Abstract
Selective logging disrupts forests, changing their structure and species composition. Long-term monitoring helps in identifying the factors influencing it and aids in designing management plans. We conducted a quantitative re-assessment of trees ≥ 30 cm girth at breast height in four 1 ha plots in logged and two 1 ha plots in adjacent unlogged compartments of Uppangala forest continuum in the Western Ghats, India to compare the structural and compositional changes after a decade (2010–2021). Altogether, four species disappeared and three species were newly recruited. Mean species richness and stem density of both the forest sites decreased. Logged plots showed a slight increase in basal area (2.5%) and biomass (5.1%), whereas unlogged plots showed a decline in basal area (3.92%) and biomass (2.9%). As compared to unlogged plots, all the demographic rates were higher for logged forest sites. Across the six individual plots, the growth rates varied significantly owing to wood density and forest strata categories. Non-metric multidimensional scaling (NMDS) identified three groups with significant difference in species composition, where logged and unlogged plots have a distinct composition except for one plot. Although species richness and stem diversity remained stable, the species composition is different 37 years after logging, and the impacts of logging are still evident in the forest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The healthy functioning of natural ecosystems depends heavily on the intricate network of interactions between biotic and abiotic elements found in tropical forests (Flores & Staal, 2022). However, anthropogenic disturbances such as logging, land-use changes, and deforestation fragment tropical forests and exacerbate the edge effect (Chazdon et al., 2009; Foley et al., 2005; Landburg et al., 2021). These disturbances trigger the positive feedback mechanism of degeneration, leading to an increase in the frequency of natural disturbances (Gibson & Sodhi, 2011). As a response to disturbances, the community begins to alter the forest’s composition to accommodate secondary forest species with the maximum and rapid rates of carbon acquisition (Poorter & Bongers, 2006; Wright et al., 2005). Secondary tropical forests are expected to expand due to future disturbances and account for more than 50% of the world’s tropical forest area (Rozendaal et al., 2019; Wang et al., 2017). The disturbed forests emerge as a place for biodiversity conservation and play an active role in acquiring above ground biomass (AGB) (Poorter et al., 2016; Ribeiro et al., 2009). Given that selectively logged forests preserve significant biodiversity, carbon, and timber stocks, it is crucial to investigate the potential of these secondary forests and assess their recovery rate. Academics, environmental organizations, stakeholders, and legislators should pay closer attention to this “middle ground” between destruction and preservation (Putz et al., 2012; Arroyo-Rodríguez et al., 2017).
In forest ecological research, the process and pace of resilience of logged forests to the primary forest are critical. After logging, the rate and direction of forest succession are heavily influenced by the severity of the disturbance and the local ecological composition of the forest (Chazdon, 2003; Pickett et al., 1987). Understanding important ecosystem processes, such as growth, mortality, and recruitment rates, which determine the structure and functioning of forests, will aid in comprehending the sensitivity of tree growth to temporal changes and predicting the recovery period (Bin et al., 2012; Carreño-Rocabado et al., 2012; Winkler et al., 2015; Anderson‐Teixeira et al., 2022). Consequently, understanding them by long-term monitoring of secondary forests is essential for decreasing the significant speculative nature of the future contributions of tropical forests to the global carbon cycle (Arora et al., 2020).
Extensive research is conducted on the plant diversity and species composition of either primary or secondary forests (Roa-Fuentes et al., 2022). However, only a few comparative studies and long-term assessments are conducted in geographically similar regions of logged/disturbed and adjacent primary forests (Blanc et al., 2009; Chazdon et al., 2009, 2016; Jeyakumar et al., 2017). The majority of comparative research concentrated on drastic immediate changes caused by logging, with only a few studies dealing with long-term consequences (> 35 years). This lack of spatiotemporally extensive data on the potential of such forests over primary forests for carbon sequestration makes it challenging to understand the fate of the carbon pool. To fill the void, ecologists have established a Tropical managed Forests Observatory network of permanent sample sites in logged forests in three tropical regions (Dalmaso et al., 2020; Sist et al., 2015; Tian et al., 2022).
However, comparable investigations of logged and unlogged forests in the Indian tropical forest are limited and hence, the present study is of critical importance. Our research area, Uppangala, was subjected to selective logging once between 1974 and 1983 (harvested eight to thirteen dipterocarp trees per hectare) after dividing the forest continuum into 28 ha compartments (Pélissier et al., 1998). Pre-logging data is necessary for estimating the recovery period and comparing the impact; however, studies are undertaken without such information by assuming that the composition of the neighboring primary forest will have a similar composition (Rozendaal et al., 2019). Our study site lacks diversity data prior to logging, but inventories were made in the unlogged parts of the forest continuum to understand the post-logging dynamics of the logged and unlogged forest sites. After the immediate comparative study, Pélissier et al. (l.c.) discovered that there was no substantial difference in the dynamics of the two forest types and, as a result, anticipated that the logged forest would resemble the unlogged forest in 20 years.
Though most of the immediate short-term studies provide the response of the forest to the logging, we require better knowledge about the long-term impact of the logging on the system and the recovery time. Determining the recovery period will help to set the rotation period for viable silvicultural practices in comparable tropical forests. Neglecting to address this issue may lead to unsustainable forest resource extraction methods (Lindenmayer et al., 2000). After 27 years of logging, the initial census indicated the presence of compositional changes in both taxonomic and functional categories of the logged forest as a residual effect of logging (Jeyakumar et al., 2017). Therefore, the current study aims to document the forest dynamics of those logged and unlogged forests to understand the natural recovery process of the logged forest 37 years after the logging operation.
Objectives
Here, we use a 10-year field study (2010–2021) to compare a primary forest that has not been logged to one that has been once selectively logged (between 1974 and 1983) to examine how selective timber harvesting affects the richness, population dynamics, and community composition of tropical forests. The study’s defined goal is to respond to the following queries:
(1) After 10 years, do logged and unlogged forests show noticeable differences in species richness, biomass stand structure, and composition?
(2) What differences exist among forest systems regarding demographic parameters?
Study site
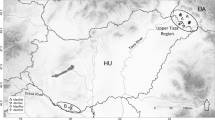
The Uppangala long-term monitoring plots (12°32′47 N latitude and 75°39′01E longitude) are situated, in the foothills of the steep western slope of the central Western Ghats, within the Pushpagiri Wildlife Sanctuary in Karnataka, India (Fig. 1). Natural vegetation of the lowland wet evergreen dipterocarp forest is identical to the west coast tropical evergreen forest type (Champion & Seth, 1968; Pascal, 1988). The annual average temperature and precipitation in this area are 22 °C (Fick & Hijmans, 2017) and around 5100 mm, respectively (rainfall data collected from Uppangala village between 1993 and 2021). Seasonal distribution of precipitation is regulated by the southwest monsoon, which receives 90% of the annual rainfall from June to October. Rainfall reaches its maximum from June to August (> 1000 mm) and minimum from December to March (< 100 mm). The soil belongs to the category of acidic and mineral-rich ferrallitic soil (Ferry, 1994; Loffeier, 1989).
Between 1974 and 1983, the forest was subdivided into segments of 28 ha each, and 237 to 359 (8–13 ha−1 individuals) big dipterocarp trees such as Dipterocarpus indicus and Vateria indica with a girth ≥ 180 cm were removed (Loffeier, 1988). To reduce damage, logged trees were pulled by elephants rather than by motorized skidding. For ease of transportation, logging activities were centered at lower elevations; however, preparations were also developed to harvest from higher elevations. In 1988, the Indian government restricted legal harvesting, and the higher elevation remained unaffected.
Methodology
In 2010 November–2011 April, four 1-hectare plots (LP1, LP2, LP3, and LP4) were established in logged forests (LP) and two 1-hectare plots (UP1 and UP2) in unlogged forests (UP) at elevations of 200–220 m, 250–270 m, 300–340 m, 380–420 m, 450–490 m, and 530–570 m respectively of the single forest continuum of floristic series — Dipterocarpus indicus-Kingiodendron pinnatifidum-Humboldtia brunonis (Jeyakumar et al., 2017; Pascal, 1988). However, UP2 was established in 1990 as a permanent sample plot. From the nearest human settlement, the individual plots are separated by distances of 0.8, 2.5, 3.0, 4.5, 5.5, and 5.7 km. Each one hectare plot was divided into 100 subplots of 10 m × 10 m dimension. Within each subplot, all trees with a girth of ≥ 30 cm at breast height (GBH) were tagged, measured, and species identified in 2010. The plots were re-inventoried from March to June, 2021.
