Abstract
The study reports concentration of 16 PAHs in sediment, water, plankton and in muscle and liver of sampled fishes from Paranagua Bay in Southern Brazil. According to the range of PAHs concentration found in sediments (ΣPAHs 15.33–133.61 µg g−1) and water (ΣPAHs 51.20–162.37 µg L−1), the area was characterized as slightly polluted. In contrast, significant concentration of PAHs was found in fish and plankton, ranging from 26.52 to 2055.00 µg g−1 and 175.41 to 2096.10 µg g−1, respectively. The concentrations were 15 times more than in sediments. Even though the concentration of PAHs in water and sediments are low, the concentration in fish tissues are alarmingly. The hypothesis of bioaccumulation is not discharged. Accumulation levels of PAHs in the biota were used to calculate biota-sediment accumulation factor (BSAF) and bioaccumulation factor (BAF). For example, fish sampled from site 11 had a BAF of 49 and BSAF of 2.3, whereas for plankton the BAF was 16.3 and BSAF 0.7. Mean values of BAF and BSAF were higher in plankton samples. It is known that PAHs are enzymatically metabolized by organisms, thus the lower BSAF in fish might have resulted from biochemical transformation. Statistical analysis indicates no significant correlation between total PAHs concentration in fish tissues and total lipid content in fishes (p > 0.1).
Accumulation in tissues is controlled by lipid content, although factors, such as spatial distribution of compounds, trophic level, and behavior of each species in the environment, must be considered. Besides, size and nutritional conditions can affect both absorption as well as metabolic process and excretion. A positive and significant correlation was found between total PAHs and trophic positions obtained from FishBase data (r2 = 0.784; p < 0.05). Thus, it seems that the area, with great influence of urban and industrial activities, and the bioaccumulation compounds, such as PAHs, depend on others factors, as well as resuspension of sediments and releasing of compounds for biota.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Among several groups of contaminants found in the marine environment, the polycyclic aromatic hydrocarbons (PAHs) are the most investigated in water, sediments, and suspend particles due to their carcinogenic properties and high level of resistance to microbiological degradation (Barakat et al. 2011; Guzzella et al. 2005). Although hundreds of PAHs compounds are known to exist in the environment, only 16 are considered as priority pollutants by US Environmental Protection Agency (USEPA). In aquatic bodies, sediment is the major sink for PAHs (Liu et al. 2012; Ranjan et al. 2012). In environment, PAHs are persistent, the degradation occurs slowly, mainly due to their persistence and hydrophobic characteristic (Lei et al. 2005).
Various PAHs are potent mutagens and carcinogens to both aquatic and terrestrial animals, including humans (Dickhut et al. 2000; Duran and Cravo-Laureau 2016). From sediments, PAHs can be transferred to organisms by accumulation or through trophic chain (Bandowe et al. 2014; Jiao et al. 2014). However, most of the studies did not consider those aquatic organisms, even in low concentration in sediments and water, where could be accumulated. Few studies examined the presence of PAHs transferred from sediment to biota (Waszak and Dabrowska 2009; Soclo et al. 2000).
Generally, monitoring programs assess the concentration of total PAHs in sediments, and most of them do not consider or estimate influence on biota. PAHs effects on fish can be shown by some pathologies in the liver and gills, corroborated by biochemical disturbances and genetic damage by biomarkers (e.g., somatic index, chemical analysis of bile, biochemical, genetic and morphological parameters). These effects showed up even at concentrations below the threshold for PAHs in sediments (Ribeiro et al. 2013; Cappello et al. 2017; Maisano et al. 2016). PAHs bioaccumulation occurs when PAHs are present in water and/or sediments and are driven by lipid content of organisms (Soclo et al. 2008). Bioaccumulation can be assessed by methods that directly measure or predict it through mathematical models based on the lipid content in the organisms and compounds properties (Gobas 1993). Noxious effects of PAHs on biota are estimated or predicted by mathematical models based on routes of exposure, lipid content of species, diet composition, and dynamic process of pollutants (Torres et al. 2014). The main advantage of using models is the small number of analysis and the possibility to assess in a short time the extension of damage. From concentration in sediments, the extension of accumulation is determined by the bioaccumulation factor (BAF) and the biota-sediment accumulation factor (BSAF) models (Jiao et al. 2014; Soclo et al. 2008).
Estuarine areas are prone to receive large amounts of PAHs from drainage and deposition of suspended air particles (Ranjan et al. 2012). Sediments from coastal marine environments concentrate most of the PAHs. Port areas also suffer from constant discharges or accidental spills of crude oil and/or derivatives from boat/ship traffic (Guzzella et al. 2005; Stout et al. 2004). Previous studies have shown that Paranagua Estuary surroundings (Southern Brazil) are suffering the impact due the socioeconomic development. In addition, the port activities have increased as well as the vessels size and capacity (Rizzi et al. 2017; Abreu-Mota et al. 2014; Froehner et al. 2010).
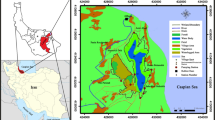
In this work, the content and distribution of PAHs in sediments and biota from Paranagua Bay, Southern Brazil (Fig. 1), were examined. The studied area is affected by local industry, port services, and soil occupation. The presence of PAHs in biota, i.e., plankton and fish (liver and muscle), were examined to understand the possible side effect, such as bioaccumulation. To understand the extension of bioaccumulation, BSAF and BAF deterministic models were used. Additionally, statistical analysis was performed in an attempt to investigate the relationship between PAHs in organisms, sediments, and among the subareas of the estuary.
