Abstract
This study aims to produce a significant quantity of fermentable sugars from tubers sourced from northern Thailand to boost bioethanol production. Bioethanol production from Amorphophallus spp. tubers was compared using biological and chemical approaches. The tubers were sliced into small pieces (1–2 cm), dried in a solar oven, and powdered before hydrolyzing with cellulase enzymes. The results revealed the fermentable sugar content of Amorphophallus spp. tuber increased from 2.6 g/L–19.01 g/L after enzymatic hydrolysis. Furthermore, under the ideal condition, the total sugar concentration was 33.22 g/L. Enzymes help to speed up the hydrolysis process, and biological methods are also less expensive and more ecologically friendly than chemical equivalents. After the alcoholic fermentation, the highest ethanol content was obtained, 8.68 ± 0.91 g/L, using S. cerevisiae for 48 h. Konjac receives little attention in the biofuel industry due to its irritating nature to the mouth and throat when swallowed, implying that competition between the fuel and food sectors is lower than for other feedstocks such as cassava and corn. Improved fermentable sugars may be used in the near future for bioethanol production to address the worldwide issue of declining fossil fuel consumption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
For several years, the primary energy sources have been non-renewable fossil fuels, oil, natural gas, and coal (Bhuyar et al., 2021; Deepanraj et al., 2017). However, these energy sources are inadequate to fulfill today's most essential requirements of societies, in particular from the environmental and public health perspectives (Mejica et al., 2021; Unpaprom et al. 2017). Biofuels have appeared to respond to ever-increasing energy demand worldwide (Nguyen et al., 2020a; Unpaprom et al. 2021; Saengsawang et al., 2020). By 2017, renewable energy only accounted for about 8% of Indonesia's primary energy mix and slightly increased to 9% in 2019. Since of the rising cost of non-renewable fuels and the increased carbon dioxide emissions (Bhuyar et al., 2019; Lawrence et al., 2011). Biofuels are made from plant materials and are primarily made up of lignocellulose and starch. Starchy tubers are more beneficial than currently accessible fuels if these materials are transformed into biofuel. Biofuels like bioethanol have been seen to gain the peak of attention as they may be acting as the substitute to fuels that are based on petroleum and may protect the reserves of oil and also may reduce the greenhouse gases and atmospheric carbon dioxide (Kausar et al., 2021; Manmai et al., 2020a; Nguyen et al., 2020b).
Konjac (Amorphophallus spp.) grows well at the edge of the forest teak, under the clumping bamboo, on the banks of the river, in thickets, and other locations up to 2500 m above sea level (Bhuyar et al., 2020; Kasno et al., 2007). Konjac is a type of plant tuber with good potential and prospects as a raw material for bioethanol production, as well as a high rate of growth. Konjac tubers have 17.5% (w/w) carbohydrate, while amylose and amylopectin include 24.50% and 75.50% (w/w), respectively (Wankhede & Sajjan, 1981). While using an Integrated bioprocessing (IBP) approach for the production of ethanol from inexpensive (alternative) starch-based substrates could reduce the cost of the process, this approach requires robust microbial hosts (such as Saccharomyces cerevisiae) that produce both -amylases and glucoamylases in sufficient quantities to ensure complete hydrolysis of the starchy substrate. This approach is currently under investigation (Favaro et al., 2019; Van Zyl et al., 2012). The method of producing bioethanol from starch-based raw materials begins with a hydrolysis step that converts starch to glucose, followed by a fermentation step that converts glucose to ethanol. Crude ethanol is produced by the fermentation process and has a concentration of roughly 8–12%. The crude ethanol can be purified by distillation, a separation procedure.
