Abstract
Climate change affects ecosystems in different ways. These effects are particularly worrying in the Neotropical region, where species are most vulnerable to these changes because they live closer to their thermal safety limits. Thus, establishing conservation priorities, particularly for the definition of protected areas (PAs), is a priority. However, some PA systems within the Neotropics are ineffective even under the present environmental conditions. Here, we test the effectiveness of a PA system, within an ecotone in northern Brazil, in protecting 24 endangered bird species under current and future (RCP8.5) climatic scenarios. We used species distribution modeling and dispersal corridor modeling to describe the priority areas for conservation of these species. Our results indicate that several threatened bird taxa are and will potentially be protected (i.e., occur within PAs). Nonetheless, the amount of protected area is insufficient to maintain the species in the ecotone. Moreover, most taxa will probably present drastic declines in their range sizes; some are even predicted to go globally extinct soon. Thus, we highlight the location of a potentially effective system of dispersal corridors that connects PAs in the ecotone. We reinforce the need to implement public policies and raise public awareness to maintain PAs and mitigate anthropogenic effects within them, corridors, and adjacent areas, aiming to conserve the richness and diversity of these already threatened species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate controls wild species distributions and ecosystem processes (Townsend et al., 2003). Current climate change is induced mainly by anthropogenic actions resulting in increasing levels of greenhouse gases in the atmosphere (Siegenthaler et al., 2005; IPCC, 2007), and ecosystems are affected differently. Entire biomes are subject to rapid modifications due to regime disturbances, colonization of new species, local extinction of populations, or even extinction of whole species, among others (Parmesan & Yohe, 2003; Thomas et al., 2004; Parmesan, 2006; Lemes & Loyola, 2013).
These effects are particularly worrisome in the Neotropical region (Williams et al., 2007). Neotropics hold several hotspots of biodiversity (Myers et al., 2000), where species are exceptionally vulnerable to climate changes (Khaliq et al., 2014; McCain, 2009; Williams et al., 2007) because they already live closer to their limit of thermal safety margins (Dillon et al., 2010; Huey et al., 2012; Sinervo et al., 2010). This fact prevents their fit development to even higher temperatures, which will likely be reached sooner than previously thought (Tewksbury et al., 2008), and will probably exceed the thermal tolerance of species very rapidly (Araújo et al., 2013).
Therefore, ongoing climate change is undoubtedly recognized as a primary concern for the establishment of conservation priorities (Thomas et al., 2004; Thuiller et al., 2005a, b), and so the definition of protected areas (PAs) (Dudley et al., 2010; Vale et al., 2018). Protected areas (PAs) play a prominent role in conservation under climate-changing scenarios, sustaining different microclimatic conditions in heterogeneous habitats and preventing species from facing extreme climatic conditions (Sunday et al., 2014). However, the establishment of most PAs in the Neotropical region was not designed under objective criteria and planning methods, resulting in an insular system of PAs (Rylands & Pinto, 1998). Such systems are only suitable for temporary insular ecosystems and are more likely to turn populations even more vulnerable and isolated, increasing the risk of local extinction (Hansen & DeFries, 2007). The creation of dispersal/ecological corridors has been recommended and successfully tested to overcome this limitation and connect isolated PAs (Crooks & Sanjayan, 2006; Haddad et al., 2003).
Dispersal corridors are habitat portions that allow movement of species between habitat patches (Beier et al., 2008). Corridors are a vital conservation management tool to increase connectivity in heterogeneous landscapes, constituted by patches of suitable and unsuitable habitats (Crooks & Sanjayan, 2006), which may promote gene flow between more or less isolated populations and mitigate other habitat fragmentation effects (Brudvig et al., 2009; Gilbert-Norton et al., 2010), such as reducing the chances of inbreeding depression (Brown et al., 2004) and decreasing rates of stochastic extinction (Fahrig & Merriam, 1994). Nonetheless, identifying corridors requires a thorough mapping of landscape permeability to the movement of target species and modeling of possible paths of organisms through that landscape (Koen et al., 2010). Connectivity models need to be developed to predict the least costly way to estimate multiple movement paths (Beier et al., 2008; Cushman et al., 2009; Pinto & Keitt, 2009), considering not only the resistance of the landscape, i.e., suitability of each habitat composing the landscape to the species movement, but also the species movement behavior and movement risk (McRae & Beier, 2007; McRae et al., 2008, 2016).
Neotropical birds constitute a group of interest for wildlife conservation, since several studies predict significant declines in their future geographic distributions (Anciães & Peterson, 2006; Foden et al., 2013; Loiselle et al., 2010; Marini et al., 2009a, b), which thus seriously threatens the world biodiversity (Lawler et al., 2009). Most bird species are found in the Neotropics, surpassing other vertebrate species. This is also one of the most threatened group of vertebrates worldwide, also with new species being discovered routinely (reviewed by Jenkins et al., 2013).
In northern Brazil, eastern Amazon is an ecotone region comprising the Amazon Forest and Cerrado, a tropical savanna. This region is excellent for a case study to understand the vulnerability of bird species to climate change. This region is among the most heterogeneous within the Amazon basin, presenting various landscapes (Ab’Saber, 1977; Mello et al., 2000; IBGE, 2013). In this ecotone, biodiversity is threatened by intensive anthropic pressure and high deforestation rates, primarily due to the massive expansion of agribusiness (Nepstad et al., 1999; Wood & Porro, 2002). It is noteworthy that 61% of the threatened bird species from the Brazilian Amazon mainly or exclusively occur in this portion of the Amazon Basin (IBAMA, 2014).
In contrast, the Cerrado is the second biome with the most threatened bird species in Brazil (Marini & Garcia, 2005). Protected areas within the eastern Amazon constitute an insular system of PAs, although an ecological corridor was recently proposed to connect some of the biggest PAs (Celentano et al., 2018). Nonetheless, Celentano et al. (2018) did not evaluate the utility of the corridor under a scenario of future climate change, and this corridor is still likely to be insufficient to protect bird species within the Cerrado portion of the ecotone, where PAs are currently misallocated (Carvalho et al., 2017). Moreover, ecotones provide opportunities to mitigate the impact of climate change on biodiversity, as populations close to these regions may have distinct genetic characteristics, pre-adapted to the physiological stress of climate change due to micro-environmental constraints in an area where climate stress is the main macro-environmental characteristic (Killeen & Solórzano, 2008; Sunday et al., 2014).
Herein, we evaluate the potential impacts of future climate change on the distribution and conservation status of 24 bird taxa currently threatened in an ecotone region within northern Brazil. First, we compare the future potential distributions of the target taxa with the current PA system to detect possible bird protection gaps under a climate change scenario. We also calculate the potential changes in the taxa distribution ranges and species richness estimates for the future. Finally, we identify possible dispersal corridors to enhance the conservation of these threatened taxa under ongoing climate change.
Methods
Study area
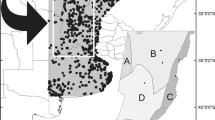
This study was performed in northern Brazil in a total area of 331,983.29 km2, corresponding to the Brazilian federal state of Maranhão. This area comprises great patches of Amazon rain forest, a large proportion of native vegetation of Cerrado, and includes small patches of Caatinga vegetation enclaves within Cerrado (Fig. 1) (INPE, 2015). Unflooded terra firme and flooded varzea rain forests, open vegetation areas named campinas, wooded savanna, mangroves, xeric shrubland, and other less represented habitats are intermixed along our study area, making this a very heterogenic region, and composing a biologically relevant ecotone area (Carvalho et al., 2017; de Oliveira et al., 2007; MMA, 2011; Olímpio et al., 2016; Serra et al., 2016). Extensive cattle ranching, logging, mineral and metallurgical extraction, agriculture, and energy production are some of the most active anthropogenic actions in the study region (FIEMA, 2009; Nepstad et al., 1999; Wood & Porro, 2002), adding to the complexity of natural habitats within the ecotone.
This study follows Carvalho et al. (2017), who delimited priority areas for conserving bird species within the study region under current climatic conditions. Therefore, we considered the same 39 PAs previously assessed (Carvalho et al., 2017), which comprise eight federal conservation units of sustainable use, four of full protection, six state conservation units of sustainable use, two of full protection areas, and 18 indigenous lands delimited and homologated by the Brazilian federal government (Fig. 1; Table 1 from Carvalho et al., 2017). Considering the perspectives in a future scenario, we added two indigenous lands under delimitation to our PA data set, Vila Real and Kanela (https://www.funai.gov.br).
Sampling
Within the ecotone study area, 41 taxa (species and subspecies) are considered threatened by the Brazilian government (IBAMA, 2014; MMA, 2008). This national list ranks regional biodiversity, such as endemic subspecies (e.g., Celeus torquatus pieteroyensi and Pteroglossus bitorquatus bitorquatus). Of these 41 taxa, 11 are marine, coastal, or migratory birds; three represent large-sized birds of prey with relatively more extensive home ranges, and three taxa have less than five independent occurrence records (IBAMA, 2014; MMA, 2008). Thus, our total data set includes 24 terrestrial, non-migratory, threatened bird species and subspecies in our ecotone study area, with more than five independent occurrences available (Table 1). Fifteen taxa are known to have distributions restricted to the Amazon biome. Four are restricted to Cerrado and Caatinga biomes (here we include Spinus yarrellii, the yellow-faced siskin, which was recently (2007–2017) observed within the Atlantic forest, on the eastern coast of Brazil; Fig. S1; WikiAves), and the other five species have widespread distributions, occurring in all biomes represented in the ecotone study area (Da Silva, 1995, 1997; Silva & Bates, 2002; IBAMA, 2014; MMA, 2008; IUCN 2018.1). All target taxa are classified as threatened on the Brazilian official list of threatened species (IBAMA, 2014; MMA, 2008), and 14 are considered globally threatened (IUCN 2018.1).
