Abstract
This study was performed on the water, bottom sediment and fish from four communities along the Asuoyeboah River, Kumasi, Ghana to explore the concentration and health risks of heavy metals. The impact of heavy metals on the ecology and aquatic toxicity was assessed with bioaccumulation factors and ecological risk indices. The sources and extent of pollution were explored with principal component analysis by applying multivariate statistics in identifying the principal sources of pollution. The levels of metals in the water samples follow a decreasing order of Pb > Fe > Zn > Cd > Cr. The water from the Asuoyeboah River may be poisoned by accumulated amounts of Cd, Fe and Pb due to the mean concentration of Fe (0.8 mg/L), Cd (0.129 mg/L) and Pb (1.759 mg/L) being greater than the World Health Organization allowed values of 0.3, 0.03 and 0.01 mg/L, respectively. Estimates of the non-carcinogenic risk assessment showed that the water and fish samples pose little or no adverse health concerns to the population. However, Cr and Pb recorded carcinogenic risk of > 10–6 indicating a possibility of cancer risk. The water quality index assessment from this study also showed that the water in the Asuoyeboah river was of very poor quality. Researchers that may need more precise results while taking into account different water quality parameters might use the water quality index and multivariate statistics. The results of this study might aid the relevant authorities in formulating policies with strict regulations to safeguard water quality, ensure effective management and reduce surface water pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aquatic toxicology studies chemical substances like trace metals, both from anthropogenic and natural sources and their effects on living organisms [1,2,3]. Heavy metals (HMs) pollution in rivers has become one of the major environmental problems in Ghana [4,5,6,7]. There are many pollutants present in river water but toxicity is only observed beyond a certain limit (permissible limit). The type of pollutants in water depends upon the nature of the agricultural, municipal and industrial activities in the environment. The different types of water pollutants may be categorized as organic, biological and inorganic. The most common inorganic water pollutants are HMs, which can be highly toxic and carcinogenic [8]. Rapid urbanisation around the Kumasi metropolis could lead to a rise in the levels of HMs in rivers [9], and the introduction of these metals into the aquatic ecosystems is on the rise with industries being the chief source of pollution [10].
Pollution is increasing in our environment yearly due to industrialisation, increase in population and technological advancement, in the last couple of years, the rate at which organisms and humans in the environment get exposed to these metals has dramatically risen due to increased industrialization [9], a chunk of the industries discharges their effluents into the open environment which may end up in rivers, eventually contaminating the water bodies. In Ghana, most of these water bodies serve as a drinking source for some people and some farmers also use the water for irrigation. The gradual deterioration of rivers and reservoirs with heavy metals usually results from poor natural sources, waste disposal, and improper fishing and farming practices [11]. Rivers are very significant to society but because they are naturally open, they can be easily polluted [12], rivers also contribute significantly to the economies of societies through irrigation for farmers and Fishing for fishermen. Around the world, anthropogenic activities and urbanization have already contributed to the pollution of rivers [13].
Water from rivers can be treated for domestic and industrial use where potassium ferrate (K2FeO4) has proven to be effective in the removal of heavy metals and natural organic matter in river water. K2FeO4 has a high metal removal capacity at increased pH’s and has coagulative, disinfected and oxidant properties that make it suitable for water treatment [14]. Ferric sulphate and chloride are effective coagulants and disinfectants in water treatment, they can remove heavy metals, oxidize inorganic and organic contaminants, disinfect microorganisms and eliminate suspended particulate matter [15], aluminium sulphate, an aluminium coagulant has also proven to be effective in water treatment [16]. Surface water like that of the Asuoyeboah river is valuable to the population along the river but could be polluted through agricultural and industrial practices [17]. River bank filtration (RBF) is an effective method for treating river water, RBF technology operates by pumping out water from drilled and bore wells along the banks of a river, during this process, there is infiltration of river water through the riverbed sediments. As the surface water travels towards the RBF well, dissolved and suspended contaminants, as well as pathogens, are potentially removed or significantly reduced in numbers, sorption, biological degradation and filtration all occur during the RBF process [18].
The health of both humans and animals depends on the water quality of an aquatic environment [19]. Heavy metals pose risks to the aquatic environment by interacting with water, fish and sediments [20,21,22,23]. Metals pose fewer risks when adsorbed to sediments but the change in some environmental factors like pH can enhance their exposure to living organisms and affect the quality of water [19]. The environment is the major receiver of all waste generated which eventually ends up in water bodies. Heavy metals may come from natural sources like wash-offs of weathered rocks [24], through environmental activities like farming and sewage disposal, these metals could end up in rivers and be uptaken by sediment and fish through leaching and irrigation could be passed on to crops. Several pollutants, such as pesticides, fertilizers, and fumes from industrial machines and vehicles that contain these pollutants can seriously affect human health throughout the food chain [25].
Metals like Cd, Pb and Cr are toxic to humans and organisms in the aquatic environment, these metals get into fishes by adsorption through the tissues and eventually bio-concentrate to levels that can be toxic [26, 27]. Biologically, this has adverse effects on the survival, growth and reproduction of aquatic organisms including fishes [28]. Zn, Fe, Mn and Cu are essential to humans at moderate concentrations but can however induce toxicity at higher concentrations [10]. Some heavy metals are usually carcinogenic, non-biodegradable and bio-accumulative [29]. Some of these heavy metals have been linked to serious disorders such as clogging of nasal mucous membranes and throat, oedema of the eyes, and reproductive and hereditary failure, monitoring these metals will therefore be critical for assessments of environmental safety, human health and remediation strategies [30]. One of the innovative remediation techniques for heavy metals is bioremediation; this technology is promising and innovative for the recovery and removal of heavy metals in polluted water and lands using living organisms and plants [31]. Microorganisms have acquired various strategies for their survival in heavy metal-polluted habitats, these organisms are known to adopt and develop several detoxification mechanisms such as biomineralization, bioaccumulation, biosorption and biotransformation [32].