Bioethanol has emerged as an instant feasible option in the face of rapidly depleting fossil fuel reserves and growing environmental concerns. In this work, Konjac tuber (Amorphophallus spp.) was used to produce bioethanol. It is known locally as Konjac, and it is a starch-based crop widely farmed in tropical nations. Because it is irritating to the mouth and throat when swallowed, it receives little attention, implying that competition between the fuel and food sectors is lower than for other feedstocks such as cassava and corn. Because of these concerns, the researchers want to assess the fermentation parameters of Konjac tuber (Amorphophallus spp.) starch for bioethanol production in terms of substrate concentration and yeast loading. Bioethanol is produced in three stages: pretreatment, hydrolysis, and fermentation. The effect of acid hydrolysis on ethanol output was investigated. This research would most likely introduce Konjac tuber (Amorphophallus spp.) as a viable bioethanol feedstock. This study aimed to investigate the effect of enzyme hydrolysis on the content of fermentable sugars and evaluate the efficacy of thermochemical pretreatment with H2SO4 at various doses to boost fermentable sugar production for efficient bioethanol production.
2 Material and methods
2.1 Sample collection
The Konjac tubers were harvested in Thailand's northwestern provinces (Fig. 1). The sampling collected the tubers from the forest areas and was brought to the laboratory for alcoholic fermentation. The Konjac tubers were cleaned adequately with tap water to remove any contaminants. The Konjac tubers were then pulverized and stored at room temperature after being chopped and dried in a solar drier. After the drying, the tubers were ground into the fine powder and further processed for hydrolysis.
2.2 Thermochemical pretreatment
Consequently, a sample of 5 g of Konjac tuber powder was weighed and mixed with 100 mL of sulfuric acid (H2SO4 96%) at different concentrations (0%, 0.2%, and 0.4%). A water bath (Julabo EcoTemp TW20) was used to heat the mixture, and three different temperatures (25 °C, 50 °C, and 75 °C) were recorded. After thermal treatment, a sample was withdrawn to determine total sugar and reducing sugar content. Acid pretreatment, including the use of diluted sulfuric acid (H2SO4) for starch-rich feedstocks, is an effective method for increasing the sugar content in raw materials destined for bioethanol synthesis (Whangchai et al., 2021; Zhang et al., 2021).
2.3 Enzymatic hydrolysis
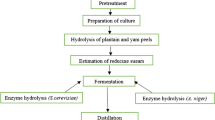
For the hydrolysis step, 35 g of Konjac powder and 700 mL of the best-resulted concentration of H2SO4 were mixed in a 1L flask and were undergone thermal treatment, followed by enzymatic hydrolysis. The pH of the pretreated mixture was measured using a potentiometer (PH700 Benchtop pH Meter) and adjusted to a range of 5 to 5.5 with a NaOH solution at [5 N] to execute the enzymatic hydrolysis stage. The hydrolysis process was initiated, and 1% of cellulose enzymes were added to the mixture. The combination was stored at 35 °C in an incubator. After 24 h, a sample was taken to determine total sugar and decreasing sugar levels. Figure 2 illustrates the procedure used to prepare Konjac tuber powder for cellulase inoculation.
2.4 Total and reducing sugar analysis
The spectrophotometry method was used to determine total sugar and reducing sugars. The phenol sulfuric acid technique was used to determine total sugar (Dubois et al., 1956). 0.5 mL of sample was mixed with 0.5 mL of phenol solution (5% w/v) in a test tube for the total sugar determination. After that, 2.5 mL of concentrated sulfuric acid (H2SO4 at 98%) was added to the mixture, and the test tubes were placed in a water bath for 15 min and then allowed to cool. Readings of absorbance at a wavelength of 540 nm with distiller water as control were recorded to calculate the total sugar content. The reducing sugar was determined by the Dinitrosalicylic acid (DNS) technique (Miller, 1959). 0.5 mL of sample was mixed with DNS reagent in a test tube and placed in boiling water for 15 min. Then, 4 mL of distiller water was added when it had cooled. The absorbance was measured at 540 nm by using distiller water as a control. Spectrometry was utilized to quantify sugar concentrations using a UV-Spectrophotometer detector DV-8000 (Drawell, Osaka, Japan).
