Abstract
In order to eliminate the effects of seawater matrix on the precise/accurate determination of elements, new and efficient analytical procedure requires. In this study, co-precipitation method based on the triethylamine (TEA)-assisted Mg(OH)2 was performed to eliminate side-effects of seawater medium on the determination with flame atomic absorption spectrometry (FAAS) prior to the preconcentration of nickel by an optimized dispersive liquid–liquid microextraction (DLLME) method. Under the optimum conditions of the presented method, the limit of detection and quantification (LOD, LOQ) values obtained for nickel were found as 16.1 and 53.8 μg kg−1, respectively. Seawater samples collected from West Antarctic region were used for real sample applications to check the accuracy and applicability of developed method, and satisfying recovery results (86–97%) were obtained. In addition to this, the digital image-based colorimetric detection system and the UV-Vis system were applied to confirm the applicability of the developed DLLME-FAAS method in other analytical systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals with an atomic mass of more than 20 and a specific gravity of more than 5 g cm−3 are called environmental pollutants that pose a serious threat to human health, agricultural production, plants, and animals (Famurewa et al., 2022; Rascio & Navari-Izzo, 2011). Contamination levels of these toxic heavy metals such as cadmium (Cd), lead (Pb), cobalt (Co), arsenic (As), mercury (Hg), copper (Cu), and nickel (Ni) in agricultural soils have increased in the last decade (Elango et al., 2022). Fossil fuels, pesticides, mining, wastewater irrigation, and the usage of fertilizer are known as sources of heavy metal contamination (He et al., 2022a; Wu et al., 2021). Heavy metals that accumulate in surface/ground waters and soil by migrating to the environment through wind erosion and flow do not decompose and enter the food chain through food consumption and are easily enriched in the human body by creating health-threatening effects (He et al., 2022b; Kumar et al., 2019). Nickel is naturally found in the earth’s crust and is commonly used in the form of batteries, electroplating coating, paint, electronic products, electric automobile, mining, coin, and aerospace industries due to its properties such as high durability, low price, corrosion resistance, and high activity (Alves et al., 2022; Bodkhe et al., 2023; Min et al., 2021; Padchasri et al., 2022). Nickel as a hard, silvery-white transition metal is an essential micronutrient for humans and has a great importance for organ development (Anyachor et al., 2022; Bodkhe et al., 2023; Kumar et al., 2021). In addition, many foods such as broccoli, canned vegetables, green beans, peas, nuts, dried fruit, cocoa, and chocolate contain nickel as ingredients (Zambelli et al., 2016). Nickel deficiency can reduce growth and decrease hemoglobin levels, in the opposite side, excessive consumption and exposure to nickel can cause harmful, carcinogenic and toxic effects resulting in lung cancer and inflammation, dysfunction of the central nervous and respiratory systems, acute pneumonitis, cardiovascular and kidney diseases, dermatitis, and eczema (Bodkhe et al., 2023; Denkhaus & Salnikow, 2002; Miglione et al., 2022; Pieczyńska et al., 2021). Nickel is known to have toxic effects on the respiratory system in fish and invertebrates (Macoustra et al., 2021). The potential of nickel toxicity is concerning in seawater due to mining activities in coastal areas, and therefore, the determination of trace levels of nickel is quite important (Sherman et al., 2021).
The determination of nickel in water samples is commonly performed by the usage of electrothermal AAS (ETAAS) and inductively coupled plasma-mass spectrometry (ICP-MS) (Khorrami et al., 2004). In the literature, flame atomic absorption spectrometry (FAAS) (Nakhaei et al., 2019), ETAAS (Bermejo-Barrera et al., 1998), ICP-optical emission spectrometry (ICP-OES) (Khorrami et al., 2006), ICP-MS (Biller & Bruland, 2012), adsorptive cathodic stripping voltammetry (AdCSV) (Aouarram et al., 2010), and also colorimetry (Yoshimura et al., 1980) have been used for the determination of nickel in seawater samples.