Tilapia is the most common fish found in the Asuoyeboah river. Metals may accumulate in fish and other pollutants because of the very close interaction with the surface that transports the chemicals when they suspend in water or solution. Fishes respire by extracting oxygen and passing large volumes of water through the gills; this may introduce some heavy metals into the tissues of the fish [33]. Metals can precipitate as oxides/hydroxides, and the hydroxide concentration is of greater importance for the mobility of metals [34], the ions of the metals can also react with the water constituents or accumulate at the bottom when the metals get into water bodies and get attached to the sediments and fishes. Heavy metals have higher availability in soil and aquatic systems but a relatively lower proportion in the atmosphere as particulate or vapour [11]. Sediment is a major deposit of several contaminants in the aquatic environment including heavy metals [29]. The mobility of these metals is greatly influenced by wind, gravity and water, these factors, therefore, affect the distribution of heavy metals in the aquatic environment [30, 35].
Food contaminated with heavy metals can reduce important nutrients in the body and may be responsible for malnutrition, gastrointestinal cancer and even reduce immunological action [36]. The ever-increasing population along the Asuoyeboah river has necessitated its proper management and conservation for sustainable development. The Asuoyeboah river was chosen for this study because the surrounding communities greatly depend on the Asuoyeboah river for industrial and domestic purposes and there is yet no official and recognize scientific study on the presence, levels and impact of heavy metal contaminants from the Asuoyeboah river. Accessing the water quality of Asuoyeboah river will provide information about the status and sustainability of a reservoir [37]. Accessing water quality provides information about the status and sustainability of a reservoir [37]. Increased domestic, municipal, agricultural and industrialisation along the river could lead to a rise in pollution levels in the water body [38]. The communities along the Asuoyeboah river greatly depend on the river for farming, as a source of fishing, washing of cars, alcohol production, mechanical works and domestic use, it is therefore necessary to access the quality of Asuoyeboah river as it largely affects the health of the population in different towns in the metropolis. This is the first scientific contribution to access the levels and address the health risks of heavy metals in the Asuoyeboah river. The study (1) focused on levels of heavy metals in fish, surface sediment and water in the Kumasi metropolis; and (2) provides information on the levels of heavy metals and their health impact on the ecology and the environment. This information will help authorities in decision-making.
2 Materials and Method
2.1 Study Area
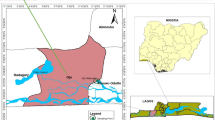
In Ghana, Asuoyeboah is a small community in the Atwima Mpouna district of the Ashanti regional capital, Kumasi. The Kumasi metropolis is located at longitude 1.30–1.37° and latitudes 6.35–6.40° covering an area of about 299 km2 with a dry and wet tropical climate [39]. The study was carried out on the Asuoyeboah river, as shown in Fig. 1.
The river flows through communities like Sepaase, Maakro and Tanoso with several agricultural and industrial activities like farming and alcohol production. The river flows from Asuoyeboah through Tanoso, Abuakwa and ends in Sepaase where it is fed with high levels of domestic waste, wash-offs of mechanical shops and hair care saloons, these could be potential pollutants to the Asuoyeboah river. Water and sediment samples were collected at four sampling sites along the river; Asuoyeboah, Tanoso, Abuakwa-Maakro and Sepaase-Besease. The Global Positioning System (GPS) location for each sampling site was recorded to be N 06° 41′ 54. 7″ W 001° 40′ 33.2″, N 06° 42′ 05.2″ W 001° 41′ 12.2″, N 06° 42′ 03.8″ W 001° 43′ 04.0″, N 06° 42′ 09.6″ W 001° 45′ 12.9″ for Asuoyeboah, Tanoso, Abuakwa-Maakro and Sepaase-Besease, respectively.
2.2 Sampling
From June to July 2020, five sampling sites were selected along the Asuoyeboah river, because during the rainy season surface water quality can be highly influenced by pollutants, such as leachate migrating into the open surface water than during the dry season. Water samples were taken from the Asuoyeboah river using properly washed buckets (washed with detergents) at two-hour intervals for 8 h at different points to form a single sample and pre-cleaned bottles (washed with detergents, rinsed and soaked in 10% nitric acid overnight) were used to collect the water samples. Before sampling at each site, sampling bottles were rinsed with deionized water and 1 mL of nitric acid was added to each bottle containing the water samples to dissolve metals that may be attached to the walls of the container and keep the metal ions in solution. Sampling was based on the USEPA guidelines for sampling surface water [40]. To minimize the potential for volatilization or biodegradation between sampling and analysis, the samples were transported to the lab and stored at 4 °C before analysis. Sediment samples were collected using a clean plastic spoon to scoop samples into zip-lock bags. Samples were picked from 6 different points to form a single (composite) sample at each sampling site to have good coverage of the average conditions of the site for the sediment and water samples, 4 samples each of sediment and water were picked for analysis. Fish samples were bought from the local fishermen and collected into a clean polyethylene bag. All 16 fish from the surface water were transported to the Kwame Nkrumah University of Science and Technology (KNUST) Fish and Nutrition Laboratory of Fisheries and Watershed Management for classification and identification. The fish were all identified as one species (Hemichromis fasciatus) belonging to the family Cichlidae.
2.3 Sample Preparation
Fish samples were homogenised using a clean sharp knife with the scales, flesh and bones removed for analysis. Sediments and fish samples were sun-dried and in an oven. The sediment samples were crushed into smaller particles with a pestle and mortar sieved using a mesh of 0.5 mm and transferred into a clean polyethylene zip-lock bag and sealed before digestion for heavy metals analysis. About 1 g each of crushed sediment and homogenized fish was weighed into a cleaned and dried digestion tube, and 1 mL of deionized water was added and swirled to mix 4 mL of nitric-perchloric acid (1:1) mixture. About 5 mL of sulphuric acid was added to the mixture and swirled to mix. The mixture was then heated on a hot plate to 200 ℃ for about 30 min. The digested sample was made up to the volume (50 mL) using deionised water after cooling [28]. It was then transferred into 50 mL pre-cleaned falcon tubes in readiness for metals analysis, 50 mL of the water sample was put in a conical flask and heated at 120 for 30 min with 20 mL nitric acid, filtered with a Whatman number 4 filter paper and topped up to 50 ml with deionized water. A Perkin Elmer flame Atomic Absorption Spectrophotometer (AAS) model AA 220 was employed for the metal analysis. The various metals were analysed at selected wavelengths with the standard solutions of all the metals and the absorbance was read as the concentration. The concentrations of some heavy metals in the water, fish and sediment mg/kg samples were measured in mg/L, mg/kg and mg/kg, respectively. The analysis was replicated and averaged; blanks and standard references were used to ensure the quality of the analysis.
