jatropha in India: A review. Renewable and Sustainable Energy Reviews, 14, 763–771. https://doi.org/10.1016/j.rser.2009.10.005
Chhabra, M., Sharma, A., & Dwivedi, G. (2017). Performance evaluation of diesel engine using rice bran biodiesel. Egyptian Journal of Petroleum, 26, 511–518. https://doi.org/10.1016/j.ejpe.2016.07.002
Dwivedi, G., Verma, P., & Sharma, M. P. (2018). Optimization of storage stability for karanja biodiesel using Box–Behnken design. Waste Biomass Valor, 9, 645–655. https://doi.org/10.1007/s12649-016-9739-2
Narula, V., Thakur, A., Uniyal, A., Kalra, S., & Jain, S. (2017). Process parameter optimization of low temperature transesterification of algae-Jatropha curcas oil blend. Energy, 119, 983–988. https://doi.org/10.1016/j.energy.2016.11.043
Verma, P., Sharma, M. P., & Dwivedi, G. (2016). Impact of alcohol on biodiesel production and properties. Renewable and Sustainable Energy Reviews,. https://doi.org/10.1016/j.rser.2015.11.048
Abdullah, S. H. Y. S., Hanapi, N. H. M., Azid, A., Umar, R., Juahir, H., Khatoon, H., & Endut, A. (2017). A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renewable and Sustainable Energy Reviews, 70, 1040–1051. https://doi.org/10.1016/j.rser.2016.12.008
Vishal, D., Dubey, S., Goyal, R., Dwivedi, G., Baredar, P., & Chhabra, M. (2020). Optimization of alkali-catalyzed transesterification of rubber oil for biodiesel production & its impact on engine performance. Renewable Energy, 158, 167–180. https://doi.org/10.1016/j.renene.2020.05.136
Chavan, S. B., Kumbhar, R. R., Madhu, D., Singh, B., & Sharma, Y. C. (2015). Synthesis of biodiesel from Jatropha curcas oil using waste eggshell and study of its fuel properties. RSC Advances, 5, 63596–63604.
Dwivedi, G., & Sharma, M. P. (2014). Prospects of biodiesel from Pongamia in India. Renewable and Sustainable Energy Reviews, 32, 114–122. https://doi.org/10.1016/j.rser.2014.01.009
Chhabra, M., Saini, B. S., & Dwivedi, G. (2019). Impact assessment of biofuel from waste neem oil. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects,. https://doi.org/10.1080/15567036.2019.1623946
Samuel, O. D., Okwu, M. O., Oyejide, O. J., Taghinezhad, E., Afzal, A., & Kaveh, M. (2020). Optimizing biodiesel production from abundant waste oils through empirical method and grey wolf optimizer. Fuel, 281, 118701. https://doi.org/10.1016/j.fuel.2020.118701
Samuel, O. D., Adekojo Waheed, M., Taheri-Garavand, A., Verma, T. N., Dairo, O. U., Bolaji, B. O., & Afzal, A. (2021). Prandtl number of optimum biodiesel from food industrial waste oil and diesel fuel blend for diesel engine. Fuel, 285, 119049. https://doi.org/10.1016/j.fuel.2020.119049
Zikri, A., Erlinawati, S. P., Agus, M., & Fathona, S. (2020). Biodiesel production from bintaro (Cerbera manghas L) seeds with potassium hydroxide as catalyst. Journal of Physics: Conference Series.