High salinity of sea and ocean waters can interfere with low concentration signals of heavy metal ions (Gilbert et al., 2023; Karadaş & Kara, 2013). Nickel is usually found at trace levels in surface waters with a maximum concentration of 100 µg L−1 (Ribas et al., 2022). The sample should be pretreated to avoid matrix effects and salt deposition/precipitation. Accordingly, different extraction and coprecipitation methods can be applied to improve the detection limit and increase sensitivity (Milne et al., 2010). Co-precipitation methods are used to pre-concentrate trace analytes and remove salt interference from ocean/sea water. For this purpose, various solutions such as gallium hydroxide (Ga(III)) (Sawatari et al., 1995), yttrium hydroxide (Y(III)) (Kagaya et al., 2007), lanthanum hydroxide (La(III)) (Toyota et al., 1983), iron hydroxide (Fe(III)) (Zhu et al., 2010), and magnesium hydroxide (Mg(II)) (Wu, 2007; Wu & Boyle, 1998) have been used in the literature. In addition, several extraction methods have been used in the literature for the pre-concentration of nickel prior to instrumental analysis such as solid-phase extraction (SPE) (Otero-Romaní et al., 2008), cloud point extraction (CPE) (Mandal & Lahiri, 2022; Zeng et al., 2012), solidified floating organic drop microextraction (SFODME) (Bidabadi et al., 2009), and deep eutectic solvent-based liquid phase microextraction (DES-LPME) (Erbas et al., 2019). In recent years, DLLME has become very popular because it provides many advantages such as cheapness, rapid application, simplicity, environmentally friendly, and high enrichment factor (Rykowska et al., 2018). The basis of the procedure consists of the formation of clouding throughout the aqueous solution with the help of injection. A mixture of extraction/dispersive solvent is rapidly injected into the aqueous phase. In this system, the disperser solvent should have high miscibility in both the aqueous and organic phase (Anthemidis & Ioannou, 2009).
The aim of this study was to develop a simple, cheap, efficient, and environmentally friendly analytical method for the accurate and sensitive determination of nickel in sea/ocean water samples. In the study, ocean water samples collected from Horseshoe Island in the Antarctic Continent throughout IV. National Antarctic Science Expedition were used. Trace levels of nickel were determined in the collected ocean water samples by FAAS using the Mg(OH)2/TEA co-precipitation method prior to dispersive liquid–liquid microextraction method (DLLME) method. The analytical performance results obtained with the digital image-based colorimetric detection system and the UV-VIS system are compared with the developed DLLME-FAAS method.
Materials and methods
Instrumentation
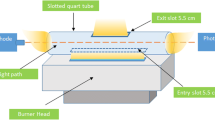
ATI UNICAM 929 AA model flame atomic absorption spectrometer was used to perform nickel absorbance measurements. All optimization studies belonging to combination of DLLME and TEA-assisted Mg(OH)2 method were carried out with 1.0 mg kg−1 nickel standard solution. Mass-based sample/standard preparation was used throughout this study. The flame is created using a combination of air and acetylene. The flow rate of the nebulizer unit was set at 8.56 mL min−1 to ensure strong sample aspiration. A deuterium (D2) lamp as background corrector was used throughout all absorbance measurements. Absorbance measurements were performed using a nickel hollow cathode lamp with a spectral bandpass of 0.20 nm and an analytical wavelength of 232.0 nm. A Hanna Instruments Edge® Multiparameter pH Meter–HI2020 was used for pH adjustment and measurements. BioBase (BKC TL5II) centrifuge device was used to direct the precipitates formed in the precipitation process to the lower part of the centrifuge tube and collect it there. Absorbance measurements of the pink-colored metal-ligand complex were performed with a Shimadzu UV120–02 model UV-Vis spectrophotometer using 1.0 cm quartz cells. Images in colorimetric measurement were taken with an Apple smartphone equipped with an 8 megapixel iSight camera with the flash lamp turned off. The smartphone is placed in front of the portable wooden rectangular box insulated from ambient light, and a recess is created in the front of the box where the smartphone’s camera is placed to view the inner box. A 1.5 m bright LED strip (12 V) is placed to the upper inside part of the box and white A4 size sheets are used placed to cover the LEDs. Another white A4 size paper was placed to the bottom part of the box and quartz cuvette including the sample/standard solution was inserted to this paper. “Color Picker” application was used to process the images. Schematic representation of the colorimetric system is given in Fig. 1.
Chemical and reagents
A total of 1000 mg L−1 nickel standard stock solution was used throughout the study and diluted with distilled water to lower concentrations. TEA (triethylamine, 99.7%) used as a co-precipitating agent was supplied from Acros Organics (Geel—Belgium). Magnesium chloride (MgCl2), potassium chloride (KCl), calcium chloride (CaCl2), sodium chloride (NaCl), sodium sulfate (Na2SO4), sodium bicarbonate (NaHCO3), boric acid (H3BO3), potassium bromide (KBr), and sodium fluoride (NaF) used to prepare synthetic seawater were obtained from Sigma-Aldrich (St. Louis, MO, USA). Hydrochloric acid (37%) used to adjust pH, disodium tetraborate used for buffering, ethanol, nitric acid, and diphenylcarbazone (DPC) were purchased from Merck. The ligand solution prepared in ethanol was stored in a tube covered with aluminum foil in the dark in order to prevent its degradation by exposure to light.