We collected occurrence records for each taxon from the literature, online databases (VertNet (http://vertnet.org/), Species Link (http://splink.cria.org.br), Global Biodiversity Information Facility (http://www.gbif.org), Wikiaves (http://www.wikiaves.com.br), xenocanto (http://www.xeno-canto.org)), and museum collections (Louisiana Museum of Natural History, Museu Paraense Emílio Goeldi, and Museu Nacional do Rio de Janeiro) (Fig. S1). Records outside the known distribution for the species were excluded based on the known distribution of the taxa (IBAMA, 2014; MMA, 2008; IUCN 2018.1). We used the geographical coordinates directly from sources or the Ornithological Gazetteer of Brazil (Paynter & Traylor, 1991). We controlled sampling bias on georeferenced data by removing duplicate records and leaving a single randomly selected record per pixel. We used a thinning technique, implemented in the package spThin (Aiello-Lammens et al., 2015), to reduce autocorrelation in the occurrence data. Afterward, we used Moran’s I and variograms that minimize spatial autocorrelation to define the thinning distance (de Andrade et al., 2020). We excluded records from years before 1970 (Fick & Hijmans, 2017; Karger et al., 2017). Bird nomenclature followed the Brazilian Ornithological Records Committee (CBRO, 2015).
Species distribution modeling procedures
For all 24 target bird taxa, first, we overlaid each data set of occurrence records on the grid of cells of 2.5 arc-min (~ 4.5 × 4.5 km), delimiting a buffer of 200 km, accounting the average mobility of the species assessed, and set around all records to define the total extent area (Fig. 2a–b). We used this same grid considering all 19 bioclimatic variables from WorldClim for the current climatic scenario and the Representative Concentration Pathway 8.5 (RCP8.5) for the future scenario (https://www.worldclim.org). To remove highly correlated variables and reduce their collinearity under each scenario, we performed pairwise Pearson correlation tests and estimated the variation inflation factor (VIF), so a subset of uncorrelated variables (r <|0.8|; VIF < 10) was used. For both scenarios, the same eight predictor variables were selected as our environmental variables annual mean temperature (BIO1), mean diurnal range (BIO2), isothermality (BIO3, annual precipitation (BIO12), precipitation of driest month (BIO14), precipitation seasonality (BIO15), precipitation of warmest quarter (BIO18), and precipitation of coldest quarter (BIO19) (Fig. 2c).We used a single future climatic scenario (Fig. 2d) because climate projections influence less future putative distributions than species distribution modeling methods (Sales et al., 2017). We chose RCP8.5, since this is the most pessimistic of the four RCPs available, representing the most severe future global climate change. It predicts continuous greenhouse emissions from anthropogenic actions until 2100 (Moss et al., 2008). Furthermore, RCP8.5 offers a focused and simplified representation of a “business-as-usual” approach to emissions, serving as a critical reference point for assessing the maximum potential impacts on species distributions, while the Shared Socioeconomic Pathways (SSPs) encompass a wider range of socio-economic development narratives coupled with climate change scenarios (IPCC, 2014, 2021). We modeled taxa distributions for the period of 2070 (average for 2061–2080) using seventeen different Atmosphere–Ocean General Circulation Models (AOGCMs): ACCESS1-0 (AC), BCC-CSM1-1 (BC), CCSM4 (CC), CNMR-CM5 (CN), GFDL-CM3 (GF), GISS-E2-R (GS), HadGEM2-AO (HD), HadGEM2-CC (HG), HadGEM2-ES (HE), INMCM4 (IN), IPSL-CM5A-LR (IP), MIROC-ESM-CHEM (MI), MIROC-ESM (MR), MIROC5 (MC), MPI-ESM-LR (MP), MRI-CGMM3 (MG), and NorESM1-M (NO) downloaded from WorldClim (https://www.worldclim.org) with the same resolution of 2.5 arc-min (Fig. 2e).
We used two methods to predict the potential distributions accounting for the number of occurrence records for each focal taxon (Fig. 2f) for SDMs for both current and future scenarios. The Jackknife approach (also known as the leave-one-out method) was used for five taxa with limited occurrence data (≤ 10 records) (Pearson et al., 2007). According to the literature, the minimum number of occurrence records should be three to five to perform such an approach (De Almeida et al., 2010; Lima-Ribeiro & Diniz-Filho, 2012). The lowest number of occurrence records was seven for our target species. Thus, we jackknifed the observations to produce occurrence subsets with n-1 occurrences for our data input. We then used these subsets to predict the distribution of the species. The occurrence record that was left out was used to evaluate the goodness-of-fit of the models. For distribution predictions to be considered reliable, p-values must be lower than 0.05, indicating that sampling bias is negligeble. Conversely, p-values greater than 0.05 indicate an unreliable outcome. For the other 20 taxa (n > 10 records), we analyzed 10 subsets of records obtained by randomly dividing the occurrences in training (70%) and testing (30%) records.
We trained all models using three SDM algorithms of machine-learning methods under both current and future climatic scenarios, respectively: maximum entropy (MaxEnt) (Phillips & Dudík, 2008; Phillips et al., 2006), was fitted using the inhomogeneous Poisson process procedure, with the linear, quadratic, product, and hinge features, default regularization values support vector machine (SVM) (Scholkopf et al., 2001), was performed with a radial basis kernel, with a constant cost value equal to one, and based on probabilities classes and random forest (RF), was tuned automatically using the ‘tuneRF’ function of the randomForest package with default values of the step factor, 500 trees, and the improvement in out-of-bag error parameter (Breiman, 2001; Prasad et al., 2006). Machine-learning algorithms were preferred because general linear models behaved poorly in representing the species distribution area (data not shown). All SDM algorithms were parameterized in R 3.4.2 (https://www.r-project.org) (Fig. 2g). MaxEnt was run using the R package maxent v.0.1.2, and the algorithm was fitted using the inhomogeneous Poisson process procedure, with the linear, quadratic, product, and hinge features and default regularization values (Phillips et al., 2017). SVM was performed using kernlab package v.0.9-25. with a radial basis kernel, a constant cost value equal to one and based on probability classes (Karatzoglou et al., 2004). Last, RF was tuned automatically using the “tuneRF” function of the randomForest R package v.4.6-12 under default values of the step factor, 500 trees, and the improvement in out-of-bag error parameters (Liaw & Wiener, 2002). These presence-absence algorithms confront true occurrence points (presence points) with points allocated in areas where the species is known not to occur (absence points). Due to the lack of absence points, we used the environmental constraint method, which randomly places samples in areas of low climatic suitability created by climate models using Bioclim (Engler et al., 2004). In this way, pseudo-absences are generated with a ratio of 1 pseudo-absence point for each presence point.
We considered the threshold that maximizes both omission and commission errors while modeling the species distributions to cut the suitability matrices of the modeled species in the modeling algorithm into presence-absence maps (ROC threshold) for all taxa. We used true skill statistics (TSS) to evaluate the metrics of our models (Allouche et al., 2006). TSS values vary from − 1 to + 1, where negative values indicate that distributions are no better than random, and values equal to + 1 represent perfect fitting between the observed and the modeled distribution. Models with TSS values reaching 0.5 or more than 0.7 were considered acceptable and excellent, respectively (Allouche et al., 2006) (Fig. 2h). This metric was calculated using 10,000 random pseudo-absences.
Two final distribution maps for each taxon were obtained with a mean ensemble of the three SDM algorithms for current and future scenarios. Future SDMs considered a mean consensual distribution map obtained from all AOGCMs. All consensus distribution maps were obtained using only reliable models (p < 0.05 after Jackknife approach) and that achieved a TSS value > 0.5, following the bootstrap method (Fig. 2h).
Estimated protected range and species richness
According to Carvalho et al. (2017) results, Cerrado PAs are particularly misallocated. Moreover, predominantly forest-dependent taxa are more likely to be found in Amazon biome PAs, while Cerrado endemics are restricted to this biome (see also Carvalho et al., 2017 predictions). Therefore, we divided our total data set into a group from the Amazon biome (Amazon taxa data set; n = 20), another grouping of taxa with occurrence in Cerrado and Caatinga (Cerrado taxa data set; n = 9), and a third data set including taxa occurring in both the Amazon, Cerrado and Caatinga (both data sets; n = 5). We predicted the proportion of PAs, estimated species richness, performed gap analysis by statistical analysis, and assessed the effectiveness of PAs in our study area for present and future scenarios for these three different data sets. We also performed statistical analysis to estimate the effectiveness of PAs, considering all study areas for taxon with gain or loss in the predicted future distribution. The modeled species richness was obtained by the sum of the final distribution estimated for each taxon (Fig. 2i) and overlaid with both the shape file of the world ecoregions (https://www.worldwildlife.org) and the shapefile of PAs within the study area (as detailed in Carvalho et al., 2017).
The effectiveness of protected areas was first analyzed using linear regressions contrasting the total distribution size predicted (independent variable) against the size predicted to be within a PA (dependent variable) for each taxon. We also used paired t-tests, suitable for dependent samples, to assess possible changes in both the estimated range and the amount of protected distribution area between present and future scenarios. For all analyses, we used a 95% confidence interval for the slope.