Materials and Methods
Study area and Samples Preparation
Surface sediments, water, and phytoplankton samples were collected in April 2012 from 17 sample sites (Fig. 1). A statement was added to the sample sites to distinguish between sediments (S), water (W), and plankton (P). Considering our previous studies, the area was divided in two subareas. Subarea 1 (including points 1–12) corresponds to the East–West Axis. Within this subarea is located Paranagua Port, city of Paranagua, and the Galheta channel (a channel of navigation). Subarea 2 corresponds to the North–South Axis and includes points 13–17, which are considered to have low human impact (Torres et al. 2014; Martins et al. 2010; Choueri et al. 2009).
The sediments were collected using a Van-Veen grab sampler. The samples were wrapped in aluminum foil and were kept at 4 °C. In the laboratory, they were frozen-dried before the extraction procedure. The plankton samples were only collected in subarea 1, using a fishing net of 20-µm mesh. Finally, the filtered extract containing the plankton was frozen. For the PAHs content, the same procedure adopted to sediments was followed. Samples of fishes were collected using a different net. Table 1 contains the characteristics of fishes examined. To determine the PAHs level in fish, the liver and muscle were removed for analysis of compounds. Most PAHs were metabolized in the liver and muscles containing the unmetabolized compounds (Soclo et al. 2008). The removed tissues were kept at − 15 °C until extraction and analysis.
Water samples were collected using a Van Dorn-type bottle and collected at a depth of 50 cm. Samples were kept at 4 °C in amber glass bottles. In the laboratory, the PAHs compounds were extracted with 60 mL of dichloromethane (twice), according the method 3510B proposed by EPA. Acenaphthylene-d8 was added as surrogate to monitor the quality of the extraction procedure. The recovery rate was higher than 76%. The extracts were combined, and the volume was reduced to approximately 1 mL in a rotatory evaporator and then dried under flux of nitrogen. The extracts were redissolved in 200 µL of hexane for further quantification by CG-MS. The chromatographic conditions are described below.
Granulometric Composition of Sediments
The granulometric composition (sand, silt, and clay) was determined by an integrated technique (sieving and laser diffraction). Briefly, samples were passed through a set of sieves with standardized mesh screens (mesh size 8, 14, 42, 80, 170, 250, 400, 500, and 635). The classification of sand, silt, and clay was obtained according the Wentworth granulometric scale (Besler and Ritter 2010). The fine grains were collected and examined with a laser analyzer, which measures the size by detecting angular diffractions from a light scanning after it has passed through the sample in an optical cell (Froehner and Martins 2008).
Total Organic Carbon
In the process of determination of total organic carbon (TOC), all samples were acid digested using 16% (v/v) hydrochloric acid to remove the carbonate fraction. Then, the organic carbon was converted to CO2 and quantified by using a TOC analyzer (Shimadzu, TOC-VCPH).
Extraction, Clean Up, and Analysis of PAHs
The extraction and analysis of PAHs in all samples were performed according to the procedure described in the existing literature (Jiao et al. 2014; Liu et al. 2012; Zakaria et al. 2002; Yunker et al. 2002). Briefly, bulk sediment (10 g) was extracted by using an accelerated solvent extractor (ASE, Dionex) in 5-min static cycles (100 °C, 4 MPa) with a mixture of dichloromethane (DCM) and acetone (1:1). Sulfur was removed from the extracts using activated copper, which was added directly after the extraction. The PAHs in the total lipid extract (TLE) was separated from other compounds by elution with hexane in columns filled with silica (3 × 4 mL). The volumes were combined and reduced to approximately 0.5 mL and completely dried under a gentle flow of nitrogen before the internal standard. The extract was redissolved with 200 µL of hexane for injection and analysis. A solution containing 16 PAHs (USEPA priority pollutants) (AccuStandard PAH Mix Z-014G) was used as a standard solution to obtain a calibration curve for quantification. The standard mixture was composed by anthracene (ANT), acenaphthylene (ANP), acenaphthene (AEN), phenanthrene (PHE), fluorene (FLU), pyrene (PYR), indene[1,2,3-cd]pyrene (IcP), benzo[a]pyrene (BaP), benzo[k]fluoranthene (BkF), benzo[b]fluoranthene (BbF), chrysene (CHR), dibenzo[a,h]anthracene (DhA), benzo[ghi]perylene (BgP), benzo[a]anthracene (BaA), fluoranthene (FLA), indene (IND), and naphthalene (NAP). Before the analysis, deuterated fluorine-d10 was added to each sample as an internal standard, whereas acenaphthylene-d8 was added before the extraction as surrogate to monitor the efficiency of the analytical protocol. The recovery rates were higher than 75% for most of all samples. All solvents were HPLC grade; silica gel was treated with DCM (24 h) and activated in an oven for (400 °C; 4 h). The PAHs were identified and quantified by chromatograph gas coupled to mass spectrometer with electron-impact ionization (GC–MS Varian model 450). The GC was equipped with a factor four VF 5MS fused silica capillary column (60 m, 0.25 mm i.e., 0.25-μm film thickness) and helium as a carrier gas with a constant flow of 1.2 mL min−1. The GC oven was programmed to increase from 40 to 120 °C (at 30 °C min−1), with an initial hold of 1 min at 40 °C and a final hold of 16 min at 280 °C. Both full scan and selected ion monitoring (SIM) modes were applied. PAHs were identified by comparing retention time with those of the appropriate authentic standards and published spectra. Quantitative analysis was performed using internal and external calibration methods.
