Abstract
The carbon storage and conservation value of old-growth tropical forests is clear, but the value of logged forest is less certain. Here we analyse >100,000 observations of individuals from 11 taxonomic groups and >2,500 species, covering up to 19 years of post-logging regeneration, and quantify the impacts of logging on carbon storage and biodiversity within lowland dipterocarp forests of Sabah, Borneo. We estimate that forests lost ca. 53% of above-ground biomass as a result of logging but despite this high level of degradation, logged forest retained considerable conservation value: floral species richness was higher in logged forest than in primary forest and whilst faunal species richness was typically lower in logged forest, in most cases the difference between habitats was no greater than ca. 10%. Moreover, in most studies >90% of species recorded in primary forest were also present in logged forest, including species of conservation concern. During recovery, logged forest accumulated carbon at five times the rate of natural forest (1.4 and 0.28 Mg C ha−1 year−1, respectively). We conclude that allowing the continued regeneration of extensive areas of Borneo’s forest that have already been logged, and are at risk of conversion to other land uses, would provide a significant carbon store that is likely to increase over time. Protecting intact forest is critical for biodiversity conservation and climate change mitigation, but the contribution of logged forest to these twin goals should not be overlooked.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forestry exports are a vital source of income to developing countries (worth > US$39 billion in 2006; Miles and Kapos 2008) and SE Asia has been prominent in this respect, with Indonesia, Malaysia and the Philippines together exporting more than 80% of all tropical timber during the latter decades of the twentieth century (Johns 1997; Table 5.2 in Gale 1998). In some regions, however, the economic benefits from forestry are now declining, because most forests outside protected areas have already been logged and are failing to recover sufficiently quickly. As a consequence, logged forests are coming under increasing pressure for conversion to other more profitable land uses (Laurance 2007).
One proposed mechanism to counter these economic incentives is to compensate developing countries for preserving carbon stores, through payments for Reduced Emissions from Deforestation and Degradation (REDD; Gullison et al. 2007; Canadell and Raupach 2008). This mechanism could also be widened to include biodiversity benefits of preserving forests intact, for instance by negotiating a premium for emissions reductions that reduce biodiversity losses, or through biodiversity agencies contributing funds to help develop REDD programs that best preserve biodiversity (Bekessy and Wintle 2008; Venter et al. 2009). However, the manner in which forests already degraded by logging should be included within such programs is uncertain (Neeff et al. 2006). Arguably, since protecting intact mature forests from destruction or degradation is the most effective way to both reduce carbon emissions and protect biodiversity (Venter et al. 2009), REDD funds should be used to expand protected areas containing mature forest rather than to preserve forest that has already been degraded (Putz and Redford 2009). Moreover there have been recent calls for a stricter definition of forests qualifying for credits (Sasaki and Putz 2009) and for more detailed ground-based information to allow funding to be targeted more effectively (Gibbs et al. 2007; Miles and Kapos 2008). Hence it is likely that protection of forests already degraded by logging will be given only a low priority by funding bodies and conservation agencies.
Within SE Asia, the island of Borneo is a centre of biodiversity and endemism (Woodruff 2010), where most remaining forests are within logging concessions (Meijaard and Sheil 2008) and timber extraction rates are among the highest globally (>150 m3 ha−1 in some cases; Putz et al. 2001; Sodhi et al. 2004). By 2010 all forest outside of conservation areas is likely to have been logged at least once, leading to severe pressure for conversion to oil palm Elaeis guinensis plantations or other uses (Laurance 2007; Wilcove and Koh 2010). Here we quantify the residual value of logged forests in northern Borneo in terms of their capacity both to support biodiversity and to store and sequester carbon. We sampled in the vicinity of Danum Valley Field Centre (DVFC) in Sabah, Malaysian Borneo, which is adjacent to a 438 km2 conservation area of unlogged forest surrounded by logged forest (Marsh and Greer 1992). Our sampling, together with published work from DVFC, includes >100,000 observations of individuals from 11 taxonomic groups and >2,500 species, covering up to 19 years of post-logging regeneration.
Study area
The Danum Valley Conservation Area (DVCA; 5°N, 117°50′E) is an area of strictly protected dipterocarp forest within the 9,730 km2 Yayasan Sabah Forest Management Area (YSFMA). Temperature (annual mean = 26.7°C) and rainfall (annual mean = 2,669 mm) in the area are typical of the moist tropics (Walsh and Newbery 1999). Most of the YSFMA, excluding the DVCA and two other large protected areas, has been logged with a modified uniform system (Whitmore 1984) in which stems of commercial species with a diameter > 60 cm were removed using high lead cable and tractor extraction methods.
