Abstract
The annual average increase in carbon dioxide (CO2) concentration is touching new heights every year. Global climate change and warming are twin outcomes of record-breaking CO2 levels. The trees outside forests (TOF) are the most promising and suitable components in the ecosystem for combating global warming via carbon (C) sequestration. Urban university campuses are the hotspot regions of TOF. We have attempted to quantify the C stock, C sequestration potential, and C credit value of dominant tree species at Maharshi Dayanand University (MDU), Rohtak. Different volumetric and biomass equations were used for biomass computation. We assessed a total of 29,442 trees (top 10) for measuring phytosociological parameters like total tree count, age, height (H), and diameter at breast height (DBH) to quantify the amount of C stored. The total C stock, C sequestration rate, and monetary value were 78.67 (Mg C ha−1), 19.05 (Mg CO2 ha−1 year−1), and 23,101.59 $ year−1, respectively. Eucalyptus globulus is the most dominant tree species on the campus and topped almost all the quantitative characteristics like total tree count (~40 %), age (25 years), density (D) (55.35 trees ha−1), and total C stock (16.06 ± 9.90 Mg C ha−1). Tree basal area (BA), D, diversity, and H positively affected the total C stocks. When the C market becomes operational, these C credits can be traded while generating additional income for the university. The results from this study can also help calculate the total C footprint of the campus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global warming, the major cause of climate change, is the most discussed topic in the scientific community, on international platforms, and among governments. Carbon dioxide (CO2) emissions, mainly due to anthropogenic actions, are further worsening the situation. The ever-increasing population not only increases the rates of CO2 emissions but will also be responsible for the degradation of ecosystems, fragmentation of urban settlements, and several other adverse effects (Dobbs et al., 2017; Grumbine, 2014; Yang et al., 2020). Globally, 50% of the world’s population is living in urban areas, and by the year 2050, this figure will be around 70% (The World Bank, 2022). Cities, particularly in tropical countries like India, Malaysia, and Indonesia, are more prone to experience the devastating consequences of climate change (Somvichian-Clausen, 2020) and are also among the major contributors to greenhouse gases (Pan et al., 2011; Saatchi et al., 2011). The progression of climate change could be slowed down by increasing carbon (C) sinks, particularly in urban spaces. In urban areas, this sink occurs in the form of vegetation, especially trees (Amoatey & Sulaiman, 2020; Salunkhe et al., 2014). These trees in urban settlements which are not part of any recorded forests are generally referred to as trees outside forests (TOF) (FSI, 2021). Through their fast growth rates, the TOF have great potential to absorb CO2 and effectively combat climate change (Bayat et al., 2012; Liu & Li, 2012). These TOF can also help India achieve its ambitious goal of climate neutrality by the year 2070 (Economic Times, 2023). Though TOF have been defined in the literature, there exists no definition for urban forests. Also, the concept of an urban forest is limited to home gardens, parks, and other green areas (Xie et al., 2019). Due to the substandard planning of green infrastructure in urban areas, many cities face several environmental challenges (Agbelade & Onyekwelu, 2020). The efficiency of trees in mitigating global warming is determined by their capacity to store C. The C concentration in a tree can be computed from the amount of biomass accumulated in the tree. The biomass is mainly present in stem wood, branches, and marginally in leaves and is generally calculated using allometric equations (Byrd et al., 2018; Chandra et al., 2011; Nandal et al., 2023).
Educational institutions, particularly university campuses, can play an important role in the process of C conservation. The universities have been termed “mini-cities” or “small-scale cities” due to the availability of all the facilities inside the campus only (Wibowo et al., 2019). The university campuses are the hub of TOF, and along with greenspaces form an integral part of the campus ecosystem (Arborday, 2022). Recently, universities are incorporating sustainable environmental education as a part of interdisciplinary studies for sensitizing students about C emissions and footprints (Robinson et al., 2018; Savageau, 2013). Also, some of the institutes have established environmental management systems for regular monitoring of the local environment (Varón-Hoyos et al., 2021). The soils on institutional campuses are another ecosystem component that provides many ecosystem services. The C storage and C sequestration are the most essential services provided by soils as they are the 2nd largest reservoir of C after oceans (Zhu et al., 2017). Soils have a higher capacity to store more C than trees as the rates of soil respiration are low (Richter et al., 2020). Besides, C sequestration trees also improve local environmental conditions and provide various socio-economic services for residents (Colding & Barthel, 2017; Liu et al., 2018). This can make a significant contribution towards campus sustainability. The C sequestration by trees and soils not only regulates the environment but can also be used for meeting the finance on campuses through C credits. A C credit generally means a tradable certificate and is part of the emission trading system.
The C credits became popular with the advent of the Clean Development Mechanism (CDM), part of the Kyoto Protocol in 2005. The CDM allows emission-reduction projects in developing countries to earn certified emission reduction (CER) credits, each equivalent to one tonne of CO2. These CERs can be traded, sold, and used by industrialized countries to meet a part of their emission reduction targets under the Kyoto Protocol. With CO2 levels roaring up to 416.55 ppm (Daily CO2, 2022), it becomes more important to take immediate steps to address climate change in the hotspots (cities). Considering climate change as a real threat, more and more campuses around the world are working for campus sustainability through green campus initiatives (Tiyarattanachai & Hollmann, 2016). Universities like Shenyang Institute of Technology (SIT) in China, Dalhousie University (USA), and Bogor Agricultural University (Indonesia) (Lavista et al., 2016; Ritchie, 2017; Wang et al., 2021) have previously worked in this direction. Several universities from India like Cotton University, Amity University, Kuvempu University, and Uttar Banga Krishi Viswavidyalaya (Nandal et al., 2019; Narayana et al., 2020; Sharma et al., 2020; Tamang et al., 2021; Yumnam & Dey, 2022) have also calculated the total C stored in campus trees. The SIT campus study provided a detailed account of campus tree structure and function and economic benefits from ecosystem services including C sequestration. Most of the other studies have not focused on the economic dimension of C sequestration by trees and soils. In addition, with the launch of the trees outside forests in India (TOFI) program, it becomes pertinent to evaluate the economic value of the dominant TOF. The study hypothesized that (a) more densely planted tree species have more C stock and (b) the phytosociological characteristics of different tree species considerably affect C sequestration rates. Thus, the study has been undertaken at Maharshi Dayanand University (MDU), Rohtak (Haryana), India, with the following primary research queries: (a) What is the C sequestration potential of trees and soils on the MDU campus? (b) What are the factors that regulate the C stock and C sequestration potential of trees on the campus? (c) What is the monetary value of C credits provided by campus trees?
Material and methods
Study site
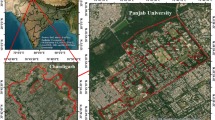
The research investigation was undertaken at MDU (Fig. 1), Rohtak (Haryana), India. The study site is located in the country’s National Capital Region (NCR). The study site lies in the Haryana state of India, one of the seven states chosen initially for the TOFI program. This program has been launched from the MDU campus itself. The university has also been awarded the cleanest and greenest government university in India in 2018. Climatically, the region lies in the sub-tropical zone. The local temperature varies from 2 to 47 °C. Annually, the region receives rainfall of around 58 cm, mainly from southwest monsoon and western disturbances. The average humidity in the region is around 65%, ranging from 13 to 98% (World Weather Online, 2023). The region is occupied by alluvial soil since the Quaternary age. The soil is mainly constituted of medium-grained sand (Central Ground Water Board, Ministry of Water Resources, RD, & GR Government of India, 2013). The campus encompasses an area of approximately 250 ha. The institution has plantations of herbs, shrubs, and trees. Along with a plant research site, there is also an herbal garden with plantations of different tree species. The campus has around 60 tree species, mainly dominated by families like Fabaceae and Moraceae. Tree species such as Eucalyptus globulus and Pongamia pinnata are abundant on the study site (Nandal et al., 2022).
Maps and spatial distribution
The study site map was generated with QGIS version 3.16 software. The spatial distribution of soil organic carbon % (SOC %) on the university campus under different tree species was constructed with the interpolation function of QGIS version 3.16 software by using the inverse weight distance method with a coefficient value of 2.0.