2.4 Quality Control and Assurance
Strict measures were put in place to ensure the quality of the study results. Analytical grade chemicals and deionized water were used for sample digestion. Glassware was washed thoroughly and cleaned using deionized water. Blank solutions for the water, sediment and fish samples were prepared and analysed. Standard solutions of the respective elements were used to prepare calibration curves to set the right instrument conditions for analysis, the detection limits were then acquired based on the slope and standard deviation of these curves.
2.5 Health Risk Assessment Methodologies
Assessment of risk is a method used to gather important information on health and other risk factors. This helps to understand the status of the population’s health and its evolvement, as well as ways to address the risks [41]. Evaluations were done for adults of 70 kg body weight and children of 15 kg body weight because this represents a large fraction of Ghana's population. The common exposure routes for water are oral and dermal absorption [42]. The following risk methodology was referred from [40]:
where EDIing is the estimated daily intake through ingestion, EDIderm is the estimated daily intake through dermal absorption in mg/kg per day (mg/kg/day), Cwater is the concentration of the metal in water (mg/L), IR is the ingestion rate (1.8 and 2.2 L/day for children and adults, respectively), EF is the exposure frequency (365 days/year), ED is the exposure duration (6 and 70 years for children and adults, respectively), AT is the averaging time (365 days/year × 6 and 70 years for children and adults, respectively), BW is the body weight (15 and 70 kg for children and adults, respectively), SA is the exposed skin area (6600 and 18,000 cm2 for children and adults, respectively), CF is the unit conversion factor (0.001 L/cm3), ET is the exposure time (1 and 0.58 h/day for children and adults, respectively); and, KP is the dermal permeability coefficient in water, (cm/h), 0.004 for Pb, 0.002 for Cr, 0.0006 for Zn and 0.001 for Cd and Fe.
The hazard quotient (HQ) is an estimate of how toxic each metal is through various exposure routes:
Rfd is the reference dose, Rfd for ingestion (RfDing) and dermal absorption (RfDderm). Hazard index (HI), a summation of the various hazard quotients to quantify the overall risk, was calculated as follows [42]:
HI > 1 is considered for potential health risk. Cancer risk (CR) was also calculated by using:
SF is the slope factor for cancer. The SF for Cd = 6.1 × 103, Pb = 8.5 and Cr = 5 × 102 all in in ug/g per day (ug/g/d−1) were referred from [41] and [43, 44]. The only available values of the SF are those of Cd, Pb and Cr. When the CR value is > 10–6, there is potential carcinogenicity [45].
2.6 Water Quality Index (WQI)
WQI measures water conditions relative to its purpose for humans using sets of reference standards to ensure compliance. WQI is assessed on the safety of water for drinking, human contact and the ecosystem [46]. In calculating the WQI, each factor was assigned a maximum weight of 5 [47]. The weighted arithmetic method was used to check the quality of the river, as shown in Table 1.
The following relations were used:
n = number of parameters used, Si = World Health Organization (WHO) standard for drinking water and Ci = amount of metal in the study area, wi = weight of a parameter and Wi = unit weight of a parameter [48]. In general, the water quality index is divided into five (5) categories: excellent water (50), good water (50–100), poor water (100–200), very poor water (200–300), and water unfit for consumption (> 300) [46].
2.7 Environmental Assessment of Sediment
Enrichment factor (EF), geoaccumulation index (Igeo) and ecological risk (Er) and indices of ecological risk (Ri) were applied to evaluate metals in the surface sediment of the Asuoyeboah river. The following equations are used:
Ri is the summation of the ecological risk factors, Er is the ecological risk factor, TR is the factor for toxic-response (Cd = 30, Pb = 5, Cr = 2 and Zn = 1). M is the amount of metal contained in the sediments and B0 is the levels from background study [49]. The Er and Ri are categories into Er < 40 = low risk, 40 < Er < 80 = medium risk, 80 < Er < 160 = Tolerable risk, 160 < Er < 320 = high risk, Er > 320 = very high risk, Ri < 150 = low risk, 150 < Ri < 300 = medium risk and 300 < Ri < 600 = very high risk [49].
2.7.1 Geoaccumulation Index
Igeo is referred to in measuring the degree of metal contamination in the environment and gives information about anthropogenic contribution [50]. The Igeo distinguishes 6 classes of quality for sediments; Igeo < 0 = not contaminated, 1 < Igeo < 1 = not contaminated to medium contamination, 2 < Igeo < 3 = medium to strong contamination and Igeo > 5 = extreme contamination [50].
M = amount of metal in the sediment, Bo = level of metal from background study. Background values of 0.005 for Zn, 12.69 for Cd, 1.29 for Cr, 4.67 for Pb and 9.27 for Fe in mg/Kg were used [51].
2.7.2 Enrichment Factor (EF)
The EF provides information on the amounts of contaminants from anthropogenic sources. It checks the quantity of waste input by human activities into the environment and the level of contamination from natural sources [52]. This is calculated by normalizing the concentration of an element in the sediment to a reference heavy metal. An element with an unaltered anthropogenic concentration [53], for surface sediment, Fe is widely used by most researchers because of its availability and how other metals are distributed [54]. The enrichment categories include; ER < 2 = very little enrichment. EF = 2—5 = medium enrichment, EF = 5—20 = severely enriched and EF > 40 = extremely enriched [55].
RE is the reference element.
2.8 Bioconcentration (BCF) and Biosediment Accumulation Factor (BSAF) of Heavy Metals in Fish
In aquatic toxicology, BCF is used to determine the concentration of a chemical substance or contaminant in an organism relative to its surrounding environment, BCF of heavy metals is the ratio of metals in water to the ratio of metals in a living organism. BCF and BSAF will be calculated for fish in the Asuoyeboah river.
BFC is in L/Kg, it is calculated by the equation:
where Cf is the concentration of metals in the fish and Cw is the concentration of metal in the water [56].
2.8.1 Biosediment Accumulation Factor
BSAF determines the excretion and absorption rate of a substance by an organism by accessing the levels of a substance in sediments to the levels in an organism. BSAF is determined by the following relation;
where Cs = the concentration of the metal in the sediments [56].