Data analysis
The species richness and density of individual plots were compared between two censuses using a student t-test. Individual tree above-ground biomass (AGB; Mg ha−1) was calculated using the general allometric equation (Eq. 1) based on tree height-H (m), diameter-D (cm), and species wood density-ρ (g cm−3) (Chave et al., 2014):
Trees with buttresses or abnormalities at the point of measurement were measured above such features. The heights of trees in the re-inventory were calculated based on height-girth linear regression equations generated from the sampled trees in the initial census. For each species, the wood density was determined from several databases (Chave et al., 2009; Jeyakumar et al., 2017; Zanne et al., 2009).
The R package BIOMASS (version 2.1.8) was used to compute aboveground biomass using the “computeAGB” function and associated uncertainty in field measurement and AGB estimation using the “AGBmonteCarlo” function (Réjou-Méchain et al., 2017). We investigated uncertainty of AGB estimation after propagating errors such as diameter measurement, wood density, tree height, and allometric model as per Réjou-Méchain et al. (l.c.). The obtained mean AGB of these approaches was illustrated in Supplementary Information (Fig. S1). We used Spearman’s rank correlation to check the relationship between biomass gain, loss, and net change. Furthermore, distribution pattern of density, basal area, and biomass across the girth classes was examined using a chi-square test between forest types and time periods. Demographic rates were calculated using the CTFS R package (version 1.00). The annual growth rate was computed (Eq. 2) as the diameter change during the time interval and later converted as girth change for the uniformity:
where T is the time elapsed between the censuses, and dbh0 and dbht are the tree diameters measured during the first and second censuses, respectively (Condit et al., 2004). Stem gain and stem loss (recruitment and mortality) were calculated as a percentage of stems lost or gained per year in each forest type. Equation (3) was used to compute the mortality rate (m):
where N is the number of living trees at the first census, S is the number of survivors at the second census, and T is the time in years (Condit et al., 1995, 1999). Subsetting trees based on their initial girth and species, mortality was determined for girth classes.
The pace at which new stems under 30 cm joined the census was known as the recruitment rate (r), and it was determined by the Eq. (4):
where N1 is the number of trees alive at the second census, S is the number of trees that survived the first census, and T is the time between the two censuses in years (Condit et al., 1999). We compared demographic rates with wood density categories and different forest strata. Based on wood density, species were categorized into low (≤ 0.5 gcm−3), medium (> 0.5 ≤ 0.72 gcm−3), and high (> 0.72 gcm−3) (as per Melo et al., 1990; Nogueira et al., 2005). According to tree height, the vertical guild/strata of the forest was categorized into four classes: lower canopy (7–15 m tall), middle canopy (15–21 m tall), upper canopy (21–37 m tall), and emergent (> 37 m tall) (Pascal, 1988). The Scheirer–Ray–Hare test, a non-parametric variant of the two-way ANOVA, was used to determine the changes in demographic rates between forest sites, functional categories (based on wood density and strata), and their interaction (Scheirer et al., 1976) followed by Dunn’s Kruskal–Wallis multiple comparisons. The obtained p-values were corrected using the Benjamini–Hochberg approach.
To determine how the community composition changed over time, we performed non-metric multidimensional scaling (NMDS) analysis on the abundance matrix of trees in 25 subplots (20 × 20 m) within each hectare. We used Bray–Curtis dissimilarity index by employing R function “metaMDS” of the vegan package (version 2.6–2) (Oksanen et al., 2022; R Core Team, 2022). Permutation-based analysis of similarities (ANOSIM) was used to determine whether species assemblages differed significantly among individual plots using “anosim” function in the vegan package in R 4.1.3 (Oksanen et al., 2022; R Core Team, 2022).
Results
Dynamics of tree species composition
Tree species richness of Uppangala forest did not change significantly (p = 0.34) 10 years after first census. By the appearance of three additional tree species new to the study site (Melicope lunu-ankenda, Margaritaria indica, and Acrocarpus fraxinifolius) and the disappearance of four species (Agrostistachys borneensis, Grewia tiliifolia, Prunus ceylanica, and Strychnos nux-vomica) which were represented by only one individual, total species richness has declined from 126 to 125 (Appendix 1). The initial census reported 116 species from 39 families and 88 genera in LP and 68 species from 28 families and 56 genera in UP (Table 1). After 10 years, the number of species in LP decreased by one due to the recruitment of five additional species and the disappearance of six other species from the sites. However, the species richness increased by two in the UP due to the addition of three species and the disappearance of one. Plot-level species richness varied in a range of 53–73 in 2010 and 51–74 species in 2021.
Changes in stem density, basal area, and biomass
After a decade, the Uppangala forest re-census recorded 3571 tree stems, a decrease of 4.51% from the previous census (3740 stems) (Table 1). The mean stem density decreased in both LP (− 2.9%) and UP (− 7.3%) and the decline was significant at the plot level (p = 0.024). Individual plot density changes in LP ranged from − 41 to 3, but varied from − 72 to − 27 in UP. The girth size class distribution of trees showed a typical reversed J-shaped curve in both the forest sites, indicating a healthy and growing forest (Fig. 2). Thirty-seven years after the logging operation, though UP harbored higher average density of Dipterocarpus indicus and two-fold of Vateria indica compared to LP, density slightly increased in LP and decreased in UP.
There was no significant difference in the distribution of individuals across various tree size classes between forest sites during the study period (p > 0.05). There are more individuals in the lower size classes in both LP and UP, and the numbers in the following size categories decreased sharply after that. The shift to the next class is what causes the decline in stems after 10 years, irrespective of the fact that recruitment in the 30–60 girth class in LP was higher than mortality. Additionally, changes in density were mostly ascribed to the shift rather than mortality in the following size groups as well. However, in UP, the initial decline is ascribed to mortality, which is two times more than the recruited stems. Additionally, the shift to the subsequent class caused alterations in stem density.
Though the number of stems declined altogether, the basal area increased slightly after a decade. Variation in number of trees and basal area was seen in both LP and UP, but the variance was higher in LP than in UP (Table 1). The general trend of size class distribution of basal area in both the forest types is similar and not significantly different across time (p > 0.05). The lower girth classes reach high basal area owing to a high density of trees, then gradually declined, except for the large girth class. The final increase in the higher girth class is due to the existence of large trees despite of their number.
Overall biomass increased by 1.91% (55.48 Mg ha−1) over a 10-year period and it varied between the forest sites (Table 1). In LP, the average biomass increased by 22.3 Mg ha−1 in 10 years; however, in UP, it decreased by 16.8 Mg ha−1. The distribution of biomass stock among size classes varied significantly between forest sites in 2010 and 2021 (x2 = 45.30, df = 9, p = 8.09E−07; x2 = 38.68, df = 9, p = 1.32E−05). However, biomass stock of various size classes did not vary significantly within the sites (p > 0.05). Between 30 and 300 cm gbh, the average biomass acquisition of LP sites ranged from 22 to 49.39 Mg ha−1 in 2010 and 22.65 to 52.93 Mg ha−1 in 2021. Whereas in UP, it ranged from 25.91 to 54.93 Mg ha−1 in 2010 and 27.53 to 52.02 Mg ha−1 in 2021. However, LP had a twofold increase and UP had a fourfold increase in biomass in trees > 300 cm gbh (Fig. 3).
To ascertain which demographic process was most influential, biomass gains and losses were tested for association with biomass net changes (Table 2). The Spearman’s rho showed a negative correlation between biomass net changes and biomass loss (rho = − 1, p < 0.05) and a positive correlation between biomass net change and biomass gain (rho = 0.94, p = 0.004). In LP, mortality and recruitment rate were similar, but in UP, mortality rate was twice as high as the recruitment rate. Therefore, the biomass loss by tree death could not be compensated by recruitment (Table 2). This paradox was due to the fact that recruited trees only belonged to 30–60 gbh class, but dead trees belonged to several size classes.
Community-wide demographic trends
Growth
At the community level, annual growth rate of LP (0.392 mm gbh) was higher than UP (0.273 mm gbh) 10 years following the first census. In LP, the growth rate increased consistently until 210 cm gbh, then decreased, and finally exhibited a high growth rate in the larger girth classes. UP depicted a unimodal rightly skewed curve, which peaked in 210–240 cm gbh class (Fig. 4). The higher and lower growth rates of the large girth class in LP and UP, respectively, can be attributed by the abundance of Lophopetalum wightianum and Syzygium gardneri individuals.