Identification of dispersal corridors
We evaluated the connectivity between PAs for the 24 threatened bird species using Linkage Mapper, which integrates least-cost path approaches with circuit theory (McRae & Kavanagh, 2011). The least cost path is the single path related to the minimum cost-weighted distance between two core areas (Adriaensen et al., 2003). This tool uses the core areas to connect as input data and a resistance surface associated with habitat variables for the target species. We used the PAs from the Cerrado biome, Amazon, or all the sets as core areas for each studied species, depending on their habitat requirements. After merging PAs that presented overlapping regions, we obtained seven core areas for the Amazon taxa (20 taxa), 14 for Cerrado’s (five species), and 16 for the whole state (four species). Reducing the number of PAs to be considered also reduces computational time and avoids software errors.
We tested two habitat variables regarding resistance surfaces: land use and climate data. For land usage, we downloaded the raster information from TerraClass Amazon (Almeida et al., 2014) and TerraClass Cerrado (INPE, 2013), both at a resolution of 2.5 arc-min (~ 4.5 × 4.5 km). Next, we assigned resistance values to each type of land use per taxa, according to our expert opinion (DLC and MPD), to quantify how much each type could act as a barrier to dispersal and quantify its degree of interference. The values applied to each land use class ranged from 1 = no resistance to 100 = barrier (Tables S1 and S2). The climate data used were previously obtained from ensembles for each species through SDM. However, the values of the ensembles ranged between 0 and 1, representing conductance. Thus, it was necessary to standardize and invert the raster values to obtain values ranging between 1 and 100, representing resistance. To do that, we applied the formula 100 – “ensemble” * 100 to each ensemble of climate rasters.
After mapping the corridors between PAs for each species, we ran the Pinchpoint Mapper tool (McRae, 2012). This tool uses circuit theory to identify pinch points within the least-cost corridors (McRae & Shah, 2009). Thus, the flow of electricity depends on the resistance value of each cell within each least-cost corridor (McRae & Shah, 2009; McRae, 2012). The least-cost corridors were clipped according to the user-specified corridor width (Dutta et al., 2016). We applied three different cutoff values depending on the ability of each species to move through a suboptimal habitat: 150 for Amazon species, characterized as rapidly accumulating cost moving through suboptimal habitat, while for Cerrado species and for more generalist species (both biomes data set) that can move easily, the cutoff values were 75 and 50, respectively.
Pinchpoint Mapper identifies sites (called “pinch points”) with the highest current densities within the least-cost corridors (McRae & Shah, 2009; Dutta et al., 2016). These locations act as bottlenecks to movement, indicating where the flow is more intensive due to the unavailability of alternative pathways. Bottlenecks may denote cover types or constraints caused by physical features adjacent to high resistance (Dutta et al., 2016). We used this analysis to find pinch points between the entire core areas and corridors network. This provides a current flow centrality measure to evaluate the importance of linkages and pinch points in maintaining connectivity in the whole landscape (Carroll et al., 2012).
Results
Species distribution models
We used 576 occurrence records for the 24 threatened bird taxa in the ecotone area studied (with 7–48 independent occurrence records). We produced potential global distribution maps for current and future scenarios for each target taxa (Figs. S2 and S3). We summarized them regarding the gain and loss area for 2070 within the study area (Fig. S4). Overall, the accuracy of the SDMs estimated for the present and future climatic scenarios was acceptable, despite some minor omission errors in three of the 24 taxa analyzed (Tables 1 and S3).
Distribution extent, protected range, and species richness
Considering the scenario for 2070, the predicted range size of suitable areas varied considerably for all target taxa, either globally or within the study area (Table 1; Figs. S3 and S4). However, these changes in size were not statistically significant while analyzing the three data sets previously described (t = 0.906, df = 19, p = 0.376 for Amazon taxa, t = − 0.622, df = 7, p = 0.553 for Cerrado taxa, and t = 0.599, df = 4, p = 0.581 for taxa occurring in both biomes). Specifically, six (out of 15 Amazon taxa), one (out of four Cerrado taxa), and two taxa (out of five taxa from both biomes) will likely increase their distribution range in the future within the ecotone area studied (Table 1). However, considering groups of taxa predicted to gain (n = 9, t = 4.959, df = 15, p = 0.0002) or lose (n = 15, t = − 3.769, df = 14, p = 0.000) a suitable area within the study area resulted in statistically significant changes in taxa distributions.
Notably, five Amazon taxa will probably go globally extinct (Psophia obscura, Celeus t. pieteroyensi, Piculus paraensis, Dendrexetastes r. paraensis, and Piprites c. grisescens), with 0–5% of its global area of distribution remaining in the future climatic scenario tested (Fig. S3). Three taxa were predicted to lose about 50% of their putative distribution within the ecotone (Table 1 and Fig. S4). For the Cerrado taxa, Cercomacra ferdinandi was predicted to go globally extinct (Fig. S3), Spinus yarrellii was predicted to disappear from the study area, and Penelope jacucaca will probably lose more than 70% of its predicted distribution (Table 1 and Fig. S4). Considering the taxa occurring in both biomes, Tinamus tao tao will lose all suitable areas within the study area, and Neomorphus geoffroyii will lose about 70% of its current range (Table 1 and Fig. S4).
The estimated protected range in the current scenario varied between 22 and 100% for the Amazon taxa, 7 and 51% for the Cerrado taxa, and 8 and 51% for the taxa occurring in both biomes. In the future scenario, the estimated protected range will likely decrease, varying between 0 and 54% for the Amazon taxa, 0 and 73% for the Cerrado taxa, and 0 and 67% for taxa distributed within both biomes (Table 2).
Our linear regressions indicated a significant positive correlation between protected range and distribution size both for Amazon (Fig. 3a, b) and Cerrado (Fig. 3c, d) threatened taxa in current and future scenarios. For the Amazon taxa, in both scenarios, for every 202.5 km2 of distribution range (10 grid cells), there was a gain of protection of 81 km2 (four grid cells; Fig. 3a). On average, 50% ± 16% of the estimated range for the Amazon taxa is currently protected in this biome, but only 32% ± 22% will be protected in the future (Figs. 3b and 4). For the Cerrado data set, we obtained the same result for the current and future scenario. For every 202.5 km2 (10 grid cells of distribution range), there was a gain of protection of only 20.25 km2 (one cell; Fig. 3c–d). On average, 18% ± 14% and 29% ± 21% of the estimated range for the Cerrado species is and will be protected in this biome, respectively (Fig. 4). Considering widespread species in both biomes, no statistically significant relationship was observed between the size of PAs and the estimated distribution of the taxa, both under current (R2 = 0.098, p = 0.608, y = 708.5546 + 0.1357*x) and future scenarios (R2 = 0.508, p = 0.176, y = 335.6479 + 0.206*x).
Distribution of protected range concerning range size within the study area for Amazon (a, b) and Cerrado (c, d) taxa data sets (see “Methods” section for details), under current (a, c) and future (b, d) climatic conditions
The highest values of estimated species richness for the current scenario (> 50%) were located in the northern and northwestern part of the Amazon area within the ecotone for the Amazon taxa (between 10 and 19 taxa) and for the species that occur in both biomes (maximum four species). For the Cerrado taxa, the highest species richness values (maximum of six species) were observed in the central, eastern, northeastern, and southern regions (Fig. 5).
In the future scenario, there was a decrease in the values of estimated species richness for the Amazon taxa (between 10 and 13 species) and those species that occur in both biomes (a maximum of three species). The highest estimated richness values were observed in the exact location of the current scenario. For the Cerrado biome, the highest values of potential richness (maximum of four species) were located in the northwestern, northeastern, and central regions of the study area. Considering the three groups, we observed decreased predicted species richness between the current and future scenarios. There was a loss of 19.744 km2 in the Amazon, 13.507 km2 in the Cerrado, and 48.398 km2 for the species from both biomes.
However, the paired t-test indicated no statistical support for the difference between the size of the estimated distribution of species richness under current and future scenarios for all data sets (Amazon taxa t = − 0.023, df = 19, p = 0.981, Cerrado taxa t = 0.000, df = 6, p = 0.999, and for the data set including those species that occur in both biomes and considering all the ecotone study area t = 0.599, df = 4, p = 0.581).
The paired t-test supported statistically significant differences between the current and future scenarios for the taxa group with loss and gain of distribution. For the taxa group with loss of distribution (t = 5.713, df = 14, p < 0.001), on average, 1586 cells were lost. On average, there was a gain of 1938 cells for the taxa group, with an increase in distribution (t = − 3.769, df = 8, p = 0.005).
Regarding estimated species richness in the protected areas, the highest values (> 50%) of protected richness in the current scenario were only 22% for the Amazon taxa, 2% for the Cerrado taxa, and 6% for the species with occurrence in both biomes. In the future scenario, the values decreased to 13% in the Amazon portion, 0% for the Cerrado taxa, and 1% for the third group (Fig. 4). There was a loss of protected distribution range of 11.198 km2 for the Amazon data set, 1.053 km2 for the Cerrado taxa, and 17.780 km2 for the species occurring in both biomes. The paired t-test supported the statistically significant differences between the current and future distribution of species richness for the Amazon and Cerrado data sets (Amazon taxa t = 3.152, df = 18, p = 0.005, losing three taxa on average; and Cerrado taxa t = 2.270, df = 21, p = 0.034, losing just one taxon on average). For the third group, the observed difference was no better than random (t = 1.598, df = 37, p = 0.118).