Methods
Carbon accumulation
To estimate aboveground biomass (AGB) we used forest monitoring plots to measure tree diameters and an allometric equation to convert these to carbon stock values. We included the tissue density of each species’ wood as this is the most important factor, beyond tree diameter, in accurately determining a tree’s carbon content (Chave et al. 2005). We used data from 20 forest inventory plots divided between the DVCA and an adjacent area in the YSFMA that was logged in 1988 and 1989 (Fig. 1; plots sampled between June 2005 and July 2006; Berry et al. 2008). These data included trees ≥ 30 cm diameter surveyed over a total area of 20 ha, and trees ≥ 5 cm diameter surveyed over a total area of 2 ha. Plots were distributed throughout a 50 km2 study area and included areas recovering from natural disturbance events such as tree falls, and so our estimate of mean above-ground biomass in intact forest is likely to be robust.
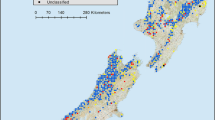
Locations of a Danum Valley Conservation Area (DVCA) and b sampling plots in unlogged forest (shaded) and logged forest (unshaded) around the Danum valley Field Centre (DVFC). Squares represent 1 ha plots where biodiversity was sampled; filled squares indicate plots that were also used for forest inventory
Estimates of wood density were obtained for 99.8% of trees sampled from an online database (ICRAF 2007) and published accounts (Köhler 1998; Suzuki 1999; Osunkoya et al. 2007). Species-level data were available for 59.8% of species. Where species-level data were not available, we used the mean for all Bornean species in the same genus (35.5% of trees) or family (5.3% of trees). Values that were reported as wood specific gravity were converted with the following equation (adapted from Chave et al. 2006): Wood Density = 1.147 (Wood Specific Gravity).
We estimated AGB per hectare for logged and unlogged forest with the allometric equation recommended for moist forests by Chave et al. (2005):
where A is the area of forest that was surveyed (ha), ρ i is the wood density (kg m−3) and D i is the diameter at breast height of tree i (m). We then assumed that 50% of biomass is carbon (Kitayama and Aiba 2002; Putz et al. 2008).
Subtracting the carbon stocks in both the amount of timber removed and the trees killed in the process (necromass or coarse woody debris) from our estimate of carbon stored in unlogged forest gives an estimate of the carbon stocks in logged forest immediately following logging. The measured carbon stocks in logged forest minus estimated stocks immediately after logging, divided by the number of years since logging, provides our estimate of the annual increase in carbon storage in logged forest. Estimates of necromass production were taken from a previous study in the YSFMA (Pinard and Putz 1996), using data for plots where logging methods were the same as in our study. However, the volume of timber extracted (154 m3 ha−1 on average) was about 60% higher than in our study and so we reduced the estimates accordingly, to estimate necromass resulting from logging in our study plots.
Biodiversity survey
We surveyed birds and ants over a 3-month period (February to April 2006) in 30 1-ha plots (Fig. 1), of which 15 were in unlogged forest and a further 15 in forest logged in 1988 or 1989. To survey birds, four 30-min point counts were carried out at each plot by one of the authors (DPE). Unknown bird vocalisations were recorded during point counts and were subsequently identified by comparison to recordings of known calls or by ornithological experts from the region. At each plot one count was carried out in early morning (06.15–09.00 h), one in late morning (09.30–12.30 h), one in late afternoon (15.30–17.00 h), and the fourth was assigned to one of the morning time-slots at random. Over-flying birds and detections outside the plot were not recorded.
For ground-dwelling ants, ten 1 m2 samples of leaf litter were collected from locations selected by stratified random sampling and separated by a minimum distance of 20 m within each plot. Sampling was carried out between 09.00 and 14.00 h, at least 24 h after any heavy rain. The litter was sieved (1 cm × 1 cm mesh) and placed in cloth bags for transportation to the laboratory where it was transferred to Winkler bags and left for 72 h to extract invertebrates (Bestelmeyer et al. 2000). Ants were identified to species where possible or assigned to morphospecies within genera by one of the authors (NBT). Voucher specimens are held at Universiti Malaysia Sabah and the California Academy of Sciences, USA.
In addition to these data, we obtained complimentary information from published papers together with data from PhD and MSc theses conducted at DVCA. This increased the range of taxa analysed while avoiding confounding factors related to studies comparing multiple sites, such as differences in the type of logging, sizes of forested areas or underlying biogeography. We examined 24 additional studies comparing primary forest in the DVCA with logged forest in the YSFMA, of which 12 studies presented information on species richness and abundances, whilst a further three studies provided autecological data on the abundances of individual species.