Sampling instruments and tools
We used a measuring tape for circumference at breast height (CBH) measurement and a Haga altimeter for measuring tree height (H). Soil samples were collected using a soil core sampler with a radius of 4.5 cm and 10 cm in H. A 2 mm sieve was used for straining soil, and an oven was used to dry soil samples.
Sampling techniques and processing
The trees were sampled for various quantitative variables like diameter at breast height (DBH), H, and basal area (BA). The tree sampling was carried out from January 2019 to December 2020. Juveniles (DBH < 4–15 cm), as well as adult (DBH > 15 cm) trees (Chaturvedi et al., 2012; Long et al., 2013), were counted. Instead of using a quadrat or plot, we manually counted all the trees on the campus. This was done to capture more precise values of tree structural attributes. Unlike natural forests, the manual and random tree plantations along with several institutional buildings did not allow for the use of the plot method. The top 10 tree species with the highest tree count were considered as dominant tree species. The trees were measured at a vertical distance of 1.37 m from the ground, i.e., breast H. The value of CBH was divided by the value of π (3.14) to get DBH. Trees with CBH~10 cm or greater (corresponding to a DBH of 4 cm) were taken into account for C stock estimation. The DBH was classified into four categories (< 9 cm, 9.1–18 cm, 18.1–27 cm, and > 27 cm). The soil samples were collected in December 2021. Soil samples were collected at four depths from 0 to 10, 10.1 to 20, 20.1 to 30, and 30.1 to 40 cm. At every depth, three sub-samples of the soil were collected and then mixed to make one composite sample. Thus, overall, 40 soil samples were collected (1 sample at each depth under 10 different tree species, accounting for 4 composite samples under a single tree species). The samples were collected at a distance corresponding to half, twice, and thrice of the canopy size of the tree, as depicted in Fig. 2 (Kokkora et al., 2022). The soil samples were sieved through a 2 mm sieve and were oven dried at 65 °C for 24 h. Bulk density (BD) was calculated with a soil core sampler using the fresh weight and oven-dried weight of soil samples at different depths with Eq. (1).
Tree quantitative parameters:
The tree density (D) and BA were calculated using the following equations:
where DBH is in meters (m).
From the tree count, various diversity indices like the species dominance index (Simpson, 1949), species diversity index (Weaver & Shannon, 1963), and species evenness index (Pielou, 1975) were calculated. The species dominance index and species diversity index are employed to determine species dominance and species richness in an ecosystem. On the other hand, the species evenness index is used to detect the degree of commonness or rarity of a species. A value of 1 on the scale indicates complete evenness, and 0 indicates no evenness.
Species dominance index:
where Ds is Simpson’s dominance index, n is the total number of trees of a particular species, and N is the total number of trees of all species.
Species diversity index:
where H′ is the Shannon-Weiner diversity index, R is the total number of tree species, and pi is the proportion of individuals belonging to the ith tree species.
Species evenness index:
where J′ is Pielou’s evenness index, H′ is the Shannon-Weiner diversity index, and S is the total number of species.
Biomass and C stock
The tree biomass was calculated by employing direct volumetric and biomass equations. All the volumetric equations were species-specific. Biomass equations on the other hand were species-specific as well as general in nature. The general biomass equation formulated by Chave et al. (2005) was used for Callistemon lanceolatus, Polyalthia longifolia, Pongamia pinnata, and Terminalia arjuna and for the rest species-specific equations were used. Already published equations in literature were used for volume and biomass. All the volumetric equations were taken from Volume Equations for Forests of India, Nepal, and Bhutan (1996), while the biomass equation for Azadirachta indica was taken from Mohamed et al. (2018). The tree volume obtained from volumetric equations was multiplied by tree-specific wood density (TSWD) (Appendix 1 - List of wood densities for tree species from tropical America, Africa, and Asia., 1997; Tamang et al., 2019). The results obtained were further multiplied by a biomass expansion factor (BEF) of 1.5 (to account for the biomass of other parts of the tree such as leaves, flowers, and fruits) (Brown & Lugo, 1992). In the case of biomass equations, it was assumed that BEF had already been considered while formulating the equations. This biomass obtained was above-ground biomass (AGB). The AGB was multiplied by 0.26 (Cairns et al., 1997; Ravindranath & Ostwald, 2007) to get below-ground biomass (BGB). The total biomass (TB) of the tree was calculated by adding AGB and BGB.
where V is the volume of the tree, ρ is the specific wood density, DBH is the diameter at breast height (cm), and H is the tree H (m).
The tree C stock is approximately half of the biomass (Eggleston et al., 2006); thus, TB was multiplied by 0.5 to get the amount of C stored in the tree. The SOC (%) was calculated using the standard protocol of Walkley and Black (1934). All the samples were analyzed in triplicates. The SOC % was converted to SOC Mg ha−1 using the formula given in Eq. (13). While processing the data, we used the mean values of BD (Online Resource 1) and SOC. The total carbon stock (TCS) was obtained by adding up the tree and soil carbon stock (SCS).
C sequestration, C credits, and monetary value
The CO2 sequestration potential of trees was obtained by multiplying the TCS value by 3.667 (44/12). Then, the obtained value of CO2 sequestration was divided by the age of the particular tree species to get the CO2 sequestration potential of trees on a per-year basis. We used the approximate age of trees from the information provided by the university administration, as precise data on the age of trees was lacking. Also, the mean value of the age was used as the planting of trees on the campus is a continuous process.
The value of 1 T of CO2 sequestered by trees equals one C credit. Therefore, to calculate C credits, we used t CO2 unit instead of Mg CO2. The total number of C credits of a tree species was divided by the age of that tree to obtain C credits on a per-year basis. The C credits were converted into monetary value. The average value of one credit was around 3.82 $/t CO2 equivalent (ClimateTrade, 2022) in 2021. For calculating the value of one C credit in terms of C alone (not CO2), we multiplied 3.82 by 3.667 (Pache et al., 2020), which turned out to be around 14 $. The value of one dollar was considered equal to 74 (avg. value in 2021) Indian rupees. A brief outline of the protocol followed in the present study is depicted in Fig. 3.
Statistical analysis
The data were subjected to one-way analysis of variance (ANOVA) to look for significant differences in values of various tree variables among different tree species. Correlation coefficient (r) and principal component analysis (PCA) were used to determine the correlation between the various tree variables and tree C stock. The PCA was also used for dimension reduction and to determine the effect of different tree variables on TCS. Based on the quantitative variables, the hierarchical cluster analysis (HCA) was used to combine or cluster the trees of different species. We used Ward’s method for clustering the data. All the significance levels were measured at p < 0.05. The statistical analysis was performed using Origin Pro and SPSS 25.0 software.