2.9 Statistical Analysis
The analysis was done with SPSS 20.0 software from IBM (Statistical Package for the Social Sciences) and Microsoft excel. A correlation method was employed to check the link and the similarity in the origin of the selected heavy metal. Multivariate methods have been used in identifying the principal sources of pollution. In the principal component analysis (PCA), Kaiser Normalization with varimax was applied. Box and whisker plots were also introduced to summarise the levels of the elements in the samples and show the distribution of these elements across the sampling sites [57].
3 Results and Discussion
The concentrations of five (5) heavy metals in water, fish and sediment samples along the Asuoyeboah river have been illustrated. Figure 2 is a box and whisker plot and a summary of the concentrations of some heavy metals in the water (mg/L), fish (mg/kg) and sediment (mg/kg) samples.
Levels for Cd, Zn, Cr, Pb and Fe in the water samples were recorded as 0.173 ± 0.043 mg/L, 0.043 ± 0.011 mg/L, 0.129 ± 0.022 mg/L, 0.800 ± 0.987 mg/L and 1.759 ± 0.470 mg/L, respectively represented in Fig. 2a. The 0.043 mg/L of Zn recorded in this current study is far lower than the 0.19 mg/L reported in the Sisa River [48]. Pb was predominantly high in the four sampling sites for the fish and water samples with an average concentration of 1.759 mg/L, which is above the world health organisation (WHO) permissible limit of 0.01 mg/L [58] for drinking water and far exceeds the 0.02–0.06 mg/L reported by Akoto et al. [59] in the Barekese Reservoir. Many other studies in Ghana have reported lower levels of lead, like the 0.75 mg/kg reported in river Birim by Osafo [60] and the 0.03 mg/kg reported by Akoto et al. [61] in the Owabi reservoir. This present study has therefore revealed higher levels of lead in the Asuoyeboah river than in other rivers in the metropolis. These levels of Pb in Asuoyeboah, Tanoso and Abuakwa-Maakro may come from the discharge of mechanical waste like batteries and cables sited along the river [62], which agrees with Danziger et al. [63]. Lead can accumulate as a result of human-generated waste and various products in our homes like batteries, pipes and ceramic, public health and environmental regulations require that sewage from communities must be treated to acceptable standards before it is released into the water bodies [64], this will help to minimize the levels of contaminants in the Asuoyeboah river. High amounts of Pb in the human body affect the brain and nervous system. Some other researchers recorded Pb levels as 0.026 mg/L in groundwater [65] and greater than 0.05 mg/L in surface water [66], which is less than the 0.987 mg/L recorded in this study. Fe recorded very high values in the sediments with a mean concentration of 14.46 ± 2.499 mg/kg as shown in Fig. 2b; Cr recorded the lowest value in the water and fish samples, while Cd recorded the lowest value for the bottom sediment. The levels of metals in the water samples follow a decreasing order of Pb > Fe > Zn > Cd > Cr. The mean concentration of Fe (0.8), Cd (0.129) and Pb (1.759) were higher than the WHO permissible of 0.3, 0.03 and 0.01 mg/L [58], respectively, in drinking water. Dadzie [67] showed that levels of Cd in river Densu were not detectable. Results from this study showed that the water from Asuoyeboah river could experience poisoning from accumulated levels of cadmium. High levels of Cd may come from domestic waste containing paint and anthropogenic waste. This agrees with Frickel, Elliott [68] and Mann et al. [69]. Cadmium in high concentration causes diarrhoea, nausea and even vomiting [70]. The information from the study indicates metals were present in all the fish collected from the Asuoyeboah river. Other studies have shown that heavy metals bioaccumulate in fishes [71]. According to the European Union (EU), the acceptable amounts of Cd and Pb in fish are 0.3 mg/kg and 0.05—0.3 mg/kg, respectively [72], comparing this to the recorded levels in fish, Pb far exceeds the acceptable levels and cadmium was within the acceptable range, as shown in Fig. 2c. Cd and Pb are non-vital elements in meals and may be harmful even at lower concentrations over time [73]. The impact of heavy metals on the population along these communities can be addressed by proper regulation from the municipal health directorate on the disposal of solid and liquid waste, proper farming practices and education on the consequences of improper waste management in these communities.
3.1 Correlation Analysis
The correlation between heavy metals was analysed by Pearson correlation matrix, as shown in Table 2. The connection between several variables can be evaluated by correlation analysis [74]. Correlation was significant at less than 0.05 levels. For the fish samples, Zn and Pb recorded a strong positive correlation of 0.816, 0.788 for Zn and Fe and 0.541 for Cd and Cr. Cd and Pb were strongly correlated in the water samples, and then Zn and Cr, Fe and Cr for the sediment were also positively correlated as shown in Table 2. The strong correlation between the metals in the fish, sediment and water samples showed they are probably released from a common origin [39], a similar source and may have a similar behaviour during the transport process [75]. The rest of the variables had negative correlations; this indicates an inverse variation between the chemical components in the fish, water and sediment. When there is no correlation between variables, it’s an indication that the variables were not bound by a common or single factor [76]. Sanitation and environmental protection authorities must therefore take steps to ensure proper disposal of domestic and industrial waste and regulate the activities of mechanics in these communities.
3.2 Principal Component Analysis
The sources and extent of metal pollution were explored with PCA [39]. Factor matrix was used to define elements grouped to a given component and factors with strong correlation were categorized as components after rotation with varimax. Table 3 shows factor loading of PCA after rotation with varimax using the concentrations of the elements in the fish, sediment and water samples in the four sampling sites.