Liver and muscle of fish and phytoplankton were lyophilized and then extracted following the same procedure described above for sediments. The amount used for extraction varied from 0.5 to 3.0 g.
Lipid proportion was determined following the procedure described by Tölgyessy and Miháliková (2015). Briefly, approximately 5 g of tissue fish was extracted with acetone/ethylacetate mixture (6:4) using vigorous shaking, and then inorganic salt (2 g MgSO4 and 0.5 g NaCl) was added and agitated. The organic phase was separated by centrifugation. An aliquot of the organic phase was air-dried and then oven-dried, and the lipid content of the fish homogenate was determined gravimetrically on a wet weight basis.
Bioaccumulation Models
To better understand the possible extension of contaminants in the trophic chain, bioaccumulation factors models were used. For this purpose, the bioaccumulation factor (BAF) and biota sediment factor accumulation (BSAF) were applied (Kwok et al. 2013). To measure the uptake of PAHs from sediments to biota, the bioaccumulation factor (BAF) was calculated. The BAF is represented by Eq. (1):
where Cf is the concentration in organisms and Cs the concentration in sediments (in ng g−1 dw).
The extension of bioaccumulation also was tested considering the lipid content of each organism. For this, a BSAF model was used according Eq. (2):
where Cf is the concentration of compound in organism (ng g−1), L is the lipid proportion in the biological tissue in %, Cs is the concentration of compound in sediment (ng g−1), and C is the organic compound in the sediment in %.
Statistical Analysis
Analysis of variance (ANOVA) was performed for the data obtained. To evaluate the significant differences between the two designated subareas of the estuary, the least significant difference (LSD) was determined by the Tukey test, at 5% level of probability. Additionally, the Tukey test was performed by ANOVA (NESTED) to check any LSD difference between environment matrices (water, sediment, and fish plankton) and PAHs groups (di, bi, tetra, penta, and hexa-aromatics rings). Normality was assessed using the Shapiro–Wilk test. Statistical correlation coefficients between total PAHs and physical–chemical characteristics of the sediments, i.e., grain size TOC and total PAHs in fish tissues and biological characteristics, i.e., percentage of lipids, total length, total weight, and trophic level, were calculated to evaluate the influence of each parameter at accumulation of PAHs. Analyses were performed using the program XLSTAT Version 2015.4.01.20116.
Results and Discussion
Granulometric Composition of Sediments
To better understand the influence of anthropogenic activities, the area was segmented in two parts: subarea 1 and subarea 2. The granulometric composition of sediments clearly showed two different areas. The inner part of estuary (sites 1, 2, and 3, subarea 1; Fig. 1) was mostly muddy, with high silt content, ranged from 41 to 46%. In contrast, sediments from the other segment (subarea 2) is composed by fine and medium sand (500–62 µm). As expected, inner parts of the estuary presented the highest content of organic carbon. In sites 1, 2, and 3 (subarea 1) varied from 85 to 69 mgC g ‒1sed and in site 17 (subarea 2) 70 mgC g ‒1sed . In general, fine and medium sand (63–500 µm) and silt (4–63 µm) fractions dominated the most samples composition, whereas clay particles (< 4 µm) were absent. The sediment samples from sites 11 and 15 presented the highest content of coarse sand (2000–500 µm), ranging from 12 to 22%, with lower TOC, 4.96 mgC g −1sed minimum.
The level of TOC in sediments is crucial to define the fate and transport of pollutants; in addition, the TOC content is usually higher for fine particles. Furthermore, a positive and strong correlation was found for TOC and silt (r2 = 0.730, p < 0.001), whereas a negative correlation was found for samples with high content of sand (r2 = ‒ 0.507, p < 0.05). Previous study in this area showed that currents and tidal are responsible for organic matter accumulation (Herrling 2003).
Distribution Levels of PAHs in Water and Sediments Samples
PAHs present very low water solubility (2–260 µg L‒1). In this research, the level of total concentration of PAHs (∑PAHs) in water samples was found low, the concentration ranged from 51.2 to 162.4 ng L−1 (Table 2), with predominance of low molecular weight compounds (naphthalene, anthracene, and fluoranthene). Water samples from sites 3 and 16 presented the highest concentration of total PAHs. Site 3 is influenced by the intense traffic of commercial ships, offloading and loading activities. Water sample from site 16 (W16) also showed ∑PAHs concentration in the same level for W3. Such high concentration is accounted for the intense traffic of small boats, including those used for local transportation driven by diesel fuel. This observation is supported by low molecular weight (LMW)/high molecular weight (HMW) PAHs ratio higher than 1, indicating petrogenic source of PAHs in water (Yunker et al. 2002).
As expected HMW PAHs are absorbed by suspended particles, whereas LMW PAHs can be present in the water in significant concentration (ASTDR 1995) HMW PAHs, i.e., chrysene, benzo(bk)fluorantene, benzo(a)pyrene, indene, dibenzo(ah)anthracene, and benzo(ghi)perylene, were found in concentrations (14.96–146.50 ug L−1). With the help of previous literature, one can say that bioaccumulation can occur even at small concentration in water (Silva et al. 2007; Gobas 1993).