Statistical analysis
For studies with more than one sample plot in each habitat, species abundances were pooled among plots to give total abundances for logged and unlogged forest. To ensure comparability among different studies, and to account for the large differences in abundance and species richness across the different taxa, all differences between the two habitats were converted to percentages of the total number of species or individuals in unlogged forest. When species abundance information was available, rarefied species richness was calculated based on the number of individuals in the habitat with the lowest abundance in that study. To determine the statistical significance of differences in species richness between the two habitat types, observed differences were then compared to the differences in 999 permutations of the species abundance data with individuals assigned to habitat types at random (Solow 1993). To investigate the possibility of local extinctions of species following logging, we also examined the proportion of species unique to unlogged forest. To avoid biases due to under-sampling (Dent and Wright 2009) we restricted this analysis to species with ≥10 individuals sampled in total. For the best-studied taxa (birds and butterflies) we also looked within these data for species that are known to be threatened (IUCN 2007) or that are endemic to Borneo or Sundaland.
Results
We estimate that intact forest stored 138 Mg C ha−1 in above-ground biomass (Table 1). Eighteen years after logging, nearby forest stored 89 Mg C ha−1, 36% lower (Table 1). The initial reduction in above-ground biomass from the removal of timber and from logging-induced mortality is calculated to have been 53%. Thus the estimated rate of carbon sequestration in logged forest between 1989 and 2007 was 1.4 Mg C ha−1 year−1 (Table 1).
Across the 12 studies of nine taxa for which species abundance data were available, logging had opposite effects on flora and fauna (Fig. 2). Herbs and trees both showed a significant increase in rarified species richness in logged forest compared to unlogged forest whereas five of the seven animal taxa declined, with birds, ants, termites and butterflies all declining significantly in at least one study (Table 2). However, with the exception of termites (Donovan et al. 2007) and canopy-dwelling butterflies (Dumbrell and Hill 2005), declines in species richness following logging were typically no greater than ca 10% (Table 2). An average of 8% (range 0–33%) of species in primary forest were not recorded in logged forest but in the best-studied taxa (birds and butterflies) no restricted range or threatened species with n ≥ 10 in primary forest was absent from logged forest. Including studies without species abundance information increased our sample from nine to 11 taxa but produced qualitatively similar results, with recorded species richness being 14% lower in logged forest than in primary forest for fish (Martin-Smith 1998) and 14–33% lower in logged forest for moths (Holloway et al. 1992; Willott 1999).
Average percentage difference in rarefied species richness between logged and unlogged forest from studies that reported species abundance information (for a full list of studies included see Table 2). Error bars indicate the 95% confidence intervals from 999 randomisations with individuals assigned to logged or unlogged forest at random
The impacts of logging on species’ abundances varied among taxa (Fig. 3), with mammals, amphibians, ground-dwelling ants, termites and herbs all decreasing by ≥20%, whilst dung beetles and arboreal ants increased by ≥20% (Table 2). At species level, the responses of primates appeared idiosyncratic; for example, gibbons (Hylobates muelleri) were present in lower numbers but orang-utans (Pongo pygmaeus) were found at much higher abundance in logged forest, suggesting that they are well suited to the conditions in regenerating forest (Table 3).
Percentage difference in species abundance between unlogged and logged forest, with error bars ± 1 SE of mean for taxa represented by more than one study (for a full list of studies see Table 2)
Discussion
Our estimate of the aboveground biomass of live trees in unlogged forest around DVFC (276 ± 29 Mg ha−1; Table 1) was similar to two previous estimates in the YSFMA (323 ± 20 and 317 ± 27 Mg ha−1; calculated from Table 3 in Pinard and Putz 1996) but was much smaller than that estimated for dipterocarp forest in southwestern Borneo (430 Mg ha−1; Paoli et al. 2008). However, the latter figure was only partly corrected for overestimation due to systematic avoidance of forest gaps, and was similar to the estimated total biomass, including belowground biomass, necromass and biomass of non-woody plants, in the YSFMA (426 Mg ha−1; Table S2 in Putz et al. 2008).