2.5 Fermentation and alcohol measurement
For the fermentation step, the hydrolyzate pH was measured and ajusted in the range of 5–5.5 before being inoculated with 1% of S. Cerevisiae. Fermentation was carried out by triplicates for 5 days and maintained at room temperature (30 ± 5 ℃). During the fermentation process, 60 mL of sample was extracted every 24 h and alcohol, total sugar, and reducing sugar were determined to follow the reaction. Ethanol yield was determined using the ebulliometry technique to compare the boiling point of a given volume of distiller water with a known volume of the broth. An ebulliometer is a simple instrument for determining the boiling point of pure substances or mixes that have been used to determine the alcohol content of wines for over a century (Cottrell, 1919; Howell & Byrne, 2014).
2.6 Statistical analysis
The statistical analysis was performed using Statgraphics Centurion 19. This study's experiments were all realized three times. The results were given as the average standard deviation of three replicates. At the 0.05 level, a significant difference was assessed.
3 Results and discussion
3.1 Optimization of the pretreatment
This study looked at the effects of different temperatures (T1 = 25 °C, T2 = 50 °C, and T3 = 75 °C) and H2SO4 concentrations (C1 = 0%, C2 = 0.2%, C3 = 0.4%) on thermochemical pretreatment of Konjac tuber. Samples were evaluated for total sugar and reduced sugar (g/L) after the pretreatment and hydrolysis processes. The findings of total sugar and reducing sugars following thermochemical pretreatment are shown in Fig. 3. At 75 °C and 0.4% deconcentration of H2SO4, the maximum concentrations of total sugars and reducing sugars were 21.6 ± 0.55 g/L and 2.396 ± 0.01 g/L, respectively, at 75 ℃ and 0.4% 0f H2SO4. Srinorakutara et al., 2006 used different concentrations of H2SO4 at different temperatures to the pretreatment cassava waste.
The experiments performed at 60 ℃ with a solution at 0.4% H2SO4 reached less than 0.5 g/L of reducing sugars, and the highest reducing sugar concentration at the same acid concentration required a temperature of 120 ℃ to reach almost 6 g/L. Nevertheless, in this study, the second-highest total sugar and reducing sugar content obtained were 20.04 ± 0.32 g/L and 2.33 ± 0.02 g/L at 50 °C, respectively. A 0.2 % H2SO4 is an additional benefit because the temperature and acid concentration is lower but with a reasonable rate of total sugars that can be broken down into fermentable sugars by enzymatic hydrolysis. Therefore, 0.2% of H2SO4 and 50 ℃ were chosen as the best conditions for the pretreatment stage. Previous studies indicate that the best conditions for the hydrolysis of wheat bran and rye bran were 121 and 130 °C, 1/8 and 1/8 W/V, 2.66 to 1.5% v/V, and 30 and 16 min, respectively (Demirel et al., 2021).
3.2 Enzymatic hydrolysis
Enzyme catalysts, acids, or a combination of both can be used in the hydrolysis process. The hydrolysis of starch to glucose has been carried out using starch as a substrate. The process of breaking down starch molecules into simpler constituent parts such as dextrin, isomaltose, maltose, and glucose is known as hydrolysis of starch (Izmirlioglu and Ali 2012). Miller, in 1959, used cold and hot acids to hydrolyze the starch in the potato starch residue stream (HCl and H2SO4). Using the Dinitrosalicylic (DNS) acid approach, the amount of glucose generated by hydrolysis was determined (Miller, 1959). For the hydrolysis step, 35 g of sample was mixed with 700 mL of a solution of H2SO4 at 0.2% for chemical pretreatment.
Meanwhile, the mixture was placed in a water bath for the thermal treatment at 50 ℃ for 2 h. After letting it cool, pH was measured and modified in a range of 5–5.5 before being infected with 7 mL (1%) of commercial cellulase and kept at 35 ℃ for 24 h to generate the hydrolysate mixture. Total sugar and reducing sugars content obtained after the complete process was found as; 27.11 ± 0.15 g/L and 21.60 ± 0.14 g/L, respectively. Hargono et al. 2016 reported 16.60 g/L as the highest fermentable sugars obtained using a blend of enzymes α-amylase and glucoamylase at 1.5% each one from Amorphophallus spp., which is lower than the reported in this study. However, enzyme hydrolysis is more beneficial based on the milder experimental conditions, the low energy requirements and low toxic subproducts and corrosion in the equipment than acid hydrolysis (Pereira et al., 2021; Rosales-Calderon & Arantes, 2019).