Jain, S., & Sharma, M. P. (2010). Biodiesel production from Jatropha curcas oil. Renewable and Sustainable Energy Reviews. https://doi.org/10.1016/j.rser.2010.07.047
Dwivedi, G., & Sharma, M. P. (2015). Application of Box–Behnken design in optimization of biodiesel yield from Pongamia oil and its stability analysis. Fuel, 145, 256–262. https://doi.org/10.1016/j.fuel.2014.12.063
Sinha, S., Agarwal, A. K., & Garg, S. (2008). Biodiesel development from rice bran oil: Transesterification process optimization and fuel characterization. Energy Conversion and Management, 49, 1248–1257. https://doi.org/10.1016/j.enconman.2007.08.010
Nakpong, P., & Wootthikanokkhan, S. (2010). High free fatty acid coconut oil as a potential feedstock for biodiesel production in Thailand. Renewable Energy, 35, 1682–1687. https://doi.org/10.1016/j.renene.2009.12.004
Jain, S., Sharma, M. P., & Rajvanshi, S. (2011). Acid base catalyzed transesterification kinetics of waste cooking oil. Fuel Processing Technology, 92, 32–38. https://doi.org/10.1016/j.fuproc.2010.08.017
Ghadge, S. V., & Raheman, H. (2006). Process optimization for biodiesel production from mahua (Madhuca indica) oil using response surface methodology. Bioresource Technology, 97, 379–384. https://doi.org/10.1016/j.biortech.2005.03.014
Thangaraj, B., Ramachandran, K. B., & Raj, S. P. (2014). Homogeneous catalytic transesterification of renewable azadirachta indica (neem) oil and its derivatives to biodiesel fuel via acid/alkaline esterification processes. IBIMA Publishing International Journal of Renewable Energy & Biofuels International Journal of Renewable Energy & Biofuels https://doi.org/10.5171/2014.515961
Veljković, V. B., Lakićević, S. H., Stamenković, O. S., Todorović, Z. B., & Lazić, M. L. (2006). Biodiesel production from tobacco (Nicotiana tabacum L.) seed oil with a high content of free fatty acids. Fuel, 85, 2671–2675. https://doi.org/10.1016/j.fuel.2006.04.015
Rashid, U., Anwar, F., Moser, B. R., & Ashraf, S. (2008). Production of sunflower oil methyl esters by optimized alkali-catalyzed methanolysis. Biomass and Bioenergy, 32, 1202–1205. https://doi.org/10.1016/j.biombioe.2008.03.001
Kumar, R., Tiwari, P., & Garg, S. (2013). Alkali transesterification of linseed oil for biodiesel production. Fuel, 104, 553–560. https://doi.org/10.1016/j.fuel.2012.05.002
Naik, B. D., & Meivelu, U. (2020). Experimental studies on sodium methoxide supported bentonite catalyst for biodiesel preparation from waste sunflower oil. Environmental Progress and Sustainable Energy.
Kamran, E., Mashhadi, H., Mohammadi, A., & Ghobadian, B. (2020). Biodiesel production from Elaeagnus angustifolia L seed as a novel waste feedstock using potassium hydroxide catalyst. Biocatalysis and Agricultural Biotechnology, 25, 101578. https://doi.org/10.1016/j.bcab.2020.101578
Jahirul, M. I., Brown, R. J., Senadeera, W., Ashwath, N., Rasul, M. G., Rahman, M. M., & O’Hara, I. M. (2015). Physio-chemical assessment of beauty leaf (Calophyllum inophyllum) as second-generation biodiesel feedstock. Energy Reports, 1, 204–215. https://doi.org/10.1016/j.egyr.2015.10.003
Leung, D. Y. C., Wu, X., & Leung, M. K. H. (2010). A review on biodiesel production using catalyzed transesterification. Applied Energy, 87, 1083–1095. https://doi.org/10.1016/j.apenergy.2009.10.006
Tariq, M., Ali, S., & Khalid, N. (2012). Activity of homogeneous and heterogeneous catalysts, spectroscopic and chromatographic characterization of biodiesel: A review. Renewable and Sustainable Energy Reviews, 16, 6303–6316. https://doi.org/10.1016/j.rser.2012.07.005