Results
Tree quantitative parameters
We documented 66 tree species on the campus. Among all the documented species, the top ten dominant tree species were selected for C stock estimation. The tree D on the campus was 139.77 trees ha−1. Amidst these, Eucalyptus globulus, with a total tree count of 13,932 (39.59%), has the highest tree count on the study site, and Dalbergia sissoo has the lowest tree count of 504 trees. The oldest plantations were of Eucalyptus globulus (25 years), closely followed by Callistemon lanceolatus (23 years), and Syzygium cumini plantations were the youngest one (10 years). The DBH (cm) ranged from 4.07 in Polyalthia longifolia to 34.39 cm in Eucalyptus globulus. Around 35% (maximum contribution) of the trees of Dalbergia sissoo were below 9 cm of DBH. In the range of 9–18 (cm) DBH, Ficus benjamina and Polyalthia longifolia were the maximum contributors, with approximately 50–55% of their total trees in this range. Around 40% of the total trees of Pongamia pinnata were present in the DBH range of 18.1–27 cm. Eucalyptus globulus and Syzygium cumini trees dominated the class with DBH greater than 27 cm. Tree species like Dalbergia sissoo, Ficus benjamina, and Polyalthia longifolia were not represented in the DBH class above 27 cm (Fig. 4). The highest mean DBH (cm) was recorded in the case of Eucalyptus globulus (21.19 ± 11.46 cm) and the lowest in Polyalthia longifolia (10.95 ± 7.63 cm). There was a huge variation in the H (m) among all the measured tree species, i.e., statistically significant at p < 0.05. A pattern similar to that of DBH was observed in the case of tree H as well with Eucalyptus globulus (14.60 ± 7.13 m) being the tallest and Polyalthia longifolia (5.19 ± 1.69 m) being the shortest. Eucalyptus globulus with a tree D of 55.35 trees ha−1 was the most densely planted tree species, and Dalbergia sissoo, on the other hand, was sparsely planted (2.00 trees ha−1). The BA of a tree was expanded to a per-hectare basis by multiplying the BA of a single tree with its D. Maximum BA was reported in Eucalyptus globulus (1.926 m2 ha−1) and minimum in Dalbergia sissoo (0.037 m2 ha−1). Overall, Simpson’s dominance index, Shannon’s diversity index, and Pielou’s evenness index values were 0.184, 2.509, and 0.598, respectively. These values suggest a moderate level of biodiversity on the campus. Relatively, the value of Simpson’s dominance index was highest in Eucalyptus globulus (0.15679) and lowest in Dalbergia sissoo (0.00020). A similar trend was also observed for Shannon’s diversity and Pielou’s evenness index (Table 1). The bold values in Table 1 are minimums.
Tree biomass and C stock
The biomass on per tree basis, obtained from volumetric and biomass equations, did not show any significant difference. The TB of these 10 tree species was 1999.39 Kg, and the mean was 199.93 Kg. The maximum AGB, BGB, and TB were recorded in Eucalyptus globulus (this was on the same lines as the 1st hypothesis of the study), having respective values of 304.65 ± 317.60 (Kg/tree), 79.21 ± 82.58 (Kg/tree), and 383.86 ± 400.18 (Kg/tree). The AGB, BGB, and TB were reported as a minimum in Polyalthia longifolia with values of 22.75 ± 29.43 (Kg/tree), 5.91 ± 7.65 (Kg/tree), and 28.66 ± 37.08 (Kg/tree), respectively.
The total AGB, BGB, and TB were also recorded in the same order as that of biomass on a per-hectare basis. A total of 31.64 Mg C ha−1 (mean 3.16 Mg C ha−1) of biomass was stored in the dominant tree species. Eucalyptus globulus was flagged as the highest biomass-accumulating species with 16.86 ± 17.58 (Mg C ha−1), 4.38 ± 4.57 (Mg C ha−1), and 21.24 ± 22.15 (Mg C ha−1) values of AGB, BGB, and TB, respectively (Table 2). Polyalthia longifolia has the least amount of biomass, with values corresponding to 0.13 ± 0.17 (Mg C ha−1) of AGB, 0.03 ± 0.04 (Mg C ha−1) of BGB, and 0.17 ± 0.21 (Mg C ha−1) of TB. The amount of biomass stored in trees was significantly different among the different tree species. Except for Eucalyptus globulus (8.43 ± 8.79 Mg C ha−1), the above-ground carbon stock (AGCS) levels were quite low and comparable with each other. Similarly, below-ground carbon stock (BGCS) amounts in all the tree species were also very less. Among various tree species, AGCS and BGCS were statistically different. Based on the amount of SOC % in soil samples, 60% of the samples had medium (0.40–0.75%) levels of SOC %. The rest 40% of soil samples under trees, viz., Callistemon lanceolatus, Eucalyptus globulus, Polyalthia longifolia, and Syzygium cumini, had low (< 0.40) levels of SOC % (Fig. 5).
The SOC (Mg C ha−1) did not follow a definite path with increasing depth. A maximum value of 13.19 (Mg C ha−1) of SOC was recorded at surface levels, i.e., 0–10 cm depth under Dalbergia sissoo (Online Resource 2). The mean peak SOC amount (9.07 ± 2.89 Mg C ha−1) was recorded in Ficus virens. The SOC values in the case of Polyalthia longifolia 2.46 ± 1.54 (Mg C ha−1) were at the trough. A significant difference was observed among the mean SOC values. These tree species stored a TCS of 78.67 (Mg C ha−1) with a mean value of 7.86 (Mg C ha−1). The maximum TCS amount was stored in Eucalyptus globulus (16.06 ± 9.90 Mg C ha−1) plantations. Apart from this, tree species like Ficus virens (10.21 ± 2.21 Mg C ha−1) and Ficus benjamina (8.03 ± 5.83 Mg C ha−1) had comparable amounts of C stocks. The least amount of TCS was recorded in Polyalthia longifolia (2.55 ± 0.81 Mg C ha−1). The TCS values varied significantly among different tree species (Table 2). The bold values in Table 2 are minimal.
C sequestration, C credits, and monetary value
The total and mean values for CO2 sequestration on a per-year basis were 19.05 (Mg CO2 ha−1 year−1) and 1.90 (Mg CO2 ha−1 year−1), respectively. In terms of the total CO2 sequestered, the Eucalyptus globulus species topped the list with a value of 58.79 ± 36.25 (Mg CO2 ha−1), followed by Ficus virens (37.37 ± 8.09 Mg CO2 ha−1). The rest of the tree species such as Ficus benjamina (29.38 ± 21.34 Mg CO2 ha−1), Dalbergia sissoo (25.98 ± 12.25 Mg CO2 ha−1), and Azadirachta indicia (25.02 ± 0.96 Mg CO2 ha−1) had equivalent values of CO2 sequestration. Callistemon lanceolatus (13.53 ± 3.74 Mg CO2 ha−1) and Polyalthia longifolia (9.33 ± 2.95 Mg CO2 ha−1) sequestered the least amount of CO2. The CO2 sequestered per year was maximum in Ficus virens (3.40 ± 0.74 Mg CO2 ha−1 year−1), while Eucalyptus globulus (2.35 ± 1.45 Mg CO2 ha−1 year−1), Syzygium cumini (2.16 ± 0.69 Mg CO2 ha−1 year−1), and Dalbergia sissoo (2.09 ± 1.52 Mg CO2 ha−1 year−1) had almost similar rates of CO2 sequestration (Table 3).
The CO2 sequestration rates were markedly different among different tree species at p < 0.05. The trees on the university campus could furnish 1663.58 C credits in 1 year. A massive number of total C credits (approximately 10 times as compared to other species) were provided by the Eucalyptus globulus (21552.87). Among other species, Ficus virens (2339.92) and Ficus benjamina (1792.25) contributed more C credits. A similar trend was observed in yearly C credits contribution where more credits were supplied by Eucalyptus globulus (862.11), and the rest of the tree species had a lesser contribution. The lowest values have been highlighted in Table 3. The monetary valuation of the tree species was calculated based on the C credits supplied by trees in 1 year. The total price of these C credits (annually) was around 23,101.59 $ (1,709,518 rupees). The Eucalyptus globulus plantations with the highest monetary value could provide 12,069.60 $ (893,151.10 rupees) in a year. Ficus virens too had a good monetary value of 2978.07 $ (220,377.53 rupees) (Fig. 6).
Correlation, PCA, and HCA
The correlation between tree variables was projected in the form of a network (Fig. 7). The thick lines of connection project more interrelationships, and the thin lines demonstrate a lesser association. The color scale depicts the value of the correlation coefficient (r) and is represented by lines connecting various tree variables in Fig. 7. The majority of the tree variables like H, DBH, age, and D were highly and positively associated with each other. Shannon’s diversity index and Pielou’s evenness index were perfectly complementing each other (r = 1). The tree BA was very closely linked with D and Simpson’s dominance index having “r” values of 0.994 and 0.992, respectively. Other than this a strong correlation was also observed between D and Simpson’s dominance index (r = 0.998). However, SCS did not show any significant coupling with other variables but feebly had a negative correlation with age, H, and Simpson’s dominance index. The “r-value” suggested that TCS was significantly correlated with the BA (r = 0.859) and D (r = 0.853). Also, a strong correlation of TCS with tree dominance (r = 0.834), diversity, and evenness of trees each having an “r” value of 0.804 was observed. Tree H also significantly affected the TCS levels. There was a weak correlation of TCS with DBH, SCS, and age (Online Resource 3).