Scheirer–Ray–Hare test revealed that growth rates differed significantly between forest sites and functional categories based on wood density and strata (Table 3). Invariably, all the three wood density categories of the unlogged plots had an almost similar growth rate (0.3 to 0.4 mm year−1) (Fig. 5a). However, in logged plots, it ranged from 0.4 to 1.0 mm year−1. The logged plot which is adjacent to the unlogged plots, LP4, showed a similar growth rate for those categories. Growth rate of various strata of each plot revealed a more or less similar pattern of significantly different and increasing trend from the lower canopy to emergent groups, except in two logged plots (LP2 and LP3) wherein the middle canopy showed a lower value when compared to the lower canopy category (Fig. 5b).
Mortality
A total of 487 trees have died during the 10-year time interval. Mean trees died in LP and UP were 82 and 79 respectively and individual plots’ tree mortality ranged from 63 to 102 in LP and 58–100 in UP (Table 2). In general, the annual mortality rate reached an equivalent level 10 years after the monitoring in both LP (0.14%) and UP (0.12%). However, death rates showed a notable variation among different size classes in different forest sites (Fig. 6). Logged and unlogged forests showed a similar trend in annual mortality rate until 120–150 gbh class. Later in LP, after the peak in 150–180 cm gbh, mortality rate constantly declined except for the 270–300 gbh class. However, in UP, all size classes exhibited a comparable mortality rate, with the exception of 150–210 and 270–300 girth classes.
Across the study plots, the mean mortality rate of species ranged 0.01–0.02 (Fig. 7a), except for LP2 and LP3. A higher mortality rate of 0.03% was recorded for LP2 and LP3 for low wood density. Also, LP2 registered a lower mortality rate for the medium and high wood density categories (Fig. 7a). However, the statistical analysis revealed that mortality rates did not differ significantly between forest sites and wood density categories (Table 3). Among the strata categories, the emergents and lower canopy of the logged forest alone showed significant difference (post hoc Dunn test; p = 0.01). Mean mortality rate for all the vertical guild categories fell under 0.03%year−1, except for the lower canopy species of plots LP2 and LP3 (Fig. 7b). One of the logged plots, LP4, had a mortality rate comparable to unlogged plots.
Recruitment
A total of 318 trees were recruited over 10 years. In LP, annual recruitment rate was twice as high (0.129% year−1) by recruiting a mean of 64.75 ha−1 trees, while UP had a rate of (0.067% year−1) by recruiting an average of 29.5 trees ha−1. The number of individuals recruited in LP ranged from 38 to 94, whereas in UP, it was 28–31 only (Table 2). Among the plots, the average recruitment rate of species exhibited a mixed tendency, with LP1, LP3, and UP2 recruiting more low wood density species than the other wood density categories (Fig. 8a). LP2 had higher recruitment of high wood density species. Plots LP4 and UP1 showed a low recruitment rate for low wood density species and it slightly increased as the value of wood density categories increased. Recruitment rates did not differ significantly between forest sites and wood density categories (Table 3). In the case of strata categories, post hoc Dunn test revealed a statistically significant difference in the recruitment rates of LP1 vs UP1 and LP2 vs UP1 (p = 0.04). Across the structural categories, all the plots had recruitment rates below 0.02%year−1 except for LP1 and LP2, wherein the upper canopy and emergent species showed higher recruitment rates, but they did not differ significantly (Fig. 8b).
Dynamics of community composition over a decade
The NMDS ordination found three slightly overlapping groups with two dimensions, and the stress (0.26) shows a weak coherence in the distribution of species composition (Fig. 9). A compositional difference is evident in the ordination pattern along the elevation from the right. LP1 is more isolated and different from the other plots, whereas LP2 and LP3, which are nearby plots, have a greater degree of similarity in species composition than LP4. Unlogged plots did not share compositional similarity with logged plots except LP4. All the plots had slight variation in the species composition after a decade. ANOSIM revealed that tree species composition significantly differed across all the individual plots (ANOSIM; Global R = 0.55, p < 0.001).
Discussion
Changes in species richness
Primary forests naturally exhibit species heterogeneity, and their assessments shed light on the ecological integrity of forests (Frelich & Reich, 1995). The two forest sites of Uppangala study area (i.e., logged and unlogged) displayed considerable species overlap with numerous generalist species, which were not unexpected given the tremendous species diversity of tropical forests. The selective removal of dominant emergent species such as Dipterocarpus indicus and Vateria indica had an impact on the tree communities. Logging opened up the canopy, providing a high-light habitat that is favorable for the growth and regeneration of species that cannot tolerate shade. The recruitment of more secondary succession/pioneer species to the logged areas is the primary reason we consistently found variations in species composition between the two forest sites. The current study provides evidence that logged forests (51–74 ha−1) have higher species richness than their unlogged counterparts (52–55 ha−1), and this finding is in line with the results of previous investigations from China, Congo and Borneo (Berry et al., 2010; Ding et al., 2012; Maicher et al., 2021). Even in the initial census, carried out 27 years after the logging activities, the number of species in Uppangala was high in logged plots. The secondary forest specialist species, however, are primarily responsible for this great richness. Even though it has a higher species richness than the UP, it will take time to return to its pre-logging state.
Logging often accompanied with a decline in the number of species in the initial few years (Cannon et al., 1998; Saiful & Latiff, 2014;). However, the temporal monitoring showed the initial decline in species richness followed by an increase to the pre-logging level (Hu et al., 2018; King & Chapman, 1983; Smith et al., 2005). Nevertheless, not all tropical secondary forests recover at the same pace. Studies have shown that even 31–48 years after the logging, the logged forests partially regained their pre-logging richness (Kartawinata et al., 1981; Chapman & Chapman, 2004; Garcia-Florez et al., 2017; Shima et al., 2018). In contrast, studies have also shown heavily logged forests regained their pre-logging level within 15 and 30 years (de Avila et al., 2015; Kariuki et al., 2006).
Dynamics of stem density, basal area, and biomass
A healthy tropical forest’s general tree size distribution trend is a reverse J-shaped curve, representing a high density in the smaller girth class. Size class distribution helps to understand the disturbance pertaining to the community and detect its regeneration pattern (Davis & Johnson, 1987; Denslow, 1995; Hitimana et al., 2004; Kigomo et al., 1990; Poorter et al., 1996). Selective harvesting has structural impacts that are still noticeable decades after harvest (Hawthorne et al., 2012). Despite this, both forest types follow the classical reverse J-shaped distribution curve, indicating healthy population, which is in line with studies conducted across tropics (Hitimana et al., 2004; Parthasarathy, 1999).
Stem density
Logging exposes forest floor to intense light, which helps to regenerate shade-intolerant pioneers (Silva et al., 1995). This will eventually increase the number of stems per unit area. However, the current study does not support the generally held assumption that logged forests are denser than unlogged forests (Ding et al., 2012). Conversely, high density of trees observed in the unlogged primary forest is also reported in yet other studies (Dionisio et al., 2018; Hall et al., 2003; Lévesque et al., 2011; Su et al., 2010). Even 37 years after the logging operation, the predicted density rebound in logged forests through recruitment that outpaces death over time did not take place. Demographic factors played a major part in this process: in LP, the effects of recruitment and mortality balanced each other out, resulting in a stem density that remained constant even after a decade. However, in UP, recruitment is considerably lesser than mortality, resulting in decreased stem density.
Basal area
The logged forest nonetheless exhibited several secondary forest structural traits, such as low basal area and above-ground biomass, compared to the unlogged forest. Reports of understocking in logged forests from Jamaica (Lévesque et al., 2011) support the current observation from the Uppangala forest. The average basal area of the unlogged forest (49.48 m2 ha−1; Table 1) falls within the range anticipated for undisturbed, well-stocked tropical mixed forests (45–55 m2 ha−1; Daniel et al., 1979; Alder & Synnott, 1992; Ding et al., 2012). In 2010, the mean basal area of logged forests was only 76.82% of unlogged forests; however, after 10 years, it increased to 81.99%. This is attributed to the increase in basal area of logged plots (1.32 m2 ha−1) and the decrease in basal area of unlogged plots (1.61 m2 ha−1) driven by the high mortality in UP that was not balanced by tree recruitment.