The presence of PAHs in rivers and estuarine waters have become common; for instance, in the Mississippi River there were ∑PAHs ranging from 62.9 to 144.7 ng L−1 (Zhang et al. 2007), Venice was reported to have the total PAHs concentration ranging from 12.4 to 266.8 ng L−1 (Manodori et al. 2006). The results found in this work are similar with both studies mentioned. The ∑PAHs values, found in Paranagua Bay, showed a distinct range compared with other areas regarding to pollution, such as Daya Bay (4228–29,325 ng L−1) (Zhou and Maskaoui 2003) and Guanabara Bay (1592 ng L−1) (Silva et al. 2007).
Presence and abundance of PAHs in sediments are frequently used to classify an area as polluted or not (Barakat, et al. 2011; Baumard et al. 1998). Sediments with ∑PAHs ranged from 0 to 100 ng g−1 are considered as unpolluted or with minimum pollution. Sediments with concentration ranges from 100 to 1000 ng g−1 are classified as moderated polluted, whilst values higher than 5000 ng g−1 represent areas extremely polluted. In this work, most of sites were classified as unpolluted, except sites 3 (133.61 ug g−1), 13 (107.5 ug g−1), and 15 (132.42 ug g−1) that were considered as moderately polluted, according the abundance of total PAHs.
The concentration of PAHs in sediment samples ranged from 15.5 to 133.6 ng g−1. However, the distribution was quite different (Table 2). As expected, the results showed distinct areas among sediment samples, high polluted and almost pristine ones. Site 3, near the port, presented the highest concentration of PAHs. On the other hand, site 9, close to Cotinga Island and Galheta navigation channel, presented the lower concentrations followed by sites 16 (36.9 ng g−1) and 17 (37.3 ng g−1), both located in subarea 2. Those results are in same level of magnitude in accordance with previous studies in some parts of Brazilian coast. For example, in the Patos Lagoon, Southern Brazil, PAHs were found ranging from 4.7 to 112.5 ng g−1 (Luz et al. 2010). The Patos Lagoon suffers influence from anthropogenic activities, especially fishing. The estuarine system of Mandau-Manguaba, Northeastern Brazil, showed PAHs ranging from 29.2 to 222.7 ng g−1 (Silva et al. 2013).
Paranagua Bay has some analogous activities with the most polluted coastal areas in Brazil, i.e., Guanabara Bay and Santos Bay, economic activities and sewage input are similar (da Silva et al. 2007). However, in those places the activity is quite intense which reflected in the level of pollution. At Guanabara Bay, there was a record of ΣPAHs values in the sediments ranging from 77 to 7751 ng g−1 and in Santos Bay concentrations higher than 15,300 ng g−1. Previous studies in the Paranagua estuary observed ∑PAHs ranging from 3.85 to 89.5 ng g−1 at North–south axis (subarea 2) of Paranagua Bay (Martins et al. 2012). In Hugli, a highly industrialized estuarine area in India, the concentration of PAHs varied from 2.5 to 1081 ng g−1 (Guzzella et al. 2005). In addition, concentration of total PAHs in sediments from Mediterranean Sea was higher than 21,600 ng g−1 (Barakat et al. 2011). The values of PAHs found in the present research confirmed an area quite preserved compared with the threshold values labeled as polluted. The PAHs content in sediments are lower than those concentrations assumed to trigger deleterious effects on biota according to sediment quality guidelines (SQG) (NOAA 1999). Table 3 shows the PAHs values for each sample and the values used as guidelines. The results are lower than the values of Effects Range-Low (ERL), Effects Range-Median (ERM), Threshold-Effects Level (TEL), and Probable-Effects Level (PEL) (Buchman 2008). According to these results, the area can be considered as unpolluted; nevertheless, histopathological studies had demonstrated mutagenic effects on some fishes collected at this estuary (Ribeiro et al. 2013).
The granulometric composition of sediment controls its content of PAHs. Besides, in marine environments physical and chemical process, water circulation and currents affect the PAHs distribution (Yunker et al. 2002; Kowalewska et al. 2003). There was a positive correlation found among PAHs and granulometric composition. PAHs showed strong positive correlation with sediments rich in silt (r2 = 0.417; p < 0.1) and negative correlation with sediments rich in medium sand (r2 = ‒0.445; p < 0.1). Differences in sorption of compounds are accounted for organic carbon referenced sorption coefficient (log Koc), however pH and ionizable capacity of soils and sediments also affect the efficiency of sorption. Thus, PAHs with high molecular weight seems to be strongly absorbed by sediments, as consequence, their mobility will be lower (Liu et al. 2012). A typical example of this behavior is the result found in site 11, composed predominantly by sand and 1.55% of TOC. The ΣPAHs for this site was only 15.5 ng g−1, even the site is located near a pollution outfall.
To distinguish the source of PAHs, the compounds were subset in LMW (< 202 g mol−1) and HMW (> 202 g mol−1). Thus, a HMW/LMW ratio lower than 1 indicates a petrogenic source of PAHs (Barakat et al. 2011; Baumard et al. 1998). The ratio values obtained indicate a mixture of sources (pyrogenic and petrogenic), except for S3 (1.4) and S9 (1.7) sites, which PAHs are mostly from pyrogenic source. In general, LMW PAHs are associated with oil. Considering the area and its activities, it was not a surprise that among 16 PAHs, lower molecular weight were predominant compounds, indicating oil spill. Such observations were in accordance with previous studies in this area (Martins et al. 2012; Froehner et al. 2010).