Alhassan, F. H., Yunus, R., Rashid, U., Sirat, K., Islam, A., Lee, H. V., & Taufiq-Yap, Y. H. (2013). Production of biodiesel from mixed waste vegetable oils using Ferric hydrogen sulphate as an effective reusable heterogeneous solid acid catalyst. Applied Catalysis, A: General, 456, 182–187. https://doi.org/10.1016/j.apcata.2013.02.019
Sheikh, R., Choi, M. S., Im, J. S., & Park, Y. H. (2013). Study on the solid acid catalysts in biodiesel production from high acid value oil. Journal of Industrial and Engineering Chemistry, 19, 1413–1419. https://doi.org/10.1016/j.jiec.2013.01.005
da Conceição, L. R. V., Carneiro, L. M., Rivaldi, J. D., & de Castro, H. F. (2016). Solid acid as catalyst for biodiesel production via simultaneous esterification and transesterification of macaw palm oil. Industrial Crops and Products, 89, 416–424. https://doi.org/10.1016/j.indcrop.2016.05.044
Yee, K. F., Lee, K. T., Ceccato, R., & Abdullah, A. Z. (2011). Production of biodiesel from Jatropha curcas L. oil catalyzed by SO42-/ZrO2 catalyst: Effect of interaction between process variables. Bioresource Technology, 102, 4285–4289. https://doi.org/10.1016/j.biortech.2010.12.048
Ramachandran, K., Sivakumar, P., Suganya, T., & Renganathan, S. (2011). Production of biodiesel from mixed waste vegetable oil using an aluminium hydrogen sulphate as a heterogeneous acid catalyst. Bioresource Technology, 102, 7289–7293. https://doi.org/10.1016/j.biortech.2011.04.100
Kafuku, G., Lam, M. K., Kansedo, J., Lee, K. T., & Mbarawa, M. (2010). Heterogeneous catalyzed biodiesel production from Moringa oleifera oil. Fuel Processing Technology, 91, 1525–1529. https://doi.org/10.1016/j.fuproc.2010.05.032
Park, Y. M., Lee, D. W., Kim, D. K., Lee, J. S., & Lee, K. Y. (2008). The heterogeneous catalyst system for the continuous conversion of free fatty acids in used vegetable oils for the production of biodiesel. Catalysis Today, 131, 238–243. https://doi.org/10.1016/j.cattod.2007.10.052
Guldhe, A., Moura, C. V. R., Singh, P., Rawat, I., Moura, E. M., Sharma, Y., & Bux, F. (2017). Conversion of microalgal lipids to biodiesel using chromium-aluminum mixed oxide as a heterogeneous solid acid catalyst. Renewable Energy, 105, 175–182. https://doi.org/10.1016/j.renene.2016.12.053
Gardy, J., Nourafkan, E., Osatiashtiani, A., Lee, A. F., Wilson, K., Hassanpour, A., & Lai, X. (2019). A core-shell SO4/Mg–Al–Fe3O4 catalyst for biodiesel production. Applied Catalysis, B: Environmental, 259, 118093. https://doi.org/10.1016/j.apcatb.2019.118093
Xie, W., Wang, H., & Li, H. (2012). Silica-supported tin oxides as heterogeneous acid catalysts for transesterification of soybean oil with methanol. Industrial and Engineering Chemistry Research, 51, 225–231. https://doi.org/10.1021/ie202262t
Sahani, S., Banerjee, S., & Sharma, Y. C. (2018). Study of’co-solvent effect’on production of biodiesel from Schleichera Oleosa oil using a mixed metal oxide as a potential catalyst. Journal of the Taiwan Institute of Chemical Engineers, 86, 42–56.
Singh, V., Yadav, M., & Sharma, Y. C. (2017). Effect of co-solvent on biodiesel production using calcium aluminium oxide as a reusable catalyst and waste vegetable oil. Fuel, 203, 360–369.
Nongbe, M. C., Ekou, T., Ekou, L., Yao, K. B., Le Grognec, E., & Felpin, F. X. (2017). Biodiesel production from palm oil using sulfonated graphene catalyst. Renewable Energy, 106, 135–141.
Zhou, Y., Noshadi, I., Ding, H., Liu, J., Parnas, R. S., Clearfield, A., & Sun, L. (2018). Solid acid catalyst based on single-layer α-zirconium phosphate nanosheets for biodiesel production via esterification. Catalysts, 8, 17.