Biomass
The results of uncertainty of above ground biomass estimation indicate a more or less similar values in each plot (Fig. S1). The least variation of 2% was found in the plot LP3 to a maximum of 12% in plots LP1 and UP2. Among the four approaches, the model using the mix of directly measured and estimated heights of trees predicted comparatively lower AGB in the all the logged plots (except LP3), while the same model predicted higher AGB for the unlogged plots. This could be due to the fact that logged plots had comparatively more short statured trees than the unlogged plots (Jeyakumar et al., 2017), which indicates indirectly the presence of structural difference between the forest types.
In the present study, the average above-ground biomass for LP and UP was 436–458 Mg ha−1 and 578–561 Mg ha−1, respectively. When compared with studies from Amazonia (298 ± 51 Mg ha−1), French Guiana (356 to 398 Mg ha−1), and Paracou (388 to 443 Mg ha−1), biomass estimated from our study is greater (Baker et al., 2004; Chave et al., 2008; Rutishauser et al., 2010). However, the logged forest plots had an average of 19% lower biomass than the unlogged primary forest (Table 1). This is comparatively higher than the other tropical forests, where only 7.1–13.4% were reported with respect to the unlogged forest (Medjibe et al., 2013). The biomass recovery is reported to take 10–40 years (Ferreira & Prance, 1999; Lévesque et al., 2011). However, a study from Africa shows even after more than 200 years, the secondary forest could recover only 57% of the pristine forest biomass (Bauters et al., 2019). Nevertheless, unlike other studies, the present study did not recover the biomass even 37 years after logging. Given that the trees in the logged forests suffered a decrease in more number of high wood density trees than in the unlogged forest, and hence, it would require more time to recuperate the biomass as found in Vietnam (Nam et al., 2018).
However, when the logged forest increased in mean biomass over 10 years, the unlogged forest lost biomass. Though the mortality and recruitment rate was similar in LP, the recruited trees could not compensate for the biomass loss. Hence, the increase in LP is attributed to the growth of the trees. While in UP, the loss of biomass is ascribed to both the high mortality rate in the large girth classes and the poor recruitment rate, which was half that of the mortality rate (Fig. 6). A negative correlation between net biomass change and biomass loss and a significant positive correlation between biomass net change and biomass gain were evident, unlike the French Guiana results, which indicate a trend toward an association with biomass loss and a complete lack of a correlation with biomass gain (Rutishauser et al., 2010).
Dynamics of demographic rates
Growth rate
The present study reveals logged forests grow faster than the unlogged forest (0.392 mm gbh for LP and 0.273 mm gbh for UP). This is in conformity with other studies (de Avila et al., 2017; Peña-Claros et al., 2008; Sist & Nguyen-Thé, 2002). Further investigation based on the functional groups (Fig. 5) determined strata as the major contributor for significant difference in growth rates between forest sites (Table 3). But the present growth rate is relatively low compared to other tropical forests (0.2–1.11 cm dbh year−1) (Dionisio et al., 2018; Silva et al., 1995). A previous study in the same forest reported a higher growth rate (2.9 mm dbh for logged compartment and 2.1 mm dbh for the unlogged compartment) (Pélissier et al., 1998). This decrease in growth rate over time is in line with reports from Amazon (Dionisio et al., 2018; Silva et al., 1995). This is probably due to the closure of canopy over some time, which might reduce the light penetration. Across the size class distribution of growth rate (Fig. 4), LP showed faster growth than the UP except in the 210–240 cm gbh category. It is expected to have a higher growth rate in high girth classes in both forest types; however, the present study only found a high growth rate in logged forests as in accordance with research from the Amazon forest (Dionisio et al., 2018).
Mortality
According to Pélissier et al. (1998) in the Uppangala forest, 6 years after logging, there was a high mortality rate (0.9% year−1) in both logged and unlogged forest types. In the present study, 37 years after logging, the mortality rate decreased to 0.14% year−1 in LP and 0.12% year−1 in UP. This mortality rate is lesser than the global mortality rate of 1.77 ± 0.24% year−1 for tropical forests (Chave et al., 2008; Phillips & Gentry, 1994). However, this trend of decreasing mortality rate over time is consistent with the studies from the Eastern Amazonia (Dionisio et al., 2018; Silva et al., 1995). The time taken to stabilize between logged and unlogged was reported to vary from 4 to 11 years (Jonkers, 1982; deGraaf, 1986; Sist & Nguyen-Thé, 2002). When the previous study by Pélissier et al. (1998) reported a comparatively higher mortality rate for the emergents and upper canopy species in logged forests, presently, the trend changed to the lower canopy of logged plots and upper canopy of unlogged forests. There was a general decrease in emergent and canopy species abundance, with the UP experiencing a higher loss of 9 to 35 trees. However, only in LP2 individuals of emergent and canopy trees have increased. Possibly, the microenvironment may have provided enough illumination for the growth of shade-intolerant canopy plants, which, in turn, may be the reason for the more number of tree deaths of the pioneer understory species, Archidendron monadelphum.
Recruitment
Light is an important factor in plant growth of tropical forests, and its availability might change the natural dynamics of forest. The structure of the canopy is altered by logging, and logging gaps receive significantly higher light than the natural forest canopy (Inada et al., 2017). It affects the recruitment rate and changes the composition. The recruitment rate in the present study is meager compared to other tropical forests world over and the previous study from the same forest. A comparative study from 28 tropical regions reported a recruitment rate of 1.65 ± 0.26% year−1 for the tropics (Phillips & Gentry, 1994). A previous analysis by Pélissier et al. (1998), conducted 6 years after the logging operation in the same forest, reported a recruitment rate almost equal to it (1.68% in logged; 1.34% in unlogged). However, the present study has a very low recruitment rate (LP = 0.12%; UP = 0.06% year−1) which corroborates the results from Brazil, where the recruitment rate decreased over time (de Avila et al., 2017). As reported in other studies, logged forest has a high recruitment rate compared to unlogged forests (Sist & Nguyen-Thé, 2002).
Dynamics of community composition
Figuring out if the species composition of two distinct forest types became increasingly similar over time and whether the depiction of the ordination could explain the tendency was one of the goals of this study. The pace of species turnover and the habitat specialization play major role in the species composition of plant community (Tian et al., 2022). Logging-induced as well as natural forest dynamics alter these processes. The logged forest has more canopy openness and has higher light availability (Palma et al., 2021). This high light attracts more pioneer/secondary succession species to the site, which are fast-growing. In the logged plots, the present study revealed the occurence of pioneer/secondary succession species such Terminalia paniculata, Aporosa lindleyana, Archidendron monadelphum, Otonephelium stipulaceum, Croton malabaricus, and Macaranga peltata and their presence and abundance changed according to the elevation (Pascal, 1988). This observation of the dominance of pioneer species in logged forests is in accordance with studies all over the tropics (Chazdon et al., 2010; de Avila et al., 2017; Ganivet et al., 2020; Katovai et al., 2016; Su et al., 2010).
Based on the results of an initial tree census, logging was the primary determinant of species assemblage, followed by elevation and spatial distance (Jeyakumar et al., 2017). Hence, the coupled effect of logging and elevation has created the microenvironment and shifted the community composition, and NMDS ordination results agreed to this distinct compositional difference between logged and unlogged forests. A more disturbed low elevation plot (LP1) has an isolated and dissimilar species composition. However, the other two plots in the logged forest, which are nearby, have a distinctly shared composition. This makes the logged forest more heterogeneous than the unlogged counterpart (Graefe et al., 2020; Maicher et al., 2021). Studies from Borneo corroborate that, despite steady species richness and diversity, logged and unlogged forests have different species compositions (Hayward et al., 2021).
However, the unlogged forest plots share a distinct and mutually exclusive species composition, which differs from the logged forest except for LP4. After a decade, though not directional, both logged and unlogged plots show a slight variation from the first census. Nevertheless, recovering species composition to the pre-logging level seems a long-term process. Seeds of shade-tolerant species do not thrive in disturbed and heavy light environmental conditions (Norden et al., 2017). The similarity between the logged and unlogged forests is contributed mainly by the generalist species, which can spread over a wide range of areas (Pitman et al., 2001). But, the primary forest specialist species are the determinants of the primary forest composition (Tian et al., 2022). Due to the selective logging of the generalist species in the logged forest sites, more secondary forest specialist species have occupied. This caused the alteration of the species composition between logged and unlogged forest sites.