Because PAHs are released and reach the environment, microorganisms can modify the distribution of PAHs, especially those with low molecular weight. To decipher the source of PAHs in sediments, different ratios among PAHs were applied, according to the ratios proposed by Yunker et al. (2002). These ratios were used: FLA/(FLA + PYR), ANT/(ANT + PHE), and BaA/(BaA + CHR). Sediments from sites 1, 2, 3, 4, 6, 8, and 12 showed contamination by oil derivatives; however, according to the results, it is more appropriate to assume a mixture of contribution (Fig. 2). For sediments collected near the port, it was already identified as influences of urban sewage (Martins et al. 2010). All samples located in the North–South of the estuary (S13, S14, S15, and S17) showed contamination associated mainly from pyrogenic and mixed sources.
Concentrations of PAHs in Plankton and Samples of Fish
Samples of plankton were collected in three different sites: P3, P6, and P11 (P denotes plankton samples and the number the location of sample site). In general, samples were constituted by phytoplankton and small fraction of zooplankton. Fish samples were collected in sites F1, F2, F3, F6, F10, and F11 (where F denotes fish samples and the number the location of sample site). In fish samples, the presence of PAHs was examined in the muscle (M) and liver (L). The ∑PAHs in plankton and fish samples were higher than those found in sediments. The ∑PAHs varied from 26.52 to 2096.08 ng g−1 in dry weight. The highest values was found in phytoplankton from site 3, and the values of total PAHs was 1569% higher than concentration in sediment of this site. This site also presented the highest value of PAHs in the water and sediment. Among fishes, samples from sites 3 and 11 (near the port activities) had the most significant level of PAHs amount in their bodies. The level of PAHs in biological tissue resulted from transfer from water, sediment, and diet. The lipid content in organisms controls the accumulation of organic compounds (Bandowe et al. 2014; Baumard et al. 1998).
Hydrophobic compounds can accumulate in organisms for long period of time (Kwok et al. 2013); the concentration in the organism is due to the environment activities and diet of each specie. Organisms that have poor biotransformation capacity (e.g., plankton) can accumulate high amounts of PAHs and other compounds (Perugini et al. 2007). Recently, samples of plankton from Seine Estuary presented 3866 ng g−1 as maximum concentration (Cailleaud et al. 2007). While in fish muscle from Ghana, the total PAHs was up to 481 ng g−1 (Bandowe et al. 2014). Muscle fishes from Hong Kong presented lower concentrations (184–194 ng g−1 dw). The presence of poly aromatic compounds in biological samples imply the existence of contaminated sites transferred through diet. The accumulation of compounds can happen even when sediments has low amount of pollutants. Different levels of concentration of PAHs can be observed in species in the same trophic chain, such difference might be attributed to the lipid content of each organism. In fish, compounds can be metabolized by biochemistry and enzymatic mechanism in response to reduce the concentration in the body (Beyer et al. 2010). Even some organisms are able to metabolize PAHs, the accumulation in the tissue with more lipids content is significant (Gobas 1993; Taniguchi et al. 2009; Maisano et al. 2016).
In this study, the highest concentration of PAHs was found in Cynoscion leiarchus (Cl) species (smooth weakfish), collected in site 11 (F11Cl), and the lower concentration was identified in muscle of Menticirrhus americanus (Ma) from site 7. Age, diet, habitat, and habits are essential to explain and understand the concentration of PAHs in fish. Smooth weakfish (C. leiarchus) is easily found in muddy habitats as well as in sand bottoms of estuarine areas (FishBase 2015). Smooth weakfish is a piscivorous species, however, macro- and micro-plankton, crustaceans, and mollusks, also form part of its diet (Chaves and Umbria 2003). Among the collected species, smooth weakfish is in the highest trophic position (TP) (4.0 ± 0.7). Trophic position indicates the trophic level occupied by animal, based on its diet, according to FishBase data (2015). The highest concentration might be a result of a biogmanigification process. Southern king croaker (M. americanus) is quite common in the Brazilian coast, found in sediments (sand and mud), feeding benthonic organisms (Rondineli et al. 2007), and it has trophic position (TP) of 3.5 ± 0.0.
The distribution of PAHs showed a distinct pattern for each matrice examined (Fig. 3). Such differences can be attributed as organic carbon content in each compartment and lipophilicity of each compound. For example, PAHs up to four benzene rings tend to be in water (17% of the total PAHs); in contrast, those PAHs with five benzene rings or more tend to be bind by sediments (39%). In biota, the accumulation is associated to the level in the trophic chain and lipid content; plankton presented 39% and fishes 38% of the total PAHs. All species analyzed were benthonic and the contact with muds is intrinsic of their habits. The bioaccumulation process is initiated by the contact with contaminated sediments. Thus, the HMW PAHs found in tissue of fish might have originated from contaminated sediments (Baumard et al. 1998). In port areas, dredging procedures often are conducted in an attempt to improve the navigation conditions. Such procedure could promote resuspension of contaminants. Usually, low molecular weight PAHs are degraded faster than high molecular weight. Thus, some of the PAH compounds are bioavailable (Perugini et al. 2007).
