Abstract
Trace element (TEs) contamination in groundwater is a key factor for health risk assessment and one of the environmental challenges linked with Ghana’s sustainable development. This study aims to investigate TE concentrations in groundwater used for drinking and domestic purposes and their potential health risk in the Kumasi Metropolis using multivariate statistics. In the study, 23 groundwater samples were analyzed for 32 TEs using Perkin Elmer Nexion 2000 Inductively Coupled Plasma Mass Spectrometer (ICP-MS). Levels of TEs recorded in the study were in order of Na > Si > Ca > K > Mg > S > Ba > Cu > Zn > Al > Sr > Li > Mn > P > Fe > B > Pb > Ni > Co > Bi > Se > Sb > Cr > As > Ti > Be > V > U > C d > T l > Mo > Hg. The study revealed that the concentration of trace and toxic elements like Al, Cu, and Pb exceeded their corresponding WHO permissible standards. Hazard index (HI) values and total likely cancer risk (TLCR) values for non-carcinogenic and carcinogenic health risks indicate that 91.3% of the total sampling sites presented health concerns to adults and children. The mean TLCR values associated with exposure to carcinogenic metals via ingestion of groundwater samples were estimated to be 2.09 × 10−4 and 4.44 × 10−4 for adults and children respectively. Exposure through the ingestion pathway was found to be more risky compared to dermal contacts. Children are particularly vulnerable to these health hazards. Pearson correlation (PC) matrix, principal component analysis (PCA), and hierarchical cluster analysis (HCA) suggested that sources of TEs in the groundwater are attributed to both anthropogenic and geogenic factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing demand for clean and accessible water resources is a critical global challenge (Khilchevskyi & Karamushka, 2021). In recent years, the increasing impact of anthropogenic activities on surface water has emerged as a critical environmental concern, particularly in urbanizing regions. Uncontrolled industrial discharges, artisanal mining, agricultural runoff, and urban sprawl have contributed to the deterioration of surface water quality, rendering it increasingly unfit for human consumption (Akoto et al., 2021). As a consequence, communities in these areas face an impending water crisis, necessitating a shift towards alternative and sustainable water sources such as groundwater.
Groundwater is an important source of drinking water and about one-third of the world’s population depends on this source (Karunanidhi et al., 2022). In the confined aquifers, groundwater is present in its purest form (Chakraborty et al., 2023). However, illegal mining, some agricultural activities, and the discharge of urban and industrial waste have contributed to the pollution of groundwater sources with trace elements (TEs). Groundwater plays an important role in strengthening the economic growth of Ghana. It is a principal source of water for many households in Kumasi (Opoku et al., 2020). Given the known health and economic consequences of drinking polluted water, identification and health risk assessment of TE pollution in groundwater sources in the context of Kumasi, becomes necessary for sustainable groundwater security.
Metalloids and metals play essential roles in various biochemical and biological functions within the human body (Crichton, 2012). These elements, including Selenium (Se), Magnesium (Mg), Silicon (Si), Phosphorus (P), Sulfur (S), Bismuth (Bi), Nickel (Ni), Zinc (Zn), Potassium (K) and Iron (Fe), are integral components of enzymes, co-factors, and structural proteins. They are involved in enzymatic reactions, cell signaling, hormone synthesis, and the maintenance of physiological balance (Bánfalvi, 2011). Though some TEs play important roles in the metabolic processes of living organisms at lower concentrations, they tend to be toxic to human life at higher concentrations (Krishan et al., 2021). The toxicity of any contaminant and its subsequent adverse effects on the environment and humans chiefly depend on its concentration and exposure pathway (Lin et al., 2016). Conversely, chronic exposure to TEs at comparatively low levels for a long period of time could be detrimental to human health since these elements can accumulate in biological systems (Morais et al., 2012). TEs like Arsenic (As), Be (Beryllium), Cd (Cadmium), Cr (Chromium), Mercury (Hg), Lead (Pb) and Ni (Nickel) are carcinogenic to humans at elevated concentrations and could cause damage to the liver, kidney, and lungs (Mulware, 2020). Alzheimer’s, Parkinsonism, and cognitive dysfunctions are human health problems associated with Copper (Cu), Fe, Pb, and Manganese (Mn) (Butterworth, 2010; Jones and Miller., 2008).
The Kumasi metropolis is situated in the Ashanti Region of Ghana. It is an industrial, economic, and cultural metropolis with a fast-growing population. The total treated water demand in Kumasi is 304,000 m3 per day (m3/day), however, the water supply system is capable of supplying 123,000 m3/day, leaving a deficit of 181,000 m3/day (Kuma et al., 2010). Thus, groundwater tends to be the available, efficient, and cost-effective alternative to supplement this demand. The groundwater in the study area is exposed to non-point and point source pollution like leachates from dumpsites, open drains, agricultural wastes, industrial waste, and illegal mining (Aboagye & Zume, 2019; Acheampong et al., 2016; Akanchise et al., 2020). Recent studies have made commendable strides in examining the presence and impact of certain TEs in groundwater sources in Kumasi, with much emphasis on TEs like As, Cd, Cr, Cu, Pb, and Zn (Boateng et al., 2019a; Myers et al., 2023; Opoku et al., 2020). While these investigations have undeniably enhanced our understanding of water quality dynamics, it is imperative to recognize that the complex interplay of TEs extends beyond the scope of these few elements. There are numerous TEs, each possessing unique chemical properties and health implications, which warrant thorough scrutiny to comprehensively evaluate the safety and sustainability of groundwater sources. Global efforts have been made to identify numerous TEs in groundwater and ascertain their health implications (Barzegar et al., 2019; Çiner et al., 2021; Mora et al., 2017; Samy et al., 2023; Selvam et al., 2017).
Informed by the collective knowledge gleaned from international efforts, the current research aims to expand the scope of TE investigations in groundwater sources of Kumasi. The study seeks to assess the potential health risk associated with a broader array of TEs, shedding light on their concentrations and interactions within the local hydrogeological context. The findings of this research will provide valuable insights into the extent and implications of TE pollution in water sources in Kumasi. The outcomes will inform policy-makers, water resource managers, and public health authorities in developing targeted interventions and policies to mitigate the risks associated with metalloid and metal contamination. This would help safeguard the health and well-being of the local population.
Materials and methods
Study area
Kumasi is a city in Ghana and serves as the capital town of the Ashanti Region. It is situated within the coordinates of 6.35°–6.40′N latitude and 1.30°–1.35′W longitude. The Kumasi metropolitan is divided into 10 sub-metros having both urban and peri-urban communities. Residents are involved in indigenous occupations like blacksmithing, traditional brewing, artisanal mining, and farming. Kumasi houses various industries like textile, food processing, wood processing, and the Suame Magazine Industrial Enclave. The landscape of Kumasi is characterized by undulating terrain with gentle slopes ranging from 50 to 150 m. The city lies within a local watershed, about 282 m high, which plays a crucial role in its hydrological dynamics (Simon et al., 2004). The geology of Kumasi is composed of metasedimentary and metavolcanic rocks of the Paleoproterozoic Birimian Supergroup formations. The Paleoproterozoic Birimian Supergroup, renowned for its geological and mineralogical richness, harbors significant implications for groundwater TE composition. It comprises a diverse array of sedimentary, volcanic, and metamorphic rocks. Its geological complexity offers a unique setting where the interaction between groundwater and rock formations profoundly influences TE concentrations (Giorgis et al., 2014). Groundwater in Birimian terrains is intricately linked to the mineralogical composition of the underlying rocks. The supergroup includes various lithologies such as metavolcanic rocks (e.g., basalt, andesite), metasedimentary rocks (e.g., shale, sandstone), and meta igneous rocks (e.g., granite, gneiss), each possessing distinct mineralogical assemblages. These minerals include sulfides (e.g., pyrite), oxides (e.g., hematite), carbonates (e.g., calcite), and silicates (e.g., quartz) (CWSA, 2009; Kortatsi, 2006; Chudasama et al., 2016; Dampare et al., 2020; Tandoh et al., 2023). The minerals play a pivotal role in controlling the geochemical behavior of TEs in groundwater through processes like dissolution, precipitation, and ion exchange. Weathering processes acting on Birimian rocks contribute significantly to groundwater mineralization. Chemical weathering of sulfide-bearing minerals like pyrite can release TEs into groundwater, affecting its quality and usability (Courtin-Nomade et al., 2009). Conversely, secondary mineral phases formed during weathering, such as secondary oxides and hydroxides, may act as sinks or sources for TEs depending on prevailing redox conditions. The Paleoproterozoic Birimian Supergroup's geological diversity and mineralogical richness exert a profound influence on groundwater TE composition. The interaction between groundwater and various rock formations within the supergroup underscores the complexity of hydrogeochemical processes.