Preparation of synthetic seawater and co-precipitation method
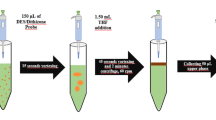
Synthetic seawater used within the scope of analytical method development studies was prepared with reference to ASTM D1141-98 guideline (Singh et al., 2020). In this context with small modifications, 24.53 g NaCl, 5.20 g MgCl2, 0.101 g KBr, 4.09 g Na2SO4, 0.201 g NaHCO3, 1.16 g CaCl2, 0.695 g KCl, 0.027 g H3BO3, and 0.003 g NaF were weighed and dissolved in de-ionized water in a 1.0 L of volumetric flask. Desalination of synthetic seawater was accomplished using a single settling step. First, the pH of 10 g standard/sample solution was adjusted to 1.60 using 1.0 M hydrochloric acid and then made up to 20 g with de-ionized water. A total of 150 µL of TEA was added to the sample solutions (pH-adjusted) and the samples were left to stand for approximately 120–180 min to complete the precipitation. The resulting solution containing colloids was centrifuged at 3000 rpm for 10 min to ensure efficient separation of the supernatant from the precipitate containing the analyte. A total of 50 µL of conc. HNO3 was applied for dissolving the precipitate obtained, and before proceeding to the extraction step, 3.0 mL of disodium tetraborate buffer solution (pH 9.0) was added to ensure the solution was buffered in order to obtain the appropriate pH value (pH 9.0) for extraction. The resulting buffered solution was made up to 10 g with de-ionized water.
Extraction procedure
A total of 1.0 mL solution of 1,5-diphenylcarbazone (0.05%, w/w) prepared in ethanol was added to 10 g of standard/sample solution and mixed by vortexing for 15 s. A mixture of extraction solvent (chloroform/300 µL) and dispersant solvent (isopropyl alcohol/2.5 mL), prepared in a separate 15 mL falcon tube, was injected into the complexed solution with a 5 mL syringe. The cloudy solution formed after the injection was vortexed for 15 s to increase the interaction between the analytes and the extraction solvent. After this process, centrifugation was carried out at 3000 rpm for 2.0 min in order to separate the aqueous and organic phases. The organic sub-phase (chloroform) containing the analytes with a volume of approximately 200 µL was transferred to a clean centrifuge tube with the help of a micropipette and evaporated at room temperature. After evaporation, 150 µL of concentrated nitric acid (65%, w/w) was added to the tubes. In order to ensure that the analytes are effectively dissolved in an acidic environment, the acidic solution containing the analytes was kept in an ultrasonic bath for two min and sent to the FAAS system.
Results and discussion
The determination of trace nickel in seawater medium was carried out using DLLME method combined with the co-precipitation method prior to the flame atomic absorption spectrometry measurement. Optimization studies of co-precipitation and DLLME methods were carried out with synthetic seawater. All parameters of the proposed method have been optimized to improve extraction efficiency and to obtain an efficient spectrophotometric measurement. Only one parameter under examination was tested at various variables in each optimization step, while the other parameters were held constant. Three replicates were evaluated and the parameter with the higher absorbance and lower standard deviation value was selected as optimum value.
FAAS optimization
FAAS systems have some drawbacks such as the low atom-light interactions of analyte atoms in the flame pathway and low nebulization efficiency (Matusiewicz, 1997). The operating parameters of the FAAS system were optimized to eliminate these disadvantages and to bring the nickel absorbance value to the highest possible value. The flow rate of the oxidizer (air) was kept constant, while the acetylene flow rate was optimized to ensure effective atomization. Acetylene flow rates were investigated while aspirating 10 mg kg−1 nickel standard solution into the flame and recording absorbance values in continuous measurement mode. Since the flow rate of the nebulizer unit could not be adjusted clearly, experiments were carried out by moving the unit providing the flow on the device to different positions until the highest absorbance value was recorded. As a result of the trials, the sample flow rate was calculated by considering the aspiration time against a certain sample amount. The optimum sample flow rate was selected as 8.56 mL min−1.