The schematic representation Fig. 4 was demonstrating the mechanism of fermentation. Pretreatment is one of the critical steps of biochemical conversion and, generally, one of the most expensive unit operations in any lignocellulosic biorefinery proposed. The primary objective of the pretreatment process is to increase polysaccharide access to hydrolytic enzymes by reducing the content of lignin and reducing crystalline cellulose content as much as possible (Behera et al., 2014; Casabar et al., 2019; Manmai et al., 2020b). The standard view is that α-amylase is the restrictive digestive enzyme that determines the digestive rate overall. In granular starch digestion, this is true: By dividing giant molecules endo-wise, -amylase provides fresh substrates for amyloglucosidase (Zhang et al., 2015). Neutralizing the hydrolyzed starch solution with NaOH was employed as a yeast growth or fermentation substrate. Thus, the highest amount of yielded fermentable sugars was employed for the fermentation step.
3.3 Ethanol yield
Fermentation is a natural process in which microbes make alcohol and organic acids such as vinegar and lactic acid from sugar (Khammee et al., 2021a, 2021b). Yeast produces bioethanol, particularly Saccharomyces spp., or bacteria, Zymomonas mobilis, fermenting sugar-containing compounds such as glucose, sucrose, or fructose. Sugar was transformed to ethanol and carbon dioxide gas following the fermentation reaction described as; C6H12O6 → 2CH3CH2OH + 2CO2 (Najafpour et al., 2004; Ramaraj et al., 2021). For ethanol production, the hydrolysate mixture was inoculated with 1% w/v S. cerevisiae. The broth was kept at room temperature (30 ± 5℃) for 5 days. The fermentation reaction was followed by measuring the alcohol content and the total sugar and reducing sugar every 24 h, as shown in Fig. 5.
The highest ethanol content was 8.68 ± 0.91 g/L, which is higher than the almost 4% v/v reported by Puškaš et al., 2020 using S. cerevisiae after 48 h of the alcoholic fermentation.
However, in the same study, the highest ethanol production, 11.92% v/v, was reported after 216 h, which means an exponential phase of 9 days compared with the 42 h needed in the present study. Following the growth phases of S. cerevisiae, the exponential phase takes part after the lag phase of adaptation. During this phase, yeast cells grow at a logarithmic rate by fermenting glucose to ethanol resulting in the correlation between the highest ethanol concentration reached and the maximum cell yeast growth during the fermentation (Busti et al., 2010; Henderson et al., 2013; Matmati & Hannun, 2008). As previously mentioned, ethanol fermentation strategy through the total use of fermentable sugar from sugarcane bagasse has been suggested. The diluted sulfuric acid pretreatment for sugar cane bagasse initially resulted in 89.5% solubilizing hemicelluloses and 82% recovering from it as monomeric sugars (xylose and arabinose) in the liquid stream (Dionisio et al. 2021). Meanwhile, ethanol measurements after 48 h presented a decrement, indicating that most of the sugar has been consumed, which means that the exponential fermentation took part during the second day. To extend the exponential fermentation and increase the ethanol yield, it is necessary to increase the content of fermentable sugars in the hydrolysate mixture before proceeding with the fermentation step (Larrea et al., 2020). Table 1 representing the various kinds of tubers utilized as the lignocellulosic biomass for bioethanol production. The results obtained from this study are much feasible compared with the literature survey data as mentioned in Table 1.