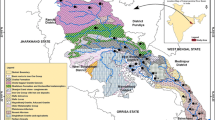
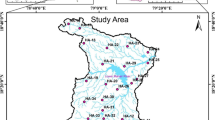
The area’s hydrogeology is dominated by the existence of fracture zones in the basement and a thick cloudy zone. The weathered zone is the aquifer’s key storage space where the rocks are leached (CWSA, 2009). Kumasi experiences a tropical savannah climate, typified by distinct wet and dry seasons. The weather remains hot throughout the year, with an average high temperature of 30.7 ºC and an average low temperature of approximately 21.5 ºC. The humidity levels in Kumasi are relatively high, with an average of 86%. Regarding soil classification, Kumasi’s soils are categorized under the Forest Ochrosol great group (Asamoa, 1973). The drainage system consists of a dense network of streams that naturally flow from the north to the south (Ahmed & Dinye, 2012). Figure 1 shows a map of the study area showing sampling sites.
Sample collection
The random sampling approach was employed to select households with groundwater as a source of water across the Kumasi metropolis, ensuring a highly representative sampling. These households had either a mechanized borehole or hand dug well. To achieve accuracy and thoroughness, the locations of these households were carefully identified and mapped, facilitating proper documentation of each sampling site. Replicate samples were collected in the morning, midday, and evening between January and July, covering the dry and wet season. The collected samples were systematically coded SL1 to SL23, representing 23 different sampling locations. SL1 was sampled from Anwomaso, SL2 from Ahinsa, SL3 from Boadi, SL4 and SL15 from Ejisu, SL5 from Oduom, SL6 from Sawaba, SL7 from Ayeduase, SL8 from Gyinase, SL9 from Esereso, SL10 form Asokore Mampong, SL11 from Duase, SL12 from Parkoso, SL13 from Atonsu Kyepatre, SL14 from Apemso, SL16 from Roman Hill, SL17 from Appiadu, SL18 and SL19 from Kotei and SL20, SL21, SL22, and SL23 all from Buokrom. During the collection of groundwater samples, taps were allowed to run for about 5 min to flush out any residues and stagnant water to obtain fresh water for analysis. About 1 L of water was collected in a plastic container and acidified with 10 mL nitric acid for preservation from each household. The acidified groundwater samples were stored at 4 ºC and analyzed within seven days of collection. The percentage recovery of analysis was between 95 and 102%.
Instrumental analysis and quality control
The US EPA Method 200.2, revision 2.8 (USEPA, 1994a) protocol for sample preparation for spectrochemical determination of total recoverable elements was employed. Sample bottles were shaken for homogeneity. Volume (1 mL) of 1:1 Nitric acid (HNO3) and Hydrochloric acid (HCl) were added to 50 mL of water samples in digestion vessels. Samples were put into a hot block preheated to 95 ºC, covered with ribbed watch glass, heated for 2 h 30 min, and made to cool. Analysis of samples was conducted with an inductively coupled mass spectrometer (ICP-MS) equipped with a micro-mist nebulizer, spray chamber, and Peltier cooler according to U.S EPA method 200.8 (USEPA, 1994b) as total water samples. TEs analyzed were Al, Antimony (Sb), As, Ba, Beryllium (Be), Bi, Boron (B), Cd, Calcium (Ca), Cobalt (Co), Cu, Cr, Fe, Pb, Li, Mg, Manganese (Mn), Hg, Molybdenum (Mo), Ni, P, K, Se, Si, Sodium (Na), Sr, S, Titanium (Ti), Thallium (Tl), U, Vanadium (V), Zn. Optimized instrumental parameters are listed in Table A1 in the supplementary file. The correlation coefficient (r2) of the calibration curves for all analytes was above 0.999. Internal standard solutions were utilized to check the signal drift during instrumental analysis. Procedural blank and reagent blank were analyzed after a batch of five samples to check for possible contamination. Laboratory control standard was analyzed after every five samples for accuracy. Matrix spike solutions were prepared at 10% intervals to obtain recoveries of trace elements. Laboratory Control Standard (LCS) and Spike samples were subjected to the same procedure used for the samples. Quality control protocols were accepted with recovery percentages > 90%. Ultra-pure trace metal grade nitric acid and hydrochloric acid with percentage purity of 69% and 37% respectively from VWR International Ltd. (United Kingdom) were used. Deionized water used in the study was generated from the Millipore ultra-pure water system (Milli-Q IQ 7015) from Millipore Co. Ltd., USA. Standard solutions of TEs and internal standard solutions were obtained from Inorganic Ventures, USA. A mixed tuning solution was obtained from Perkin Elmer, USA. Ultra-pure grade of argon gas (99.99% pure) was used as carrier gas. The hot block digestion system used was SC180 from Environmental Express Ltd., USA. Nexion 2000–B Inductively Coupled Plasma Mass Spectrometer from Perkin Elmer, USA, was used for TE speciation.
Health risk assessment
Health risk assessment was conducted by the approach of the United States Environmental Protection Agency’s Risk Assessment Guidance for Superfund (EPA, 2001). Two forms of risks constitute health risk assessment based on this approach; carcinogenic health risk and non-carcinogenic health risk.
In this study, the major routes of exposure of the population to TEs in samples are through ingestion and dermal contact. The population in this study exposed to pollution of these elements in samples was adults and children; hence, health risk assessment was conducted in both classes of population.
In the determination of non-carcinogenic health risks associated with contamination, Eqs. (1) to (4) below were used for the calculation. Chronic Daily Intake (CDI) and Dermal Absorbed Dose (DAD) are both measured in mg/kg-day, whereas Hazard Quotient (HQ) and Hazard Index (HI) are unitless. In this assessment, RFDingestion/dermal used is for chronic reference doses. According to the US EPA (EPA 2001), RfDs (Reference Dose) are daily estimated levels of exposure to a metal that is considered safe for the human population, including vulnerable groups, over a lifetime; SF is the slope factor; Kp is the permeability coefficient. Values of parameters in Eqs. 1 to 4 and their respective SI units are given in Tables 1 and 2.
Values of HI are estimates representing the sum of the potential non-carcinogenic risk of two or more metals associated with pollution. A potential for non-carcinogenic health risk exists when HI ≥ 1 (EPA, 2001). Long-term accumulation of heavy metals in humans is due to inadequate mechanisms for their elimination (Jaishankar et al., 2014). There are different routes of exposure: ingestion, dermal and inhalation. The ingestion and dermal routes of exposure were considered in the present study.
Carcinogenic health risk in this study was determined using Eqs. 5 and 6 below.
From Table 2 above, values of the cancer slope factor for dermal exposure (CSFdermal) were calculated using Eq. (7) below (EPA, 2001). ABSGI represents the Gastrointestinal Absorption Factor (dimensionless). Values of parameters in Eqs. 5 to 7 are given in Table 2.
In a carcinogenic risk assessment, values of TLCR within the ranges of 1 × 10−6 to 1 × 10−4 imply there is no potential for carcinogenic health risk associated with exposure (EPA, 2001). Values of TLCR that fall out of this range indicate a potential for carcinogenic health risk.