Identification of dispersal corridors
On a broader scale, corridors predicted from both models of surface resistance (land use and climate) are similar. They have more than one possible corridor between various pairs of core groups of PAs (Figs. 6 and S5). Pinch points are observed between the central and northwest regions in the Amazon region, connecting Araribóia, Awa, and Caru indigenous lands with Gurupi Biological Reserve and APA das Reentrâncias Maranhenses. Within the Cerrado, pinch values were observed in a center-southwest direction between the central block of indigenous lands (Kanela, Porquinhos, Cana Brava/Guajajara, Lagoa Comprida, and Urucu Juruá) toward Araribóia and Krikati, and in a center-northeast direction, with high values between the same central block toward the Environmental Protection Area of Morro dos Garapenses.
Discussion
Species distribution models, protected range, and species richness
Here, we modeled the current potential distribution of all data-sufficient terrestrial, non-migratory, threatened bird species and subspecies from an ecotone area in northern Brazil, comprising Amazon and Cerrado biomes, with Caatinga enclaves. We also compared these current putative distributions with those projected into the future, in 2070, under the most pessimistic climate prediction model (https://www.worldclim.org).
In the current scenario, although we have combined different species distribution modeling algorithms, we observed the same patterns Carvalho et al. (2017) obtained for the 14 common target taxa. Thus, despite some minor omission errors, overall, our SDMs accurately represented the known distributions of the target taxa (IBAMA, 2014; MMA, 2008; IUCN 2018.1). This uncertainty is also inherent in using SDMs to evaluate the effects of climate change on the geographic distribution of species (Anderson, 2013; Diniz-Filho et al., 2009; Stoklosa et al., 2015; Tessarolo et al., 2014). Nonetheless, contrary to current SDMs, there is no such simple way to validate future models, and different databases or modeling approaches can produce different results (Wiens et al., 2009). Moreover, we found different distribution patterns for species sharing ecological requirements within our study area (discussed below). However, we obtained consistent results independently across all algorithms used for each taxon. Therefore, we support the idea that using our SDMs supplies essential biological and ecological considerations and is adequate to direct practical conservation actions (Hannah et al., 2007, Heller & Zavaleta, 2009, Guisan et al., 2006).
According to our predictions, most of the bird taxa analyzed will probably have drastic declines in their range sizes within the ecotone area and in their global distributions. Some taxa are predicted to go globally extinct soon, widely impacting species richness levels across the ecotone. We used the future scenario that predicted a significant increase in greenhouse gas emissions (https://www.worldclim.org). Therefore, dramatic changes are expected. Several other studies using different predictive models have observed similar alarming decreases in the species distributions due to climate change, not only for birds in tropical regions worldwide (Marini et al., 2009a, b; Sekercioglu et al., 2012) but also for other vertebrate groups (Mesquita et al., 2013; Vasconcelos, 2014; Bozinovic et al., 2011; Sheth & Angert, 2014; Ribeiro et al., 2016). Therefore, predicting species extinctions in the tropics is unsurprising (Anciães & Peterson, 2006; Sekercioglu et al., 2008; Sinervo et al., 2010).
However, although we used the most pessimistic future scenario, some threatened bird taxa were also predicted to significantly increase their distributions in the ecotone area evaluated (and globally). The survival of species depends on them keeping pace with climate changes through rapid adaptation to the new climatic conditions or colonizing new areas of suitable habitat (Sinervo et al., 2010). As our models are based only on abiotic conditions, and no test of future biological adaptation was performed, a putative increase in suitable area availability may better explain the dispersal variation observed for Pyrrhura coerulescens, Pyrilia vulturina, Pteroglossus b. bitorquatus, Hylopezus paraensis, Dendrocincla m. badia, and Tunchiornis ochraceiceps (Amazon taxa), Xiphocolaptes falcirostris (Cerrado), Celeus obrieni, and Lophornis goldii (occurrence in both biomes), which showed a dispersal pattern with distribution gain higher than loss.
Notwithstanding, this trend toward increasing their distribution ranges in the future might not be reassuring for these currently threatened birds. Amazon forest taxa usually have high specificity for forested habitats (Jolly et al., 2015), and tropical forests are more vulnerable and likely to disappear than savannas or grasslands due to climate change (Anjos & Toledo, 2018). These forests have a narrow thermal tolerance (Perez et al., 2016), are less resistant to climate stress, and have significant exposure to new climatic conditions (Anjos & Toledo, 2018; Holmgren et al., 2013; Seddon et al., 2016). Within the eastern Amazon, due to the ongoing precipitation decrease and increasing temperature indices, a replacement of the rain forest with more open savanna-like vegetation is expected (Hilker et al., 2014; Malhi et al., 2008; Oyama & Nobre, 2003; Seidl et al., 2017). However, despite the resemblance of this vegetation to a Cerrado vegetation type, most Cerrado tree species are predicted to lose at least 50% of their current distribution by 2055 due to climate change (Siqueira & Peterson, 2003). Furthermore, past climate changes in the eastern Amazon likely transformed the composition of the forest rather than the vegetation physiognomy (Cheng et al., 2013).
Regarding the amount of protected range for each taxon within the ecotone, our linear regressions indicate that for both Amazon and Cerrado bird taxa, a more comprehensive range corresponds to a higher amount of PA, and this trend will be maintained in the future. Nonetheless, to evaluate the effectiveness of PAs, species representativeness in those areas should be considered to vary with the extent of their occurrence. For instance, narrowly distributed species (< 1000 km2) should have their whole ranges fully protected (100%), widespread species (< 250,000 km2) must have at least 10% of their ranges within PAs, and species with ranges of intermediate size should have intermediate protection (Rodrigues et al., 2004a). The majority of our threatened taxa have current potential distributions within the ecotone greater than 5000 km2 (5.204–196.243 km2), thus needing a protection range of about 80 to 10%, respectively (Rodrigues et al., 2004a), but have between 70 and 8% of protected potential ranges. Thus, we confirm that current PA systems within the study area are ineffective in protecting threatened bird species (Carvalho et al., 2017).
Under the future climatic model, the predicted ranges vary dramatically, significantly increasing or decreasing. No trend was detected for gain or loss in the predicted future distribution of the target taxa related to their current distributions being restricted (or not) to either of the biomes within the ecotone. Some species will disappear from the ecotone, while others will reach between 2.025 and 69.032 km2 for those predicted to lose area, corresponding to a percentage of the recommended protected area of 10–100% (Rodrigues et al., 2004a). Between 3.1367 and 254.138 km2 should be considered for species that will increase their distributions by 10–20% of the recommended area within PAs (Rodrigues et al., 2004a). Protected potential ranges will likely reach 45% and 34% of distribution included in PAs for taxa, whose ranges will decrease and increase, respectively. Thus, in both time frames, the percentage of the potential protected area estimated is often lower than the recommended level (Rodrigues et al., 2004b), suggesting that bird taxa are not and will not be effectively protected within the ecotone.
Identification of dispersal corridors and conservation planning concerns
Corridors in this study indicate areas with low values of anthropogenic disturbance that can provide ecological connectivity between sizable protected core areas (PCAs). The maintenance or further decrease of human impact along corridors should be part of a conservation strategy plan for the ecotone. This is because a well-connected network of PAs can ensure the opportunity for the movement of wild fauna (Belote et al., 2016; Gaston et al., 2008) and, consequently, enable the long-term persistence of species (Christie & Knowles, 2015, Watson et al., 2017).
The location of most of our corridors coincides with our SDM results, Celentano et al. (2018) Gurupi corridor, and with the priority areas previously indicated for endemic and threatened bird taxa (Carvalho et al., 2017). However, our corridor models highlight several common pinch points for the analyzed species, showing numerous common bottleneck locations (Dutta et al., 2016). These locations of unavailable alternative pathways make perfect sense, considering all threats the ecotone faces. Within the Brazilian Amazon, the eastern portion has the highest deforestation rates, leading to a rapid fragmentation of forest cover (Lees et al., 2006, Michalski et al., 2008, Aldrich et al., 2012). The Amazon region studied here is included in a broader region popularly known as the “Arc of Deforestation,” one of the regions most impacted by human pressures, and is expected to lose 40% of its natural habitat for logging and cattle-raising by the year 2050 (Fearnside, 2001; Malhi et al., 2008; Soares-Filho et al., 2006). Within the northern Cerrado, the loss of natural vegetation has been pressured by agribusiness, with an increase of 86% for the planted area between 2005 and 2014 (a national average of 29%) (Lahsen et al., 2016).
The eastern portion of the Brazilian Amazon presents only around 1.7% of its PA (Da Silva et al., 2005). Among these, 1.7% of Important Bird Areas (IBAs) shelter threatened and near-threated species populations (MMA, 2003, Lees et al., 2012, De Lucca et al., 2009). However, these PAs also have been suffering illegal occupations for deforestation, selective logging, and burning by squatters and other landowners (Rylands & Pinto, 1998, Couto, 2004, De Lucca et al., 2009, Oren & Roma, 2011). These anthropic pressures are accounted for, creating drier and warmer microhabitats that are more susceptible to fire, inducing local and regional climate instability, and changing water regimes (Malhi et al., 2008; Nepstad et al., 2008). The interaction of deforestation and climate change may be more catastrophic here than each of these isolated impacts (Mantyka-Pringle et al., 2012), making the taxa inhabiting this region extremely vulnerable to extinction (Ribeiro et al., 2016). Thus, more effective control of human occupations and activities is crucial.