4 Conclusion
An efficient amount of bioethanol can be generated from Konjac tubers (Amorphophallus spp.). The thermochemical pretreatment procedure was used to improve enzyme accessibility and to achieve high sugar concentrations with great success. The chemical composition of the treatments varies with temperature and acid concentration. Obtained results showed that the conditions for the effective pretreatment of Konjac tubers were at 50 ℃ and H2SO4 at 0.2% to archive the highest concentration of fermentable sugars after the enzyme hydrolysis of 21.60 ± 0.14 g/L. The optimized conditions for thermochemical pretreatment followed by enzyme hydrolysis were conducted to enhance ethanol production with a concentration of 8.68 ± 0.91 g/L after 48 h of fermentation. As a result, the tubers of Amorphophallus spp. can be used as a feedstock for bioethanol production. The food vs fuel competition is gaining much attention through the lignocellulosic bioenergy advancements. The use of non-edible starchy tubers may be utilized for next-generation bioenergy production.
References
Ademiluyi, F. T., Mepba, H. D. (2013). Yield and properties of ethanol biofuel produced from different whole cassava flours. International Scholarly Research Notices.
Behera, S., Arora, R., Nandhagopal, N., & Kumar, S. (2014). Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renewable and Sustainable Energy Reviews, 36, 91–106.
Ben Taher, I., Fickers, P., Chniti, S., & Hassouna, M. (2017). Optimisation of enzymatic hydrolysis and fermentation conditions for improved bioethanol production from potato peel residues. Biotechnology Progress, 33(2), 397–406.
Bhuyar, P., Sathyavathi, S., Math, R., Maniam, G. P., & Govindan, N. (2020). Production of bioethanol from starchy tuber (Amorphophallus commutatus) and antimicrobial activity study of its extracts. African Journal of Biological Sciences, 2(2), 70–76.
Bhuyar, P., Sundararaju, S., Rahim, M. H. A., Ramaraj, R., Maniam, G. P., Govindan, N. (2019). Microalgae cultivation using palm oil mill effluent as growth medium for lipid production with the effect of CO 2 supply and light intensity. Biomass Conversion and Biorefinery, p. 1–9.
Bhuyar, P., Trejo, M., Dussadee, N., Unpaprom, Y., Ramaraj, R., & Whangchai, K. (2021). Microalgae cultivation in wastewater effluent from tilapia culture pond for enhanced bioethanol production. Water Science and Technology. https://doi.org/10.2166/wst.2021.194
Busti, S., Coccetti, P., Alberghina, L., & Vanoni, M. (2010). Glucose signaling-mediated coordination of cell growth and cell cycle in Saccharomyces cerevisiae. Sensors, 10(6), 6195–6240.
Casabar, J. T., Unpaprom, Y., & Ramaraj, R. (2019). Fermentation of pineapple fruit peel wastes for bioethanol production. Biomass Conversion and Biorefinery, 9, 761–765.
Cottrell, F. G. (1919). On the determination of boiling points of solutions. Journal of the American Chemical Society, 41(5), 721–729.
Deepanraj, B., Srinivas, M., Arun, N., Sankaranarayanan, G., & Abdul Salam, P. (2017). Comparison of jatropha and karanja biofuels on their combustion characteristics. International Journal of Green Energy, 14(15), 1231–1237.
Demirel, F., Germec, M., & Turhan, I. (2021). Fermentable sugars production from wheat bran and rye bran: Response surface model optimisation of dilute sulfuric acid hydrolysis: Optimisation of dilute acid hydrolysis conditions of wheat and rye brans. Environmental Technology, p. 1–33.
Dionísio, S. R., Santoro, D. C. J., Bonan, C. I. D. G., Soares, L. B., Biazi, L. E., Rabelo, S. C., Ienczak, J. L. (2021). Second-generation ethanol process for integral use of hemicellulosic and cellulosic hydrolysates from diluted sulfuric acid pretreatment of sugarcane bagasse. Fuel, 304, 121290.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356.
Favaro, L., Jansen, T., & van Zyl, W. H. (2019). Exploring industrial and natural Saccharomyces cerevisiae strains for the bio-based economy from biomass: the case of bioethanol. Critical Reviews in Biotechnology, 39(6), 800–816.