Conclusion
Our study offers important information on the species richness, structural attributes, biomass, demographic rates, and community compositional changes in logged and unlogged forests after 10 years in the tropical wet evergreen dipterocarp forest, Uppangala, central Western Ghats. A considerable shift in forest structure and composition was observed, a decade after the initial inventory. The logged forest displayed a higher species richness relative to the unlogged forest. However, due to resource extraction, logged forests have lower stem density, basal area, and above-ground biomass per hectare than unlogged forests. Nevertheless, basal area and biomass increased after a decade in logged forests, but decreased in the unlogged forest sites. We found that variations in the demographic parameters of trees, which varied based on tree size class, species, and type of forest, all of which caused changes in biomass. Although the death rate was similar in both forest types, the difference in recruitment rate explains the loss in biomass in the unlogged forest over the research period.
In tropical lowland wetevergreen forests of Uppangala censused after 10 years, both the logged and unlogged forests exhibited a significantly different and mutually and exclusive composition. However, restoration of species composition of the logged forest should not be the main objective of restoration, especially in tropical forests with significant carbon reserves. So, biomass recovery should be incorporated into management plans since secondary forests in tropical climates have enormous carbon storage and sequestration potential.
This information may help in developing conservation strategies and action plans for additional forest areas with comparable characteristics. More thorough and further long-term monitoring are needed to assess the capacity for carbon sequestration in the present state of climate change and human disturbances. A 10-year observation is insufficient while tracking a long-term process. Results such as the increased species richness in the logged plots are in line with other studies. However, other results, such as lower stem density in logged forests and declining biomass in the unlogged forest, were unanticipated, and they could be exclusive to the current location and could only be uncovered through long-term study. Continual monitoring of permanent plots in tropical forests and similar forests that have been understudied would help better understand the processes of forest dynamics, implementation of bioresource extraction strategies, and conservation of species.
Data availability
The datasets generated during and /or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alder, D., & Synnott, T. J. (1992). Permanent sample plot techniques for mixed tropical forest. Oxford Forestry Institute. Retrieved July 15, 2022, from http://www.fao.org/sustainable-forest-management/toolbox/tools/tool-detail/en/c/340781/
Anderson-Teixeira, K. J., Herrmann, V., Rollinson, C. R., Gonzalez, B., Gonzalez-Akre, E. B., Pederson, N., Alexander, M. R., Allen, C. D., Alfaro-Sánchez, R., Awada, T., Baltzer, J. L., Baker, P. J., Birch, J. D., Bunyavejchewin, S., Cherubini, P., Davies, S. J., Dow, C., Helcoski, R., Kašpar, J., Zuidema, P. A. (2022). Joint effects of climate, tree size, and year on annual tree growth derived from tree-ring records of ten globally distributed forests. Global Change Biology, 28(1), 245–266. https://doi.org/10.1111/gcb.15934
Arora, V. K., Katavouta, A., Williams, R. G., Jones, C. D., Brovkin, V., Friedlingstein, P., Schwinger, J., Bopp, L., Boucher, O., Cadule, P., Chamberlain, M. A., Christian, J. R., Delire, C., Fisher, R. A., Hajima, T., Ilyina, T., Joetzjer, E., Kawamiya, M., Koven, C., & C.,... Ziehn, T. (2020). Carbon–concentration and carbon–climate feedbacks in CMIP6 models and their comparison to CMIP5 models. Biogeosciences, 17(16), 4173–4222. https://doi.org/10.5194/bg-2019-473
Arroyo-Rodríguez, V., Melo, F. P., Martínez-Ramos, M., Bongers, F., Chazdon, R. L., Meave, J. A., Norden, N., Santos, B. A., Leal, I. R., & Tabarelli, M. (2017). Multiple successional pathways in human-modified tropical landscapes: New insights from forest succession, forest fragmentation and landscape ecology research. Biological Reviews, 92(1), 326–340. https://doi.org/10.1111/brv.12231
Baker, T. R., Phillips, O. L., Malhi, Y., Almeida, S., Arroyo, L., Di Fiore, A., Erwin, T., Higuchi, N., Killeen, T. J., Laurance, S. G., Laurance, W. F., Lewis, S. L., Monteagudo, A., Neill, D. A., Vargas, P. N., Pitman, N. C. A., Silva, N. M. J., & Vasquez Martinez, R. (2004). Increasing biomass in Amazonian forest plots. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 359(1443), 353–365. https://doi.org/10.1098/rstb.2003.1422
Bauters, M., Vercleyen, O., Vanlauwe, B., Six, J., Bonyoma, B., Badjoko, H., Hubau, W., Hoyt, A., Boudin, M., Verbeeck, H., & Boeckx, P. (2019). Long-term recovery of the functional community assembly and carbon pools in an African tropical forest succession. Biotropica, 51(3), 319–329. https://doi.org/10.1111/btp.12647
Berry, N. J., Phillips, O. L., Lewis, S. L., Hill, J. K., Edwards, D. P., Tawatao, N. B., Ahmad, N., Magintan, D., Khen, C. V., Maryati, M., Ong, R. C., & Hamer, K. C. (2010). The high value of logged tropical forests: Lessons from northern Borneo. Biodiversity and Conservation, 19(4), 985–997. https://doi.org/10.1007/s10531-010-9779-z
Bin, Y., Lin, G., Li, B., Wu, L., Shen, Y., & Ye, W. (2012). Seedling recruitment patterns in a 20 ha subtropical forest plot: Hints for niche-based processes and negative density dependence. European Journal of Forest Research, 131(2), 453–461. https://doi.org/10.1007/s10342-011-0519-z
Blanc, L., Echard, M., Herault, B., Bonal, D., Marcon, E., Chave, J., & Baraloto, C. (2009). Dynamics of aboveground carbon stocks in a selectively logged tropical forest. Ecological Applications, 19(6), 1397–1404. https://doi.org/10.1890/08-1572.1
Cannon, C. H., Peart, D. R., & Leighton, M. (1998). Tree species diversity in commercially logged Bornean rainforest. Science, 281(5381), 1366–1368. https://doi.org/10.1126/science.281.5381.1366
Carreño-Rocabado, G., Peña-Claros, M., Bongers, F., Alarcón, A., Licona, J. C., & Poorter, L. (2012). Effects of disturbance intensity on species and functional diversity in a tropical forest. Journal of Ecology, 100(6), 1453–1463. https://doi.org/10.1111/j.1365-2745.2012.02015.x
Champion, H.G., & Seth, S.K. (1968). A revised survey of forest types of India. Manager of publications.
Chapman, C. A., & Chapman, L. J. (2004). Unfavorable successional pathways and the conservation value of logged tropical forest. Biodiversity and Conservation, 13(11), 2089–2105. https://doi.org/10.1023/B:BIOC.0000040002.54280.41
Chave, J., Coomes, D., Jansen, S., Lewis, S. L., Swenson, N. G., & Zanne, A. E. (2009). Towards a worldwide wood economics spectrum. Ecology Letters, 12(4), 351–366.