We estimate that forests around DVFC lost 53% of AGB as a result of logging, which is consistent with losses indicated by other studies in Borneo (Pinard and Cropper 2000; Putz et al. 2008). Despite this high level of degradation, however, logged forest retained considerable residual value in terms of conserving biodiversity. Floral species richness was higher in logged forest than in unlogged forest, probably reflecting increased landscape-scale heterogeneity resulting from spatial variation in the intensity of disturbance due to logging (Hill and Hamer 2004; Berry et al. 2008). In contrast, faunal species richness was typically lower in logged forest, but in most cases the difference between habitats was no greater than ca 10%. This difference may be underestimated if canopy species not recorded in unlogged forest descend to lower heights in logged forest. In our study, however, there was little difference in the heights of the canopy in logged and unlogged forest (38 and 42 m, respectively in 1999–2000; Table 3 in Hamer et al. 2003) and so we are confident that this was not a problem. Species richness does not take account of changes in species composition but typically >90% of species recorded in primary forest were also present in logged forest, including species of conservation concern. In addition, there was no consistent pattern of changes in abundance following logging. These data accord with studies elsewhere indicating that whilst logged forest undoubtedly has lower conservation value than primary forest, logging has fewer adverse consequences for biodiversity than is often assumed (Dunn 2004; Meijaard et al. 2005; Dent and Wright 2009). A recent meta-analysis of SE Asian data indicated that the sensitivity of biodiversity to forest disturbance was moderately high (Sodhi et al. 2009). However, that analysis included clear-felling, fire and conversion to agriculture, all of which have much greater impacts than logging on biodiversity (Lawton et al. 1998; Barlow et al. 2007).
Following the initial loss of biomass due to logging, regenerating forests re-accumulated AGB at an average of 1.8% of this lost biomass each year (calculated from data in Table 1). Primary forests have also accumulated biomass over recent decades (Baker et al. 2004; Lewis et al. 2009) and in Borneo, AGB in Lambir Hills National Park increased by 0.2% year−1 between 1990 and 2000 (Chave et al. 2008). Against this background, the gain in AGB attributable to post-logging regeneration was about 2.4 Mg ha−1 year−1, equivalent to 1.6% of AGB lost through logging. These data suggest that even if the rate of recovery remained constant, the carbon deficit due to logging of these forests would persist for around 60–65 years. In practice, however, the annual rate of growth is likely to decline as the forest matures, and our data are compatible with a time-span of 120 years suggested by Pinard and Cropper (2000) from a physiologically based model of forest gap dynamics.
We estimate that during recovery, logged forest accumulated carbon in AGB at five times the rate of natural forest (1.4 and 0.3 Mg C ha−1 year−1, respectively). These are conservative estimates of total carbon accumulation because they do not include below-ground biomass, which would increase these totals by around 37% (Phillips et al. 2008), or increases in soil carbon stocks within logged forest as it recovers. Allowing the continued regeneration of extensive areas of Borneo’s forest that have already been logged, and are at risk of conversion to other land uses, would thus provide a significant carbon store that is likely to increase over time, providing a valuable contribution to meeting carbon dioxide mitigation targets. Moreover, this accumulation could be accelerated by management to rehabilitate logged forest, including planting of native tree species and cutting of climbers that retard tree regeneration and growth (Putz et al. 2001; Kettle 2010). Concern has been expressed that such carbon-based conservation could undermine the conservation of biodiversity (Putz and Redford 2009). However, recent evidence from our study area suggests that such rehabilitation can also benefit the recovery of biodiversity within logged forests (Edwards et al. 2009).
All IPCC emissions scenarios assume large reductions in carbon emissions from tropical deforestation over the coming decades (Nakicenovic et al. 2000). To achieve this aim, parties to the United Nations Framework Convention on Climate Change will need to design schemes that make the long-term protection of forests socially and economically attractive. The commitment by signatories to the Convention on Biodiversity to reduce the rate of biodiversity loss also requires concerted action to reduce tropical deforestation (Laurance 2007). Both these important policy objectives are more attainable through proper consideration of the full value of logged forest. We therefore urge that an emphasis on expanding the protection of intact forests should not result in the much more immediate threats to forests already degraded by logging being overlooked. In this respect we echo the sentiments of Meijaard and Sheil (2007) that a logged forest in Borneo is much better than no forest at all, and we urge that preventing logged forests being converted to oil palm or other crops should be a priority for policy makers and conservationists in Southeast Asia.