Statistical analysis
Descriptive statistics was used to determine the minimum, mean, maximum, and standard deviations. Principal component analysis (PCA) was used to reduce the dimensionality of the data to make it more interpretative. PCA was employed to study the factor extraction at eigenvalues greater than 1. Varimax rotation was combined with Kaiser Normalization. Rotation converged in 6 iterations. Pearson correlation (PC) analysis was employed to determine the relationship and association between the TEs and provided details of their sources. The change in the magnitude of a variable is associated with the change in magnitude of another variable in the same or opposite direction. Hierarchical cluster analysis (HCA) was employed to identify relatively homogeneous groups of the variables. The dendrogram was obtained by employing Ward’s method. Descriptive statistics, PCA, correlation, and HCA were all performed using SPSS 24.
Results and discussion
Trace element levels in groundwater samples
There were significant levels of essential elements in the water samples analyzed such as Ca, Na, Mg, and K with mean concentrations of 5.02 ± 4.96 mg/L, 12.25 ± 12.59 mg/L, 1.52 ± 2.21 mg/L, and 4.47 ± 3.83 mg/L respectively as shown in Table 3. High concentrations of Ca and Mg could cause hardness in water with a resulting change in taste (Shehzadi et al., 2015). Na and K are crucial in maintaining the balance of fluids and the contraction of muscles in the human system. However, excessive levels of Na could contribute to hypertension (Epstein & Sowers, 1992). The levels of As, Cd, Cr, Hg, Ni, Pb, and Mn observed in this study were well within the acceptable limits set by the World Health Organization (WHO, 2017) as presented in Table 3. However, some water samples in this study exhibited elevated levels of Cu (0.10 ± 0.34 mg/L), exceeding the WHO permissible limit of 0.05 mg/L. This finding conforms to studies conducted by (Akoto et al., 2022), where groundwater samples had Cu levels exceeding WHO limit. Notably, sample SL17 from Appiadu recorded the highest levels of Cu (1.65 mg/L) and Pb (0.03 mg/L) as shown in Fig. 2. The elevated levels of Cu recorded in the water could adversely affect the health of consumers over time (Mathew & Krishnamurthy, 2015). Studies have reported that elevated Cu in drinking water could be due to water stagnation within copper pipes used for water transportation (Dietrich et al., 2004; Lagos et al., 2001; Zietz et al., 2003). It could be inferred that elevated Cu concentrations in the groundwater samples may be attributed to water stagnation within copper pipes used for groundwater transportation. Ingesting excessive amounts of Cu can lead to symptoms such as nausea, vomiting, diarrhea, stomach issues, and headaches and could cause liver damage. Cu toxicity can also manifest as neurological symptoms like headaches, dizziness, and confusion (Ortiz et al., 2020).
Elevated Pb concentration (0.03 mg/L) recorded in SL17 makes groundwater from that area unfit for consumption. This finding is a cause for concern and comparable with similar cases observed in the Oti landfill site by (Boateng et al., 2019b) and from hostels around Kwame Nkrumah University of Science and Technology by (Akoto et al., 2022). Pb can impact the central nervous system, resulting in neurological disorders, memory issues, and reduced coordination. Additionally, prolonged exposure to Pb can harm the kidneys and hinder their proper function over an extended period (Collin et al., 2022). In pregnant women, Pb exposure may lead to reproductive issues, affecting fertility and negatively impacting the developing fetus (Ettinger & Wengrovitz, 2010).
The concentration of Al obtained from the test samples ranged from 0.001 to 0.304 mg/L, with a mean concentration of 0.03 ± 0.08 mg/L. However, Al concentration for SL13 (0.30 mg/L) was above the safe limit of 0.2 mg/L set by WHO. This result is a cause for concern. Al is a toxic element and has sparked debate over potential health repercussions due to its suspected link to Alzheimer’s disease or dialysis encephalopathy (Srinivasan et al., 1999). Groundwater in SL13 may not be safe for drinking and domestic purposes due to high levels of Al. Studies conducted by some researchers (Edmunds & Kinniburgh, 1986; Hart et al., 2021; Soveri, 1992) attributed the high Al content of groundwater to the acidification of aquifers caused by acid rains. Thus, high levels of Al observed may be linked to acid rains.
Mean concentrations obtained for Tl, U, and V were relatively low and did not exceed recommended values (0.002, 0.03, 0.05 mg/L respectively) set by WHO (2017). Lower levels of these TEs found suggest that they do not constitute a health concern; nevertheless, at higher concentrations, they may be harmful. As a highly toxic element, Tl is listed as an EPA priority pollutant. According to reports, Tl is more acutely poisonous than Cd, Cu, Hg, Pb, and Zn in mammals (Cheam, 2001; Nriagu, 1998; Peter & Viraraghavan, 2005; Repetto et al., 1998). Prolonged exposure to high levels of uranium could result in cancer, chronic kidney diseases, and renal dysfunction (Chiba & Fukuda, 2005; Prat et al., 2009). The US Geological Survey (USGS) conducted a study of trace elements in rock, unconsolidated materials, and groundwater in Southern California and found that levels of U and V were at concentrations of concern for public health (Izbicki et al., 2023). Health risks posed by exposure to high concentrations of V may include cirrhosis, renal stone disease, distal renal tubular acidosis, hypokalemic periodic paralysis, and cancer (Al-Saleh, 1996; Barceloux & Barceloux, 1999).
Co, Fe, and Mo values obtained were appreciably low in all tested samples. They are considered essential elements to human health at concentration ranges of 0.1–1 µg/L and 10–100 µg/L for Co and Fe respectively (Foster et al., 2006). Co has also been proven to promote plant growth in several species but it is detrimental to humans, terrestrial and aquatic animals, and plants at high concentrations (Bruland et al., 1991). Recent research (Kuiper et al., 2015; Leybourne & Cameron, 2008; Sehar et al., 2013) highlights specific aquifers with excessive Mo levels all around the world, accentuating the need for a health-based guideline to be established. Unlike elevated concentrations of Mo observed in these studies (Kuiper et al., 2015; Leybourne & Cameron, 2008; Sehar et al., 2013), analysis of the results obtained from the groundwater samples is comparably low. Thus, concentrations of these elements pose no health risk in groundwater samples. Mean concentrations of Mn, Se, and Zn from the study were significantly lower and were well within the acceptable limit set by WHO (2017). These elements are also essential elements and play vital roles in the human body (Al-Fartusie & Mohssan, 2017). Low concentrations of these essential TEs below dietary intake pose no health risk in the groundwater samples.
Metalloids including B, Si, and Sb were detected in some test samples. The study reveals that B concentrations averaged 0.001 ± 0.002 mg/L, suggesting that all the groundwater samples were below the safe limit (2.4 mg/L) set by WHO (2017). The chemical characteristics of B in groundwater are chiefly controlled by hydrogeochemical factors such as semi-arid climatic conditions, the alkaline nature of water, water–rock interaction, intense weathering, ion exchange, mineral dissolution, and precipitation (Zango et al., 2019). Si is known to have potential therapeutic effects (Holtzclaw et al., 1984). Currently, there is no known drinking quality guideline established for Si. Levels of Si observed in the study ranged from 3.39 to 27.24 mg/L with a mean concentration of 11.267 ± 6.256 mg/L. The result obtained from the study is in agreement with the findings of other researchers (Vetrimurugan et al., 2017) who obtained a mean Si concentration of 9.820 ± 7.010 mg/L.
As it stands, there is no drinking water quality guideline established for Be by WHO. It is rarely found in drinking water at concentrations of health concern. However, mean Be concentrations obtained in this study were lower than the limits for drinking water (0.004 mg/L) recommended by ATSDR (2002). Ba is present in trace amounts in igneous and sedimentary rocks. It is usually found as BaSO4 and as BaCO3 to a lesser extent. Thus, the presence of Ba in groundwater is inevitable. In the present study, the average Ba concentration (0.51 ± 0.13 mg/L) is below the safe limits set by WHO (2017). Thus groundwater in the study area is safe with respect to this element.