Hargono, H., Jos, B., Kumoro, A. C., Haryani, K. (2019). Low Temperature Enzymatic Hydrolysis (LTEH) and Fermentation for Bioethanol Generation from Suweg (Amorphophallus campanulatus B) Starch. In Journal of Physics: Conference Series IOP Publishing. p. 012035
Henderson, C. M., Lozada-Contreras, M., Jiranek, V., Longo, M. L., & Block, D. E. (2013). Ethanol production and maximum cell growth are highly correlated with membrane lipid composition during fermentation as determined by lipidomic analysis of 22 saccharomyces cerevisiae strains. Applied and Environmental Microbiology, 79(1), 91–104.
Howell, G., & Byrne, S. (2014). Ebulliometry for measuring alcohol in wine: improving your accuracy. Australian and New Zealand Grapegrower and Winemaker, 608, 77–80.
Izmirlioglu, G., & Demirci, A. (2012). Ethanol production from waste potato mash by using Saccharomyces cerevisiae. Applied Sciences, 2(4), 738–753.
Kasno, A., Trustinah, M. Anwari, and B. Swasono, (2007). The prospect of Konjac as a food ingredient is currently famine. http://balitkabi.litbang.deptan.go.id.
Kausar, F., Irfan, M., Shakir, H. A., Khan, M., Ali, S., & Franco, M. (2021). Challenges in Bioethanol Production: Effect of Inhibitory Compounds. Bioenergy Research: Basic and Advanced Concepts. Springer, Singapore. p. 119–154.
Khammee, P., Ramaraj, R., Whangchai, N., Bhuyar, P., & Unpaprom, Y. (2021a). The immobilisation of yeast for fermentation of macroalgae Rhizoclonium sp. for efficient conversion into bioethanol. Biomass Conversion and Biorefinery, 11, 827–835.
Khammee, P., Unpaprom, Y., Chaichompoo, C., Khonkaen, P., & Ramaraj, R. (2021b). Appropriateness of waste jasmine flower for bioethanol conversion with enzymatic hydrolysis: sustainable development on green fuel production. 3 Biotech. https://doi.org/10.1007/s13205-021-02776-x
Larrea, F. A., Salazar, S., Andino, C., Ona, D., Benalcazar, M., Mora, J., Alvarez-Barreto, J. F. (2020). "Comparison of bioethanol production of starches from different andean tubers. Chem. Eng. Trans.
Lawrence, P., Mathews, P. K., & Deepanraj, B. (2011). The effect of Prickly poppy methyl ester blends on CI engine performance and emission characteristics. American Journal of Environmental Sciences, 7(2), 145–149.
Magnaye, R. C., Alagar, S. S., Pagsinohin, C. M., & Magnaye, M. L. Y. A. (2015). Evaluation of Fermentation Parameters of Elephant foot Yam (Amorphophalluspaeoniifolius) for Bioethanol Production. Asia Pacific Journal of Multidisciplinary Research, 3(4).
Manmai, N., Unpaprom, Y., Ponnusamy, V. K. & Ramaraj, R. (2020b). Bioethanol production from the comparison between optimization of sorghum stalk and sugarcane leaf for sugar production by chemical pretreatment and enzymatic degradation. Fuel, 278, 118262.
Manmai, N., Unpaprom, Y., & Ramaraj, R. (2020a). Bioethanol production from sunflower stalk: application of chemical and biological pretreatments by response surface methodology (RSM). Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-020-00602-7
Matmati, N., & Hannun, Y. A. (2008). Thematic review series: sphingolipids. ISC1 (inositol phosphosphingolipid-phospholipase C), the yeast homologue of neutral sphingomyelinases. Journal of lipid research, 49(5), 922–928.
Mejica, G. F. C., Unpaprom, Y., Whangchai, K., & Ramaraj, R. (2021). Cellulosic-derived bioethanol from Limnocharis flava utilizing alkaline pretreatment. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-020-01218-7
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428.
Najafpour, G., Younesi, H., & Ismail, K. S. K. (2004). Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Bioresource Technology, 92(3), 251–260.
Nguyen, T.V.T., Unpaprom, Y., Manmai, N., Whangchai, K. and Ramaraj, R., (2020a). Impact and significance of pretreatment on the fermentable sugar production from low-grade longan fruit wastes for bioethanol production. Biomass Conversion and Biorefinery, p.1–13.