The first principal component, PC1 showed loadings that were high for Zn (0.943), Cd (0.951) and Fe (0.812) in the water samples with a variance of 61.1% in Table 3, indicating they may be from a common source like the factory wastes from the alcohol produced along the Asuoyeboah river. Cr (0.958) was the only highly loaded metal in the second principal component (PC2) in the water samples, with a variance of 30.2%. The PC1 for Zn, Cr, Pb and Fe were highly loaded in the sediment, contributing 52.1% of the total variation as shown in Table 3. This signals a similarity in their pollution sources in the Asuoyeboah river [59]. PC2 in the sediment contributed 31.7% and was dominated by Cd and Cr with high loadings. Sewage, paint, pigments, waste generated by residents and weathering of rock materials could account for the levels of Cr in PC2 [77]. Anthropogenic activities such as farming could be the primary source of Cd contamination in Asuoyeboah river [78], smelting of metals and plastics contributes a lot of cadmium to the environment [25] and therefore the waste from these activities must be channelled into the Asuoyeboah river. Zn and Cd also dominated the PC1 of the metals in the fish and contributed to 67.7% of the total variation. Cr, Fe, Zn, and Cd in the Asuoyeboah river may result from deposits from the atmosphere, industrial waste, and sewage. Cadmium recorded in the Asuoyeboah river results from waste from domestic sources containing paint [79], proper disposal of these wastes could curb this menace.
3.3 Health Risk Assessment
Table 4 shows the assessed risk (non-carcinogenic) of the water and fish samples of the Asuoyeboah river. The HQ and HI did not exceed the safety limit (unity).
This indicates that the water and fish of the Asuoyeboah river pose little or no health concerns. The CR was only calculated for Cd, Cr and Pb as represented in Table 5, Fe and Zn gave no values of cancer slope by the Integrated Risk Information System [80]. A CR value of > 10–6 is considered to have potential carcinogenic risks [40]. The results showed Cd has no potential cancer risk. However, Cr and Pb recorded values greater than 1 in a million, both for adults and children through ingestion (Table 5). This indicates that the water and consumption of fish are likely to pose significant cancer risks to the population along the Asuoyeboah river.
Table 6 shows the calculated indices of water quality. The results in Table 6 ranged from 69.87 to 92.01% showing the water was poor in quality. Farming activities that generate and feed waste into the Asuoyeboah river may have contributed to the poor state of the water [81]. Also, the rate of flow of the water may have played a role in its poor quality as this river continues to flow through different communities [82].
3.4 Ecological Risk
Table 7 shows the calculated Er and Ri. From the results, Cr, Pb and Cd had very low ecological risks, But Zn had very high Er and Ri, showing considerable risks and low overall risks (Ri).
This study suggests that Cr, Cd, Pb and Fe pose minimal ecological risks in the Asuoyeboah river. The levels of Zn, however, indicate a considerable potential ecological risk. Some fish can accumulate Zn in their bodies and they can biomagnify up the food chain. High amounts of Zn in the soil can negatively affect the activities of microorganisms and plants, have the potential to seriously slow down the breakdown of organic matter, and increase the acidity of water [83].
The calculated Igeo indices of HMs are in Table 7. The results showed the sediment was not contaminated with Cr, Cd, Pb and Fe. Zn, however, recorded very high Igeo indices in the four sampling sites showing that the sediments are extremely contaminated with Zn. The Zn amounts in this study were far less than the 81.93 mg/kg recorded in a study conducted in Indonesia [75].
The calculated values of EF for metals in the sediment are given in Table 7 on comparing to the enrichment categories shows that Cr, Fe, Pb and Cd in the four sampling sites were less enriched. This suggests there is less anthropogenic contribution to the concentrations of these trace metals and maybe from natural processes [75]. However, Zn was moderately enriched in Asuoyeboah and severely enriched in Abuakwa-Maakro and Sepaase-Besease. This suggests that other sources may have introduced high amounts of Zn, which agree with the report by Sutherland et al. [84]. When the EF is between 0.5 and 1.5, the amount of the heavy metal in the sediment may occur by nature, as is the case of Fe in this current study, Land run-off, biota and sewage are responsible for the enrichment when the EF is greater than 1.5 [85].
3.5 Bioconcentration and Biosediment Accumulation Factors of Heavy Metals in Fishes
BCAF and BCA were calculated to assess the transfer of heavy metals from sediment and water to fish. A BCF/BSAF > 1 is an indication that metals can accumulate in immoderate amounts in the fish and induce some level of toxicity to consumers [56]. From Table 8, Fe and Cr had a BCA value of less than one. This indicates almost little or no bioaccumulation of these heavy metals from the river water on the fishes, Cd, Pb and Zn however exceeded 1 indicating a high absorption of metals into the fishes from the water and a potential accumulative metal toxicity especially Zn as a result of high bioaccumulation from the Asuoyeboah river.
Very strong bioaccumulation factors were recorded for Cd, Zn and Pb from the sediment; most of the heavy metals (Cd, Zn and Pb) were therefore transferred to the fishes from the sediment and the sediment contributed significantly to the levels of these metals in the fishes of the Asuoyeboah river [56].
4 Conclusion
Heavy metal concentrations (Pb, Fe, Zn, Cd and Cr) were examined in fish, surface water and surface sediments collected from four sampling points along the Asuoyeboah river, Kumasi. This work aimed to assess the health risk assessment and heavy metal pollution status based on the multi indicators evaluation methods. Furthermore, multivariate statistics were used to infer the possible correlations between these studied heavy metals. This study revealed considerable metal contamination in the Asuoyeboah river. The results showed sediment recorded very high concentrations of Fe. The concentration of elements in the surface sediment of the Asuoyeboah river was higher than the levels recorded in the water and fish samples. The health risk assessment indicated no risk to consume fish and water in the study area because the estimated daily intakes of these five HMs were all much lower than their respective recommend values. The target hazard quotient values indicated no non-carcinogenic risk to those consuming fish and water in the study area. The average annual cancer risk suggested carcinogenic risk for Pb and Cr. Zinc recorded very high geoaccumulation indices in the four sampling sites showing that the sediments are extremely contaminated with Zn. The water quality index from this study also revealed that the water in the Asuoyeboah river was of very poor quality, contact with this water can therefore pose adverse health effects to the population. Based on multivariate data, anthropogenic activity is often the primary source of HMs in the Asuoyeboah River, therefore regulating the source discharge and sewage interception will be recommended for proper management measures. This study will serve as a guide for the relevant authorities to implement proper regulations and measures to help address the risk posed by heavy metals from the Asuoyeboah river.
Data Availability
Data available on request from the authors.