Sr was detected in some groundwater samples. Sr levels were in the concentration range of 0.006–0.18 mg/L, with a mean concentration of 0.049 ± 0.052 mg/L. Compared to reports by Zhang et al. (2018) and Peng et al. (2021) who had 0.34 mg/L and 0.360 mg/L respectively as mean concentrations of Sr in drinking water, levels of Sr in this study are in trace amounts and would pose no health risk with regards to this element. Investigations by Peng et al, (2021) revealed a positive correlation of Sr to rickets in children. Thus, efforts should be put in place to involve Sr in the scope of drinking water supervision.
Bi was not actually detected in the majority of the groundwater samples tested. Bi is usually categorized among the group of elements that are conventionally known as poor elements due to their rarity. It is considered to have low toxicity to humans, thus, it is used extensively in pharmaceutics (Wang et al., 2019).
The mean concentration of P obtained is 0.018 ± 0.050 mg/L. The presence of P in drinking water is not considered a threat to human hazards. Nevertheless, the odor and color of water could be affected to a greater extent by P. It could hasten the growth of algae and aquatic vegetation, resulting in eutrophication and deterioration of municipal water supplies (Matson et al., 1997).
Sulfur concentrations were in the range of 0.70 to 7.42 mg/L with a mean concentration of 1.196 ± 1.198 mg/L. It exists in nature and is present in soil, plants, food, and water but usually in the form of organosulfur compounds and sulfides. There is no drinking water quality standard established for elemental S. It has low toxicity in humans. Nevertheless, ingesting high levels of S could lead to severe burning sensations or diarrhea (Barsan, 2007).
Lithium is listed by the UESPA as the fifth Unregulated Contaminant Monitoring Rule (UCMR5) (USEPA, 2012). There is insufficient exposure data available for Li in drinking water. It is used in pharmaceutics for the treatment of bipolar disorder (Cipriani et al., 2013). Various studies (Barjasteh-Askari et al., 2020; Ishii & Terao, 2018; Kessing et al., 2017) have revealed an inverse relationship between intake of trace Li in drinking water with suicide, depression, violent crime rates, and incidence of dementia and Alzheimer's disease. Li in drinking water is not regulated in many countries, but the United States Geological Survey (USGS) in collaboration with USEPA gives a non-regulatory Health-Based Screen Level (HBSL) of 0.01 mg/L (Sharma et al., 2022) (Table 4).
Pearson’s correlation analysis
The obtained correlation coefficients are shown in Table 5 below. A strong correlation (0.85) was observed between As and P. This observation is consistent with the report by Acharyya et al. (2000), who observed a strong correlation between As and P and attributed the release of As to excessive use of phosphate fertilizers. It is well known that phosphate encourages the growth of sediment biota and also acts as an eluant for As from sediment (Granéli, 1999). A study by Manning and Martens (1996) indicated that 1 mM KH2PO4 solution leaches up to 150 µg/g As from Fe-coated quartz grains. As was detected in ground water from SL1 (Anwomaso), SL5 (Oduom), SL11 (Duase), and SL23 (Buokrom). There are various farmland areas with vegetables like Cabbage, Lettuce, and Onion in these areas. This strong correlation could be due to the use of phosphate fertilizers. Thus, the source of As and P in the groundwater sample could be purely anthropogenic. A strong correlation was found between Ba and Mn (0.77), Ba and Ni (0.72), Ba and Na (0.74), Ba and Co (0.88), and Ba and Tl (0.80). A strong correlation (0.89) between Cu and Zn may be attributed to the corrosion of pipe materials and coating during groundwater transportation which highlights anthropogenic sources (Sadiq et al., 1989). This observation conforms to the strong correlation of 0.85 and 0.84 between Cu and Zn obtained by Suresh et al. (2012) and Ogbaran and Uguru (2021) in their study. A very strong correlation (0.98) between Cu and Pb indicates common influential factors, common sources, and similar contamination pathways for the trace elements. The use of corroded plumbing systems and industrial discharges from auto mechanic shops could be a major source, emphasizing that sources of Cu and Pb are largely anthropogenic (Chang et al., 2022; Owamah et al., 2023). The distribution of Cu and Pb may be attributed to geogenic sources. There was a strong correlation (0.80) between Mn and Ni, while a very strong correlation (0.94) was observed between Mn and Co. A strong correlation (0.87) was observed between Ni and Co. Pb and Zn showed a very strong correlation with a correlation coefficient of 0.95. There was a strong correlation (0.80) between Sr and Pb. K showed a strong correlation of 0.72 and 0.79 with Na and Tl respectively. Mg also showed a significant correlation (0.80 and 0.79) with Na and Co respectively. Al exhibits weak significant correlations with Ba (0.277), Mn (0.343), Sr (0.626), Ca (0.529), K (0.289), Na (0.556), and Co (0.268). These associations suggest an array of common sources, either geogenic or anthropogenic origin. A positive weak significant correlation between As and Si (0.533) suggests silicate mineral dissolution which is essential in controlling geogenic As contamination in groundwater as suggested by Alam et al., 2014. Moreover, the weak significant correlation between As and V (0.210) could be attributed to the presence of continental sediments containing volcanic ash in the aquifer. The weak correlation between Cu and Ni (0.264) and Li (0.335) could be attributed to sulfide minerals containing both copper and nickel and lithium-bearing minerals associated with copper deposits. Mn reveals weak significant correlations between Sr (0.543), Ca (0.239), K (0.292), Na (0.513), and Tl (0.403). These relationships indicate that a variety of sources are involved with the groundwater pollutants in the study area.
Principal component analysis
PCA identified 5 principal components (PC) with a cumulative variance of 82.79% (Table 4). PC1 explains 36.20% of the total variance, indicating high PC loading for Ba, Mn, Ni, Sr, Mg, Na, Co, and Tl. This indicates that PC1 captures a substantial portion of the variability present in the original data. While it does not explain all of the variance, it's significant enough to be considered a meaningful component. The high loading of TEs on PC1, such as Ba, Mn, Ni, Sr, Mg, Na, Co, and Tl, suggests that these elements are strongly correlated with each other within the dataset. In other words, when PC1 increases or decreases, these elements tend to increase or decrease together. The presence of these TEs in high-loading groups can provide insights into the geochemical processes occurring in the ground water system. Ba and Sr are often associated with carbonate minerals and can indicate the influence of carbonate dissolution or precipitation. Mn and Ni could be indicative of redox processes, such as oxidation–reduction reactions, occurring in the groundwater. Na and Mg are major ions commonly found in groundwater and are influenced by factors like water–rock interactions. Co and Tl may have various natural and anthropogenic sources and can be indicative of specific geological or environmental influences. Strong positive loadings of these TEs may imply leaching of sediments rich in greywacke. PC2 (Cu, Ni, Pb, Zn, Li) had a variance of 20.12%. While this is less than PC1, it still captures a significant portion of the variability present in the original data. PC2 contributes unique information beyond what is explained by PC1. Cu, Ni, Pb, and Zn are commonly associated with mining activities, industrial processes, or urban runoff. Their presence may indicate anthropogenic influences on groundwater quality. PC3 which loaded with Sr, Al, Ca, Mg, Na, and Li had a variance of 10.98%. Sr and Ca are often associated with carbonate minerals and can indicate the influence of carbonate dissolution or precipitation. Mg and Na are mainly influenced by water–rock interactions. Al may originate from natural sources such as clay minerals or anthropogenic sources such as industrial activities or acid mine drainage. PC4 (Ba, K, Mg, Na, Si, Tl, V) had a variance of 8.66%. Si is abundant in the Earth’s crust and can be present in groundwater due to the weathering of silicate minerals like quartz and feldspar. It is also commonly introduced into groundwater through natural weathering processes. K can enter groundwater through agricultural activities like fertilization. Weathering of potassium-rich minerals in the soil and rocks can also release potassium into groundwater. V occurs naturally in various minerals and rocks, with concentrations in groundwater influenced by geological factors. Anthropogenic sources like industrial discharge and combustion of fossil fuels can also contribute to elevated levels of V in groundwater. The variance of PC5 which is loaded with As, P, Si, and V was 6.83%. Figure 3 shows the loading plot for the first three PCs. The loading plot displays the physical properties and spatial distribution of the trace elements while highlighting the role that each variable played. Variables that are close together indicate the strength of their correlation.