Statistical analysis indicates no significant correlation between total PAHs concentration in fish tissues and total lipid content in fishes (p > 0.1).
Accumulation in tissues are controlled by lipid content, although factors, such as spatial distribution of compound, trophic level, and behavior of each species in the environment, must be considered. Besides, size and nutritional conditions can affect both absorption as well as metabolic process and excretion. A positive and significant correlation was found between total PAHs and trophic positions obtained from FishBase data (r2 = 0.784; p < 0.05).
The BAF values obtained ranged from 0.3 (site S7) to 49 (site S11; Table 4). The TOC in sediments might support such variation together with granulometric composition (Baumard et al. 1998). The result is not surprising, because the more hydrophobic the compound is, the more tightly it will bind to the sediments containing higher organic matter, resulting in less partition from sediment to water and therefore lower bioavailability to accumulation by organisms. Site 11, which presented the highest BAF value for sample F11Cl, is composed predominately of sand (55%) and lower TOC (0.5%). The high value of BAF also suggests that PAHs are more available to be taken by organism than other sites. In contrast, site 1, which had presented the highest percentage of silt and fine grain, showed the lowest BAF value (0.8). Biological characteristics of each species can be determinant in accumulation of organic compounds; the species Cynoscion leiarchus (F11C1M) is the top selected trophic chain and has a diet extremely piscivorous. For plankton, the BAF was 16.3.
The capacity of accumulation by organisms also was examined considering the lipid content of each species. The BSAF varied from 0.1 (F1Ma) to 13.5 (P3). Mean values of BAF and BSAF were higher in plankton samples (Fig. 4). It is known that PAHs are enzymatically metabolized by organisms. Thus, the lower BSAF in fish might have resulted from biochemical transformation.
To exclude variation due to different behaviors among species, such as feeding habits, the results of BAF and BSAF were determined to C. spixii sampled in sites 3 and 6, wherein the individuals presented similar characteristics of size and weight. It was observed that bioaccumulation factors from site 6 (F6Cs) were higher than values from site 3 (F3Cs), (BAFs = 2.2 and 0.71 and BSAFs = 13.4 and 6.09, respectively). The hydrodynamic characteristics (tidal and eddy current) are the major difference between these sites. While station 3 is in a more protected area, station 6 is located in a region with more intense tidal currents near to the port shipping channel where dredging activities occur frequently. Thus, it seems that the area with greatest influence of urban and industrial activities, as station 3, bioaccumulation compounds, such as PAHs, depend on others factors as well as resuspension of sediments and releasing of compounds for biota.
Conclusions
PAHs pollution levels in water, sediment, and biota were investigated. The levels of total PAHs of water and sediments represent a slightly contaminated area; the concentrations found in plankton and in fish tissues were much higher. The concentration of PAHs in sediments were proportional to TOC.
There was no significant difference between the subareas (1 and 2) of the estuary respect to total concentration. However, PAHs distribution in sediments near to Paranagua Harbour and to the navigation channel were related with mixed combustion and petrogenic sources, showing the influence of this activity at the estuary, whereas the sites located at North–South axis of the estuary showed more influence of pyrogenic sources.
Plankton and benthic fishes exhibited preference in accumulate penta and hexa-aromatic rings compounds in their tissues, and they were the most abundant compounds in sediments. The mathematical models used to examine the extension of contaminants in biota showed bioaccumulation of PAHs in some species. Values obtained in organisms were more than 1000% higher than in sediments, clearly representing a potential bioaccumulation process. Even the concentration of contaminant in water and sediments label the area as unpolluted. The values of BAF and BSAF showed that the bioaccumulation of PAHs is evident at the estuary, and the sediments could represent the main source of contamination.
References
Abreu-Mota MA, Barboza CAM, Bícego M, Martins CC (2014) Sedimentary biomarkers along contamination gradient in a human-impacted sub-estuary in Southern Brazil: a multi-parameter approach based on spatial and seasonal variability. Chemosphere 103:156–163
Agency for Toxic Substances and Disease Registry (ASTDR) (1995) Toxicological profile for Polycyclic Aromatic Hydrocarbons. U.S. Department of Health and Human Services. Public Health Service
Bandowe BAM, Bigalke M, Boamah L, Nyarko E, Saalia FK, Wilcke W (2014) Polycyclic aromatic compounds (PAHs and oxygenated PAHs) and trace metals in fish species from Ghana (West Africa): bioaccumulation and health risk assessment. Environ Int 65:135–146