The Cerrado biome is one of the 35 biodiversity hotspots of the world (Myers et al., 2000) and is one of the most impacted by anthropogenic disturbance (Azevedo et al., 2016; Salazar et al., 2015). Considering the entire biome, the Cerrado has already lost 50% of its native vegetation for agricultural and cattle-rise industries (MMA, 2015). In a future scenario of climate change, as mentioned above, the Cerrado is considered more resilient due to the already substantial seasonal variations in moisture and temperature, which characterize the biome (Anjos & Toledo, 2018). However, in the future scenario, the dry season would occupy most of the year, probably favoring fires and reducing tree coverage, a critical temperature, and soil humidity regulator (Bustamante et al., 2012; Cochrane & Barber, 2009). Additionally, the PA system in the Cerrado is highly inefficient (Carvalho et al., 2017). Only 2.2% of Cerrado extent is protected (well below the 10% established by the Convention on Biological Diversity, 1992), and most PAs are misallocated (Ratter et al., 1997; Klink & Machado, 2005; MMA, 2011). In this portion of the ecotone, establishing new PAs and dispersal corridors are the more immediate conservation actions we recommend.
Thus, due to the significant number of threats, and their synergetic impact on species richness and diversity, we emphasized the creation of a corridor system for maintenance of the already threatened bird diversity of this ecotonal area (Lima & Gascon, 1999, Peres, 2005, Hawes et al., 2008, Lees & Peres, 2006; Barlow et al., 2010). We also highlight the establishment of effective policy actions, as the maintenance and recovery of natural or low-anthropized areas guarantee connectivity between the protected area system and the conservation of richness and diversity in this region.
Data availability
The data sets and other supplementary figures and tables are available online.
References
Ab’Saber, A. N. (1977). Espaços ocupados pela expansão dos climas secos na América do Sul, por ocasião dos períodos glaciais quaternários. Universidade de São Paulo/Instituto de Geografia.
Adriaensen, F., Chardon, J. P., De Blust, G., Swinnen, E., Villalba, S., Gulinck, H., & Matthysen, E. (2003). The application of ‘“least-cost”’ modelling as a functional landscape model. Landscape and Urban Planning, 64, 233–247. https://doi.org/10.1016/S0169-2046(02)00242-6
Aiello‐Lammens, M. E., Boria, R. A., Radosavljevic, A., Vilela, B., & Anderson, R. P. (2015). spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography, 38(5), 541–545. https://doi.org/10.1111/ecog.01132
Aldrich, S., Walker, R., Simmons, C., Caldas, M., & Perz, S. (2012). Contentious land change in the Amazon’s arc of deforestation. Annals of the Association of American Geographers, 102, 103–128. https://doi.org/10.1080/00045608.2011.620501
Allouche, O., Tsoar, A., & Kadmon, R. (2006). Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). Journal of Applied Ecology, 43, 1223–1232. https://doi.org/10.1111/j.1365-2664.2006.01214.x
Almeida, C., Silva, M., Lobo, F. D. L., Farias, T. P., Gomes, A., Costa, L. C., & Escada, M. I. S. (2014). TerraClass: classificação dos padrões de uso e cobertura da terra da Amazônia Legal. Cenários para a Amazônia: clima, biodiversidade e uso da terra, pp. 137–147.
Anciães, M., & Peterson, A. T. (2006). Climate change effects on Neotropical manakin diversity based on ecological niche modeling. The Condor, 108, 778–791.
Anderson, R. P. (2013). A framework for using niche models to estimate impacts of climate change on species distributions. Annals of the New York Academy of Sciences, 1297, 8–28. https://doi.org/10.1111/nyas.12264
Anjos, L. J. S., & de Toledo, P. M. (2018). Measuring resilience and assessing vulnerability of terrestrial ecosystems to climate change in South America. PLoS One, 13, e0194654. https://doi.org/10.1371/journal.pone.0194654
Araújo, M. B., Ferri-Yáñez, F., Bozinovic, F., Marquet, P. A., Valladares, F., & Chown, S. L. (2013). Heat freezes niche evolution. Ecology Letters, 16, 1206–1219. https://doi.org/10.1111/ele.12155
Azevedo, J. A., Valdujo, P. H., & Nogueira, C. de C. (2016). Biogeography of anurans and squamates in the Cerrado hotspot: Coincident endemism patterns in the richest and most impacted savanna on the globe. Journal of Biogeography, 43(12), 2454–2464. https://doi.org/10.1111/jbi.12803
Beier, P., Majka, D. R., & Spencer, W. D. (2008). Forks in the road: Choices in procedures for designing wildland linkages. Conservation Biology, 22, 836–851. https://doi.org/10.1111/j.1523-1739.2008.00942.x
Belote, R. T., Dietz, M. S., McRae, B. H., Theobald, D. M., McClure, M. L., Irwin, G. H., et al. (2016). Identifying corridors among large protected areas in the United States. PLoS One, 11, e0154223. https://doi.org/10.1371/journal.pone.0154223
Bozinovic, F., Calosi, P., & Spicer, J. I. (2011). Physiological correlates of geographic range in animals. Annual Review of Ecology, Evolution, and Systematics, 42, 155–179.
Breiman, L. (2001). Random forests. Machine Learning, 45, 5–32. https://doi.org/10.1023/A:1010933404324
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., & West, G. B. (2004). Toward a metabolic theory of ecology. Ecology, 85(7), 1771–1789.
Brudvig, L. A., Damschen, E. I., Tewksbury, J. J., Haddad, N. M., & Levey, D. J. (2009). Landscape connectivity promotes plant biodiversity spillover into non-target habitats. Proceedings of the National Academy of Sciences, 106(23), 9328–9332. https://doi.org/10.1073/pnas.0809658106
Bustamante, M. M. C., Nardoto, G. B., Pinto, A. S., Resende, J. C. F., Takahashi, F. S. C., & Vieira, L. C. G. (2012). Potential impacts of climate change on biogeochemical functioning of Cerrado ecosystems. Brazilian Journal of Biology, 72, 655–671. https://doi.org/10.1590/S1519-69842012000400005
Carroll, C., McRae, B., & Brookes, A. (2012). Use of linkage mapping and centrality analysis across habitat gradients to conserve connectivity of gray wolf populations in western North America. Conservation Biology, 26, 78–87. https://doi.org/10.1111/j.1523-1739.2011.01753.x
Carvalho, D. L., Sousa-Neves, T., Cerqueira, P. V., Gonsioroski, G., Silva, S. M., Silva, D. P., & Santos, M. P. D. (2017). Delimiting priority areas for the conservation of endemic and threatened Neotropical birds using a niche-based gap analysis. PLoS One, 12, e0171838. https://doi.org/10.1371/journal.pone.0171838. Public Library of Science.
CBD. (1992). Convention on biological diversity. https://www.cbd.int. Accessed 15 July 2018
CBRO. (2015). Comitê Brasileiro de Ornitologia. Listas das aves do Brasil. https://www.cbro.org.br. https://doi.org/10.1007/BF03544294. Accessed 10 Mar 2018
Celentano, D., Miranda, M. V. C., Mendonça, E. N., Rousseau, G. X., Muniz, F. H., Loch, V. do C., Varga, I. van D., Freitas, L., Araújo, P., & Narvaes, I. da S. (2018). Desmatamento, degradação e violência no “Mosaico Gurupi”: A região mais ameaçada da Amazônia. Estudos Avançados, 32, 315–339. https://doi.org/10.5935/0103-4014.20180021. SciELO Brasil.
Cheng, H., Sinha, A., Cruz, F. W., Wang, X., Edwards, R. L., d’Horta, F. M., Ribas, C. C., Vuille, M., Stott, L. D., & Auler, A. S. (2013). Climate change patterns in Amazonia and biodiversity. Nature Communications, 4, 1411. https://doi.org/10.1038/ncomms2415. Nature Publishing Group.
Christie, M. R., & Knowles, L. L. (2015). Habitat corridors facilitate genetic resilience irrespective of species dispersal abilities or population sizes. Evolutionary Applications, 8, 454–463. https://doi.org/10.1111/eva.12255
Cochrane, M. A., & Barber, C. P. (2009). Climate change, human land use and future fires in the Amazon. Global Change Biology, 15, 601–612. https://doi.org/10.1111/j.1365-2486.2008.01786.x
Couto, R. G. (2004). Atlas de conservação da natureza brasileira - Unidades Federais. Metalivros.
Crooks, K. R., & Sanjayan, M. (2006). Connectivity conservation: Maintaining connections for nature. Conservation Biology, Series Cambridge, 14, 1.
Cushman, S. A., McKelvey, K. S., & Schwartz, M. K. (2009). Use of empirically derived source-destination models to map regional conservation corridors. Conservation Biology, 23, 368–376. https://doi.org/10.1111/j.1523-1739.2008.01111.x
Da Silva, J. M. C. (1995). Birds of the cerrado region, South America. Steenstrupia, 21, 69–92. https://doi.org/10.1017/S0959270900001052
Da Silva, J. M. C. (1997). Endemic bird species and conservation in the Cerrado region, South America. Biodiversity and Conservation, 6, 435–450. https://doi.org/10.1023/A:1018368809116
Da Silva, J. M. C., & Bates, J. M. (2002). Biogeographic patterns and conservation in the South American Cerrado: A tropical savanna hotspot. BioScience, 52, 225–233. https://doi.org/10.1641/0006-3568(2002)052[0225:BPACIT]2.0.CO;2
Da Silva, J. M. C., Rylands, A. B., Fonseca, D., & Gustavo, A. B. (2005). The fate of the Amazonian areas of endemism. Conservation Biology, 19, 689–694. https://doi.org/10.1111/j.1523-1739.2005.00705.x
De Almeida, M. C., Cortes, L. G., & De Marco Junior, P. (2010). New records and a niche model for the distribution of two Neotropical damselflies: Schistolobos boliviensis and Tuberculobasis inversa (Odonata: Coenagrionidae). Insect Conservation and Diversity, 3(4), 252–256. https://doi.org/10.1111/j.1752-4598.2010.00096.x
de Andrade, A. F. A., Velazco, S. J. E., & Júnior, P. D. M. (2020). ENMTML: An R package for a straightforward construction of complex ecological niche models. Environmental Modelling & Software, 125, 104615. https://doi.org/10.1016/j.envsoft.2019.104615
De Luca, A. C., Develey, P. F., Bencke, G. A., & Goerck, J. M. (2009). Áreas Importantes para a Conservação das Aves no Brasil. Parte II-Amazônia, Cerrado e Pantanal. SAVE Brasil.