The tree parameters were dominantly explained in the 1st two principal components (PCs). These both PCs had an eigenvalue greater than 1 and accounted for 86.04% of the variance. Except for SCS, all other tree variables along with TCS were explained in the 1st PC itself. The SCS was present in the 2nd PC (Fig. 8).
The PCA also confirmed that D and BA have more bearing on the TCS as compared to other variables (Online Resource 4). Based on the measured values of tree parameters, two clusters were generated from HCA. All the tree species except Eucalyptus globulus (in the 2nd cluster) were combined in the 1st cluster (Fig. 9).
Contribution of different tree species
Eucalyptus globulus was the leading tree species that contributed around 20% to the TCS. Ficus species contributed approximately 10–12% towards TCS values. Callistemon lanceolatus and Polyalthia longifolia contributed less than 5% to the TCS.
The % values of contribution towards monetary value varied widely from 66.66 in Eucalyptus globulus to 0.55% in Polyalthia longifolia. Apart from this, each tree species contributed less than 10% towards monetary value (Fig. 10).
Discussion
The quantifiable characteristics of a tree-like DBH, H, BA, and diversity largely affect tree ecology. The study demonstrates that every tree species contributes differently to biomass and C stock. The variation in tree variables and the effect of these variables on tree biomass and C stock have been described in the following section.
Phytosociological characteristics of trees
The total number of tree species (66) recorded from the university campus was identical to the tree species documented at Tripura University Campus (Deb et al., 2016), India, and Shenyang Institute of Technology (Wang et al., 2021), China. The tree species were less as compared to the Pondicherry University campus (139 species) (Sundarapandian et al., 2014) and Dalhousie University campus (71 species) (Ritchie, 2017) and quite higher than other campuses (Table 4).
The ten dominant tree species account for 85% of the total tree cover on the campus which is more as compared to SIT (China) where dominant species accounted for 76.5% of the total tree cover. Unlike other institutional campuses where the number of trees of different species was planted in comparable numbers, the MDU campus has huge plantations of Eucalyptus globulus (39.59%). The precise data on the age of plantations on the other campuses were not available. The DBH values of trees from other campuses suggested that the plantations on the MDU campus were quite young (Islam, 2013; Yumnam & Dey, 2022). The DBH range (4.07–34.39 cm) in the present study was quite low as compared to trees at Cotton University (up to 142 cm) but was comparable to trees of Tripura University (around 35–40 cm). This indicates that the stems are thinner on the university campus. The H of trees ranged from 3.6 to 23.7 m. The tree H measurement data were not recorded by studies conducted in other universities. The H of the trees was quite similar to the trees of the University of Pennsylvania (Bassett, 2015). This huge variation in tree H may be due to differences in plantation timings and other management practices (Amoatey & Sulaiman, 2020). In terms of tree D (139.77 trees ha−1), the MDU campus fared better, except Agroforestry and Environmental Science Sher Bangla Agricultural University which had an exceptionally good D of 1096.88 tree ha−1 (Islam, 2013) and Cotton University (370 tree ha−1) (Yumnam & Dey, 2022). The total BA of the dominant tree species was 3.38 m2 ha−1. This value was very less in comparison with trees at Agroforestry and Environmental Science Sher Bangla Agricultural University which had a BA of 32.48 m2 ha−1. Contrary to this, the BA in Tripura University (1.95 m2 ha−1) and Pondicherry University (2.94 m2 ha−1) (Sundarapandian et al., 2014) was in agreement with our results. This was attributed to the lower DBH values of the trees on the campus. The individual diversity indices were not so significant for comparison and also, we did not find any such study on university campuses that has taken diversity indices into account. Therefore, we compared the total value of these indices with values from natural forests for validation. The tree dominance levels (2.50) and tree evenness (6.209) levels were lower than trees in Eastern Ghats in India with dominance and evenness of 3.87 and 10.75, respectively (Naidu & Kumar, 2016). The individual index values were used to study the relationship between tree diversity and C stock.
Factors affecting biomass and C accumulation in trees
The biomass and C stock in urban forests is mainly accumulated in the form of vegetation, litter, and SCS. We measured the biomass and C stock from two components, i.e., vegetation and soil. The TB (31.64 Mg C ha−1) and C stock (78.67 Mg C ha−1) in the present study were quite high as compared to Tripura University where the biomass storage capacity of the trees was around 11 Mg C ha−1 and the C stock was 5.36 Mg C ha−1 (Deb et al., 2016). The TCS values of the present study were faintly closer to C stocks at Jnana Bharathi Campus, Bangalore University (Kumar et al., 2015). Despite having smaller DBH, the trees of the MDU campus stored more biomass and C; this was probably due to the large number of trees on campus. The DBH of trees is the main factor that regulates C storage in trees. The tree biomass is determined using stem volume (measured using stem diameter), thus generally, the higher the DBH, the more the biomass and C content (Kumar et al. 2021a; Rizvi et al., 2011). This was true in the case of Eucalyptus globulus where the mean DBH and biomass values were highest. In this study, Azadirachta indica despite having the 2nd highest DBH (Table 1) had less mean TB (Table 2) as compared to Syzygium cumini (269.48 ± 268.63 Kg/tree) and Ficus virens (261.83 ± 280.28 Kg/tree). Although DBH has some sort of bearing on the TCS in PCA (Fig. 8), the correlation coefficient proved that there is no direct correlation between TCS and DBH (Fig. 7 and Online Resource 3). This anomaly was also due to a lower % of trees in higher diameter classes (Fig. 4). This reduced the overall DBH of the trees. Apart from DBH, the volumetric and biomass equations also greatly affect the C stocks. As mentioned above, certain tree species with more DBH and H had low C content; this was mainly attributed to the use of different equations for different trees (Manaye et al., 2019). In our study, as compared to DBH, tree H was more correlated with TCS (Fig. 7). Eucalyptus globulus species with an overall good tree H stored more C, and Polyalthia longifolia with the least H had the least amount of C stock. Various studies have shown a direct and positive relationship between H and TCS (Chauhan et al., 2020). Our results also flag a significant positive correlation between tree H and TCS (Figs. 7 and 8). The age of plantations is also a critical parameter that affects the C storage capacity of trees. A good tree age structure may help reduce the adverse effects of tree mortality from establishment and construction works (Millward & Sabir, 2010). The older stands tend to have more C as compared to the young stands (Martínez-Sánchez et al., 2015; Rana et al., 2020). Even though this was applicable in the case of Eucalyptus globulus which has the highest biomass and C accumulation (Table 2) but this hypothesis proved to be wrong in the case of some tree species like Callistemon lanceolatus (23 years old) and Polyalthia longfolia (16 years old), where TCS was less than 4 Mg C ha−1. This was confirmed with correlation (r) analysis (Fig. 7) and PCA (Fig. 8). The tree D is a key component that affects the overall TCS in the vegetation (Kumar et al. 2021b). Ficus benjamina regardless of less TB on a per-tree basis as compared to Syzygium cumini had more AGCS and BGCS due to a high tree D. A very high value of tree D of Eucalyptus globulus (55.35) helped to store the tree species around 7 times more C than Polyalthia longifolia (Table 2). Day et al. (2014) also demonstrated a linear and positive effect of stand D on TCS. Our study also confirmed this, as tree D was the 2nd most (1st being BA) strongly correlated tree variable with TCS (Fig. 7). Besides D, the tree BA is another major phytosociological parameter that controls the amount of C content in trees. While the BA itself is assessed from DBH but here the BA was calculated on a per-hectare basis where tree D came into play. This reduced the effect of DBH on TCS and increased the impact of the BA on TCS. The correlation level between BA and TCS was almost identical as in the case of stand D and TCS (Fig. 7). A similar trend was observed by Amoatey and Sulaiman (2020) and Nowak and Crane (2002) in urban trees of Oman and the USA. The selection effects (dominant species with special traits increase tree biomass) and complementary effects (diversified ecosystems with more species have different niche requirements that promote resource partitioning, which enhances resource utilization efficiency that ultimately increases productivity) are the two aspects of species richness that seems to affect the tree biomass (Cardinale et al., 2007; Kaushal & Baishya, 2021; Loreau & Hector, 2001). Our results also complement these findings, and correlation analysis proves diversity variable as a good predictor of tree biomass and C stock (Fig. 7). Of all the measured biodiversity indices, Simpon’s dominance index has a more pronounced effect on TCS (Fig. 7 and Online Resource 3). The majority of the investigations on SCS are limited to surface soils (up to 10 cm) only. The value of SCS in our study ranged from 0.87 Mg C ha−1 (30.1–40 cm) under Polyalthia longifolia to 13.19 Mg C ha−1 (up to 10 cm) under Dalbergia sissoo (Online Resource 2). In context with the upper range of SCS, these results were similar to SCS values obtained in the Tezpur University campus (Saha & Handique, 2022), Assam (India), and Pondicherry University (Sundarapandian et al., 2014), India, that had SCS values up to 15.24 Mg C ha−1 and 12.56 Mg C ha−1, respectively. The SCS followed a non-uniform pattern with an increase in soil depth. These results were contrary to results obtained by Kurien et al. (2021) and Subashree et al. (2019) in the Western Ghats (India) where there was a uniform decrease in SCS with an increase in soil sampling depth. This anomaly in our study could be linked to a high disturbance in the university campus in the form of routine uprooting and replanting of plants, water availability, and water permeability of the soil and other physical parameters of soil. Besides this tree litterfall, soil erosion and soil transportation also greatly affects the SCS values (de Nijs & Cammeraat, 2020). A comparison of C stocks in different universities is depicted in Table 4. The above-stated different phytosociological parameters affect the tree C stock in different ways, and this also supports the 2nd hypothesis of the study.