Co-precipitation system optimization
Co-precipitation methods are effective strategies to preconcentrate the trace elements in seawater while removing the salt matrix. Co-precipitation with Mg(OH)2, which is among these approaches, has become popular for ultra-trace analysis as it uses Mg(II) found in sea water without the need for any additives. The aqueous solution also includes significant ions like Na, Ca, and K, which causes in interference effects (Arslan et al., 2018). Hence, TEA supported Mg(OH)2 co-precipitation method was used for the desalination of seawater samples. Two main factors which are the volumes of TEA and conc. HNO3 affecting the co-precipitation process were optimized. Due to the presence of Mg(II) in seawater samples, tests were performed with different volumes of TEA (50, 100, 150, and 200 µL) to determine their co-precipitation activities. After the TEA solution was added, the solution was shaken by hand for 3.0 s and allowed to stand for approximately 2–3 h to complete precipitation. The resulting colloidal solution was centrifuged at 3000 rpm for 10 min and the supernatant was carefully decanted. In addition to this, optimum amount of nitric acid was also investigated to dissolve the precipitate. First trying was achieved by 10 µL of concentrated nitric acid and then increasing volumes of nitric acid were tried till the precipitate was dissolved completely. Finally, after the precipitate obtained was dissolved with 50 µL of concentrated nitric acid (optimum volume), 3.0 mL of buffer solution was added to provide the necessary buffering for the extraction step and made up to 10 g with distilled water. No precipitation was observed at 50 µL of TEA. Although the absorbance values were close to each other for other TEA volumes, 150 µL was selected as the optimum one owing to high reproducibility and low volume.
Optimization of the metal-ligand interactions
The pH of the sample has a critical importance on the formation and stability of the metal-ligand complex. Therefore, buffer solutions at different pHs between 6.0 and 10.0 were tested to monitor the effects on the complexation of between nickel and ligand. It was observed that the complexation was achieved with the best efficiency by using a pH 9.0 borax buffer solution and the highest absorbance value was obtained for nickel at this pH value.
The concentration of the complexing ligand is important parameter that needs to be optimized as it affects the distribution ratio of the metal-ligand complex in the sample/standard solution. Here, the relationship between complexation efficiency and ligand concentration was checked by 0.010, 0.020, 0.05, and 0.10% (w/w) diphenylcarbozone (DPC). While absorbance values increased sharply at ligand concentrations from 0.010 to 0.05%, a decrease was observed in absorbance values at higher concentrations. Considering the experimental data, 0.05% was chosen as the optimum ligand concentration.
The effect of ligand volume on complexing was investigated by testing different volumes, including 0.75, 1.0, 1.5, and 2.0 mL, while keeping the concentration of the ligand solution constant at 0.05% (w/w). No ligand addition was also investigated and there was no analytical signal observed. This clearly showed that determination was not performed without adding ligand and the signals obtained were not due to possible precipitation of analyte at the optimum pH. The ligand volume of 1.0 mL was chosen as the optimum value because it gives the highest average absorbance value and repeatability. Finally, vortex times of 0.0, 15.0, 30, 45, and 60 s were tested to determine the effect of mixing time period on complexation after ligand addition. Considering the experimental data, it was seen that the mixing time did not affect the complexing efficiency, but 15.0 s was chosen as the optimum mixing time period to ensure a homogeneous mixture.
Optimization of the DLLME method
The selection of the appropriate extraction solvent and dispersive solvent for the analytes and then the determination of the suitable amounts for the extraction efficiency were carried out by optimization studies. A good extraction solvent must have some properties such as not being miscible with the aqueous sample, having a different density from the aqueous solution, being soluble in the dispersive solvent, and providing good solubility for the analyte. The organic solvent used in DLLME can be both low-density and high-density. Highly volatile, low-density solvents collected on the surface of the aqueous sample can lead to low sensitivity due to their reproducibility and impact on the final volume after extraction (Chormey & Bakırdere, 2018). Therefore, organic solvents such as chloroform (1.49 g cm−3), 1–2, dichloroethane (1.25 g cm−3), and dichloromethane (1.33 g cm−3), whose densities are greater than the aqueous solution, were studied as extraction solvents. In this optimization step, extraction efficiency was tested by mixing 400 µL of each extraction solvent with 1.5 mL of ethanol as dispersive solvent. No phase formation was observed for dichloromethane. Chloroform was chosen as the extraction solvent, which provided the highest average absorbance value. In the next step, 200, 250, 300, and 400 µL chloroform amounts were tried to determine the amount of extraction solvent and 300 µL was chosen as the optimum value in terms of extraction efficiency.
The extraction solvent should effectively disperse in the aqueous solution and collect the analytes that is why the dispersive solvent is very important in the way of extraction efficiency. Therefore, some organic solvents such as ethanol, methanol, and isopropyl alcohol were tried as dispersive solvent. The optimum value in terms of repeatability and average absorbance value was recorded from isopropyl alcohol. In order to determine the amount of dispersive solvent, the amounts of 1.5, 2.0, 2.5, and 3.0 mL of isopropyl alcohol were tried and 2.5 mL was obtained as the optimum value.