Singh, V., Bux, F., & Sharma, Y. C. (2016). A low cost one pot synthesis of biodiesel from waste frying oil (WFO) using a novel material, β-potassium dizirconate (β-K2Zr2O5). Applied Energy, 172, 23–33.
Borges, M. E., & Díaz, L. (2012). Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renewable and Sustainable Energy Reviews, 16, 2839–2849. https://doi.org/10.1016/j.rser.2012.01.071
Boey, P. L., Maniam, G. P., & Hamid, S. A. (2011). Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chemical Engineering Journal, 168, 15–22. https://doi.org/10.1016/j.cej.2011.01.009
Chavan, S. B., Kumbhar, R. R., Kumar, A., & Sharma, Y. C. (2015). Study of biodiesel blends on emission and performance characterization of a variable compression ratio engine. Energy & Fuels, 29, 4393–4398.
Narula, V., Khan, M. F., Negi, A., Kalra, S., Thakur, A., & Jain, S. (2017). Low temperature optimization of biodiesel production from algal oil using CaO and CaO/Al2O3 as catalyst by the application of response surface methodology. Energy, 140, 879–884. https://doi.org/10.1016/j.energy.2017.09.028
Teo, S. H., Rashid, U., & Taufiq-Yap, Y. H. (2014). Biodiesel production from crude Jatropha curcas oil using calcium based mixed oxide catalysts. Fuel, 136, 244–252. https://doi.org/10.1016/j.fuel.2014.07.062
Kaur, M., & Ali, A. (2011). Lithium ion impregnated calcium oxide as nano catalyst for the biodiesel production from karanja and jatropha oils. Renewable Energy, 36, 2866–2871. https://doi.org/10.1016/j.renene.2011.04.014
Takase, M., Zhang, M., Feng, W., Chen, Y., Zhao, T., Cobbina, S. J., & Wu, X. (2014). Application of zirconia modified with KOH as heterogeneous solid base catalyst to new non-edible oil for biodiesel. Energy Conversion and Management, 80, 117–125. https://doi.org/10.1016/j.enconman.2014.01.034
Li, Y., Qiu, F., Yang, D., Li, X., & Sun, P. (2011). Preparation, characterization and application of heterogeneous solid base catalyst for biodiesel production from soybean oil. Biomass and Bioenergy, 35, 2787–2795. https://doi.org/10.1016/j.biombioe.2011.03.009
Sun, C., Qiu, F., Yang, D., & Ye, B. (2014). Preparation of biodiesel from soybean oil catalyzed by Al–Ca hydrotalcite loaded with K2CO3 as heterogeneous solid base catalyst. Fuel Processing Technology, 126, 383–391. https://doi.org/10.1016/j.fuproc.2014.05.021
Hernández-Hipólito, P., Juárez-Flores, N., Martínez-Klimova, E., Gómez-Cortés, A., Bokhimi, X., Escobar-Alarcón, L., & Klimova, T. E. (2015). Novel heterogeneous basic catalysts for biodiesel production: Sodium titanate nanotubes doped with potassium. Catalysis Today, 250, 187–196. https://doi.org/10.1016/j.cattod.2014.03.025
Mahesh, S. E., Ramanathan, A., Begum, K. M. M. S., & Narayanan, A. (2015). Biodiesel production from waste cooking oil using KBr impregnated CaO as catalyst. Energy Conversion and Management, 91, 442–450. https://doi.org/10.1016/j.enconman.2014.12.031
Wang, B., Li, S., Tian, S., Feng, R., & Meng, Y. (2013). A new solid base catalyst for the transesterification of rapeseed oil to biodiesel with methanol. Fuel, 104, 698–703. https://doi.org/10.1016/j.fuel.2012.08.034
Tantirungrotechai, J., Thepwatee, S., & Yoosuk, B. (2013). Biodiesel synthesis over Sr/MgO solid base catalyst. Fuel, 106, 279–284. https://doi.org/10.1016/j.fuel.2013.01.028
Sahani, S., Roy, T., & Sharma, Y. C. (2020). Studies on fast and green biodiesel production from an indigenous nonedible Indian feedstock using single phase strontium titanate catalyst. Energy Conversion and Management, 203, 112180. https://doi.org/10.1016/j.enconman.2019.112180
Yadav, M., & Sharma, Y. C. (2018). Process optimization and catalyst poisoning study of biodiesel production from kusum oil using potassium aluminum oxide as efficient and reusable heterogeneous catalyst. Journal of Cleaner Production, 199, 593–602.