C sequestration potential, C credits, and Monetary value of trees
Urban vegetation was generally not considered for C cycle modeling and was considered a source of C emissions (Churkina et al., 2010; Tang et al., 2016). Regardless of the previous views on urban vegetation, our study demonstrated that urban plantations act as a C sink. The CO2-mitigating potential of trees is directly connected with the amount of C stored in them. The trees on the MDU campus sequester 19.05 Mg CO2 ha−1 year−1. This rate of CO2 sequestration was quite high as compared to street trees in Beijing (China) where the CO2 sequestration rate was only 0.5 Mg C ha−1 year−1 (Tang et al., 2016). The values obtained in the present study were closer to the urban trees of Philadelphia which sequestered 13.6 ± 0.2 Mg C ha−1 year−1.
The differences in CO2 sequestration rates may be due to mowing, precipitation, and growth rates (Nowak et al., 2013). The C credits were calculated from the CO2 sequestration rates of vegetation. These C credits can be a source of income for people, institutions, and countries (O’Donoghue & Shackleton, 2013). To the best of our knowledge, we could not find any study on university campuses that involved the concept of C credits, though some studies evaluated the monetary value of C stored by vegetation using other methods. The total value of these C credits (452,634.6 $ and 23,101.59 $ year−1) was quite high as compared to SIT campus, Fushun (China), where the monetary value for CO2 reduction was 128,360 $ (total) and 15,785 $ (annual). This huge difference was probably linked to the use of different methods, i.e., i-Tree eco application for C stock calculation (on SIT campus). Furthermore, the differences could also arise due to the use of the different monetary values of 1 C credit. On the other hand, the economic value of Retezat National Park in Romania (170,607 $ year−1) (Pache et al., 2020) was quite high compared to the MDU campus. This was attributed to very high rates of CO2 sequestration.
It is worthwhile, to note that in the present study, Eucalyptus globulus outcompeted other tree species in almost all the quantifiable parameters. This is due to several factors like a very high growth rate, very long H, and branching pattern initiating from a greater vertical distance, thus making it less vulnerable to pruning. Though, Dalbergia sissoo is also a fast-growing tree species and could contribute more to reducing CO2 with more efficiency on the campus. Since Dalbergia sissoo could not make it into the top 3 species with the highest contribution towards C stock, this indicates poor management practices. A similar study reported the same trend due to lower DBH values (Deve & Parthiban, 2014). Azadirachta indica is known to store huge amounts of C (Times of India, 2016), but in this study, it occupied the 5th spot in terms of C storage capacity. A couple of factors like the location of this species along the walking paths (more anthropogenic disturbance) and less soil C stock may have reduced the magnitude of C stocks. The other younger plantations of Syzygium cumini and Ficus virens have greater potential to sequester more C and provide more ecosystem services on the campus.
Although tree D on the campus is moderate, this is mainly contributed by Eucalyptus globulus plantations. Increasing the D of other tree species with higher C-capturing capacity such as Azadirachta indica and Dalbergia sissoo may enhance the overall capacity of campus trees to sequester C. In addition to this, management practices like extending the rotation period of stands (reduces soil disturbance), preferring native trees for plantation (apart from C sequestration, native trees also provide numerous ecosystem services), cutting dead trees into pieces (rather than letting them decay) for sustainable use, and harvesting during the winter season (instead of rainy season) may aid in amplifying C stocks on campus.
Limitations of the study
We did not estimate the C stock values for shrubs and herbs. This is one of the limitations of the present study. This was done because around 90% of the biomass is stored in trees (Singh et al., 2011). Furthermore, regular pruning and trimming of grasses and shrubs lead to enormous loss of biomass. We planted litter traps under these tree species in January 2020 for the collection of litterfall on a monthly basis. Yet, we could only collect the litterfall for January and February 2020. This was because of the implementation of a nationwide lockdown due to COVID-19. So, this is also one of the two limitations of this study.
Conclusions and recommendations
Our study assessed the C stocks, C sequestration rates, and monetary worth of the top 10 dominant tree species on the MDU campus. The campus is overly dependent on Eucalyptus globulus species which may cause tree management issues. All the trees on the campus are still young (< 25 years) and have lower DBH and BA. Tree D (139.77 ha−1) on the campus is moderately high, and tree diversity lies in the lower to moderate range of the diversity spectrum. A total of 78.67 Mg C ha−1 C is stored in trees on the MDU campus. Trees like Eucalyptus globulus with a larger diameter and BA stored around 20% of the total tree C on the campus. Tree BA, D, and diversity positively influenced the tree C stocks. The top 10 tree species sequestered 19.05 Mg CO2 ha−1 year−1 and have 32,331.04 C credits from inception. These trees also supply 1663.58 C credits annually. Based on the value of these C credits, campus trees sequestered CO2 that has a net worth of 452,634.60 $ in the international market. Additionally, the campus can earn 23,101.59 $ annually by selling the C credits. To maintain or enhance the rates of CO2 sequestration, a tree database can be maintained on the campus. This database should primarily address tree mortality as trees are planted in huge numbers but are not looked after. This can be achieved with proper tree management practices that are species-specific. From our analysis, we suggest that these management practices can radically enhance the C stocks in Dalbergia sissoo. Furthermore, the results from this study will help this green and clean campus in better management of trees and take a step ahead in its mission of environmental sustainability. Additionally, the onsite composting of the plant litter will have dual benefits in the form of reduced methane emissions and a clean campus. This will also help the university campus to manage some of its finances and take part in C trading in the near future. The results obtained herein could also help educational institutions particularly in the Global South (low- and middle-income countries of the world) for preliminary modeling of C sequestration rates and C neutrality pathways.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Change history
11 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10661-023-11994-0
Abbreviations
- CO2 :
-
Carbon dioxide
- TOF:
-
Trees outside forests
- MDU:
-
Maharshi Dayanand University
- DBH:
-
Diameter at breast height
- Mg C ha−1 :
-
Megagram carbon per hectare
- Mg CO2 ha−1 year−1 :
-
Megagram carbon dioxide per hectare
- C:
-
Carbon
- CDM:
-
Clean Development Mechanism
- CER:
-
Certified emission reduction
- TOFI:
-
Trees outside forests in India
- NCR:
-
National Capital Region
- SOC %:
-
Soil organic carbon %
- CBH:
-
Circumference at breast height
- H:
-
Height
- BA:
-
Basal area
- BD:
-
Bulk density
- D:
-
Density
- TSWD:
-
Tree-specific wood density
- BEF:
-
Biomass expansion factor
- AGB:
-
Above-ground biomass
- BGB:
-
Below-ground biomass
- TB:
-
Total biomass
- PCA:
-
Principal component analysis
- TCS:
-
Total carbon stock
- SCS:
-
Soil carbon stock
- HCA:
-
Hierarchical cluster analysis
- AGCS:
-
Above-ground carbon stock
- BGCS:
-
Below-ground carbon stock
References
Agbelade, A. D., & Onyekwelu, J. C. (2020). Tree species diversity, volume yield, biomass and carbon sequestration in urban forests in two Nigerian cities. Urban Ecosystems, 23(5), 957–970. https://doi.org/10.1007/s11252-020-00994-4
Amoatey, P., & Sulaiman, H. (2020). Quantifying carbon storage potential of urban plantations and landscapes in Muscat, Oman. Environment, Development and Sustainability, 22(8), 7969–7984. https://doi.org/10.1007/s10668-019-00556-5
Appendix 1 - List of wood densities for tree species from tropical America, Africa, and Asia. (1997). https://www.fao.org/3/w4095e/w4095e0c.htm. Accessed 13 October 2022
Arborday. (2022). Tree Campus Higher Education at arborday.org. https://www.arborday.org/programs/tree-campus-higher-education/. Accessed 25 December 2022
Bassett, C. G. (2015). The environmental benefits of trees on an urban university campus. Master of Environmental Studies Capstone Projects., 66.