Chave, J., Olivier, J., Bongers, F., Châtelet, P., Forget, P. M., van der Meer, P., Natalia Norden, N., Riéra, B., & Charles-Dominique, P. (2008). Above-ground biomass and productivity in a rain forest of eastern South America. Journal of Tropical Ecology, 24(4), 355–366. https://doi.org/10.1017/S0266467408005075
Chave, J., Réjou-Méchain, M., Búrquez, A., Chidumayo, E., Colgan, M. S., Delitti, W. B., Duque, A., Eid, T., Fearnside, P. M., Goodman, R. C., Henry, M., Martínez-Yrízar, A., Mugasha, W. A., Muller-Landau, H. C., Mencuccini, M., Nelson, B. W., Ngomanda, A., Nogueira, E. M., Ortiz-Malavassi, D., & Vieilledent, G. (2014). Improved allometric models to estimate the aboveground biomass of tropical trees. Global Change Biology, 20(10), 3177–3190. https://doi.org/10.1111/gcb.12629
Chazdon, R. L. (2003). Tropical forest recovery: Legacies of human impact and natural disturbances. Perspectives in Plant Ecology, Evolution and Systematics, 6(1–2), 51–71. https://doi.org/10.1078/1433-8319-00042
Chazdon, R. L., Broadbent, E. N., Rozendaal, D. M., Bongers, F., Zambrano, A. M. A., Aide, T. M., Balvanera, P., Becknell, J. M., Boukili, V., Brancalion, P. H. S., Craven, D., Almeida-Cortez, J. S., Cabral, G. A. L., Jong, B. D., Denslow, J. S., Dent, D. H., DeWalt, S. J., Dupuy, J. M., Durán, S. M., & Poorter, L. (2016). Carbon sequestration potential of second-growth forest regeneration in the Latin American tropics. Science Advances, 2(5), e1501639. https://doi.org/10.1126/sciadv.1501639
Chazdon, R. L., Finegan, B., Capers, R. S., Salgado-Negret, B., Casanoves, F., Boukili, V., & Norden, N. (2010). Composition and dynamics of functional groups of trees during tropical forest succession in northeastern Costa Rica. Biotropica, 42(1), 31–40. https://doi.org/10.1111/j.1744-7429.2009.00566.x
Chazdon, R. L., Peres, C. A., Dent, D., Sheil, D., Lugo, A. E., Lamb, D., Stork, N. E., & Miller, S. E. (2009). The potential for species conservation in tropical secondary forests. Conservation Biology, 23(6), 1406–1417. https://doi.org/10.1111/j.1523-1739.2009.01338.x
Condit, R., Aguilar, S., Hernandez, A., Perez, R., Lao, S., Angehr, G., Hubbell, S. P., & Foster, R. B. (2004). Tropical forest dynamics across a rainfall gradient and the impact of an El Nino dry season. Journal of Tropical Ecology, 20(1), 51–72. https://doi.org/10.1017/S0266467403001081
Condit, R., Ashton, P. S., Manokaran, N., LaFrankie, J. V., Hubbell, S. P., & Foster, R. B. (1999). Dynamics of the forest communities at Pasoh and Barro Colorado: comparing two 50–ha plots. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 354(1391), 1739–1748. https://doi.org/10.1098/rstb.1999.0517
Condit, R., Hubbell, S. P., & Foster, R. B. (1995). Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecological Monographs, 65(4), 419–439. https://doi.org/10.2307/2963497
Dalmaso, C. A., Marques, M. C., Higuchi, P., Zwiener, V. P., & Marques, R. (2020). Spatial and temporal structure of diversity and demographic dynamics along a successional gradient of tropical forests in southern Brazil. Ecology and Evolution, 10(7), 3164–3177. https://doi.org/10.1002/ece3.5816
Daniel, T. W., Helms, J. A., & Baker, F. S. (1979). Principles of silviculture. McGraw-Hill Book Company.
Davis, L. S., & Johnson, K. N. (1987). Forest management. McGraw-Hill Book Company.
de Avila, A. L., Ruschel, A. R., de Carvalho, J. O. P., Mazzei, L., Silva, J. N. M., Lopes, J. D. C., Araujo, M. M., Dormann, C. F., & Bauhus, J. (2015). Medium-term dynamics of tree species composition in response to silvicultural intervention intensities in a tropical rain forest. Biological Conservation, 191, 577–586. https://doi.org/10.1016/j.biocon.2015.08.004
de Avila, A. L., Schwartz, G., Ruschel, A. R., & do Carmo Lopes, J., Silva, J. N. M., de Carvalho, J. O. P., Dormann, C. F., Mazzei, L., Soares, M. H. M., & Bauhus, J. (2017). Recruitment, growth and recovery of commercial tree species over 30 years following logging and thinning in a tropical rain forest. Forest Ecology and Management, 385, 225–235. https://doi.org/10.1016/j.foreco.2016.11.039
deGraaf, N. R. (1986). A silvicultural system for natural regeneration of tropical rain forest in Suriname. Wageningen University and Research.
Denslow, J. S. (1995). Disturbance and diversity in tropical rain forests: The density effect. Ecological Applications, 5(4), 962–968. https://doi.org/10.2307/2269347
Ding, Y., Zang, R., Liu, S., He, F., & Letcher, S. G. (2012). Recovery of woody plant diversity in tropical rain forests in southern China after logging and shifting cultivation. Biological Conservation, 145(1), 225–233. https://doi.org/10.1016/j.biocon.2011.11.009
Dionisio, L. F. S., Schwartz, G., & do Carmo Lopes, J., & de Assis Oliveira, F. (2018). Growth, mortality, and recruitment of tree species in an Amazonian rainforest over 13 years of reduced impact logging. Forest Ecology and Management, 430, 150–156. https://doi.org/10.1016/j.foreco.2018.08.024
Ferreira, L. V., & Prance, G. T. (1999). Ecosystem recovery in terra firme forests after cutting and burning: A comparison on species richness, floristic composition and forest structure in the Jaú National Park, Amazonia. Botanical Journal of the Linnean Society, 130(2), 97–110. https://doi.org/10.1111/j.1095-8339.1999.tb00514.x
Ferry, B. (1994). Etude des humus forestiers de la région des Ghâts occidentaux: facteurs climatiques, édaphiques et biologiques intervenant dans le stockage de la matière organique du sol. Institut Français de Pondichéry.
Fick, S. E., & Hijmans, R. J. (2017). WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37(12), 4302–4315. https://doi.org/10.1002/joc.5086
Flores, B. M., & Staal, A. (2022). Feedback in tropical forests of the Anthropocene. Global Change Biology. https://doi.org/10.1111/gcb.16293
Foley, J. A., DeFries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., Chapin, F. S., Coe, M. T., Daily, G. C., Gibbs, H. K., Helkowski, J. H., Holloway, T., Howard, E. A., Kucharik, C. J., Monfreda, C., Patz, J. A., Prentice, C., Ramankutty, N., & Snyder, P. K. (2005). Global consequences of land use. Science, 309(5734), 570–574. https://doi.org/10.1126/science.1111772
Frelich, L. E., & Reich, P. B. (1995). Spatial patterns and succession in a Minnesota southern-boreal forest. Ecological Monographs, 65(3), 325–346. https://doi.org/10.2307/2937063
Ganivet, E., Unggang, J., Bodos, V., Demies, M., Ling, C. Y., Sang, J., & Bloomberg, M. (2020). Assessing tree species diversity and structure of mixed dipterocarp forest remnants in a fragmented landscape of north-western Borneo, Sarawak. Malaysia. Ecological Indicators, 112, 106117. https://doi.org/10.1016/j.ecolind.2020.106117
Garcia Florez, L., Vanclay, J. K., Glencross, K., & Nichols, J. D. (2017). Understanding 48 years of changes in tree diversity, dynamics and species responses since logging disturbance in a subtropical rainforest. Forest Ecology and Management, 393, 29-39. https://doi.org/10.1016/j.foreco.2017.03.012
Gibson, L., & Sodhi, N. S. (2011). Habitats at risk: A step forward, a step back. Science, 331(6021), 1137–1137. https://doi.org/10.1126/science.331.6021.1137-b
Graefe, S., Rodrigo, R., Cueva, E., Butz, P., Werner, F. A., & Homeier, J. (2020). Impact of disturbance on forest structure and tree species composition in a tropical dry forest of South Ecuador. Ecotropica, 22, 202002–202002. https://doi.org/10.30427/ecotrop202002
Hall, J. S., Harris, D. J., Medjibe, V., & Ashton, P. M. S. (2003). The effects of selective logging on forest structure and tree species composition in a Central African forest: Implications for management of conservation areas. Forest Ecology and Management, 183(1–3), 249–264. https://doi.org/10.1016/S0378-1127(03)00107-5
Hawthorne, W. D., Sheil, D., Agyeman, V. K., Juam, M. A., & Marshall, C. A. M. (2012). Logging scars in Ghanaian high forest: Towards improved models for sustainable production. Forest Ecology and Management, 271, 27–36. https://doi.org/10.1016/j.foreco.2012.01.036
Hayward, R. M., Banin, L. F., Burslem, D. F., Chapman, D. S., Philipson, C. D., Cutler, M. E., Reynolds, G., Nilus, R., & Dent, D. H. (2021). Three decades of post-logging tree community recovery in naturally regenerating and actively restored dipterocarp forest in Borneo. Forest Ecology and Management, 488, 119036. https://doi.org/10.1016/j.foreco.2021.119036
Hitimana, J., Kiyiapi, J. L., & Njunge, J. T. (2004). Forest structure characteristics in disturbed and undisturbed sites of Mt. Elgon Moist Lower Montane Forest, western Kenya. Forest Ecology and Management, 194(1–3), 269–291. https://doi.org/10.1016/j.foreco.2004.02.025
Hu, J., Herbohn, J., Chazdon, R. L., Baynes, J., Wills, J., Meadows, J., & Sohel, M. S. I. (2018). Recovery of species composition over 46 years in a logged Australian tropical forest following different intensity silvicultural treatments. Forest Ecology and Management, 409, 660–666. https://doi.org/10.1016/j.foreco.2017.11.061
Inada, T., Kitajima, K., Hardiwinoto, S., & Kanzaki, M. (2017). The effect of logging and strip cutting on forest floor light condition and following change. Forests, 8(11), 425. https://doi.org/10.3390/f8110425
Jeyakumar, S., Ayyappan, N., Muthuramkumar, S., & Rajarathinam, K. (2017). Impacts of selective logging on diversity, species composition and biomass of residual lowland dipterocarp forest in central Western Ghats. India. Tropical Ecology, 58(2), 315–330.