Dillon, M. E., Wang, G., & Huey, R. B. (2010). Global metabolic impacts of recent climate warming. Nature, 467, 704–706. https://doi.org/10.1038/nature09407
Diniz-Filho, J. A. F., Bini, L. M., Rangel, T. F., Loyola, R. D., Hof, C., et al. (2009). Partitioning and mapping uncertainties in ensembles of forecasts of species turnover under climate change. Ecography, 32, 897–906. https://doi.org/10.1111/j.1600-0587.2009.06196.x
Dudley, N., Stolton, S., Belokurov, A., Krueger, L., Lopoukhine, N., MacKinnon, K., Sekhran, N., et al. (2010). Natural solutions: Protected areas helping people cope with climate change. Natural solutions: protected areas helping people cope with climate change.
Dutta, T., Sharma, S., McRae, B. H., Roy, P. S., & DeFries, R. (2016). Connecting the dots: mapping habitat connectivity for tigers in central India. Regional Environmental Change, 16, 53–67.
Engler, R., Guisan, A., & Rechsteiner, L. (2004). An improved approach for predicting the distribution of rare and endangered species from occurrence and pseudo-absence data. Journal of applied ecology, 41(2), 263–274. https://doi.org/10.1111/j.0021-8901.2004.00881.x
Fahrig, L., & Merriam, G. (1994). Conservation of fragmented populations. Conservation biology, 8(1), 50–59. https://doi.org/10.1046/j.1523-1739.1994.08010050.x
Fearnside, P. M. (2001). Soybean cultivation as a threat to the environment in Brazil. Environmental Conservation, 28, 23–38. https://doi.org/10.1017/S0376892901000030
FIEMA. Federação das Indústrias do Estado do Maranhão. (2009). Plano Estratégico de Desenvolvimento Industrial do Maranhão.https://www.fiema.org.br. Accessed 09 July 2013
Fick, S. E., & Hijmans, R. J. (2017). WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. International journal of climatology, 37(12), 4302–4315. https://doi.org/10.1002/joc.5086
Foden, W. B., Butchart, S. H., Stuart, S. N., Vié, J. C., Akçakaya, H. R., Angulo, A., Donner, S. D., et al. (2013). Identifying the world’s most climate change vulnerable species: A systematic trait-based assessment of all birds, amphibians and corals. PLoS One, 8, e65427. https://doi.org/10.1371/journal.pone.0065427
Gaston, K. J., Jackson, S. F., Cantú-Salazar, L., & Cruz-Piñón, G. (2008). The ecological performance of protected areas. Annual Review of Ecology Evolution and Systematics, 39, 93–113.
Gilbert‐Norton, L. Y. N. N. E., Wilson, R., Stevens, J. R., & Beard, K. H. (2010). A meta‐analytic review of corridor effectiveness. Conservation biology, 24(3), 660–668. https://doi.org/10.1111/j.1523-1739.2010.01450.x
Guisan, A., Broennimann, O., Engler, R., Vust, M., Yoccoz, N. G., Lehmann, A., et al. (2006). Using niche-based models to improve the sampling of rare species. Conservation Biology, 20, 501–511. https://doi.org/10.1111/j.1523-1739.2006.00354.x
Haddad, N. M., Bowne, D. R., Cunningham, A., Danielson, B. J., Levey, D. J., Sargent, S., & Spira, T. (2003). Corridor use by diverse taxa. Ecology, 84, 609–615. https://doi.org/10.1890/0012-9658(2003)084[0609:CUBDT]2.0.CO;2
Hannah, L., Midgley, G., Andelman, S., Araújo, M., Hughes, G., Martinez-Meyer, E., & Williams, P. (2007). Protected area needs in a changing climate. Frontiers in Ecology and the Environment, 5, 131–138. https://doi.org/10.1890/1540-9295(2007)5[131:PANIAC]2.0.CO;2
Hansen, A. J., & DeFries, R. (2007). Ecological mechanisms linking protected areas to surrounding lands. Ecological Applications, 17, 974–988. https://doi.org/10.1890/05-1098
Heller, N. E., & Zavaleta, E. S. (2009). Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biological Conservation, 142, 14–32. https://doi.org/10.1016/j.biocon.2008.10.006
Hilker, T., Lyapustin, A. I., Tucker, C. J., Hall, F. G., Myneni, R. B., Wang, Y., et al. (2014). Vegetation dynamics and rainfall sensitivity of the Amazon. PNAS, 111, 16041–16046. https://doi.org/10.1073/pnas.1404870111
Holmgren, M., Hirota, M., van Nes, E. H., & Scheffer, M. (2013). Effects of interannual climate variability on tropical tree cover. Nature Climate Change, 3, 755–758. https://doi.org/10.1038/nclimate1906. Nature Publishing Group.
Huey, R. B., Kearney, M. R., Krockenberger, A., Holtum, J. A., Jess, M., & Williams, S. E. (2012). Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philosophical Transactions of the Royal Society b: Biological Sciences, 367, 1665–1679. https://doi.org/10.1098/rstb.2012.0005
IBAMA. (2014). Instituto Brasileiro do Meio Ambiente, Ministério do Meio Ambiente. Lista das espécies da fauna brasileira ameaçada de extinção. Brasilia. https://www.ibama.gov.br. Accessed 12 July 2018
IBGE. (2013). Instituto Brasileiro de Geografia e Estatística. Mapas temáticos do Brasil. mapas.ibge.gov.br.
INPE. (2013). Instituto Nacional de Pesquisa Espacial. Projeto TerraClass Cerrado. Brasília. http://www.inpe.br/cra/projetos_pesquisas/terraclass2014.php. Accessed 20 Mar 2018
INPE. (2015). Instituto Nacional de Pesquisa Espacial. Taxa estimada do desmatamento da Amazônia Legal para período ago/2014 –jul/2015. Brasília. https://www.obt.inpe.br
IPCC. (2007). Intergovernmental Panel on Climate Change 2007. Climate change: Impacts, adaptation, and vulnerability. IPCC, Cambridge University Press, Cambridge.
IPCC. (2014). Climate change 2014: Synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC.
IPCC. (2021). Climate change 2021: The physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.IUCN (2017) The IUCN Red List of Threatened Species. https://www.iucnredlist.org
Jenkins, C. N., Pimm, S. L., & Joppa, L. N. (2013). Global patterns of terrestrial vertebrate diversity and conservation. Proceedings of the National Academy of Sciences, 110, E2602–E2610. https://doi.org/10.1073/pnas.1302251110. National Academy Sciences.
Jolly, W. M., Cochrane, M. A., Freeborn, P. H., Holden, Z. A., Brown, T. J., Williamson, G. J., et al. (2015). Climate-induced variations in global wildfire danger from 1979 to 2013. Nature Communications. Nature Publishing Group, 6, 1–11. https://doi.org/10.1038/ncomms8537
Karatzoglou, A., Smola, A., Hornik, K., & Zeileis, A. (2004). kernlab-an S4 package for kernel methods in R. Journal of statistical software, 11, 1–20. https://doi.org/10.18637/jss.v011.i09
Karger, D., Conrad, O., Böhner, J. et al. (2017). Climatologies at high resolution for the earth’s land surface areas. Sci Data 4, 170122. https://doi.org/10.1038/sdata.2017.122
Khaliq, I., Hof, C., Prinzinger, R., Bohning-Gaese, K., & Pfenninger, M. (2014). Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proceedings of the Royal Society b: Biological Sciences, 281, 20141097. https://doi.org/10.1098/rspb.2014.1097
Killeen, T. J., & Solórzano, L. A. (2008). Conservation strategies to mitigate impacts from climate change in Amazonia. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 363, 1881–1888. https://doi.org/10.1098/rstb.2007.0018
Klink, C. A., & Machado, R. B. (2005). Conservation of the Brazilian cerrado. Conservation Biology, 19, 707–713. https://doi.org/10.1111/j.1523-1739.2005.00702.x
Koen, E. L., Garroway, C. J., Wilson, P. J., & Bowman, J. (2010). The effect of map boundary on estimates of landscape resistance to animal movement. PLoS One, 5, e11785. https://doi.org/10.1371/journal.pone.0011785
Lahsen, M., Bustamante, M. M., & Dalla-Nora, E. L. (2016). Undervaluing and overexploiting the Brazilian Cerrado at our peril. Environment: Science and Policy for Sustainable Development, 58, 4–15. https://doi.org/10.1080/00139157.2016.1229537
Lawler, J. J., Shafer, S. L., White, D., Kareiva, P., Maurer, E. P., Blaustein, A. R., & Bartlein, P. J. (2009). Projected climate-induced faunal change in the Western Hemisphere. Ecology, 90, 588–597. https://doi.org/10.1890/08-0823.1
Lees, A. C., & Peres, C. A. (2006). Rapid avifaunal collapse along the Amazonian deforestation frontier. Biological Conservation, 133, 198–211. https://doi.org/10.1016/j.biocon.2006.06.005
Lees, A. C., Moura, N. G., Santana, A., Aleixo, A., Barlow, J., Berenguer, E., et al. (2012). Paragominas: A quantitative baseline inventory of an eastern Amazonian avifauna. Revista Brasileira de Ornitologia, 20, 93–118.