Roy, T., Sahani, S., & Sharma, Y. C. (2020). Green synthesis of biodiesel from Ricinus communis oil (castor seed oil) using potassium promoted lanthanum oxide catalyst: Kinetic, thermodynamic and environmental studies. Fuel, 274, 117644. https://doi.org/10.1016/j.fuel.2020.117644
Sahani, S., Roy, T., & Chandra Sharma, Y. (2019). Clean and efficient production of biodiesel using barium cerate as a heterogeneous catalyst for the biodiesel production; kinetics and thermodynamic study. Journal of Cleaner Production, 237, 117699. https://doi.org/10.1016/j.jclepro.2019.117699
Yadav, M., Chavan, S. B., Singh, R., Bux, F., & Sharma, Y. C. (2019). Experimental study on emissions of algal biodiesel and its blends on a diesel engine. Journal of the Taiwan Institute of Chemical Engineers, 96, 160–168.
Singh, R., Kumar, A., & Chandra Sharma, Y. (2019). Biodiesel production from microalgal oil using barium–calcium–zinc mixed oxide base catalyst: Optimization and kinetic studies. Energy & Fuels, 33, 1175–1184. https://doi.org/10.1021/acs.energyfuels.8b03461
Banerjee, S., Sahani, S., & Sharma, Y. C. (2019). Process dynamic investigations and emission analyses of biodiesel produced using Sr–Ce mixed metal oxide heterogeneous catalyst. Journal of Environmental Management, 248, 109218.
Sahani, S., & Sharma, Y. C. (2018). Economically viable production of biodiesel using a novel heterogeneous catalyst: Kinetic and thermodynamic investigations. Energy Conversion and Management, 171, 969–983.
Yadav, M., Singh, V., & Sharma, Y. C. (2017). Methyl transesterification of waste cooking oil using a laboratory synthesized reusable heterogeneous base catalyst: Process optimization and homogeneity study of catalyst. Energy Conversion and Management, 148, 1438–1452.
Chouhan, A. P. S., & Sarma, A. K. (2011). Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renewable and Sustainable Energy Reviews, 15, 4378–4399. https://doi.org/10.1016/j.rser.2011.07.112
Shan, R., Lu, L., Shi, Y., Yuan, H., & Shi, J. (2018). Catalysts from renewable resources for biodiesel production. Energy Conversion and Management, 178, 277–289. https://doi.org/10.1016/j.enconman.2018.10.032
Pandit, P. R., & Fulekar, M. H. (2019). Biodiesel production from microalgal biomass using CaO catalyst synthesized from natural waste material. Renewable Energy, 136, 837–845. https://doi.org/10.1016/j.renene.2019.01.047
Al-Muhtaseb, A. H., Jamil, F., Al-Haj, L., Zar Myint, M. T., Mahmoud, E., Ahmad, M. N. M. … Rafiq, S. (2018). Biodiesel production over a catalyst prepared from biomass-derived waste date pits. Biotechnology Reports, 20, https://doi.org/10.1016/j.btre.2018.e00284
Abu-Jrai, A. M., Jamil, F., Al-Muhtaseb, A. H., Baawain, M., Al-Haj, L., Al-Hinai, M., & Rafiq, S. (2017). Valorization of waste Date pits biomass for biodiesel production in presence of green carbon catalyst. Energy Conversion and Management, 135, 236–243. https://doi.org/10.1016/j.enconman.2016.12.083
Zhao, C., Lv, P., Yang, L., Xing, S., Luo, W., & Wang, Z. (2018). Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Conversion and Management, 160, 477–485. https://doi.org/10.1016/j.enconman.2018.01.059
Mansir, N., Teo, S. H., Rabiu, I., & Taufiq-Yap, Y. H. (2018). Effective biodiesel synthesis from waste cooking oil and biomass residue solid green catalyst. Chemical Engineering Journal, 347, 137–144. https://doi.org/10.1016/j.cej.2018.04.034