Hierarchical cluster analysis of trace elements
The obtained dendrogram shows three major different clusters with several groups. Cu, Pb, Zn, and Li have good similarities and are located in cluster A (colored blue). Cu, Pb, and Zn are considered chalcophile elements and usually form covalent bonds with sulfides (Haque et al., 1982). The association of these TEs may be due to sulfide deposits in the aquifer. As, P, Si, and V are in cluster 2. An association is found between As and V (cluster B, red-colored) since V usually combines with other elements for As (García & Ruiz, 2002). Cluster C has three different groups. Group 1(colored yellow) of cluster C consists of Mn, Co, and Ni. These elements are usually found in ultramafic rock aquifers in low concentrations (Rodríguez-Pacheco et al., 2005). Group 2 (colored green) is made up of Ba, Tl, and K, and Group 3 (colored purple) comprises Mg, Na, Sr, Al, and Ca, which are also grouped. There is no clear spatial difference among these groups. Figure 4 shows the dendrogram of TEs with significant concentrations which were compared in order to identify similarities and metal distributions of the investigated sampling locations. The related elements would have the same mobility entrance into the environment and similar sources of origin. Sources of TEs are both geogenic and anthropogenic (Fig. 5).
Health risk assessment
Non-carcinogenic health risk was calculated by values obtained for the Hazard Index (HI) using the USEPA RAGs format. According to Ametepey et al. (2018), HI is the total of all the potential adverse health effects associated with human exposure to two or more metals. Values of HI ≥ 1 indicate the possibility of a non-carcinogenic health risk. When computed for adults with regards to oral exposure, 34.8% (SL1, 3, 7, 9, 13, 17, 18, and 19) of groundwater water from total sampling sites had HI ≥ 1. Considering oral exposure in children, 52.2% (SL1, 2, 3, 5, 7, 9, 10, 13, 16, 17, 18, and 19) of all the groundwater from the total sampling site had H ≥ 1. Both adults and children are exposed to non-carcinogenic health risks when they drink groundwater from SL1, 3, 7, 9, 13, 17, 18, and 19. When exposed via the dermal route, adults as well as children had HI values less than one, implying the absence of any potential non-cancer health risks. Hazard index values of groundwater samples through oral and dermal pathways are shown in Table 6.
The estimated total likely cancer risk (TLCR) through oral and dermal exposure routes for groundwater samples in the study are shown in Table 7. The results suggested that adult exposure to groundwater water from sampling sites SL1, 4, 5, 7, 8, 10, 13, 16, 17, 19, 20, 22, and 23 could result in carcinogenic health risks, according to the oral assessment. TLCR values for these sampling sites exceeded the upper limit (1 × 10−4). Children's exposure to water from sampling sites SL 1–5, 7–20, 22, and 23 raises concerns with regard to potential cancer risks via ingestion. The TLCR revealed that water from sampling locations HS1, 4, 5, 7,8, 10, 13, 16, 17, 19, and 20 poses cancer hazards in both adult and child exposure via ingestion. The mean TLCR values associated with exposure to carcinogenic metals via ingestion of groundwater samples were estimated to be 2.09 × 10−4 and 4.44 × 10−4 for adults and children respectively. Only SL7 with a TLCR value of 2.38 × 10−4 via the dermal pathway indicated concerns about cancer risks in adults, whereas SL 7 (7.04 × 10−4), SL 10 (1.3 × 10−4), SL 13 (1.21 × 10−4), SL16 (2.27 × 10−4), and SL 22 (1.19 × 10−4) prompted concerns in children. SL7 sampling site posed cancer hazards to both adults and children exposed through dermal contact.
Groundwater water from only two sampling sites (SL6 and SL21) posed no possible health hazards to both population classes via both ingestion and dermal exposure routes. Groundwater water from the other sampling sites (91.3%) presented (either carcinogenic, non-carcinogenic, or both) health risks through ingestion, dermal exposure routes, or both. These health risks have a negative impact on the health of both adults and children. Adult exposure to water from five sampling sites (HS1, 7, 13, 17, 18, and 19) posed both cancer and non-cancer risks, whereas child exposure to water from twelve sampling sites (HS1, 2, 3, 5, 7, 9, 10, 13, 16, 17, 18, and 19) posed both cancer and non-cancer hazards. Sampling locations posing health concerns on child exposure were larger than on adult exposure for both types of health risks. Needless to say, children in this population are more vulnerable to these potential health risks. Children's vulnerability to trace elements in contaminated groundwater stems from their higher intake relative to body weight, ongoing growth and development processes, increased absorption rates, potential neurotoxic effects, cumulative health risks, and compromised immune responses. In addition, children’s biological systems, including their metabolic, detoxification, and excretory processes, are still in the developmental stage. This means their bodies may not be as efficient or capable as adult systems in handling and removing toxins and other harmful substances. These factors highlight the importance of protecting children from exposure to contaminated drinking water to safeguard their health and development (NRC, 1993; WHO, 2010). A similar study conducted by Akoto, et al. (2019) and Jonathan, et al. (2023) revealed concerns about potential health dangers posed by numerous water sources in Kumasi to the community.
Remediation of trace elements in groundwater
Remediation of trace element concentrations in groundwater involves strategies to either prevent further contamination or mitigate existing contamination. This research emphasizes the need to develop appropriate water treatment methods, monitoring programs, and public awareness initiatives to provide safe drinking water. One remedial technique that would help with TE concentration in groundwater is source control. It would be very imperative to identify and control the sources of contamination to prevent further introduction of trace elements into groundwater. This may involve regulating industrial discharge, controlling agricultural runoff, and implementing measures to manage waste disposal sites effectively. Membrane filtration implements nanofiltration or reverse osmosis membranes to remove trace elements from groundwater. These membrane filtration techniques can effectively reject heavy metals and other trace elements based on size and charge differences (Chauhan et al., 2023). Chemical precipitation could be used. For instance application of ferric chloride for the precipitation of trace elements like As from groundwater. Ferric chloride can effectively form insoluble precipitates with As, facilitating its removal through filtration (Moreira et al., 2023). Phytoremediation utilizes plants with hyperaccumulation properties for the remediation of trace elements in groundwater. Certain plant species can absorb and accumulate high concentrations of trace elements, helping to remediate contaminated groundwater through processes like phytoextraction. Some plants commonly used for Phytoremediation include water hyacinth, Indian mustard, sunflower, willow tree, ferns, and sedum spp. (Mudgal et al., 2023).
Conclusion
Trace elements contamination of groundwater used for drinking and domestic purposes and its health impact on the health of people in the Kumasi metropolis of Ghana have been evaluated. A total of 23 water samples were collected and analyzed for trace elements. Results revealed that concentrations of Al, Cu, and Pb were above WHO permissible limits of drinking water. Groundwater from Appiadu and Atonsu Kyepatre may be unsuitable for drinking and domestic purposes. To explore the relationship between trace elements and determine their possible sources, multivariate analysis was employed. It indicated that sources of trace elements are both anthropogenic and geogenic. Assessment of human health risk through ingestion and dermal exposure routes were characterized for adult and children population groups. Hazard Indices obtained reveal that consumption of groundwater from the study area poses non-carcinogenic risks to both adults and children. Children are more vulnerable to these health risks. Total likely cancer risk values obtained revealed that continuous consumption of trace element-contaminated drinking water may increase cancer risk. The findings in this study would be useful for developing the management plan for proper utilization of water resources in the Kumasi metropolis.
Data availability
Data is available upon request.