References
Rand G, Wells P, McCarty L (2020) Introduction to aquatic toxicology. Fundamentals of aquatic toxicology. CRC Press, pp 3–67
Kumar V, Agrawal S, Bhat SA, Américo-Pinheiro JHP, Shahi SK, Kumar S (2022) Environmental impact, health hazards, and plant-microbes synergism in remediation of emerging contaminants. Clean Chem Eng 2:100030. https://doi.org/10.1016/j.clce.2022.100030
Becher J, Englisch C, Griebler C, Bayer P (2022) Groundwater fauna downtown—drivers, impacts and implications for subsurface ecosystems in urban areas. J Contam Hydrol 248:104021. https://doi.org/10.1016/j.jconhyd.2022.104021
Ewusi A, Sunkari ED, Seidu J, Coffie-Anum E (2022) Hydrogeochemical characteristics, sources and human health risk assessment of heavy metal dispersion in the mine pit water–surface water–groundwater system in the largest manganese mine in Ghana. Environ Technol Innov 26:102312. https://doi.org/10.1016/j.eti.2022.102312
Hagan GB, Minkah R, Yiran GAB, Dankyi E (2022) Assessing groundwater quality in peri-urban Accra, Ghana: implications for drinking and irrigation purposes. Groundw Sustain Dev 17:100761. https://doi.org/10.1016/j.gsd.2022.100761
Sunkari ED, Seidu J, Ewusi A (2022) Hydrogeochemical evolution and assessment of groundwater quality in the Togo and Dahomeyan aquifers, Greater Accra Region Ghana. Environ Res 208:112679. https://doi.org/10.1016/j.envres.2022.112679
Akoto O, Samuel A, Gladys L, Sarah OAA, Apau J, Opoku F (2022) Assessment of groundwater quality from some hostels around Kwame Nkrumah University of Science and Technology. Sci Afr 17:e01361. https://doi.org/10.1016/j.sciaf.2022.e01361
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling—an overview. RSC Adv 2(16):6380–6388
Zhuang Q, Li G, Liu Z (2018) Distribution, source and pollution level of heavy metals in river sediments from South China. CATENA 170:386–396
Fisher RM, Gupta V (2021) Heavy metals. StatPearls. StatPearls Publishing, Chennai
Manzoor J, Sharma M, Wani KA (2018) Heavy metals in vegetables and their impact on the nutrient quality of vegetables: a review. J Plant Nutr 41(13):1744–1763
He L, Gao B, Luo X, Jiao J, Qin H, Zhang C, Dong Y (2018) Health risk assessment of heavy metals in surface water near a uranium tailing pond in Jiangxi Province South China. Sustainability 10(4):1113
Borges RC, Dos Santos FV, Caldas VG, Lapa CMF (2015) Use of geographic information system (GIS) in the characterization of the Cunha Canal, Rio de Janeiro, Brazil: effects of the urbanization on water quality. Environ Earth Sci 73(3):1345–1356
Lim M, Kim M-J (2010) Effectiveness of potassium ferrate (K2FeO4) for simultaneous removal of heavy metals and natural organic matters from river water. Water, Air, Soil Pollut 211(1):313–322
Jiang JQ, Lloyd B (2002) Progress in the development and use of ferrate(VI) salt as an oxidant and coagulant for water and wastewater treatment. Water Res 36(6):1397–1408. https://doi.org/10.1016/s0043-1354(01)00358-x
Konieczny K, Sąkol D, Płonka J, Rajca M, Bodzek M (2009) Coagulation—ultrafiltration system for river water treatment. Desalination 240(1–3):151–159
Kumari S, Kumar RN (2021) River water treatment using electrocoagulation for removal of acetaminophen and natural organic matter. Chemosphere 273:128571
Ahmed AKA, Marhaba TF (2017) Review on river bank filtration as an in situ water treatment process. Clean Technol Environ Policy 19(2):349–359
Miranda LS, Wijesiri B, Ayoko GA, Egodawatta P, Goonetilleke A (2021) Water-sediment interactions and mobility of heavy metals in aquatic environments. Water Res 202:117386
Pappoe C, Palm LMND, Denutsui D, Boateng CM, Danso-Abbeam H, Serfor-Armah Y (2022) Occurrence of microplastics in gastrointestinal tract of fish from the Gulf of Guinea Ghana. Mar Pollut Bull 182:113955. https://doi.org/10.1016/j.marpolbul.2022.113955
Khan MA, Khan N, Ahmad A, Kumar R, Singh A, Chaurasia D, Neogi S, Kumar V, Bhargava PC (2022) Potential health risk assessment, spatio-temporal hydrochemistry and groundwater quality of Yamuna river basin Northern India. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.136880
Mohiuddin M, Hossain MB, Ali MM, Kamal Hossain M, Habib A, Semme SA, Rakib MRJ, Rahman MA, Yu J, Al-Sadoon MK, Gulnaz A, Arai T (2022) Human health risk assessment for exposure to heavy metals in finfish and shellfish from a tropical estuary. J King Saud Univ Sci 34(4):102035. https://doi.org/10.1016/j.jksus.2022.102035
Adani P, Sawale AA, Nandhagopal G (2022) Bioaccumulation of heavy metals in the food components from water and sediments in the coastal waters of Kalpakkam, Southeast coast of India. Environ Nanotechnol Monit Manag 17:100627. https://doi.org/10.1016/j.enmm.2021.100627
Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN (2019) Mechanism and health effects of heavy metal toxicity in humans. Poisoning in the modern world—new tricks for an old dog? IntechOpen
Sharma V, Singh P (2015) Heavy metals pollution and it’s effect on environment and human health. Int J Recent Sci Res 6(12):7752–7755
Ahmadi A, Moore F, Keshavarzi B, Shahimi H, Hooda PS (2022) Bioaccumulation of selected trace elements in some aquatic organisms from the proximity of Qeshm Island ecosystems: human health perspective. Mar Pollut Bull 182:113966. https://doi.org/10.1016/j.marpolbul.2022.113966
Souza-Araujo Jd, Hussey NE, Hauser-Davis RA, Rosa AH, Lima MdO, Giarrizzo T (2022) Human risk assessment of toxic elements (As, Cd, Hg, Pb) in marine fish from the Amazon. Chemosphere 301:134575. https://doi.org/10.1016/j.chemosphere.2022.134575