Lemes, P., & Loyola, R. D. (2013). Accommodating species climate-forced dispersal and uncertainties in spatial conservation planning. PLoS One, 8, e54323. https://doi.org/10.1371/journal.pone.0054323
Lima, D. M., Martínez, C., & Raíces, D. S. L. (2014). An avifaunal inventory and conservation prospects for the Gurupi Biological Reserve, Maranhão, Brazil. Revista Brasileira de Ornitologia-Brazilian Journal of Ornithology, 22, 317–340. https://doi.org/10.1007/BF03544270
Lima-Ribeiro, M. S., & Diniz-Filho, J. A. F. (2012). Modelando a distribuição geográfica das espécies no passado: Uma abordagem promissora em paleoecologia. Revista Brasileira de Paleontologia, 15, 371–385.
Liaw, A., & Wiener, M. (2002). Classification and regression by randomForest. R news, 2(3), 18–22.
Loiselle, B. A., Graham, C. H., Goerck, J. M., & Ribeiro, M. C. (2010). Assessing the impact of deforestation and climate change on the range size and environmental niche of bird species in the Atlantic forests, Brazil. Journal of Biogeography, 37, 1288–1301. https://doi.org/10.1111/j.1365-2699.2010.02285.x
Malhi, Y., Roberts, J. T., Betts, R. A., Killeen, T. J., Li, W., & Nobre, C. A. (2008). Climate change, deforestation, and the fate of the Amazon. Science, 319, 169–172. https://doi.org/10.1126/science.1146961
Mantyka-Pringle, C. S., Martin, T. G., & Rhodes, J. R. (2012). Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Global Change Biology, 18, 1239–1252. https://doi.org/10.1111/j.1365-2486.2011.02593.x
Marini, M. A., & Garcia, F. I. (2005). Bird conservation in Brazil. Conservation Biology, 19, 665–671. https://doi.org/10.1111/j.1523-1739.2005.00706.x
Marini, M. A., Barbet-Massin, M., Lopes, L. E., & Jiguet, F. (2009a). Predicted climate-driven bird distribution changes and forecasted conservation conflicts in a Neotropical savanna. Conservation Biology, 23, 1558–1567. https://doi.org/10.1111/j.1523-1739.2009.01258.x
Marini, M. A., Barbet-Massin, M., Lopes, L. E., & Jiguet, F. (2009b). Major current and future gaps of Brazilian reserves to protect Neotropical savanna birds. Biological Conservation, 142, 3039–3050. https://doi.org/10.1016/j.biocon.2009.08.002
McCain, C. M. (2009). Vertebrate range sizes indicate that mountains may be “higher” in the tropics. Ecology Letters, 12, 550–560. https://doi.org/10.1111/j.1461-0248.2009.01308.x
McRae, B. (2012). Pinchpoint Mapper connectivity analysis software. The Nature Conservancy, Fort Collins.
McRae, B. H., & Beier, P. (2007). Circuit theory predicts gene flow in plant and animal populations. PNAS (USA), 104, 19885–19890. https://doi.org/10.1073/pnas.0706568104
McRae, B. H., Dickson, B. G., Keitt, T. H., & Shah, V. B. (2008). Using circtuit theory to model connectivity in ecology, evolution, and conservation. Ecology, 89, 2712–2724. https://doi.org/10.1890/07-1861.1
McRae, B. H., & Kavanagh, D. M. (2011). Linkage mapper connectivity analysis software. The Nature Conservancy, Seattle WA.
McRae, B. H. & Shah, V. B. (2009). Circuitscape users’ guide online. The University of California, Santa Barbara. Available: http://www.circuitscape.org
McRae, B. H., Shah, V., & Edelman, A. (2016). Circuitscape: Modeling landscape connectivity to promote conservation and human health. The Nature Conservancy, 14, 14. https://doi.org/10.13140/RG.2.1.4265.1126
Mello, C. F., Mochel, F. R., Silveira, O. F. M., Santos, V. F., Prost, M. T., Mendes, A., et al. (2000). IBAMA: Diagnóstico para avaliação e ações prioritárias para conservação da biodiversidade da zona costeira-estuarina dos Estados do Piauí, Maranhão, Pará e Amapá.
Mesquita, P. C. M. D., Pinheiro-Mesquita, S. F., & Pietkzac, C. (2013). Are common species endangered by climate change? Habitat suitability projections for the royal ground snake, Liophis reginae (Serpentes, Dipsadidae). Northwestern Journal of Zoology, 9, 51–56.
Michalski, F., Peres, C. A., & Lake, I. R. (2008). Deforestation dynamics in a fragmented region of southern Amazonia: Evaluation and future scenarios. Environmental Conservation, 35, 93–103. https://doi.org/10.1017/S0376892908004864
MMA. (2003). Ministério do Meio Ambiente. Áreas prioritárias para a conservação, utilização sustentável e repartição de benefícios da biodiversidade Brasileira. Brasília.
MMA. (2008). Ministério do Meio Ambiente. Monitoramento do Desmatamento dos Biomas Brasileiros. https://www.mapas.mma.gov.br
MMA. (2011). Ministério do Meio Ambiente. Plano de ação para a prevenção e controle do desmatamento no estado no Maranhão.
MMA. (2015). Ministério do Meio Ambiente. Mapeamento do uso e cobertura do Cerrado: Projeto TerraClass Cerrado 2013/MMA/SBF.
Moss, R., Babiker, M., Brinkman, S., Calvo, E., Carter, T., Edmonds, J., Hibbard, K., et al. (2008). Towards new scenarios for analysis of emissions, climate change, impacts, and response strategies. Intergovernmental Panel on Climate Change, Geneva, Switzerland. Journal of the Japan Institute of Energy, 92, 189–195. 2013.
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. https://doi.org/10.1038/35002501
Nepstad, D. C., Verssimo, A., Alencar, A., Nobre, C., Lima, E., Lefebvre, P., Cochrane, M., et al. (1999). Large-scale impoverishment of Amazonian forests by logging and fire. Nature, 398, 505. https://doi.org/10.1038/19066
Nepstad, D. C., Stickler, C. M., Filho, B. S., & Merry, F. (2008). Interactions among Amazon land use, forests and climate: Prospects for a near-term forest tipping point. Philosophical Transactions of the Royal Society b: Biological Sciences, 363, 1737–1746. https://doi.org/10.1098/rstb.2007.0036
Olímpio, A. P. M., Ventura, M. C. D. S., Mascarenhas, M. D. J. O., Nascimento, D. C. D., Andrade, F. A. G. D., Fraga, E. D. C., & Barros, M. C. (2016). Bat fauna of the Cerrado savanna of eastern Maranhão, Brazil, with new species occurrences. Biota Neotropica, 16. https://doi.org/10.1590/1676-0611-BN-2015-0089
Oliveira, T. G. D., Gerude, R. G., Júnior, S., & de Sousa, J. (2007). Unexpected mammalian records in the state of Maranhão. Boletim do Museu Paraense Emílio Goeldi Ciências Naturais, 2, 23–32.
Oren, D. C., & Roma, J. C. (2011). Composição e vulnerabilidade da avifauna da Amazônia Maranhense, Brasil. In M. B. Martins & T. G. Oliveira (Eds.), Amazônia Maranhense - diversidade e conservação (pp. 221–248). Museu Paraense Emílio Goeldi.
Oyama, M. D., & Nobre, C. A. (2003). A new climate-vegetation equilibrium state for tropical South America. Geophysical Research Letters, 30, 23. https://doi.org/10.1029/2003GL018600
Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics, 37, 637–669.
Parmesan, C., & Yohe, G. (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421, 37. https://doi.org/10.1038/nature01286
Paynter, R. A., & Traylor, M. A. (1991). Ornithological gazetteer of Brazil. Bird Department, Museum of Comparative Zoology, Harvard University.
Pearson, R. G., Raxworthy, C. J., Nakamura, M., & Townsend Peterson, A. (2007). Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. Journal of Biogeography, 34, 102–117. https://doi.org/10.1111/j.1365-2699.2006.01594.x. Wiley Online Library.
Perez, T. M., Stroud, J. T., & Feeley, K. J. (2016). Thermal trouble in the tropics. Science, 80(351), 1392–1393. https://doi.org/10.1126/science.aaf3343
Phillips, S. J., & Dudík, M. (2008). Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography, 31, 161–175. https://doi.org/10.1111/j.0906-7590.2008.5203.x
Phillips, S. J., Anderson, R. P., & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190, 231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026
Phillips, S. J., Anderson, R. P., Dudík, M., Schapire, R. E., Blair, M. E. (2017). Opening the black box: an open-source release of Maxent. Ecography, 40, 887–893. https://doi.org/10.1111/ecog.03049
Pinto, N., & Keitt, T. H. (2009). Beyond the least-cost path: Evaluating corridor redundancy using a graph-theoretic approach. Landscape Ecology, 24, 253–266. https://doi.org/10.1007/s10980-008-9303-y
Prasad, A. M., Iverson, L. R., & Liaw, A. (2006). Newer classification and regression tree techniques: Bagging and random forests for ecological prediction. Ecosystems, 9, 181–199. https://doi.org/10.1007/s10021-005-0054-1
Ratter, J. A., Ribeiro, J. F., & Bridgewater, S. (1997). The Brazilian cerrado vegetation and threats to its biodiversity. Annals of Botany, 80, 223–230. https://doi.org/10.1006/anbo.1997.0469
Ribeiro, B. R., Sales, L. P., De Marco, P., Jr., & Loyola, R. (2016). Assessing mammal exposure to climate change in the Brazilian Amazon. PLoS One, 11, e0165073. https://doi.org/10.1371/journal.pone.0165073
Rodrigues, A. S. L., Akcakaya, H. R., Andelman, S. J., Bakarr, M. I., Boitani, L., Brooks, T. M., Chanson, J. S., Fishpool, L. D. C., Da Fonseca, G. A. B., & Gaston, K. J. (2004a). Global gap analysis: Priority regions for expanding the global protected-area network. BioScience, 54, 1092–1100. https://doi.org/10.1641/0006-3568(2004)054[1092:GGAPRF]2.0.CO;2. Oxford University Press.