References
Aboagye, D., & Zume, J. T. J. A. G. R. (2019). Assessing groundwater quality in peri-urban localities of Kumasi. Ghana., 38(4), 390–405.
Acharyya, S. K., Lahiri, S., Raymahashay, B. C., & Bhowmik, A. (2000). Arsenic toxicity of groundwater in parts of the Bengal basin in India and Bangladesh: The role of Quaternary stratigraphy and Holocene sea-level fluctuation. Environmental Geology, 39, 1127–1137.
Acheampong, F., Akenten, J. W., Imoro, R., Agbesie, H. R., Abaye, D. J. J. o. H., & Pollution. (2016). Evaluation of heavy metal pollution in the Suame Industrial Area, Kumasi, Ghana. 6(10), 56-63.
Ahmed, A., & Dinye, R. D. (2012). Impact of land use activities on Subin and Aboabo Rivers in Kumasi Metropolis. Journal of Water Resources and Environmental Engineering, 4(7), 241–251.
Akanchise, T., Boakye, S., Borquaye, L. S., Dodd, M., & Darko, G. J. S. A. (2020). Distribution of heavy metals in soils from abandoned dump sites in Kumasi. Ghana., 10, e00614.
Akoto, O., Gyimah, E., Zhan, Z., Xu, H., & Nimako, C. (2019). Evaluation of health risks associated with trace metal exposure in water from the Barekese reservoir in Kumasi, Ghana. Human and Ecological Risk Assessment: An International Journal.
Akoto, O., Adopler, A., Tepkor, H. E., & Opoku, F. J. G. f. S. D. (2021). A comprehensive evaluation of surface water quality and potential health risk assessments of Sisa river, Kumasi. 15, 100654. https://doi.org/10.1016/j.gsd.2021.100654
Akoto, O., Samuel, A., Gladys, L., Sarah, O. A. A., Apau, J., & Opoku, F. (2022). Assessment of groundwater quality from some hostels around Kwame Nkrumah University of Science and Technology. 17, e01361. https://doi.org/10.1016/j.sciaf.2022.e01361
Alam, M. S., Wu, Y., & Cheng, T. (2014). Silicate minerals as a source of arsenic contamination in groundwater. Water, Air, & Soil Pollution, 225, 1–15.
Al-Fartusie, F. S., & Mohssan, S. N. (2017). Essential trace elements and their vital roles in human body. Indian Journal of Advances in Chemical Science, 5(3), 127–136.
Al-Saleh, I. A. (1996). Trace elements in drinking water coolers collected from primary schools, Riyadh, Saudi Arabia. Science of the Total Environment, 181(3), 215–221.
Ametepey, S. T., Cobbina, S. J., Akpabey, F. J., Duwiejuah, A. B., & Abuntori, Z. N. (2018). Health risk assessment and heavy metal contamination levels in vegetables from Tamale Metropolis. Ghana. International Journal of Food Contamination, 5(1), 1–8.
Asamoa, G. K. (1973). Particle size and free iron oxide distribution in some latosols and groundwater laterites of Ghana. Geoderma, 10(4), 285–297.
ASTDR. (2002). Toxicological profile for beryllium.
Bánfalvi, G. (2011). Heavy metals, trace elements and their cellular effects. Cellular effects of heavy metals, 3–28.
Barceloux, D. G., & Barceloux, D. (1999). Vanadium. Journal of Toxicology: Clinical Toxicology, 37(2), 265–278.
Barjasteh-Askari, F., Davoudi, M., Amini, H., Ghorbani, M., Yaseri, M., Yunesian, M., . . . Lester, D. (2020). Relationship between suicide mortality and lithium in drinking water: A systematic review and meta-analysis. Journal of affective disorders, 264, 234–241.
Barsan, M. E. (2007). NIOSH pocket guide to chemical hazards. Department of Health and Human Services. Center for Disease Control and Prevention, DHHS (NIOSH). Publication, (2005–149).
Barzegar, R., Asghari Moghaddam, A., Adamowski, J., & Nazemi, A. H. (2019). Assessing the potential origins and human health risks of trace elements in groundwater: A case study in the Khoy plain. Iran, 41, 981–1002.
Boateng, T. K., Opoku, F., & Akoto, O. (2019a). Heavy metal contamination assessment of groundwater quality: A case study of Oti landfill site. Kumasi. Applied Water Science, 9(2), 33.
Boateng, T. K., Opoku, F., & Akoto, O. J. A. w. s. (2019b). Heavy metal contamination assessment of groundwater quality: a case study of Oti landfill site, Kumasi. 9(2), 33.
Bruland, K. W., Donat, J. R., & Hutchins, D. A. (1991). Interactive influences of bioactive trace metals on biological production in oceanic waters. Limnology and Oceanography, 36(8), 1555–1577.
Butterworth, R. F. J. N. r. (2010). Metal toxicity, liver disease and neurodegeneration. Neurotoxicity Research 18, 100–105. https://doi.org/10.1007/s12640-010-9185-z
Chakraborty, S., Rajak, C., & Das, S. J. J. o. C. C. (2023). The contamination of groundwater and possible remedial measures in the Coastal Zone of Purba Medinipur, India. 27(4), 29.
Chang, L., Lee, J. H., & Fung, Y. (2022). Prediction of lead leaching from galvanic corrosion of lead-containing components in copper pipe drinking water supply systems. Journal of Hazardous Materials, 436, 129169.
Chauhan, M. S., Rahul, A. K., Shekhar, S., & Kumar, S. (2023). Removal of heavy metal from wastewater using ion exchange with membrane filtration from Swarnamukhi river in Tirupati. Materials Today: Proceedings, 78, 1–6.
Cheam, V. (2001). Thallium contamination of water in Canada. Water Quality Research Journal, 36(4), 851–877.
Chiba, M., & Fukuda, S. (2005). Uranium in environmental water and its health effects. Transactions of the American Nuclear Society, 93, 832–834.
Chudasama, B., Porwal, A., Kreuzer, O. P., & Butera, K. (2016). Geology, geodynamics and orogenic gold prospectivity modelling of the Paleoproterozoic Kumasi Basin, Ghana, West Africa. Ore Geology Reviews, 78, 692–711.
Çiner, F., Sunkari, E. D., & Şenbaş, B. A. (2021). Geochemical and multivariate statistical evaluation of trace elements in groundwater of Niğde Municipality, South-Central Turkey: implications for arsenic contamination and human health risks assessment. 80, 164-182. https://doi.org/10.1007/s00244-020-00759-2
Cipriani, A., Hawton, K., Stockton, S., & Geddes, J. R. (2013). Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. Bmj, 346. https://doi.org/10.1136/bmj.f3646
Collin, M. S., Venkatraman, S. K., Vijayakumar, N., Kanimozhi, V., Arbaaz, S. M., Stacey, R. S., ... & Swamiappan, S. (2022). Bioaccumulation of lead (Pb) and its effects on human: A review. Journal of Hazardous Materials Advances, 7, 100094.
Courtin-Nomade, A., GrOSBOIS, C., Marcus, M. A., FAKrA, S. C., BenY, J.-M., & Foster, A. L. (2009). The weathering of a sulfide orebody: speciation and fate of some potential contaminants. The Canadian Mineralogist, 47(3), 493-508. https://doi.org/10.3749/canmin.47.3.493
Crichton, R. R. (2012). Biological inorganic chemistry: a new introduction to molecular structure and function: Elsevier.
CWSA, C. W. a. S. A. (2009). Rural Water Supply Programme IV (RWSPIV) in Ashanti Region - Report on Borehole Construction in Amansie Central District. 3 - 4 (Unpublished).
Dampare, S. B., Shibata, T., Asiedu, D. K., Okano, O., Osae, S. K., Atta-Peters, D., & Sakyi, P. A. (2020). Ultramafic–mafic and granitoids supra-subduction magmatism in the southern Ashanti volcanic belt, Ghana: Evidence from geochemistry and Nd isotopes. Geological Journal, 55(4), 2495–2531.