Bayat, A. T., van Gils, H., & Weir, M. (2012). Carbon stock of European beech forest; a case at m. Pizzalto, Italy. APCBEE Procedia, 1, 159–168.
Brown, S., & Lugo, A. E. (1992). Aboveground biomass estimates for tropical moist forests of the Brazilian Amazon. Interciencia Caracas, 17(1), 8–18.
Byrd, K. B., Ballanti, L., Thomas, N., Nguyen, D., Holmquist, J. R., Simard, M., & Windham-Myers, L. (2018). A remote sensing-based model of tidal marsh aboveground carbon stocks for the conterminous United States. ISPRS Journal of Photogrammetry and Remote Sensing, 139, 255–271.
Cairns, M. A., Brown, S., Helmer, E. H., & Baumgardner, G. A. (1997). Root biomass allocation in the world’s upland forests. Oecologia, 111(1), 1–11.
Cardinale, B. J., Wright, J. P., Cadotte, M. W., Carroll, I. T., Hector, A., Srivastava, D. S., et al. (2007). Impacts of plant diversity on biomass production increase through time because of species complementarity. Proceedings of the National Academy of Sciences, 104(46), 18123–18128. https://doi.org/10.1073/pnas.0709069104
Central Ground Water Board, Ministry of Water Resources, RD & GR Government of India. (2013). https://cgwb.gov.in/AQM/Haryana%20Report.html. Accessed 30 May 2023
Chandra, I. A., Seca, G., & Hena, M. A. (2011). Aboveground biomass production of Rhizophora apiculata Blume in Sarawak mangrove forest. American Journal of Agricultural and Biological Sciences, 6(4), 469–474. https://doi.org/10.3844/ajabssp.2011.469.474
Chaturvedi, R. K., Raghubanshi, A. S., & Singh, J. S. (2012). Biomass estimation of dry tropical woody species at juvenile stage. The Scientific World Journal, 2012. https://doi.org/10.1100/2012/790219
Chauhan, M., Kumar, M., & Kumar, A. (2020). Impact of carbon stocks of Anogeissus latifolia on climate Change and socioeconomic development: A case study of Garhwal Himalaya, India. Water, Air, & Soil Pollution, 231(8), 436. https://doi.org/10.1007/s11270-020-04803-8
Chave, J., Andalo, C., Brown, S., Cairns, M. A., Chambers, J. Q., Eamus, D., et al. (2005). Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia, 145(1), 87–99. https://doi.org/10.1007/s00442-005-0100-x
Churkina, G., Brown, D. G., & Keoleian, G. (2010). Carbon stored in human settlements: The conterminous United States. Global Change Biology, 16(1), 135–143. https://doi.org/10.1111/j.1365-2486.2009.02002.x
ClimateTrade. (2022, June 16). What influences carbon offset pricing? ClimateTrade. https://climatetrade.com/what-influences-carbon-offset-pricing/. Accessed 14 October 2022
Colding, J., & Barthel, S. (2017). The role of university campuses in reconnecting humans to the biosphere. Sustainability, 9(12), 2349. https://doi.org/10.3390/su9122349
Daily CO2. (2022). Daily CO2. https://www.co2.earth/daily-co2. Accessed 8 November 2022
Day, M., Baldauf, C., Rutishauser, E., & Sunderland, T. C. (2014). Relationships between tree species diversity and above-ground biomass in Central African rainforests: Implications for REDD. Environmental Conservation, 41(1), 64–72. https://doi.org/10.1017/S0376892913000295
de Nijs, E. A., & Cammeraat, E. L. (2020). The stability and fate of soil organic carbon during the transport phase of soil erosion. Earth-Science Reviews, 201, 103067. https://doi.org/10.1016/j.earscirev.2019.103067
Deb, D., Deb, S., Debbarma, J., & Datta, B. K. (2016). Tree species richness and carbon stock in Tripura University Campus, Northeast India. Journal of Biodiversity Management & Forestry, 5(4), 1–7. https://doi.org/10.4172/2327-4417.1000167
Deve, K. D., & Parthiban, K. T. (2014). Growth performance and variability of Dalbergia sissoo Roxb. clones. Electronic Journal of. Plant Breeding, 5(2), 317–321.
Dobbs, C., Nitschke, C., & Kendal, D. (2017). Assessing the drivers shaping global patterns of urban vegetation landscape structure. Science of the Total Environment, 592, 171–177. https://doi.org/10.1016/j.scitotenv.2017.03.058
Economic Times. (2023). India can achieve its goal of being carbon neutral much before its target of 2070: IMF MD, ET EnergyWorld. https://energy.economictimes.indiatimes.com/news/renewable/india-can-achieve-its-goal-of-being-carbon-neutral-much-before-its-target-of-2070-imf-md/98152360. Accessed 30 May 2023
Eggleston, S., Buendia, L., Miwa, K., Ngara, T., & Tanabe, K. (2006). IPCC guidelines for national greenhouse gas inventories.
FSI. (2021, January 13). Forest survey of India . India State of Forest Report... - Google Scholar. https://scholar.google.com/scholar?q=Forest%20Survey%20of%20India%20.%20India%20State%20of%20Forest%20Report%202017;%20Ministry%20of%20Environment%20and%20Forests:%20Dehradun,%20India.%20http:fsi.nic.inisfr2017isfr-forest-cover-2017.pdf%20. Accessed 13 January 2021
Grumbine, R. E. (2014). Assessing environmental security in China. Frontiers in Ecology and the Environment, 12(7), 403–411. https://doi.org/10.1890/130147
Islam, M. S. (2013). Estimation of biomass carbon stock at three plantation sites in Sher-e-Bangla Agricultural University campus (PhD Thesis). Dept. of Agroforestry & Environmental Science.
Kaushal, S., & Baishya, R. (2021). Stand structure and species diversity regulate biomass carbon stock under major Central Himalayan forest types of India. Ecological Processes, 10(1), 1–18. https://doi.org/10.1186/s13717-021-00283-8
Kokkora, M. I., Vrahnakis, M., & Kleftoyanni, V. (2022). Soil quality characteristics of traditional agroforestry systems in Mouzaki area, central Greece. Agroforestry Systems, 1–15. https://doi.org/10.1007/s10457-022-00746-7
Kumar, A., Tewari, S., Singh, H., Kumar, P., Kumar, N., Bisth, S., et al. (2021a). Biomass accumulation and carbon stock in different agroforestry systems prevalent in the Himalayan foothills, India. Current Science, 120(6), 1083–1088.