Bhatia, S. K., Gurav, R., Choi, T. R., Kim, H. J., Yang, S. Y., Song, H. S., & Yang, Y. H. (2020). Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresource Technology, 302, 122872. https://doi.org/10.1016/j.biortech.2020.122872
Bastos, R. R. C., da Luz, A. P., Corrêa, P. T. S., da Luz, G. N., da Rocha Filho, J. R., & Zamian. (2020). L.R.V. da Conceição, Optimization of biodiesel production using sulfonated carbon-based catalyst from an amazon agro-industrial waste. Energy Conversion and Management, 205, 112457. https://doi.org/10.1016/j.enconman.2019.112457
Yaşar, F. (2019). Biodiesel production via waste eggshell as a low-cost heterogeneous catalyst: Its effects on some critical fuel properties and comparison with CaO. Fuel, 255, 115828. https://doi.org/10.1016/j.fuel.2019.115828
Mendonça, I. M., Paes, O. A. R. L., Maia, P. J. S., Souza, M. P., Almeida, R. A., Silva, C. C., & de Freitas, F. A. (2019). New heterogeneous catalyst for biodiesel production from waste tucumã peels (Astrocaryum aculeatum Meyer): Parameters optimization study. Renewable Energy, 130, 103–110. https://doi.org/10.1016/j.renene.2018.06.059
Sai, B. A. V. S. L., Subramaniapillai, N., Khadhar Mohamed, M. S. B., & Narayanan, A. (2020). Optimization of continuous biodiesel production from rubber seed oil (RSO) using calcined eggshells as heterogeneous catalyst. Journal of Environmental Chemical Engineering, 8, 103603. https://doi.org/10.1016/j.jece.2019.103603
Tang, Z. E., Lim, S., Pang, Y. L., Ong, H. C., & Lee, K. T. (2018). Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renewable and Sustainable Energy Reviews, 92, 235–253. https://doi.org/10.1016/j.rser.2018.04.056
Mardhiah, H. H., Ong, H. C., Masjuki, H. H., Lim, S., & Pang, Y. L. (2017). Investigation of carbon-based solid acid catalyst from Jatropha curcas biomass in biodiesel production. Energy Conversion and Management, 144, 10–17. https://doi.org/10.1016/j.enconman.2017.04.038
Saravanan Arumugamurthy, S., Sivanandi, P., Pandian, S., Choksi, H., & Subramanian, D. (2019). Conversion of a low value industrial waste into biodiesel using a catalyst derived from brewery waste: An activation and deactivation kinetic study. Waste Management, 100, 318–326. https://doi.org/10.1016/j.wasman.2019.09.030
Thushari, I., Babel, S., & Samart, C. (2019). Biodiesel production in an autoclave reactor using waste palm oil and coconut coir husk derived catalyst. Renewable Energy, 134, 125–134. https://doi.org/10.1016/j.renene.2018.11.030
Fatimah, I., Rubiyanto, D., Taushiyah, A., Najah, F. B., Azmi, U., & Sim, Y. L. (2019). Use of ZrO 2 supported on bamboo leaf ash as a heterogeneous catalyst in microwave-assisted biodiesel conversion. Sustainable Cities and Society, 12, 100129. https://doi.org/10.1016/j.scp.2019.100129
Lathiya, D. R., Bhatt, D. V., & Maheria, K. C. (2018). Synthesis of sulfonated carbon catalyst from waste orange peel for cost effective biodiesel production. Bioresource Technology Reports, 2, 69–76. https://doi.org/10.1016/j.biteb.2018.04.007
Bora, A. P., Dhawane, S. H., Anupam, K., & Halder, G. (2018). Biodiesel synthesis from Mesua ferrea oil using waste shell derived carbon catalyst. Renewable Energy, 121, 195–204. https://doi.org/10.1016/j.renene.2018.01.036