Barakat AO, Mostafa A, Wade TL, Sweet ST, Sayed NB (2011) Distribution and characteristics of PAHs in sediments from the Mediterranean coastal environment of Egypt. Mar Pollut Bull 62(9):1969–1978
Baumard P, Budzinski H, Garrigues P (1998) Concentrations of PAHs (polycyclic aromatic hydrocarbons) in various marine organisms in relation to those in sediments and to trophic level. Mar Pollut Bull 36(12):951–960
Besler H, Ritter M (2010) A note on granulometric sand types and the Wentworth classification. Z Geomorphol 54:443–454
Beyer J, Jonsson G, Porte C, Krahn MM, Ariese F (2010) Analytical methods for determining metabolites of polycyclic aromatic hydrocarbon (PAH) pollutants in fish bile: a review. Environ Toxicol Pharmacol 30(3):224–244
Buchman MF (2008) Screening quick reference tables. NOAA OR&R Report 08-1, Seattle WA, Office of Response and Restoration Division, National Oceanic and Atmospheric Administration
Cailleaud K, Forget-Leray J, Souissi S, Hilde D, LeMenach K, Budzinski H (2007) Seasonal variation of hydrophobic organic contaminant concentrations in the water-column of the Seine Estuary and their transfer to a planktonic species Eurytemora affinis (Calanoida, copepod). Part 1: PCBs and PAHs. Chemosphere 70:270–280
Cappello T, Maisano M, Mauceri A, Fasulo S (2017) 1H NMR-based metabolomics investigation on the effects of petrochemical contamination in posterior adductor muscles of caged mussel Mytilus galloprovincialis. Ecotoxicol Environ Saf 142:417–422
Chaves PTC, Umbria SC (2003) Changes in the diet composition of transitory fishes in coastal systems, estuary and continental shelf. Braz Arch Biol Technol 46:41–46
Choueri RB, Cesar A, Torres RJ (2009) Integrated sediment quality assessment in Paranagua Estuarine System, Southern Brazil. Ecotoxicol Environ Saf 72:1824–1831
Dickhut RM, Canuel EA, Gustafson KE, Liu K, Arzayus KM, Walker SE, Edgecombe G, Gaylor MO, MacDonald EH (2000) Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay region. Environ Sci Technol 34(21):4635–4640. http://pubs.acs.org/doi/abs/10.1021/es000971e?journalCode=esthag-es000971eAF2
Duran R, Cravo-Laureau C (2016) Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. FEMS Microbiol Rev 40(6):814–830
FishBase (2015) A global information system on fishes. https://www.fishbase.de/home.htm. Accessed 15 Sept 2015
Froehner S, Martins RF (2008) Assessment of fate and bioaccumulation of benzo(a)pyrene by computer modeling. Quim Nova 31:1089–1093
Froehner S, Maceno M, Botelho D, Luz EC, Machado KS (2010) Distribution of polycyclic aromatic hydrocarbon in marine sediments and their potential toxic effect. Environ Monit Assess 168:205–213
Gobas FAPC (1993) A model for predicting the bioaccumulation of hydrophobic organic chemicals in aquatic food-webs: application to Lake Ontario. Ecol Model 69(1–2):1–17
Guzzella L, Roscioli C, Viganò L (2005) Evaluation of the concentration of HCH, DDT, HCB, PCB and PAH in the sediments along the lower stretch of Hugli estuary, West Bengal, northeast India. Environ Int 31(4):523–534
Herrling G (2003) Development of a numerical model for the Paranagua Bay in Brazil. Master Dissertation, University of Kiel
Jiao W, Wang T, Lu Y, Chen W, He Y (2014) Ecological risks of polycyclic hydrocarbons found in coastal sediments along the northern shores of the Bohai Sea (China). Chem Ecol 30(6):501–512
Kowalewska G, Konat-stepowicz J, Wawrzyniak-wydrowska B, Szymczak-zyła MS (2003) Transfer of organic contaminants to the Baltic in the Odra Estuary. Mar Pollut Bull 46(6):703–718
Kwok CK, Liang Y, Leung SY, Wang H, Dong YH, Young L, Giesy JP, Wong MH (2013) Biota–sediment accumulation factor (BSAF), bioaccumulation factor (BAF), and contaminant levels in prey fish to indicate the extent of PAHs and OCPs contamination in eggs of waterbirds. Environ Sci Pollut Res 20:8425–8434
Lei L, Khodadoust A, Suidan MT, Tabak HH (2005) Biodegradation of sediment-bound PAHs in field contaminated sediment. Water Res 39(2–3):349–361
Liu Y, Yu N, Li Z, Wei Y, Ma L, Zhao J (2012) Sedimentary record of PAHs in the Liangtan River and its relation to socioeconomic development of Chongqing, Southwest China. Chemosphere 89:893–899
Luz LP, Sanches Filho PJ, Sousa EEH, Kerstner T, Caramão EB (2010) Evaluation of surface sediment contamination by polycyclic aromatic hydrocarbons in colony Z3—(Patos Lagoon, Brazil). Microchem J 96(1):161–166
Maisano M, Cappello T, Oliva S, Natalotto A, Giannetto A, Parrino V, Battaglia P, Romeo T, Salvo A, Spanò N, Maucer A (2016) PCB and OCP accumulation and evidence of hepatic alteration in the Atlantic bluefin tuna, Thunnus thynnus, from the Mediterranean Sea. Mar Environ Res 121:40–48
Manodori L, Gambaro A, Piazza R (2006) PCBs and PAHs in sea-surface microlayer and sub-surface water samples of the Venice Lagoon (Italy). Mar Pollut Bull 52(2):184–192
Martins CC, Braun JAF, Seyffert BH, Machado EC, Fillmann G (2010) Anthropogenic organic matter inputs indicated by sedimentary fecal steroids in a large South American tropical estuary (Paranagua estuarine system, Brazil). Mar Pollut Bull 60(11):2137–2143