Kumar, M., Nandini, N., Vijay Kumar, M., & Raghavendra, M. (2015). Urbanization and green spaces—a study on Jnana Bharathi Campus, Bangalore University. In W. Leal Filho, N. Muthu, G. Edwin, & M. Sima (Eds.), Implementing Campus Greening Initiatives: Approaches, Methods and Perspectives (pp. 351–358). Springer International Publishing. https://doi.org/10.1007/978-3-319-11961-8_26
Kumar, M., Kumar, A., Kumar, R., Konsam, B., Pala, N. A., & Bhat, J. A. (2021b). Carbon stock potential in Pinus roxburghii forests of Indian Himalayan regions. Environment, Development and Sustainability, 23(8), 12463–12478. https://doi.org/10.1007/s10668-020-01178-y
Kurien, V. T., Thomas, E., Prasanth Narayanan, S., & Thomas, A. P. (2021). Soil organic carbon pool under selected tree plantations in the Southern Western Ghats of Kerala, India. Tropical Ecology, 62(1), 126–138. https://doi.org/10.1007/s42965-020-00137-y
Lavista, L., Prasetyo, L. B., & Hermawan, R. (2016). Dynamics change of the above carbon stocks in Bogor Agricultural University, Darmaga campus. Procedia Environmental Sciences, 33, 305–316. https://doi.org/10.1016/j.proenv.2016.03.081
Liu, C., & Li, X. (2012). Carbon storage and sequestration by urban forests in Shenyang, China. Urban Forestry & Urban Greening, 11(2), 121–128. https://doi.org/10.1016/j.ufug.2011.03.002
Liu, Q., Zhang, Y., Lin, Y., You, D., Zhang, W., Huang, Q., et al. (2018). The relationship between self-rated naturalness of university green space and students’ restoration and health. Urban Forestry & Urban Greening, 34, 259–268. https://doi.org/10.1016/j.ufug.2018.07.008
Long, W., Zang, R., Ding, Y., & Huang, Y. (2013). Effects of competition and facilitation on species assemblage in two types of tropical cloud forest. PLoS One, 8(4), e60252. https://doi.org/10.1371/journal.pone.0060252
Loreau, M., & Hector, A. (2001). Partitioning selection and complementarity in biodiversity experiments. Nature, 412(6842), 72–76. https://doi.org/10.1038/35083573
Manaye, A., Negash, M., & Alebachew, M. (2019). Effect of degraded land rehabilitation on carbon stocks and biodiversity in semi-arid region of Northern Ethiopia. Forest Science and Technology, 15(2), 70–79. https://doi.org/10.1080/21580103.2019.1592787
Martin, N. A., Chappelka, A. H., Loewenstein, E. F., & Keever, G. J. (2012). Comparison of carbon storage, carbon sequestration, and air pollution removal by protected and maintained urban forests in Alabama, USA. International Journal of Biodiversity Science, Ecosystem Services & Management, 8(3), 265–272. https://doi.org/10.1080/21513732.2012.712550
Martínez-Sánchez, J. L., Tigar, B. J., Cámara, L., & Castillo, O. (2015). Relationship between structural diversity and carbon stocks in humid and sub-humid tropical forest of Mexico. Écoscience, 22(2–4), 125–131. https://doi.org/10.1080/11956860.2016.1169384
Millward, A. A., & Sabir, S. (2010). Structure of a forested urban park: Implications for strategic management. Journal of Environmental Management, 91(11), 2215–2224. https://doi.org/10.1016/j.jenvman.2010.06.006
Mohamed, M. B., Rao, G. R., Keerthika, A., Gupta, D. K., & Shukla, A. K. (2018). Allometric relationships for biomass and carbon estimation of neem (Azadirachta indica A. Juss) plantations in dryland of Hyderabad, Telangana. International Journal of Bio-resource and Stress Management, 9(1), 37–43. https://doi.org/10.23910/ijbsm/2018.9.1.3c0526a
Naidu, M. T., & Kumar, O. A. (2016). Tree diversity, stand structure, and community composition of tropical forests in Eastern Ghats of Andhra Pradesh, India. Journal of Asia-Pacific Biodiversity, 9(3), 328–334. https://doi.org/10.1016/j.japb.2016.03.019
Nandal, A., Singh, N., Yadav, S. S., Rao, A. S., & Yadav, V. S. (2019). Carbon stock assessment of selected tree species in Maharshi Dayanand University Campus, Rohtak (Haryana) India. Indian Journal of Ecology, 46(2).
Nandal, A., Yadav, S. S., Khuroo, A. A., Rao, A. S., Singh, N., & Chhikara, A. (2022). Assessing diversity and ecosystem services of trees in educational institutions: A case study of a university campus from the Global South. Arboricultural Journal. https://doi.org/10.1080/03071375.2022.2092349
Nandal, A., Yadav, S. S., Rao, A. S., Meena, R. S., & Lal, R. (2023). Advance methodological approaches for carbon stock estimation in forest ecosystems. Environmental Monitoring and Assessment, 195(2), 315. https://doi.org/10.1007/s10661-022-10898-9
Narayana, J., Shashidhar, N., & A., & Savinaya, M. S. (2020). Carbon Sequestration Potential of Trees in Kuvempu University Campus Forest Area, Western Ghats, Karnataka. In N. Roy, S. Roychoudhury, S. Nautiyal, S. K. Agarwal, & S. Baksi (Eds.), Socio-economic and eco-biological dimensions in resource use and conservation: Strategies for sustainability (pp. 303–312). Springer International Publishing. https://doi.org/10.1007/978-3-030-32463-6_15
Nowak, D. J., & Crane, D. E. (2002). Carbon storage and sequestration by urban trees in the USA. Environmental Pollution, 116(3), 381–389. https://doi.org/10.1016/S0269-7491(01)00214-7
Nowak, D. J., Greenfield, E. J., Hoehn, R. E., & Lapoint, E. (2013). Carbon storage and sequestration by trees in urban and community areas of the United States. Environmental Pollution, 178, 229–236. https://doi.org/10.1016/j.envpol.2013.03.019
O’Donoghue, A., & Shackleton, C. M. (2013). Current and potential carbon stocks of trees in urban parking lots in towns of the Eastern Cape, South Africa. Urban Forestry & Urban Greening, 12(4), 443–449. https://doi.org/10.1016/j.ufug.2013.07.001
Pache, R.-G., Abrudan, I. V., & Niță, M.-D. (2020). Economic valuation of carbon storage and sequestration in Retezat National Park, Romania. Forests, 12(1), 43. https://doi.org/10.3390/f12010043
Pan, Y., Birdsey, R. A., Fang, J., Houghton, R., Kauppi, P. E., Kurz, W. A., et al. (2011). A large and persistent carbon sink in the world’s forests. Science, 333(6045), 988–993. https://doi.org/10.1126/science.1201609
Pielou, E. C. (1975). Ecological diversity New York. Wiley.
Rana, K., Kumar, M., & Kumar, A. (2020). Assessment of annual shoot biomass and carbon storage potential of Grewia optiva: An approach to combat climate change in Garhwal Himalaya. Water, Air, & Soil Pollution, 231(9), 450. https://doi.org/10.1007/s11270-020-04825-2
Ravindranath, N. H., & Ostwald, M. (2007). Carbon inventory methods: Handbook for greenhouse gas inventory, carbon mitigation and roundwood production projects (Vol. 29). Springer Science & Business Media.
Richter, S., Haase, D., Thestorf, K., & Makki, M. (2020). Carbon pools of Berlin, Germany: Organic carbon in soils and aboveground in trees. Urban Forestry & Urban Greening, 54, 126777. https://doi.org/10.1016/j.ufug.2020.126777
Ritchie, Y. (2017). Investigating the carbon sequestration and storage capacity of trees in a university campus environment. Earth and Environmental Sciences Undergraduate Honours Theses. http://hdl.handle.net/10222/76521
Rizvi, R. H., Dhyani, S. K., Yadav, R. S., & Singh, R. (2011). Biomass production and carbon stock of poplar agroforestry systems in Yamunanagar and Saharanpur districts of northwestern India. Current Science, 736–742.