System analytical performance
All parameters, including the instrumental system and extraction parameters that affect the sensitivity of the developed method, were determined by optimization studies and the optimum values are listed in Table 1. In order to determine the analytical performance of the DLLME-FAAS system under optimum conditions, a series of nickel-containing standards was extracted under optimum conditions and analyses were performed in the FAAS system. According to the obtained data, the calibration plot was obtained by using the absorbance values versus the concentrations. The detection limit of the analyte was calculated as 244 µg kg−1. The analytical performance values of the developed method are shown in Table 2.
The proposed method is easy to apply and cheap. In addition, the method exhibited rapid response and wide linear range with high precision for the detection of nickel compared to other examples in the literature (Table 2).
Colorimetry and UV-VIS applications
Calibration curve for the analyte was developed using the mean green (G) color intensity of measurements. The relationship between increasing concentration of standard solutions and color intensity of the complex solution in pink color is shown in Fig. 2 as a logarithmic function. The average color intensities of the three selected points were recorded for each image obtained with triplicate shots.
The same standards were also analyzed in UV-VIS instrument. Working with the wavelength (λmax) at which the maximum absorbance value obtained has great importance for accurate and reliable results in spectrophotometric measurements. Therefore, the wavelength range of 900–190 nm was scanned using a pink colored metal-ligand complex solution versus a blank solution. The maximum absorbance value was obtained at 520 nm. Calibration studies were carried out in the range of 0.125–6.0 mg kg−1. The results obtained are shown in Fig. 3.
Comparing the calibration curves obtained with the digital image-based colorimetric detection system and the UV-Vis system, it was confirmed that the developed DLLME-FAAS method can be easily used in other analytical systems.
Real sample applications
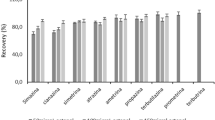
Within the scope of recovery studies, real ocean water samples brought from the Antarctic region were used to check the applicability of the DLLME-FAAS method combined with the selected co-precipitation method to real samples. Complex matrix, seawater, was selected as real sample for checking the applicability of the developed method. Four seawater samples taken from different places were analyzed in recovery studies. Initially, the samples were analyzed to confirm the presence or absence of the nickel in the seawater samples. No detectable nickel absorbance signal was observed in the unspiked sample according to the method’s detection limit. Then, nickel standard was added to each seawater sample at a final concentration of 75, 100, 150, and 200 µg kg−1.
Recovery values for each seawater sample were calculated based on the calibration plot obtained from synthetic seawater and nickel standards prepared at different concentrations. In Table 3, the recovery percentages for each sample can be seen. Since the synthetic seawater matrix and the matrix of the Antarctic samples are congruent, this has increased the accuracy of the developed method for nickel. It is clear that this combination gave the accurate results with high reproducibility by reducing/eliminating the matrix effect.
Conclusion
In this study, a simple, cheap analytical technique for trace nickel determination in seawater medium was presented. TEA-assisted Mg(OH)2 based co-precipitation method was chosen to eliminate the side effects belonging to seawater matrix to achieve accurate quantification of nickel. TEA was used as a co-precipitation reagent for nickel determination. DLLME method was also used to preconcentrate of treated seawater samples in terms of its nickel content. Measurement of nickel absorbance values was obtained by a FAAS system. The digital image-based colorimetric detection system and the UV-Vis system were used to ensure the applicability of the developed DLLME-FAAS method in other analytical systems. The detection limit was recorded as 16.1 μg kg−1 with the combination of DLLME and TEA-assisted Mg(OH)2 method prior to FAAS determination which is competitive with more expensive analytical systems in terms of sensitivity. In order to make sure the developed method was applicable to real seawater samples brought from Antarctic Region, recovery studies were achieved. Results showed that quantification of nickel was performed in the matrix selected with high precision and accuracy.
Data availability
Data will be available on reasonable request.
References
Alves, A. C. F., Saiki, P. T. O., da Silva Brito, R., Scalize, P. S., & Rocha, T. L. (2022). How much are metals for next-generation clean technologies harmful to aquatic animal health? A study with cobalt and nickel effects in zebrafish (Danio rerio). Journal of Hazardous Materials Advances, 8, 100160.
Anthemidis, A. N., & Ioannou, K.-I.G. (2009). Recent developments in homogeneous and dispersive liquid–liquid extraction for inorganic elements determination. A Review. Talanta, 80(2), 413–421.