Jonkers, W. B. J. (1982). Options for silviculture and management of the mixed dipterocarp forest of Sarawak. Forest Department Sarawak.
Kariuki, M., Kooyman, R. M., Smith, R. G. B., Wardell-Johnson, G., & Vanclay, J. K. (2006). Regeneration changes in tree species abundance, diversity and structure in logged and unlogged subtropical rainforest over a 36-year period. Forest Ecology and Management, 236(2–3), 162–176. https://doi.org/10.1016/j.foreco.2006.09.021
Kartawinata, K., Abdulhadi, R., & Partomihardjo, T. (1981). Composition and structure of a lowland dipterocarp forest at Wanariset, East Kalimantan. Malaysian Forester, 44, 397–406.
Katovai, E., Sirikolo, M., Srinivasan, U., Edwards, W., & Laurance, W. F. (2016). Factors influencing tree diversity and compositional change across logged forests in the Solomon Islands. Forest Ecology and Management, 372, 53–63. https://doi.org/10.1016/j.foreco.2016.03.052
Kigomo, B. N., Savill, P. S., & Woodell, S. R. (1990). Forest composition and its regeneration dynamics; A case study of semi-deciduous tropical forests in Kenya. African Journal of Ecology, 28(3), 174–188. https://doi.org/10.1111/j.1365-2028.1990.tb01151.x
King, G. C., & Chapman, W. S. (1983). Floristic composition and structure of a rainforest area 25yr after logging. Australian Journal of Ecology, 8(4), 415–423. https://doi.org/10.1111/j.1442-9993.1983.tb01338.x
Landburg, G., Amatamsir, C., Muys, B., & Hermy, M. (2021). Medium and long term effects of logging systems on forest structure and composition in the tropical rainforest of Suriname. Journal of Forest Research, 26(5), 328–335. https://doi.org/10.1080/13416979.2021.1913305
Lévesque, M., McLaren, K. P., & McDonald, M. A. (2011). Recovery and dynamics of a primary tropical dry forest in Jamaica, 10 years after human disturbance. Forest Ecology and Management, 262(5), 817–826. https://doi.org/10.1016/j.foreco.2011.05.015
Lindenmayer, D. B., Margules, C. R., & Botkin, D. B. (2000). Indicators of biodiversity for ecologically sustainable forest management. Conservation Biology, 14(4), 941–950. https://doi.org/10.1046/j.1523-1739.2000.98533.x
Loffeier, M. E. (1988). Reconstitution après exploitation sélective en forêt sempervirente du Coorg (Inde). I: Méthodes et résultats préliminaires d’une étude floristique et structurale. Acta Oecologica/oecologia Generalis, 9, 69–87.
Loffeier, M. E. (1989). Sylviculture et sylvigenèse en forêt dense sempervirente du Coorg (sud-ouest de l’Inde), Institut Français de Pondichéry.
Maicher, V., Clark, C. J., Harris, D. J., Medjibe, V. P., & Poulsen, J. R. (2021). From town to national park: Understanding the long-term effects of hunting and logging on tree communities in Central Africa. Forest Ecology and Management, 499, 119571. https://doi.org/10.1016/j.foreco.2021.119571
Medjibe, V. P., Putz, F. E., & Romero, C. (2013). Certified and uncertified logging concessions compared in Gabon: Changes in stand structure, tree species, and biomass. Environmental Management, 51(3), 524–540. https://doi.org/10.1007/s00267-012-0006-4
Melo, J. E., Coradin, V. T. R., & Mendes, J. C. (1990). Classes de densidade de madeira para a Amazonia Brasileira. In Anais Do Congresso Florestal Brasileiro, 6, 695–699.
Nam, V. T., Anten, N. P., & van Kuijk, M. (2018). Biomass dynamics in a logged forest: The role of wood density. Journal of Plant Research, 131(4), 611–621. https://doi.org/10.1007/s10265-018-1042-9
Nogueira, E. M., Nelson, B. W., & Fearnside, P. M. (2005). Wood density in dense forest in central Amazonia. Brazil. Forest Ecology and Management, 208(1–3), 261–286. https://doi.org/10.1016/j.foreco.2004.12.007
Norden, N., Boukili, V., Chao, A., Ma, K. H., Letcher, S. G., & Chazdon, R. L. (2017). Opposing mechanisms affect taxonomic convergence between tree assemblages during tropical forest succession. Ecology Letters, 20(11), 1448–1458. https://doi.org/10.1111/ele.12852
Oksanen, J., Simpson, G. L., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O'Hara, R. B., Solymos, P., Stevens, M. H. H., Szoecs, E., Wagner, H., Barbour, M., Bedward, M., Bolker, B., Borcard, D., Carvalho, G., Chirico, M., De Caceres, M., Durand, S., Weedon, J. (2022). Vegan: Community ecology package. R package version 2.6–2.
Palma, A. C., Goosem, M., Fensham, R. J., Goosem, S., Preece, N. D., Stevenson, P. R., & Laurance, S. G. (2021). Dispersal and recruitment limitations in secondary forests. Journal of Vegetation Science, 32(1), e12975. https://doi.org/10.1111/jvs.12975
Parthasarathy, N. (1999). Tree diversity and distribution in undisturbed and human-impacted sites of tropical wet evergreen forest in southern Western Ghats. India. Biodiversity and Conservation, 8(10), 1365–1381. https://doi.org/10.1023/A:1008949407385
Pascal, J. P. (1988). Wet evergreen forests of the Western Ghats of India: Ecology, structure, floristic composition and succession. Institut francais de Pondichery.