Robinson, O. J., Tewkesbury, A., Kemp, S., & Williams, I. D. (2018). Towards a universal carbon footprint standard: A case study of carbon management at universities. Journal of Cleaner Production, 172, 4435–4455. https://doi.org/10.1016/j.jclepro.2017.02.147
Saatchi, S., Marlier, M., Chazdon, R. L., Clark, D. B., & Russell, A. E. (2011). Impact of spatial variability of tropical forest structure on radar estimation of aboveground biomass. Remote Sensing of Environment, 115(11), 2836–2849. https://doi.org/10.1016/j.rse.2010.07.015
Saha, A., & Handique, S. (2022). Variation of soil organic carbon in a university campus in temperate north-eastern India. Environmental Challenges, 7, 100493. https://doi.org/10.1016/j.envc.2022.100493
Salunkhe, O., Khare, P. K., Sahu, T. R., & Singh, S. (2014). Above ground biomass and carbon stocking in tropical deciduous forests of state of Madhya Pradesh, India. Taiwania, 59(4). https://doi.org/10.6165/tai.2014.59.4.353
Savageau, A. E. (2013). Let’s get personal: Making sustainability tangible to students. International Journal of Sustainability in Higher Education, 14(1), 15–24. https://doi.org/10.1108/14676371311288921
Sharma, R., Pradhan, L., Kumari, M., & Bhattacharya, P. (2020). Assessment of carbon sequestration potential of tree species in amity university campus Noida. Environmental Sciences Proceedings, 3(1), 52. https://doi.org/10.3390/IECF2020-08075
Simpson, E. H. (1949). Measurement of diversity. Nature, 163(4148), 688–688. https://doi.org/10.1038/163688a0
Singh, V., Tewari, A., Kushwaha, S. P., & Dadhwal, V. K. (2011). Formulating allometric equations for estimating biomass and carbon stock in small diameter trees. Forest Ecology and Management, 261(11), 1945–1949. https://doi.org/10.1016/j.foreco.2011.02.019
Somvichian-Clausen, A. (2020, November 19). These are the 10 countries most at risk from the effects of climate change. The Hill. Text. https://thehill.com/changing-america/sustainability/climate-change/526684-these-are-the-10-countries-most-at-risk-from/. Accessed 7 November 2022
Subashree, K., Dar, J. A., & Sundarapandian, S. (2019). Variation in soil organic carbon stock with forest type in tropical forests of Kanyakumari Wildlife Sanctuary, Western Ghats, India. Environmental Monitoring and Assessment, 191(11), 690. https://doi.org/10.1007/s10661-019-7881-6
Sundarapandian, S. M., Amritha, S., Gowsalya, L., Kayathri, P., Thamizharasi, M., Dar, J. A., et al. (2014). Biomass and carbon stock assessments of woody vegetation in Pondicherry University campus, Puducherry. International Journal of Environmental Biology, 4(2), 87–99.
Tamang, B., Pala, N., Sarkar, B., Shukla, G. V., & Chakravarty, S. (2019). Wood specific gravity of some tree species in sub-tropical humid climate in India. Indian Forester, 146.
Tamang, B., Pala, N. A., Shukla, G., Rashid, M., Rather, M. M., Bhat, J. A., et al. (2021). Trees outside forest (TOFs) aids in mitigating global climatic change through carbon sequestration: Example from academic institutional landscapes. Acta Ecologica Sinica, 41(4), 351–357. https://doi.org/10.1016/j.chnaes.2021.06.007
Tang, Y., Chen, A., & Zhao, S. (2016). Carbon storage and sequestration of urban street trees in Beijing, China. Frontiers in Ecology and Evolution, 4. https://doi.org/10.3389/fevo.2016.00053 Accessed 7 November 2022.
The World Bank. (2022). Overview. World Bank. Text/HTML. https://www.worldbank.org/en/topic/urbandevelopment/overview. Accessed 7 November 2022
Times of India. (2016). Teak absorbs max CO2 from air, helps check global warming | Ahmedabad News - Times of India. https://timesofindia.indiatimes.com/city/ahmedabad/Teak-absorbs-max-CO2-from-air-helps-check-global-warming/articleshow/51721842.cms. Accessed 24 January 2021
Tiyarattanachai, R., & Hollmann, N. M. (2016). Green campus initiative and its impacts on quality of life of stakeholders in green and non-green campus universities. SpringerPlus, 5(1), 84. https://doi.org/10.1186/s40064-016-1697-4
Varón-Hoyos, M., Osorio-Tejada, J., & Morales-Pinzón, T. (2021). Carbon footprint of a university campus from Colombia. Carbon Management, 12(1), 93–107. https://doi.org/10.1080/17583004.2021.1876531
Volume Equations for Forests of India, Nepal, and Bhutan. (1996). Forest Survey of India. Ministry of Environment & Forests, Government of India.
Walkley, A., & Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Science, 37(2). https://doi.org/10.1097/00010694-193401000-00003
Wang, X., Wang, Y., Qu, X., Huang, B., Li, Z., Sun, J., et al. (2021). Urban trees in university campus: Structure, function, and ecological values. Environmental Science and Pollution Research, 1–16. https://doi.org/10.1007/s11356-021-13841-6
Weaver, W., & Shannon, C. E. (1963). The mathematical theory of communication. University of Illinois Press Urbana.
Wibowo, A., Yussof, M. M., Hamzah, T. A. A., & Salleh, K. O. (2019). Urban heat signature impact on university campus. In IOP Conference Series: Earth and Environmental Science (Vol. 338, p. 012027). IOP Publishing.
World Weather Online. (2023). World Weather Online. WorldWeatherOnline.com. https://www.worldweatheronline.com/rohtak-weather/haryana/in.aspx. Accessed 29 May 2023
Xie, Q., Yue, Y., Sun, Q., Chen, S., Lee, S.-B., & Kim, S. W. (2019). Assessment of ecosystem service values of urban parks in improving air quality: A case study of Wuhan, China. Sustainability, 11(22), 6519. https://doi.org/10.3390/su11226519
Yang, C., Zhang, C., Li, Q., Liu, H., Gao, W., Shi, T., et al. (2020). Rapid urbanization and policy variation greatly drive ecological quality evolution in Guangdong-Hong Kong-Macau Greater Bay Area of China: A remote sensing perspective. Ecological Indicators, 115, 106373. https://doi.org/10.1016/j.ecolind.2020.106373
Yumnam, J. Y., & Dey, N. (2022). Biomass and carbon stock of trees growing in Cotton University, Guwahati, Assam, India. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 1–7. https://doi.org/10.1007/s40011-022-01365-z
Zhu, W., Egitto, B. A., Yesilonis, I. D., & Pouyat, R. V. (2017). Soil carbon and nitrogen cycling and ecosystem service in cities. In Urban soils (pp. 121–136). CRC Press.
Acknowledgements
The first author would like to thank the Council of Scientific & Industrial Research (CSIR), New Delhi, for financial help. The corresponding author is thankful to the University Grants Commission (UGC), New Delhi, SERB-DST, Govt. of India, New Delhi, DST-FIST, New Delhi, and Haryana State Council for Science and Technology (HSCST) for the financial support.
Author information
Authors and Affiliations
Contributions
Abhishek Nandal: conceptualization, investigation, data curation, and written original draft. Surender Singh Yadav: conceptualization, supervision, validation, and review and editing. Arun Jyoti Nath: conceptualization and review and editing.
Corresponding author
Ethics declarations
Ethical approval
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and are aware that with minor exceptions; no changes can be made to authorship once the paper is submitted.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nandal, A., Yadav, S.S. & Nath, A.J. Trees outside forests as climate change mitigation champions: evaluating their carbon sequestration potential and monetary value in Maharshi Dayanand University, Rohtak (Haryana), India. Environ Monit Assess 195, 995 (2023). https://doi.org/10.1007/s10661-023-11597-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11597-9