Anyachor, C. P., Dooka, D. B., Orish, C. N., Amadi, C. N., Bocca, B., Ruggieri, F., …, Orisakwe, O. E. (2022). Mechanistic considerations and biomarkers level in nickel-induced neurodegenerative diseases: An updated systematic review. IBRO Neuroscience Reports, 13, 136–146
Aouarram, A., Galindo-Riaño, M., García-Vargas, M., Stitou, M., El Yousfi, F., & Espada-Bellido, E. (2010). An efficient approach to designing and optimizing the analysis of Ni (II) by AdCSV in seawater. Talanta, 82(5), 1749–1756.
Arslan, Z., Oymak, T., & White, J. (2018). Triethylamine-assisted Mg (OH) 2 coprecipitation/preconcentration for determination of trace metals and rare earth elements in seawater by inductively coupled plasma mass spectrometry (ICP-MS). Analytica Chimica Acta, 1008, 18–28.
Bermejo-Barrera, P., Moreda-Piñeiro, J., Moreda-Piñeiro, A., & Bermejo-Barrera, A. (1998). Direct electrothermal atomic absorption spectrometry determination of nickel in sea water using multiple hot injection and Zeeman correction. Talanta, 45(5), 807–815.
Bidabadi, M. S., Dadfarnia, S., & Shabani, A. M. H. (2009). Solidified floating organic drop microextraction (SFODME) for simultaneous separation/preconcentration and determination of cobalt and nickel by graphite furnace atomic absorption spectrometry (GFAAS). Journal of Hazardous Materials, 166(1), 291–296.
Biller, D. V., & Bruland, K. W. (2012). Analysis of Mn, Fe Co, Ni, Cu, Zn, Cd, and Pb in seawater using the Nobias-chelate PA1 resin and magnetic sector inductively coupled plasma mass spectrometry (ICP-MS). Marine Chemistry, 130, 12–20.
Bodkhe, G. A., Khandagale, D. D., More, M. S., Deshmukh, M. A., Ingle, N. N., Sayyad, P. W., …, Tsai, M.-L. (2023). Ag@ MOF-199 metal organic framework for selective detection of nickel ions in aqueous media. Ceramics International, 49(4), 6772–6779.
Chormey, D. S., & Bakırdere, S. (2018). Principles and recent advancements in microextraction techniques. Comprehensive analytical chemistry (Vol. 81, pp. 257–294). Netherlands: Elsevier.
Denkhaus, E., & Salnikow, K. (2002). Nickel essentiality, toxicity, and carcinogenicity. Critical Reviews in Oncology/hematology, 42(1), 35–56.
Elango, D., Devi, K. D., Jeyabalakrishnan, H. K., Rajendran, K., Haridass, V. K. T., Dharmaraj, D., …, Mishra, R. (2022). Agronomic, breeding, and biotechnological interventions to mitigate heavy metal toxicity problems in agriculture. Journal of Agriculture and Food Research, 10, 100374.
Erbas, Z., Soylak, M., Yilmaz, E., & Dogan, M. (2019). Deep eutectic solvent based liquid phase microextraction of nickel at trace level as its diethyldithiocarbamate chelate from environmental samples. Microchemical Journal, 145, 745–750.
Famurewa, A. C., Renu, K., Eladl, M. A., Chakraborty, R., Myakala, H., El-Sherbiny, M., …, Wanjari, U. R. (2022). Hesperidin and hesperetin against heavy metal toxicity: Insight on the molecular mechanism of mitigation. Biomedicine & Pharmacotherapy, 149, 112914.
Gilbert, M., Ho, P., Whitmore, L., & Shiller, A. (2023). Automated determination of gallium in seawater using seaFAST pre-concentration and high-resolution inductively-coupled plasma mass spectrometry. Analytica Chimica Acta, 1241, 340799.
He, Y., Wang, Y., Mao, G., Liang, C., & Fan, M. (2022a). Ratiometric fluorescent nanoprobes based on carbon dots and multicolor CdTe quantum dots for multiplexed determination of heavy metal ions. Analytica Chimica Acta, 1191, 339251.
He, Z., Xu, Y., Yang, X., Shi, J., Wang, X., Jin, Z., …, Pan, X. (2022b). Passivation of heavy metals in copper–nickel tailings by in-situ bio-mineralization: A pilot trial and mechanistic analysis. Science of the total environment, 838, 156504.
Kagaya, S., Miwa, S., Mizuno, T., & Tohda, K. (2007). Rapid coprecipitation technique using yttrium hydroxide for the preconcentration and separation of trace elements in saline water prior to their ICP-AES determination. Analytical Sciences, 23(8), 1021–1024.