Nguyen, T. V. T., Unpaprom, Y., Tandee, K., Whangchai, K., & Ramaraj, R. (2020b). Physical pretreatment and algal enzyme hydrolysis of dried low-grade and waste longan fruits to enhance its fermentable sugar production. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-020-01176-0
Pereira, L. M. S., Milan, T. M., and Tapia-Blácido, D. R. (2021). Using Response Surface Methodology (RSM) to optimise 2G bioethanol production: A review. Biomass and Bioenergy, 151, 106166.
Puškaš, V. S., Miljić, U. D., Djuran, J. J., & Vučurović, V. M. (2020). The aptitude of commercial yeast strains for lowering the ethanol content of wine. Food Science & Nutrition, 8(3), 1489–1498.
Ramaraj R., Bhuyar, P., Intarod, K., Sameechaem, N., & Unpaprom, Y. (2021). Stimulation of natural enzymes for germination of mimosa weed seeds to enhanced bioethanol production. 3 Biotech, 11, 307.
Rosales-Calderon, O., & Arantes, V. (2019). A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnology for Biofuels, 12(1), 1–58.
Saengsawang, B., Bhuyar, P., Manmai, N., Ponnusamy, V. K., Ramaraj, R., & Unpaprom, Y. (2020). The optimisation of oil extraction from macroalgae, Rhizoclonium sp. by chemical methods for efficient conversion into biodiesel. Fuel, 274, 117841.
Srinorakutara, T., Kaewvimol, L., & Saengow, L. A. (2006). Approach of cassava waste pretreatments for fuel ethanol production in Thailand. Journal of Science Research Chula University, 31(1), 77–84.
Unpaprom, Y., Pimpimol, T., Whangchai, K., & Ramaraj, R. (2021). Sustainability assessment of water hyacinth with swine dung for biogas production, methane enhancement, and biofertilizer. Biomass Conversion and Biorefinery, 11(3), 849–860.
Van Zyl, W. H., Bloom, M., & Viktor, M. J. (2012). Engineering yeasts for raw starch conversion. Applied Microbiology and Biotechnology, 95(6), 1377–1388.
Villadiego-del Villar, A. E., Sarmiento-Zea, N., León-Pulido, J., & Rojas-Pérez, L. C. (2021). Bioethanol production from yam (Dioscorea Rotundata) Using simultaneous saccharification and fermentation (SSF). TecnoLógicas, 24(50), 116–125.
Wankhede, D. B., & Sajjan, S. U. (1981). Isolation and physico-chemical properties of starch extracted from yam, elephant (Amorphophallus campanulatus). Starch-Stärke, 33(5), 153–157.
Whangchai, K., Inta, W., Unpaprom, Y., Bhuyar, P., Adoonsook, D., & Ramaraj, R. (2021). Comparative analysis of fresh and dry free-floating aquatic plant Pistia stratiotes via chemical pretreatment for second-generation (2G) bioethanol production. Bioresource Technology Reports, 14, 100651.
Zhang, B., Dhital, S., & Gidley, M. J. (2015). Densely packed matrices as rate determining features in starch hydrolysis. Trends in Food Science & Technology, 43(1), 18–31.
Zhang, B., Khushik, F. A., Zhan, B., and Bao, J. (2021). Transformation of lignocellulose to starch‐like carbohydrates by organic acid-catalysed pretreatment and biological detoxification. Biotechnology and Bioengineering.
Acknowledgements
The authors want to extend special thanks to the Faculty of Science, Energy Research Center, School of Renewable Energy, Maejo University, Chiang Mai, Thailand, for the research facilities to complete this experimental study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhuyar, P., Shen, MY., Trejo, M. et al. Improvement of fermentable sugar for enhanced bioethanol production from Amorphophallus spp. tuber obtained from northern Thailand. Environ Dev Sustain 24, 8351–8362 (2022). https://doi.org/10.1007/s10668-021-01786-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-021-01786-2