Rodrigues, A. S. L., Andelman, S. J., Bakarr, M. I., Boitani, L., Brooks, T. M., Cowling, R. M., Fishpool, L. D. C., Da Fonseca, G. A. B., Gaston, K. J., & Hoffmann, M. (2004b). Effectiveness of the global protected area network in representing species diversity. Nature, 428, 640–643. https://doi.org/10.1038/nature02422
Rylands, A. B., & Pinto, L. P. de S. (1998). Conservação da biodiversidade na Amazônia brasileira: uma análise do sistema de unidades de conservação. Fundação Brasileira para o Desenvolvimento Sustentável.
Salazar, A., Baldi, G., Hirota, M., Syktus, J., & McAlpine, C. (2015). Land use and land cover change impacts on the regional climate of non-Amazonian South America: A review. Global and Planetary Change, 128, 103–119. https://doi.org/10.1016/j.gloplacha.2015.02.009
Sales, L. P., Neves, O. V., De Marco, P., Jr., & Loyola, R. (2017). Model uncertainties do not affect observed patterns of species richness in the Amazon. PLoS One, 12, e0183785. https://doi.org/10.1371/journal.pone.0183785
Scholkopf, B., Platt, J. C., Shawe-Taylor, J., Smola, A. J., & Williamson, R. C. (2001). Estimating the support of a highdimensional distribution. Neural Computation, 13, 1443–1471. https://doi.org/10.1162/089976601750264965
Seddon, A. W., Macias-Fauria, M., Long, P. R., Benz, D., & Willis, K. J. (2016). Sensitivity of global terrestrial ecosystems to climate variability. Nature, 531, 229–232. https://doi.org/10.1038/nature16986. Nature Publishing Group.
Seidl, R., Thom, D., Kautz, M., Martin-Benito, D., Peltoniemi, M., Vacchiano, G., et al. (2017). Forest disturbances under climate change. Nature Climate Change, 7, 395–402. https://doi.org/10.1038/nclimate3303. Nature Publishing Group.
Sekercioglu, C. H., Schneider, S. H., Fay, J. P., & Loarie, S. R. (2008). Climate change, elevational range shifts, and bird extinctions. Conservation Biology, 22, 140–150. https://doi.org/10.1111/j.1523-1739.2007.00852.x
Sekercioglu, C. H., Primack, R. B., & Wormworth, J. (2012). The effects of climate change on tropical birds. Biological Conservation, 148, 1–18. https://doi.org/10.1016/j.biocon.2011.10.019
Serra, F. C. V., Lima, P. B., & Almeida Junior, E. B. D. (2016). Species richness in restinga vegetation on the eastern Maranhão State, Northeastern Brazil. Acta Amazonica, 46, 271–280. https://doi.org/10.1590/1809-4392201504704
Sheth, S. N., & Angert, A. L. (2014). The evolution of environmental tolerance and range size: A comparison of geographically restricted and widespread Mimulus. Evolution, 68, 2917–2931. https://doi.org/10.1111/evo.12494
Siegenthaler, U., Stocker, T. F., Monnin, E., Lüthi, D., Schwander, J., Stauffer, B., Raynaud, D., Barnola, J.-M., Fischer, H., Masson-Delmotte, V., & Jouzel, J. (2005). Stable carbon cycle-climate relationship during the Late Pleistocene. Science, 310, 1313–1317. https://doi.org/10.1126/science.1120130
Sinervo, B., Mendez-De-La-Cruz, F., Miles, D. B., Heulin, B., Bastiaans, E., Villagrán-Santa Cruz, M., & Gadsden, H. (2010). Erosion of lizard diversity by climate change and altered thermal niches. Science, 328, 894–899. https://doi.org/10.1126/science.1184695
Siqueira, M. F. D., & Peterson, A. T. (2003). Consequences of global climate change for geographic distributions of cerrado tree species. Biota Neotropica, 3, 1–14. https://doi.org/10.1590/S1676-06032003000200005
Soares-Filho, B. S., Nepstad, D. C., Curran, L. M., Cerqueira, G. C., Garcia, R. A., Ramos, C. A., & Schlesinger, P. (2006). Modelling conservation in the Amazon basin. Nature, 440, 520. https://doi.org/10.1038/nature04389
Stoklosa, J., Daly, C., Foster, S. D., Ashcroft, M. B., & Warton, D. I. (2015). A climate of uncertainty: Accounting for error in climate variables for species distribution models. Methods in Ecology and Evolution, 6, 412–423. https://doi.org/10.1111/2041-210X.12217
Sunday, J. M., Bates, A. E., Kearney, M. R., Colwell, R. K., Dulvy, N. K., Longino, J. T., et al. (2014). Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. PNAS, 111, 5610–5615. https://doi.org/10.1073/pnas.1316145111
Tessarolo, G., Rangel, T. F., Araujo, M. B., Hortal, J. J., et al. (2014). Uncertainty associated with survey design in species distribution models. Diversity and Distributions, 20, 1258–1269. https://doi.org/10.1111/ddi.12236
Tewksbury, J. J., Huey, R. B., & Deutsch, C. A. (2008). Putting the heat on tropical animals. Science, 320, 1296. https://doi.org/10.1126/science.1159328
Thomas, C. D., Cameron, A., Green, R. E., Bakkenes, M., Beaumont, L. J., et al. (2004). Extinction risk from climate change. Nature, 427, 145–148. https://doi.org/10.1038/nature02121
Thuiller, W., Lavorel, S., Araújo, M. B., Sykes, M. T., & Prentice, I. C. (2005a). Climate change threats to plant diversity in Europe. PNAS (USA), 102, 8245–8250. https://doi.org/10.1073/pnas.0409902102
Thuiller, W., Richardson, D. M., Pysek, P., Midgley, G. F., Hughes, G. O., & Rouget, M. (2005b). Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Global Change Biology, 11, 2234–2250. https://doi.org/10.1111/j.1365-2486.2005.001018.x
Townsend, C. R., Begon, M., & Harper, J. L. (2003). Essentials of ecology. Blackwell Science.
Vale, M. M., Souza, T. V., Alves, M. A. S., & Crouzeilles, R. (2018). Planning protected areas network that are relevant today and under future climate change is possible: The case of Atlantic Forest endemic birds. PeerJ, 6, e4689. https://doi.org/10.7717/peerj.4689
Vasconcelos, T. S. (2014). Tracking climatically suitable areas for an endemic Cerrado snake under climate change. Natureza and Conservação, 12, 47–52. https://doi.org/10.4322/natcon.2014.009
Watson, D. M., Doerr, V. A., Banks, S. C., Driscoll, D. A., van der Ree, R., Doerr, E. D., & Sunnucks, P. (2017). Monitoring ecological consequences of efforts to restore landscape-scale connectivity. Biological Conservation, 206, 201–209. https://doi.org/10.1016/j.biocon.2016.12.032
Wiens, J. A., Stralberg, D., Jongsomjit, D., Howell, C. A., & Snyder, M. A. (2009). Niches, models, and climate change: Assessing the assumptions and uncertainties. Proceedings of the National Academy of Sciences, 106, 19729–19736. https://doi.org/10.1073/pnas.0901639106
Williams, J. W., Stephen, T. J., & John, E. K. (2007). Projected distributions of novel and disappearing climates by 2100 AD. Proceedings of the National Academy of Sciences, 104, 5738–5742. https://doi.org/10.1073/pnas.0606292104
Wood, C. H., & Porro, R. (2002). Deforestation and land use in the Amazon. University Press of Florida.
Acknowledgements
We acknowledge J. V. Remsen (LSUMNH), M. Raposo (MNRJ), Josué (MNRJ), A. Aleixo (MPEG), F. Lima (MPEG), FNJV for access to the information about occurrence records, and all the birdwatchers for giving away their occurrence records in the WikiAves and Xeno-canto databases. We also acknowledge the comments and valuable input of two anonymous reviwers that helped to improve this manuscript. We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for DLC’s PhD scholarship (process number 1663127). DPS received a productivity grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (process number: 304494/2019-4), and SMS received a post-doctoral grant (process number 150657/2017-0). MPDS received a productivity grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (process number: 310976/2020-0).
Funding
DLC was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (PhD scholarship; process number 1663127). SMS received a post-doctoral grant (process number 150657/2017-0).
Author information
Authors and Affiliations
Contributions
D.L.C. wrote the main manuscript text with feedback from S.M.S., D.P.S., and M.P.D.S.; D.L.C., T.S.N., and G.G.R. performed the analyses; D.L.C. and T.S.N. prepared the figures; M.P.D.S. conceptualized and supervised the research, and all authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Carvalho, D.L., Silva, S.M., Sousa-Neves, T. et al. Predicting the future of threatened birds from a Neotropical ecotone area. Environ Monit Assess 196, 61 (2024). https://doi.org/10.1007/s10661-023-12174-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-12174-w