Martins CC, Bícego MC, Figueira RCL, Angelli JLF, Combi T, Gallice WC, Mansur AV, Nardes E, Rocha ML, Wisnieski E, Cheschim LMM, Ribeiro AP (2012) Multi-molecular markers and metals as traces of organic matter inputs and contamination status from an Environmental Protection Area in the SW Atlantic (Laranjeiras Bay, Brazil). Sci Total Environ 417–418:158–168
NOAA (1999) Sediment quality guidelines developed for the national status and trends program. http://www.noaa.gov/. Accessed 20 April 2016
Perugini M, Visciano P, Giammarino A, Manera M, Di Nardo W, Amorena M (2007) Polycyclic aromatic hydrocarbons in marine organisms from the Adriatic Sea, Italy. Chemosphere 66:1904–1910
Ranjan RK, Routh J, Ramanathan AL, Klump JV (2012) Polycyclic aromatic hydrocarbon fingerprints in the Pichavaram mangrove–estuarine sediments, southeastern India. Org Geochem 53:88–94
Ribeiro CAO, Katsumiti A, França P, Maschio J, Zandoná E, Cestari MM, Vicari T, Roche H, Assis HCS, Neto Filipak F (2013) Biomarkers responses in fish (Atherinella brasiliensis) of Paranagua Bay, Southern Brazil, for assessment of pollutant effects. Braz J Oceanogr 61(1):1–11
Rizzi J, Pérez-Albaladejo E, Fernandes D, Contreras J, Froehner S, Porte C (2017) Characterization of quality of sediments from Paranaguá Bay (Brazil) by combined in vitro bioassays and chemical analyses. Environ Toxicol Chem 36(7):1811–1819
Rondineli GR, Braga FMDS, Tutui SLDS, Bastos GCC (2007) Diet of Menticirrhus americanus (Linnaeus, 1758) and Cynoscion jamaicensis (Vaillant and Bocourt, 1883) (Pisces, Sciaenidae) in the State of Sao Paulo, Southeastern Brazil. Bol Inst Pesca 33(2):221–228
Silva TF, Azevedo DDA, Neto FRDA (2007) Distribution of polycyclic aromatic hydrocarbons in surface sediments and waters from Guanabara Bay, Rio de Janeiro, Brazil. J Braz Chem Soc 18(3):628–637
Silva TR, Lopes SRP, Spörl G, Knoppers BA, Azevedo DA (2013) Evaluation of anthropogenic inputs of hydrocarbons in sediment cores from a tropical Brazilian estuarine system. Microchem J 109:178–188
Soclo H, Garrigues PH, Ewald M (2000) Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: case studies in Cotonou (Benin) and Aquitaine (France) areas. Mar Pollut Bull 40(5):387–396
Soclo HH, Budzinski H, Garrigues P, Matsuzawa S (2008) Biota accumulation of polycyclic aromatic hydrocarbon in Benin coastal waters. Polycycl Aromat Comp 28:112–127
Stout S, Uhler A, Emsbo-Mattingly S (2004) Comparative evaluation of background anthropogenic hydrocarbons in surficial sediments from nine urban waterways. Environ Sci Technol 38:2987–2994
Taniguchi S, Montone RC, Bícego MC, Colabuono FI, Weber RR, Sericano JL (2009) Chlorinated pesticides, polychlorinated biphenyls and polycyclic aromatic hydrocarbons in the fat tissue of seabirds from King George Island, Antarctica. Mar Pollut Bull 58(1):129–133
Tölgyessy P, Miháliková Z (2015) Rapid determination of total lipids in fish samples employing extraction/partitioning with acetone/ethyl acetate solvent mixture and gravimetric quantification. Food Control 60:44–49
Torres RJ, Cesar A, Pastor VA, Pereira CDS, Choueri RB, Cortez FS, Morais RD, Abessa DMS, Nascimento MRL, Morais CR, Fadini PD, Del Valls Casillas TA, Mozeto AA (2014) A critical comparison of different approaches to sediment-quality assessments in the santos estuarine system in Brazil. Arch Environ Contam Toxicol 68:132–147
Waszak I, Dabrowska H (2009) Persistent organic pollutants in two fish species of Percidae and sediment from the Sulejowski Reservoir in central Poland. Chemosphere 75(9):1135–1143
Yunker MB, MacDonald RW, Vingarzan R, Mitchell RH, Goyettte D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zakaria MP, Takada H, Tsutsumi S, Ohno K, Yamada J, Kouno E, Kumatra H (2002) Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs. Environ Sci Technol 36:1907–1918
Zhang S, Zhang Q, Darisaw S, Ehie O, Wang G (2007) Simultaneous quantification of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and pharmaceuticals and personal care products (PPCPs) in Mississippi river water, in New Orleans, Louisiana, USA. Chemosphere 66(6):1057–1069
Zhou JL, Maskaoui K (2003) Distribution of polycyclic aromatic hydrocarbons in water and sediments of Daya Bay, China. Environ Pollut 121:269–281
Acknowledgements
SF thanks CNPq (National Council for Scientific and Technological Development) for the financial resources, Process 442422/2014-8, and 31138/2015-0. The authors thank the anonymous reviewers that help to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Froehner, S., Rizzi, J., Vieira, L.M. et al. PAHs in Water, Sediment and Biota in an Area with Port Activities. Arch Environ Contam Toxicol 75, 236–246 (2018). https://doi.org/10.1007/s00244-018-0538-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-018-0538-6






 ), sediments (
), sediments (
 ), plankton (
), plankton (
 ) and fish (
) and fish (
 ) sampled at Paranaguá Bay. The highest means significantly different were with the asterisk symbol (*)
) sampled at Paranaguá Bay. The highest means significantly different were with the asterisk symbol (*)