Darko G, Azanu D, Logo NK (2016) Accumulation of toxic metals in fish raised from sewage-fed aquaculture and estimated health risks associated with their consumption. Cogent Environ Sci 2(1):1190116
Cimboláková I, Uher I, Laktičová KV, Vargová M, Kimáková T, Papajová I (2020) Heavy metals and the environment. Environ Factors Affect Hum Heal 10
Jia Z, Li S, Wang L (2018) Assessment of soil heavy metals for eco-environment and human health in a rapidly urbanization area of the upper Yangtze Basin. Sci Rep 8(1):3256. https://doi.org/10.1038/s41598-018-21569-6
Dixit R, Malaviya D, Pandiyan K, Singh UB, Sahu A, Shukla R, Singh BP, Rai JP, Sharma PK, Lade H (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7(2):2189–2212
Elekwachi CO, Andresen J, Hodgman TC (2014) Global use of bioremediation technologies for decontamination of ecosystems. J Bioremediat Biodegrad 5(4):1
Javed M, Usmani N (2011) Accumulation of heavy metals in fishes: a human health concern. Int J Environ Sci 2(2):659–670
Akannam PO (2020) Flame atomic absorption spectrophotometric determination of heavy metals in some agricultural soils of Kwali Area Council, Abuja Nigeria. J Environ Sci Public Health 4(3):216–228
Kwaansa-Ansah EE, Nti SO, Opoku F (2019) Heavy metals concentration and human health risk assessment in seven commercial fish species from Asafo Market Ghana. Food Sci Biotechnol 28(2):569–579. https://doi.org/10.1007/s10068-018-0485-z
Singh J, Kalamdhad AS (2011) Effects of heavy metals on soil, plants, human health and aquatic life. Int J Res Chem Environ 1(2):15–21
Guo Z, Boeing WJ, Borgomeo E, Xu Y, Weng Y (2021) Linking reservoir ecosystems research to the sustainable development goals. Sci Total Environ 781:146769
Boateng TK, Opoku F, Acquaah SO, Akoto O (2015) Pollution evaluation, sources and risk assessment of heavy metals in hand-dug wells from Ejisu-Juaben Municipality Ghana. Environ Syst Res 4(1):18. https://doi.org/10.1186/s40068-015-0045-y
Darko G, Dodd M, Nkansah MA, Aduse-Poku Y, Ansah E, Wemegah DD, Borquaye LS (2017) Distribution and ecological risks of toxic metals in the topsoils in the Kumasi metropolis Ghana. Cogent Environ Sci 3(1):1354965
USEPA (2014) Sediment Sampling. United States Environmental Protection Agency, Georgia, United States, www.epa.gov/sites/production/files/2015-06/documents/Sediment-Sampling.pdf. (2014) www.epa.gov/sites/production/files/2015
Hashmi MZ, Yu C, Shen H, Duan D, Shen C, Lou L, Chen Y (2014) Concentrations and human health risk assessment of selected heavy metals in surface water of the Siling Reservoir Watershed in Zhejiang Province China. Pol J Environ Stud 23(3):801–811
Zhong W, Zhang Y, Wu Z, Yang R, Chen X, Yang J, Zhu L (2018) Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotoxicol Environ Saf 157:343–349
Shao Y, Wang J, Wu H, Liu J, Aksay IA, Lin Y (2010) Graphene based electrochemical sensors and biosensors: a review. Electroanalysis 22(10):1027–1036
Kavcar P, Sofuoglu A, Sofuoglu SC (2009) A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int J Hyg Environ Health 212(2):216–227
WHO (2017) Guidelines for drinking-water quality, vol 1. World Health Organization
Kumar GP, Chandrasekhar T, Murty BS (2017) Assessment of groundwater quality status based on water quality index method in two coastal villages, Konada and Chintapalli of Vizianagaram district in Andhra Pradesh India. J Indian Chem Soc 94:1–9
Şener Ş, Şener E, Davraz A (2017) Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci Total Environ 584:131–144
Akoto O, Adopler A, Tepkor HE, Opoku F (2021) A comprehensive evaluation of surface water quality and potential health risk assessments of Sisa river Kumasi. Groundw Sustain Dev 15:100654. https://doi.org/10.1016/j.gsd.2021.100654
Tian K, Wu Q, Liu P, Hu W, Huang B, Shi B, Zhou Y, Kwon B-O, Choi K, Ryu J (2020) Ecological risk assessment of heavy metals in sediments and water from the coastal areas of the Bohai Sea and the Yellow Sea. Environ Int 136:105512
Wardani N, Prartono T, Sulistiono S (2020) Sediments quality based on geo-accumulation index in heavy metals (Pb, Cu, and Cd) of cengkok coastal waters Banten bay. Jurnal Pendidikan IPA Indonesia 9(4):574–582
Darko G, Boakye S, Akoto O, Rammika M, Gyamfi O (2015) Distribution and potential risks of heavy metals in fish, water and sediment. Environ Sci 11:74–82
Olubunmi FE, Olorunsola OE (2010) Evaluation of the status of heavy metal pollution of sediment of Agbabu bitumen deposit area Nigeria. Eur J Sci Res 41(3):373–382
Ganugapenta S, Nadimikeri J, Chinnapolla SRRB, Ballari L, Madiga R, Nirmala K, Tella LP (2018) Assessment of heavy metal pollution from the sediment of Tupilipalem Coast, southeast coast of India. Int J Sediment Res 33(3):294–302
Williams JA, Antoine J (2020) Evaluation of the elemental pollution status of Jamaican surface sediments using enrichment factor, geoaccumulation index, ecological risk and potential ecological risk index. Mar Pollut Bull 157:111288
Barbieri M, Sappa G, Vitale S, Parisse B, Battistel M (2014) Soil control of trace metals concentrations in landfills: a case study of the largest landfill in Europe, Malagrotta, Rome. J Geochem Explor 143:146–154
Sanou A, Coulibaly S, Coulibaly M, N’Goran-N’dri S, Célestin Atse B (2021) Assessment of heavy metal contamination of fish from a fish farm by bioconcentration and bioaccumulation factors. Egypt J Aquat Biol Fish 25(1):821–841
Qu M, Wang Y, Huang B, Zhao Y (2018) Source apportionment of soil heavy metals using robust absolute principal component scores-robust geographically weighted regression (RAPCS-RGWR) receptor model. Sci Total Environ 626:203–210