Karadaş, C., & Kara, D. (2013). On-line preconcentration and determination of trace elements in waters and reference cereal materials by flow injection–FAAS using newly synthesized 8-hydroxy-2-quinoline carboxaldehyde functionalized Amberlite XAD-4. Journal of Food Composition and Analysis, 32(1), 90–98.
Khorrami, A. R., Hashempur, T., Mahmoudi, A., & Karimi, A. R. (2006). Determination of ultra trace amounts of cobalt and nickel in water samples by inductively coupled plasma-optical emission spectrometry after preconcentration on modified C18-silica extraction disks. Microchemical Journal, 84(1–2), 75–79.
Khorrami, A. R., Naeimi, H., & Fakhari, A. R. (2004). Determination of nickel in natural waters by FAAS after sorption on octadecyl silica membrane disks modified with a recently synthesized Schiff’s base. Talanta, 64(1), 13–17.
Kumar, A., Jigyasu, D. K., Subrahmanyam, G., Mondal, R., Shabnam, A. A., Cabral-Pinto, M., …, Fagodiya, R. K. (2021). Nickel in terrestrial biota: Comprehensive review on contamination, toxicity, tolerance and its remediation approaches. Chemosphere, 275, 129996.
Kumar, S., Prasad, S., Yadav, K. K., Shrivastava, M., Gupta, N., Nagar, S., …, Yadav, S. (2019). Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches-A review. Environmental research, 179, 108792.
Macoustra, G. K., Jolley, D. F., Stauber, J. L., Koppel, D. J., & Holland, A. (2021). Speciation of nickel and its toxicity to Chlorella sp. in the presence of three distinct dissolved organic matter (DOM). Chemosphere, 273, 128454.
Mandal, S., & Lahiri, S. (2022). A review on extraction, preconcentration and speciation of metal ions by sustainable cloud point extraction. Microchemical Journal, 175, 107150.
Matusiewicz, H. (1997). Atom trapping and in situ preconcentration techniques for flame atomic absorption spectrometry. Spectrochimica Acta Part b: Atomic Spectroscopy, 52(12), 1711–1736.
Miglione, A., Di Lorenzo, R., Grumetto, L., Spinelli, M., Amoresano, A., Laneri, S., & Cinti, S. (2022). An integrated electrochemical platform empowered by paper for fast nickel detection in cosmetics. Electrochimica Acta, 434, 141332.
Milne, A., Landing, W., Bizimis, M., & Morton, P. (2010). Determination of Mn, Fe Co, Ni, Cu, Zn, Cd and Pb in seawater using high resolution magnetic sector inductively coupled mass spectrometry (HR-ICP-MS). Analytica Chimica Acta, 665(2), 200–207.
Min, H.-K., Kweon, S., Kim, Y. W., An, H., Jo, D., Park, E. D., …, Park, M. B. (2021). Atomically dispersed nickel species in a two-dimensional molecular sieve: Origin of high activity and stability in dry reforming of methane. Applied Catalysis B: Environmental, 298, 120627.
Nakhaei, J. M., Jamali, M. R., Sohrabnezhad, S., & Rahnama, R. (2019). In-syringe solvent-assisted dispersive solid phase extraction followed by flame atomic absorption spectrometry for determination of nickel in water and food samples. Microchemical Journal, 144, 88–92.
Otero-Romaní, J., Moreda-Pineiro, A., Bermejo-Barrera, P., & Martin-Esteban, A. (2008). Synthesis, characterization and evaluation of ionic-imprinted polymers for solid-phase extraction of nickel from seawater. Analytica Chimica Acta, 630(1), 1–9.
Padchasri, J., Montreeuppathum, A., Siriroj, S., Poo-arporn, Y., Pinitsoontorn, S., Songsiriritthigul, P., …, Chanlek, N. (2022). Effect of Ni content in lithium nickel borate glasses: Structure and electrochemical studies. Journal of Alloys and Compounds, 911, 164994.
Pieczyńska, J., Płaczkowska, S., Sozański, R., Skórska, K., & Sołtysik, M. (2021). Effect of nickel on red blood cell parameters and on serum vitamin B12, folate and homocysteine concentrations during pregnancy with and without anemia. Journal of Trace Elements in Medicine and Biology, 68, 126839.
Rascio, N., & Navari-Izzo, F. (2011). Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Science, 180(2), 169–181.
Ribas, T. C., Mesquita, R. B., & Rangel, A. O. (2022). Use of dimethylglyoxime for the spectrophotometric flow-based determination of nickel in natural waters. Talanta Open, 6, 100132.