Sandouqa, A., Al-Hamamre, Z., & Asfar, J. (2019). Preparation and performance investigation of a lignin-based solid acid catalyst manufactured from olive cake for biodiesel production. Renewable Energy, 132, 667–682. https://doi.org/10.1016/j.renene.2018.08.029
Endut, A., Abdullah, S. H. Y. S., Hanapi, N. H. M., Hamid, S. H. A., Lananan, F., Kamarudin, M. K. A., & Khatoon, H. (2017). Optimization of biodiesel production by solid acid catalyst derived from coconut shell via response surface methodology. International Biodeterioration and Biodegradation, 124, 250–257. https://doi.org/10.1016/j.ibiod.2017.06.008
Farabi, M. S. A., Ibrahim, M. L., Rashid, U., & Taufiq-Yap, Y. H. (2019). Esterification of palm fatty acid distillate using sulfonated carbon-based catalyst derived from palm kernel shell and bamboo. Energy Conversion and Management, 181, 562–570. https://doi.org/10.1016/j.enconman.2018.12.033
De, S., Zhang, J., Luque, R., & Yan, N. (2016). Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy and Environmental Science, 9, 3314–3347. https://doi.org/10.1039/C6EE02002J
Yang, R., Du, X., Zhang, X., Xin, H., Zhou, K., Li, D., & Hu, C. (2019). Transformation of jatropha oil into high-quality biofuel over Ni–W bimetallic catalysts. ACS Omega. https://doi.org/10.1021/acsomega.9b00375
Kwong, T. L., & Yung, K. F. (2015). Heterogeneous alkaline earth metal–transition metal bimetallic catalysts for synthesis of biodiesel from low grade unrefined feedstock. RSC Advances, 5, 83748–83756. https://doi.org/10.1039/C5RA13819A
Farooq, M., Ramli, A., Naeem, A., & Saleem khan, M. (2016). Effect of different metal oxides on the catalytic activity of γ-Al 2 O 3 –MgO supported bifunctional heterogeneous catalyst in biodiesel production from WCO. RSC Advances, 6, 872–881. https://doi.org/10.1039/C5RA18146A
Abdulkareem, A. S., Kariim, I., Bankole, M. T., Tijani, J. O., Abodunrin, T. F., & Olu, S. C. (2017). Synthesis and characterization of tri-metallic Fe–Co–Ni catalyst supported on CaCO3 for multi-walled carbon nanotubes growth via chemical vapor deposition technique. Arabian Journal for Science and Engineering, 42, 4365–4381. https://doi.org/10.1007/s13369-017-2478-2
KAMAL, N. B. M. (2018). Preparation, characterization and mechanistic study of alumina supported calcium oxide based catalysts in transesterification of refined cooking oil. Universiti Teknologi Malaysia
Turkkul, B., Deliismail, O., & Seker, E. (2020). Ethyl esters biodiesel production from Spirulina sp. and Nannochloropsis oculata microalgal lipids over alumina-calcium oxide catalyst. Renewable Energy, 145, 1014–1019.
Zhang, Q., Zhang, Y., Deng, T., Wei, F., Jin, J., & Ma, P. (2020). Sustainable production of biodiesel over heterogeneous acid catalysts. In Biomass, Biofuels, Biochemicals (pp. 407–432). Elsevier.
Branco-Vieira, M., Mata, T. M., Martins, A. A., Freitas, M. A. V., & Caetano, N. S. (2020). Economic analysis of microalgae biodiesel production in a small-scale facility. Energy Reports, 6, 325–332. https://doi.org/10.1016/j.egyr.2020.11.156
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dwivedi, G., Jain, S., Shukla, A.K. et al. Impact analysis of biodiesel production parameters for different catalyst. Environ Dev Sustain (2022). https://doi.org/10.1007/s10668-021-02073-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10668-021-02073-w