WHO (2011) Guidelines for drinking-water quality. WHO Chron 38(4):104–108
Akoto O, Gyimah E, Zhan Z, Xu H, Nimako C (2019) Evaluation of health risks associated with trace metal exposure in water from the Barekese reservoir in Kumasi, Ghana. Hum Ecol Risk Assess: An Int J 26(4):1134–1148
Osafo E (2011) The Effect of Small Scale Gold Mining activities on the Birim River in the East Akim Municipality of the Eastern Region. Dissertation, Kwame Nkrumah University of Science and Technology, Ghana
Akoto O, Bruce T, Darko D (2008) Heavy metals pollution profiles in streams serving the Owabi reservoir. Afr J Environ Sci Technol 2(11):354–359
Mor S, Ravindra K, Dahiya R, Chandra A (2006) Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ Monit Assess 118(1–3):435–456
Danziger J, Mukamal KJ, Weinhandl E (2021) Associations of community water lead concentrations with hemoglobin concentrations and erythropoietin-stimulating agent use among patients with advanced CKD. J Am Soc Nephrol 32(10):2425–2434
Jiang JQ, Panagoulopoulos A, Bauer M, Pearce P (2006) The application of potassium ferrate for sewage treatment. J Environ Manag 79(2):215–220. https://doi.org/10.1016/j.jenvman.2005.06.009
Kortatsi B (2004) Hydrochemistry of groundwater in the mining area of Tarkwa-Prestea, Ghana. PhD, University of Ghana, Ghana
Kuma JS (2004) Is groundwater in the Tarkwa gold mining district of Ghana potable? Environ Geol 45(3):391–400
Dadzie ES (2012) Assessment of heavy metal contamination of the Densu River, Weija from leachate. Kwame Nkrumah University of Science and Technology
Frickel S, Elliott JR (2008) Tracking industrial land use conversions: a new approach for studying relict waste and urban development. Organ Environ 21(2):128–147
Mann S, Rate A, Gilkes R (2002) Cadmium accumulation in agricultural soils in Western Australia. Water, Air, Soil Pollut 141(1–4):281–297
Ashraf MW (2012) Levels of heavy metals in popular cigarette brands and exposure to these metals via smoking. Sci World J 2012:1–5
Mohammadi M, Sary AA, Khodadadi M (2011) Determination of heavy metals in two barbs, Barbus grypus and Barbus xanthopterus in Karoon and Dez Rivers, Khoozestan Iran. Bull Environ Contam Toxicol 87(2):158–162
Commission E (2006) Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union 364 (365–324)
Azaman F, Juahir H, Yunus K, Azid A, Kamarudin MKA, Toriman ME, Mustafa AD, Amran MA, Hasnam CNC, Saudi ASM (2015) Heavy metal in fish: analysis and human health—a review. J Teknol 77(1):61–69
Balachandran KK, Raj CL, Nair M, Joseph T, Sheeba P, Venugopal P (2005) Heavy metal accumulation in a flow restricted, tropical estuary. Estuar Coast Shelf Sci 65(1–2):361–370
Effendi H, Kawaroe M, Lestari DF (2016) Ecological risk assessment of heavy metal pollution in surface sediment of Mahakam Delta, East Kalimantan. Procedia Environ Sci 33:574–582
Suresh G, Ramasamy V, Meenakshisundaram V, Venkatachalapathy R, Ponnusamy V (2011) Influence of mineralogical and heavy metal composition on natural radionuclide concentrations in the river sediments. Appl Radiat Isot 69(10):1466–1474
Mukate S, Panaskar D, Wagh V, Muley A, Jangam C, Pawar R (2018) Impact of anthropogenic inputs on water quality in Chincholi industrial area of Solapur, Maharashtra, India. Groundw Sustain Dev 7:359–371
Boateng TK, Opoku F, Akoto O (2019) Heavy metal contamination assessment of groundwater quality: a case study of Oti landfill site Kumasi. Appl Water Sci 9(2):33. https://doi.org/10.1007/s13201-019-0915-y
Yan C, Zhuang T, Bai J, Wen X, Lu Q, Zhang L (2020) Assessment of as, cd, Zn, Cu and Pb pollution and toxicity in river wetland sediments and artificial wetland soils affected by urbanization in a Chinese delta. Wetlands 40(6):2799–2809
Persad AS, Cooper GS (2008) Use of epidemiologic data in Integrated Risk Information System (IRIS) assessments. Toxicol Appl Pharmacol 233(1):137–145
Bodrud-Doza M, Islam AT, Ahmed F, Das S, Saha N, Rahman MS (2016) Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Sci 30(1):19–40
Wątor K, Zdechlik R (2021) Application of water quality indices to the assessment of the effect of geothermal water discharge on river water quality–case study from the Podhale region (Southern Poland). Ecol Indic 121:107098
Meers E, Unamuno V, Du Laing G, Vangronsveld J, Vanbroekhoven K, Samson R, Diels L, Geebelen W, Ruttens A, Vandegehuchte M (2006) Zn in the soil solution of unpolluted and polluted soils as affected by soil characteristics. Geoderma 136(1–2):107–119
Sutherland RA, Tolosa CA, Tack F, Verloo M (2000) Characterization of selected element concentrations and enrichment ratiosin background and anthropogenically impacted roadside areas. Arch Environ Contam Toxicol 38(4):428–438
Zhang J, Liu C (2002) Riverine composition and estuarine geochemistry of particulate metals in China—weathering features, anthropogenic impact and chemical fluxes. Estuar Coast Shelf Sci 54(6):1051–1070
Acknowledgements
The Researchers are grateful to the Department of Chemistry, Kwame Nkrumah University of Science and Technology, for the use of its facilities for this study and Opoku Adomako Kwabena of the Geomatic engineering department for his assistance with the study area design.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jonathan, A., Opoku, F. & Akoto, O. Health Risk Assessment, Bioaccumulation Factors and Ecological Indices of Heavy Metals in Sediment, Fish and Water Along Asuoyeboah River, Kumasi: A Case Study. Chemistry Africa 6, 1103–1115 (2023). https://doi.org/10.1007/s42250-022-00524-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00524-z