Roldan, P., Alcântara, I., Rocha, J. C., Padilha, C., & Padilha, P. (2004). Determination of copper, iron, nickel and zinc in fuel kerosene by FAAS after adsorption and pre-concentration on 2-aminothiazole-modified silica gel. Eclética Química, 29, 33–40.
Rykowska, I., Ziemblińska, J., & Nowak, I. (2018). Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. Journal of Molecular Liquids, 259, 319–339.
Saçmacı, Ş., & Saçmacı, M. (2017). A new procedure for determination of nickel in some fake jewelry and cosmetics samples after dispersive liquid–liquid microextraction by FAAS. Applied Organometallic Chemistry, 31(11). https://doi.org/10.1002/aoc.4081
Sawatari, H., Fujimori, E., & Haraguchi, H. (1995). Multi-element determination of trace elements in seawater by gallium coprecipitation and inductively coupled plasma mass spectrometry. Analytical Sciences, 11(3), 369–374.
Sherman, S., Chen, W., Blewett, T., Smith, S., Middleton, E., Garman, E., …, McGeer, J. (2021). Complexation reduces nickel toxicity to purple sea urchin embryos (Strongylocentrotus purpuratus), a test of biotic ligand principles in seawater. Ecotoxicology and Environmental Safety, 216, 112156.
Singh, A., Dayu, X., Ituen, E., Ansari, K., Quraishi, M., Kaya, S., & Lin, Y. (2020). Tobacco extracted from the discarded cigarettes as an inhibitor of copper and zinc corrosion in an ASTM standard D1141–98 (2013) artificial seawater solution. Journal of Materials Research and Technology, 9(3), 5161–5173.
Toyota, Y., Okabe, S., Kanamori, S., & Kitano, Y. (1983). The determination of Mn, Fe, Ni, Cu and Zn in seawater by atomic absorption spectrometry after coprecipitation with lanthanum hydroxide. Journal of the Oceanographical Society of Japan, 38, 357–361.
Wu, J. (2007). Determination of picomolar iron in seawater by double Mg (OH) 2 precipitation isotope dilution high-resolution ICPMS. Marine Chemistry, 103(3–4), 370–381.
Wu, J., & Boyle, E. A. (1998). Determination of iron in seawater by high-resolution isotope dilution inductively coupled plasma mass spectrometry after Mg (OH) 2 coprecipitation. Analytica Chimica Acta, 367(1–3), 183–191.
Wu, J., Zhou, Q., Huang, R., Wu, K., & Li, Z. (2021). Contrasting impacts of mobilisation and immobilisation amendments on soil health and heavy metal transfer to food chain. Ecotoxicology and Environmental Safety, 209, 111836.
Yoshimura, K., Toshimitsu, Y., & Ohashi, S. (1980). Ion-exchanger colorimetry—VI Microdetermination of nickel in natural water. Talanta, 27(9), 693–697.
Zambelli, B., Uversky, V. N., & Ciurli, S. (2016). Nickel impact on human health: An intrinsic disorder perspective. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1864(12), 1714–1731.
Zeng, C., Xu, X., Zhou, N., & Lin, Y. (2012). Synergistic enhancement effect of room temperature ionic liquids for cloud point extraction combined with UV-vis spectrophotometric determination nickel in environmental samples. Spectrochimica Acta Part a: Molecular and Biomolecular Spectroscopy, 94, 48–52.
Zhu, Y., Inagaki, K., Haraguchi, H., & Chiba, K. (2010). On-line elution of iron hydroxide coprecipitate carrier for determination of REEs in natural water by mix-gas ICP-MS. Journal of Analytical Atomic Spectrometry, 25(3), 364–369.
Funding
This work with a grant number of 119Z846 was supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
İrem Zehra Kublay: formal analysis, investigation, methodology, validation, visualization, and roles/writing — original draft. Elif Seda Koçoğlu: formal analysis, methodology, validation, and roles/writing — original draft. Sude Oflu: formal analysis, methodology, validation, and roles/writing — original draft. Büşra Arvas: methodology, validation, and roles/writing — original draft. Çiğdem Yolaçan: methodology, validation, and roles/writing — original draft. Sezgin Bakırdere: conceptualization, investigation, methodology, supervision, validation, and writing — review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kublay, İ.Z., Koçoğlu, E.S., Oflu, S. et al. Trace nickel determination in seawater matrix using combination of dispersive liquid–liquid microextraction and triethylamine-assisted Mg(OH)2 method. Environ Monit Assess 195, 861 (2023). https://doi.org/10.1007/s10661-023-11435-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11435-y