Dietrich, A., Glindemann, D., Pizarro, F., Gidi, V., Olivares, M., Araya, M., . . . Younos, T. (2004). Health and aesthetic impacts of copper corrosion on drinking water. Water Science and Technology, 49(2), 55–62.
Edmunds, W. M., & Kinniburgh, D. G. (1986). The susceptibility of UK groundwaters to acidic deposition. Journal of the Geological Society, 143(4), 707–720.
Epstein, M., & Sowers, J. R. (1992). Diabetes mellitus and hypertension. Hypertension, 19(5), 403–418.
Ettinger, A. S., & Wengrovitz, A. M. (2010). Guidelines for the identification and management of lead exposure in pregnant and lactating women.
Foster, S., Kemper, K., Tuinhof, A., Koundouri, P., Nanni, M., & Garduno, H. (2006). Natural Goroundwater Quality Hazards, avoiding problems and formulating mitigation strategies.
García, M. H., & Ruiz, L. F. (2002). Presencia de arsénico de origen natural en las aguas subterráneas del acuífero detrítico del Terciario de Madrid. Boletín Geológico y Minero, 113(2), 119–130.
Giorgis, I., Bonetto, S., Giustetto, R., Lawane, A., Pantet, A., Rossetti, P., . . . Vinai, R. (2014). The lateritic profile of Balkouin, Burkina Faso: geochemistry, mineralogy and genesis. Journal of African Earth Sciences, 90, 31–48.
Granéli, W. (1999). Internal phosphorus loading in Lake Ringsjön. Nutrient reduction and biomanipulation as tools to improve water quality: The Lake Ringsjön story, 19–26.
Haque, M. A., Subramanian, V., & Gibbs, R. J. (1982). Copper, lead, and zinc pollution of soil environment. Critical Reviews in Environmental Science and Technology, 12(1), 13–68.
Hart, K. A., Kennedy, G. W., & Sterling, S. M. (2021). Distribution, drivers, and threats of aluminum in groundwater in Nova Scotia. Canada. Water, 13(11), 1578.
Holtzclaw, H. F., Nebergall, W. H., & Robinson, W. R. (1984). College chemistry: with qualitative analysis: DC Heath.
Ishii, N., & Terao, T. (2018). Trace lithium and mental health. Journal of Neural Transmission, 125, 223–227.
Izbicki, J. A., Groover, K. D., & Seymour, W. A. (2023). Arsenic, chromium, uranium, and vanadium in rock, alluvium, and groundwater, Mojave River and Morongo Areas, western Mojave Desert, southern California (2328–0328). Retrieved from
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60.
Jonathan, A., Opoku, F., & Akoto, O. (2023). Health Risk Assessment, Bioaccumulation Factors and Ecological Indices of Heavy Metals in Sediment, Fish and Water Along Asuoyeboah River, Kumasi: A Case Study. Chemistry Africa, 6(2), 1103–1115.
Jones, D. C., & Miller, G. W. (2008). The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochemical Pharmacology 76(5), 569–581. https://doi.org/10.1016/j.bcp.2008.05.010
Karunanidhi, D., Subramani, T., Srinivasamoorthy, K., & Yang, Q. J. E. R. (2022). Environmental chemistry, toxicity and health risk assessment of groundwater: Environmental persistence and management strategies. In (pp. 113884): Elsevier.
Kessing, L. V., Gerds, T. A., Knudsen, N. N., Jørgensen, L. F., Kristiansen, S. M., Voutchkova, D., . . . Andersen, P. K. (2017). Association of lithium in drinking water with the incidence of dementia. JAMA psychiatry, 74(10), 1005–1010.
Khilchevskyi, V., & Karamushka, V. (2021). Global Water Resources: Distribution and Demand. In Clean Water and Sanitation (pp. 1–11): Springer.
Kortatsi, B. (2006). Concentration of trace metals in boreholes in the Ankobra Basin, Ghan. West African Jornal of Applied Ecology, 10(1).
Krishan, G., Taloor, A. K., Sudarsan, N., Bhattacharya, P., Kumar, S., Ghosh, N. C., . . . Mittal, S. J. G. f. S. D. (2021). Occurrences of potentially toxic trace metals in groundwater of the state of Punjab in northern India. 15, 100655.
Kuiper, N., Rowell, C., & Shomar, B. (2015). High levels of molybdenum in Qatar’s groundwater and potential impacts. Journal of Geochemical Exploration, 150, 16–24.
Kuma, S. J., Owusu, O. R., & Gawu, S. K. (2010). Evaluating the water supply system in Kumasi, Ghana. European Journal of Scientific Research, 40(4), 506–514.
Lagos, G. E., Cuadrado, C. A., & Letelier, M. V. (2001). Aging of copper pipes by drinking water. Journal-American Water Works Association, 93(11), 94–103.
Leybourne, M. I., & Cameron, E. M. (2008). Source, transport, and fate of rhenium, selenium, molybdenum, arsenic, and copper in groundwater associated with porphyry–Cu deposits, Atacama Desert. Chile. Chemical Geology, 247(1–2), 208–228.
Lin, Q., Liu, E., Zhang, E., Li, K., & Shen, J. J. C. (2016). Spatial distribution, contamination and ecological risk assessment of heavy metals in surface sediments of Erhai Lake, a large eutrophic plateau lake in southwest China. 145, 193–203. https://doi.org/10.1016/j.catena.2016.06.003
Manning, B. A., & Martens, D. A. (1996). Speciation of arsenic (III) and arsenic (V) in sediment extracts by high-performance liquid chromatography− hydride generation atomic absorption spectrophotometry. Environmental Science & Technology, 31(1), 171–177.
Mathew, B., & Krishnamurthy, N. B. (2015). Health effects caused by metal contaminated ground water. International Journal of Advances in Scientific Research, 1(2), 60–64.
Matson, P. A., Parton, W. J., Power, A. G., & Swift, M. J. (1997). Agricultural Intensification and Ecosystem Properties. Science, 277(5325), 504–509. https://doi.org/10.1126/science.277.5325.504
Mora, A., Mahlknecht, J., Rosales-Lagarde, L., & Hernández-Antonio, A. (2017). Assessment of major ions and trace elements in groundwater supplied to the Monterrey metropolitan area, Nuevo León. Mexico., 189, 1–15.
Morais, S., Costa, F. G., & Pereira, M. d. L. (2012). Heavy metals and human health. 10(1), 227–245. https://doi.org/10.5772/29869
Moreira, V. R., Costa, F. C., Moser, P. B., Guimarães, R. N., Santos, L. V., de Paula, E. C., & Amaral, M. C. (2023). Ultrafiltration with in-line coagulation (FeCl3) to enhance arsenic removal and improve drinking water quality: From bench to pilot-scale. Chemical Engineering Journal, 472, 145063.
Mudgal, V., Raninga, M., Patel, D., Ankoliya, D., & Mudgal, A. (2023). A review on phytoremediation: Sustainable method for removal of heavy metals. Materials Today: Proceedings, 77, 201–208.
Mulware, S. J. (2020). Toxicity of Heavy Metals. A. Subject in Review., 6(2), 30–43.
Myers, R. A., Gyimah, E., Gbemadu, K., Osei, B., & Akoto, O. J. S. A. (2023). Appraising groundwater quality and the associated health risks of heavy metal contamination at Suame magazine. 21, e01794.
NRC. (1993). Pesticides in the diets of infants and children. (Division on Earth Life Studies; Commission on Life Sciences; Committee on Pesticides in the Diets of Infants Children).
Nriagu, J. O. (1998). Thallium in the Environment. Wiley. ISBN: 0471177555
Ogbaran, N., Uguru, H. (2021). Assessment of groundwater quality around an active dumpsite using pollution index. Civil Engineering Research Journal, 11(3). https://doi.org/10.19080/CERJ.2021.11.555814.