Pélissier, R., Pascal, J. P., Houllier, F., & Laborde, H. (1998). Impact of selective logging on the dynamics of a low elevation dense moist evergreen forest in the Western Ghats (South India). Forest Ecology and Management, 105(1–3), 107–119. https://doi.org/10.1016/S0378-1127(97)00275-2
Peña-Claros, M., Fredericksen, T. S., Alarcón, A., Blate, G. M., Choque, U., Leaño, C., Licona, J. C., Mostacedo, B., Pariona, W., Villegas, Z., & Putz, F. E. (2008). Beyond reduced-impact logging: Silvicultural treatments to increase growth rates of tropical trees. Forest Ecology and Management, 256(7), 1458–1467. https://doi.org/10.1016/j.foreco.2007.11.013
Phillips, O. L., & Gentry, A. H. (1994). Increasing turnover through time in tropical forests. Science, 263(5149), 954–958. https://doi.org/10.1126/science.263.5149.954
Pickett, S. T. A., Collins, S. L., & Armesto, J. J. (1987). Models, mechanisms and pathways of succession. The Botanical Review, 53(3), 335–371. https://doi.org/10.1007/BF02858321
Pitman, N. C., Terborgh, J. W., Silman, M. R., Núñez, V., & P., Neill, D. A., Cerón, C. E., Palacios, W. A., & Aulestia, M. (2001). Dominance and distribution of tree species in upper Amazonian terra firme forests. Ecology, 82(8), 2101–2117. https://doi.org/10.1890/0012-9658(2001)082[2101:DADOTS]2.0.CO;2
Poorter, L., Bongers, F., van Rompaey, R. S., & de Klerk, M. (1996). Regeneration of canopy tree species at five sites in West African moist forest. Forest Ecology and Management, 84(1–3), 61–69. https://doi.org/10.1016/0378-1127(96)03736-X
Poorter, L., & Bongers, F. (2006). Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology, 87(7), 1733–1743. https://doi.org/10.1890/0012-9658(2006)87[1733:LTAGPO]2.0.CO;2
Poorter, L., Bongers, F., Aide, T. M., Almeyda Zambrano, A. M., Balvanera, P., Becknell, J. M., Boukili, V., Brancalion, P. H. S., Broadbent, E. N., Chazdon, R. L., Craven, D., de Almeida-Cortez, J. S., Cabral, G. A. L., de Jong, B. H. J., Denslow, J. S., Dent, D. H., DeWalt, S. J., Dupuy, J. M., Durán, S. M., & Rozendaal, D. (2016). Biomass resilience of neotropical secondary forests. Nature, 530(7589), 211–214. https://doi.org/10.1038/nature16512
Putz, F. E., Zuidema, P. A., Synnott, T., Peña-Claros, M., Pinard, M. A., Sheil, D., Vanclay, J. K., Sist, P., Gourlet-Fleury, S., Griscom, B., Palmer, J., & Zagt, R. (2012). Sustaining conservation values in selectively logged tropical forests: The attained and the attainable. Conservation Letters, 5(4), 296–303. https://doi.org/10.1111/j.1755-263X.2012.00242.x
R Core Team. (2022). R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. Retrieved July 21, 2022, from https://www.R-project.org/
Réjou-Méchain, M., Tanguy, A., Piponiot, C., Chave, J., & Hérault, B. (2017). Biomass: An r package for estimating above-ground biomass and its uncertainty in tropical forests. Methods in Ecology and Evolution, 8(9), 1163–1167. https://doi.org/10.1111/2041-210X.12753
Ribeiro, M. C., Metzger, J. P., Martensen, A. C., Ponzoni, F. J., & Hirota, M. M. (2009). The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for Conservation. Biological Conservation, 142(6), 1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021
Roa-Fuentes, L., Villamizar-Peña, L. A., Mantilla-Carreño, J. A., & Jaramillo, M. A. (2022). Functional diversity and species diversity in flooded and unflooded tropical forests. Acta Oecologica, 114, 103814. https://doi.org/10.1016/j.actao.2022.103814
Rozendaal, D. M., Bongers, F., Aide, T. M., Alvarez-Dávila, E., Ascarrunz, N., Balvanera, P., Becknell, J. M., Bentos, T. V., Brancalion, P. H. S., Cabral, G. A. L., Calvo-Rodriguez, S., Chave, J., César, R. G., Chazdon, R. L., Condit, R., Dallinga, J. S., de Almeida-Cortez, J. S., de Jong, B., de Oliveira, A., Poorter, L. (2019). Biodiversity recovery of neotropical secondary forests. Science Advances, 5(3), eaau3114. https://doi.org/10.1126/sciadv.aau3114
Rutishauser, E., Wagner, F., Herault, B., Nicolini, E. A., & Blanc, L. (2010). Contrasting above-ground biomass balance in a neotropical rain forest. Journal of Vegetation Science, 21(4), 672–682. https://doi.org/10.1111/j.1654-1103.2010.01175.x
Saiful, I., & Latiff, A. (2014). Effects of selective logging on tree species composition, richness and diversity in a hill dipterocarp forest in Malaysia. Journal of Tropical Forest Science, 188–202.
Scheirer, C. J., Ray, W. S., & Hare, N. (1976). The analysis of ranked data derived from completely randomized factorial designs. Biometrics, 32, 429–434. https://doi.org/10.2307/2529511
Shima, K., Yamada, T., Okuda, T., Fletcher, C., & Kassim, A. R. (2018). Dynamics of tree species diversity in unlogged and selectively logged Malaysian Forests. Scientific Reports, 8(1), 1–8. https://doi.org/10.1038/s41598-018-19250-z
Silva, J. N. M., de Carvalho, J. D., & do Ca Lopes, J., De Almeida, B. F., Costa, D. H. M., de Oliveira, L. D., Vanclay, J. K., & Skovsgaard, J. P. (1995). Growth and yield of a tropical rain forest in the Brazilian Amazon 13 years after logging. Forest Ecology and Management, 71(3), 267–274. https://doi.org/10.1016/0378-1127(94)06106-S
Sist, P., & Nguyen-Thé, N. (2002). Logging damage and the subsequent dynamics of a dipterocarp forest in East Kalimantan (1990–1996). Forest Ecology and Management, 165(1–3), 85–103. https://doi.org/10.1016/S0378-1127(01)00649-1
Sist, P., Rutishauser, E., Peña-Claros, M., Shenkin, A., Hérault, B., Blanc, L., Baraloto, C., Baya, F., Benedet, F., da Silva, K. E., Descroix, L., Ferreira, J. N., Gourlet-Fleury, S., Guedes, M. C., Harun, I. B., Jalonen, R., Kanashiro, M., Krisnawati, H., & Kshatriya, M.,& Yamada, T. (2015). The Tropical managed Forests Observatory: A research network addressing the future of tropical logged forests. Applied Vegetation Science, 18(1), 171–174. https://doi.org/10.1111/avsc.12125
Smith, R. G. B., Nichols, J. D., & Vanclay, J. K. (2005). Dynamics of tree diversity in undisturbed and logged subtropical rainforest in Australia. Biodiversity and Conservation, 14(10), 2447–2463. https://doi.org/10.1007/s10531-004-0215-0
Su, D., Yu, D., Zhou, L., Xie, X., Liu, Z., & Dai, L. (2010). Differences in the structure, species composition and diversity of primary and harvested forests on Changbai Mountain, Northeast China. Journal of Forest Science, 56(6), 285–293.
Tian, L., Letcher, S. G., Ding, Y., & Zang, R. (2022). A ten-year record reveals the importance of tree species’ habitat specialization in driving successional trajectories on Hainan Island. China. Forest Ecology and Management, 507, 120027. https://doi.org/10.1016/j.foreco.2022.120027
Wang, F., Ding, Y., Sayer, E. J., Li, Q., Zou, B., Mo, Q., Yingwen Li, Y., Lu, X., Tang, J., Zhu, W., & Li, Z. (2017). Tropical forest restoration: Fast resilience of plant biomass contrasts with slow recovery of stable soil C stocks. Functional Ecology, 31(12), 2344–2355. https://doi.org/10.1111/1365-2435.12925
Winkler, E., Marcante, S., & Erschbamer, B. (2015). Demography of the alpine pioneer species Saxifraga aizoides in different successional stages at the glacier foreland of the Rotmoosferner (Obergurgl, Otztal, Austria). Tuexenia, 35, 267–283.
Wright, I. J., Reich, P. B., Cornelissen, J. H., Falster, D. S., Groom, P. K., Hikosaka, K., Lee, W., Lusk, C. H., Niinemets, U., Oleksyn, J., Osada, N., Poorter, H., Warton, D. I., & Westoby, M. (2005). Modulation of leaf economic traits and trait relationships by climate. Global Ecology and Biogeography, 14(5), 411–421. https://doi.org/10.1111/j.1466-822x.2005.00172.x
Zanne, A. E., Lopez-Gonzalez, G., Coomes, D. A., Ilic, J., Jansen, S., Lewis, S. L., Miller, R. E., Swenson, N. G., Wiemann, M. C., & Chave, J. (2009). Global wood density database. Retrieved March 1, 2022, from https://www.worldagroforestry.org/output/wood-density-database?kid=8
Acknowledgements
We thank the Karnataka Forest Department for permission to carry out research work. VKW is indebted to Mr. Obayya and Mr. Manoj, Mr. Barathan for their assistance during the fieldwork. We thank the director of the French Institute of Pondicherry and Pondicherry University for their facilities and support for the research. We also thank two anonymous reviewers for their valuable suggestions.
Funding
This study was funded by Council of Scientific and Industrial Research (CSIR), Ministry of Science and Technology of India (grant no. 09/559(0147)/2019-EMR-I) through the Junior Research Fellowship.
Author information
Authors and Affiliations
Contributions
VKW, NP, and NA designed the study. VKW and NA contributed to the field work. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethical approval
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wilson, V.K., Ayyappan, N. & Parthasarathy, N. Decadal forest dynamics in logged and unlogged sites at Uppangala, Western Ghats, India. Environ Monit Assess 195, 66 (2023). https://doi.org/10.1007/s10661-022-10706-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10706-4