Opoku, P. A., Anornu, G. K., Gibrilla, A., Owusu-Ansah, E. d.-G. J., Ganyaglo, S. Y., & Egbi, C. D. J. G. f. s. d. (2020). Spatial distributions and probabilistic risk assessment of exposure to heavy metals in groundwater in a peri-urban settlement: case study of Atonsu-Kumasi, Ghana. Groundwater for Sustainable Development 10, 100327.
Ortiz, J. F., Cox, Á. M., Tambo, W., Eskander, N., Wirth, M., Valdez, M., & Niño, M. (2020). Neurological manifestations of Wilson's disease: pathophysiology and localization of each component. Cureus, 12(11).
Owamah, H. I., Akpoedafe, T. O., Ikpeseni, S. C., Atikpo, E., Orugba, H. O., & Oyebisi, S. (2023). Spatial distribution of heavy metals in groundwater around automobile workshops in a popular Niger-Delta University town, Nigeria. Journal of Engineering and Applied Science, 70(1), 79.
Peng, H., Yao, F., Xiong, S., Wu, Z., Niu, G., & Lu, T. (2021). Strontium in public drinking water and associated public health risks in Chinese cities. Environmental Science and Pollution Research, 28(18), 23048–23059. https://doi.org/10.1007/s11356-021-12378-y
Peter, A. L. J., & Viraraghavan, T. (2005). Thallium: A review of public health and environmental concerns. Environment International, 31(4), 493–501.
Prat, O., Vercouter, T., Ansoborlo, E., Fichet, P., Perret, P., Kurttio, P., & Salonen, L. (2009). Uranium speciation in drinking water from drilled wells in southern Finland and its potential links to health effects. Environmental Science & Technology, 43(10), 3941–3946.
Repetto, G., Del Peso, A., Repetto, M., & Nriagu, J. O. (1998). Thallium in the Environment. Advances in Environmental Science and Technology, 29, 167–199.
Rodríguez-Pacheco, R. L., Candela, L., Proenza, J., Hidalgo, M., & Salvado, V. (2005). Natural and anthropogenic origin of chromium, nickel and manganese in groundwater in the Moa region (eastern Cuba). Groundwater and Human Development. AA Balkema Publishers, The Netherlands, pág, 101–117.
Sadiq, M., Alam, I., El-Mubarek, A., & Al-Mohdhar, H. M. (1989). Preliminary evaluation of metal pollution from wear of auto tires. Bulletin of Environmental Contamination and Toxicology, 42(5), 743–748.
Samy, A., Eissa, M., Shahen, S., Said, M. M., & Abou Shahaba, R. M. (2023). Geochemistry and assessment of groundwater resource in coastal arid region aquifer (Dahab delta, South Sinai, Egypt). Beni-Suef University Journal of Basic and Applied Sciences 12(1), 1–21.
Sehar, S., Naz, I., Ali, N., & Ahmed, S. (2013). Analysis of elemental concentration using ICP-AES and pathogen indicator in drinking water of Qasim Abad, District Rawalpindi, Pakistan. Environmental Monitoring and Assessment, 185, 1129–1135.
Selvam, S., Venkatramanan, S., Sivasubramanian, P., Chung, S., & Singaraja, C. (2017). Geochemical characteristics and evaluation of minor and trace elements pollution in groundwater of Tuticorin City. Tamil Nadu, India Using Geospatial Techniques., 90, 62–68.
Sharma, N., Westerhoff, P., & Zeng, C. (2022). Lithium occurrence in drinking water sources of the United States. Chemosphere, 305, 135458.
Shehzadi, R., Rafique, H., Abbas, I., Sohl, M. A., Ramay, S. M., Mahmood, A., & Sohl, M. N. (2015). Assessment of drinking water quality of Tehsil Alipur. Pakistan. Desalination and Water Treatment, 55(8), 2253–2264.
Simon, D., McGregor, D., & Nsiah-Gyabaah, K. (2004). The changing urban-rural interface of African cities: Definitional issues and an application to Kumasi. Ghana. Environment and Urbanization, 16(2), 235–248.
Soveri, J. (1992). Acidifying effects on groundwater. In Studies in Environmental Science (Vol. 50, pp. 135–143). Elsevier.
Srinivasan, P. T., Viraraghavan, T., & Subramanian, K. S. (1999). Aluminium in drinking water: An overview. Water Sa, 25(1), 47–55.
Suresh, G., Sutharsan, P., Ramasamy, V., & Venkatachalapathy, R. (2012). Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicology and Environmental Safety, 84, 117–124.
Tandoh, K. K., Gbedemah, S. F., Doku, M.-R.S., Adams, S. J., & Brako, B. A. (2023). Petrographic and structural analysis of Paleoproterozoic Birimian granitoids and associated rocks of Boankra areas in the Kumasi Basin of Ghana. Arabian Journal of Geosciences, 16(1), 8.
U.S. Environmental Protection Agency. (2001). Risk Assessment guidance for superfund: volume III-part a. Process for Conducting Probabilistic Risk Assessment. III.
U.S. Environmental Protection Agency. (2007). Integrated Risk Information System (IRIS). Chemical Assessement Summary: National Center for Environmental Assessment.
U.S. Environmental Protection Agency. (2012). Revisions to the Unregulated Contaminant Monitoring Rule (UCMR 3) for Public Water Systems. Federal Register.
U.S. Environmental Protection Agency. (1989). Risk assessment guidance for superfund. Volume I. Human health evaluation manual (Part A). Washington, DC: U.S. Environmental Protection Agency, Office of Emergency and Remedial Response. EPA5401859002.
U.S. Environmental Protection Agency. (1994a). Method 200.2, Revision 2.8, Sample preparation procedure for spectrochemical determination of total recoverable elements. 2.8.
U.S. Environmental Protection Agency. (1994b). Method 200.8, Revision 5.4, Determination of trace elements in water and wastes by inductively coupled plasma - mass spectrometry. 5.4.
Vetrimurugan, E., Brindha, K., Elango, L., & Ndwandwe, O. M. (2017). Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Applied Water Science, 7, 3267–3280.
Wang, R., Li, H., & Sun, H. (2019). Bismuth: environmental pollution and health effects. In Encyclopedia of Environmental Health (pp. 415–423): Elsevier.
WHO. (2010). Protecting children's health in a changing environment: report of the fifth ministerial conference on environment and health: World Health Organization. Regional Office for Europe.
WHO. (2017). Guidelines for drinking-water quality: fourth edition incorporating first addendum (4th ed + 1st add ed.). Geneva: World Health Organization.
Zango, M. S., Sunkari, E. D., Abu, M., & Lermi, A. (2019). Hydrogeochemical controls and human health risk assessment of groundwater fluoride and boron in the semi-arid North East region of Ghana. Journal of Geochemical Exploration, 207, 106363.
Zhang, H., Zhou, X., Wang, L., Wang, W., & Xu, J. (2018). Concentrations and potential health risks of strontium in drinking water from Xi’an, Northwest China. Ecotoxicology and Environmental Safety, 164, 181–188. https://doi.org/10.1016/j.ecoenv.2018.08.017
Zietz, B. P., de Vergara, J. D., & Dunkelberg, H. (2003). Copper concentrations in tap water and possible effects on infant’s health—Results of a study in Lower Saxony. Germany. Environmental Research, 92(2), 129–138.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Michael Yaw Peasah: conceptualization, analysis, and interpretation of data, writing—original draft. Jonathan Awewomom: methodology, interpretation of data, writing—original draft. Richard Osae: formal analysis, data curation, writing—review, and editing. Eric Selorm Agorku: supervision, project administration, writing—review and editing. All the authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All authors have read, understood, and complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the instructions for authors.
Consent for publication
All authors carefully read and approved the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peasah, M.Y., Awewomom, J., Osae, R. et al. Trace elements determination and health risk assessment of groundwater sources in Kumasi Metropolis, Ghana. Environ Monit Assess 196, 857 (2024). https://doi.org/10.1007/s10661-024-13024-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-13024-z