References
Ahmed A (2001) Frugivores and fruit production in primary and logged tropical rain forests. PhD Thesis, University Kebangsaan Malaysia, Selangor, Malaysia
Baker TR, Phillips OL, Mahli Y, Almeida S, Arroyo L, Fiore A et al (2004) Increasing biomass in Amazonian forest plots. Phil Trans R Soc Lond B 359:353–365
Barlow J, Gardner TA, Araujo IS, Avila-Pires TC, Bonaldo AB, Costa JE et al (2007) Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc Natl Acad Sci 104:18555–18560
Bekessy SA, Wintle BA (2008) Using carbon investment to grow the biodiversity bank. Conserv Biol 22:510–513
Berry NJ, Phillips OL, Ong R, Hamer KC (2008) Impacts of selective logging on diversity of trees across a Bornean rain forest landscape: the importance of spatial scale. Landsc Ecol 23:915–929
Bestelmeyer BT, Agosti D, Alonso LE, Brandao CRF, Brown WL Jr, Delabie JHC (2000) Field techniques for the study of ground-dwelling ants: an overview, description, and evaluation. In: Agosti D, Majer J, Alonso E, Schultz TR et al (eds) Ants: standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington, pp 122–144
Canadell JG, Raupach MR (2008) Managing forests for climate change mitigation. Science 320:1456–1457
Chave J, Andalo C, Brown S, Cairos MA, Chambers JQ, Eamus D, Fölster H, Fromard F, Higuchi N, Kira T, Lescure J-P, Nelson BW, Ogawa H, Puig H, Riéra B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99
Chave J, Muller-Landau HC, Baker TR, Easdale TA, ter Steege H, Webb CO (2006) Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecol Appl 16:2356–2367
Chave J, Condit R, Muller-Landau HC, Thomas SC, Ashton PS, Bunyavejchewin S et al (2008) Assessing evidence for a pervasive alteration in tropical tree communities. PLoS Biol 6:e45. doi:10.1371/journal.pbio.0060045
Colón CP (2002) Ranging behaviour and activity of the Malay civet (Viverra tangalunga) in a logged and an unlogged forest in Danum Valley, east Malaysia. J Zool Lond 257:473–485
Davis AJ, Holloway JD, Huijbregts H, Krikken J, Kirk-Spriggs AH, Sutton SL (2001) Dung beetles as indicators of change in the forests of northern Borneo. J Appl Ecol 38:593–616
Delaney AM (2007) Diversity in seedlings and saplings in logged and primary forest on Borneo. MRes Thesis, University of Leeds, Leeds, UK
Dent DH, Wright SJ (2009) The future of tropical species in secondary forests: a quantitative review. Biol Conserv. doi:10.1016/jbiocon.2009.05.035
Donovan SE, Georgianne JK, Griffiths RH, Winder L (2007) The spatial pattern of soil-dwelling termites in primary and logged forest in Sabah, Malaysia. Ecol Entomol 32:1–10
Dumbrell AJ, Hill JK (2005) Impacts of selective logging on canopy and ground assemblages of tropical forest butterflies: implications for sampling. Biol Conserv 125:123–131
Dunn RR (2004) Managing the tropical landscape: a comparison of the effects of logging and forest conversion to agriculture on ants, birds, and lepidoptera. For Ecol Manag 191:215–224
Edwards DP, Ansell FA, Ahmad AH, Nilus R, Hamer KC (2009) The value of rehabilitated logged rainforest for birds. Conserv Biol 23:1628–1633
Gale FP (1998) The tropical timber trade regime. MacMillan Press, London
Gibbs HK, Brown S, Niles JO, Foley JA (2007) Monitoring and estimating tropical forest carbon stocks: making REDD a reality. Environ Res Lett 2:045023
Gullison RE, Frumhoff PC, Canadell JG, Field CB, Nepstad DC, Hayhoe K, Avissar R, Curran LM, Friedlingstein P, Jones CD, Nobrell C (2007) Tropical forests and climate policy. Science 316:985–986
Hamer KC, Hill JK, Benedick S, Mustaffa N, Sherratt TN, Maryati M, Chey VK (2003) Ecology of butterflies in natural and selectively-logged forests of northern Borneo: the importance of habitat heterogeneity. J Appl Ecol 40:150–162
Heydon MJ, Bulloh P (1997) Mouse deer densities in a tropical rainforest: the impact of selective logging. J Appl Ecol 34:484–496
Hill JK, Hamer KC (2004) Determining impacts of habitat modification on diversity of tropical forest fauna: the importance of spatial scale. J Appl Ecol 41:744–754
Holloway JD, Kirk-Spriggs AH, Chey VK (1992) The response of some rain forest insect groups to logging and conversion to plantation. Phil Trans R Soc B 335:425–436
ICRAF (2007) Wood density database. World Agroforestry Centre, Nairobi, Kenya. http://www.worldagroforestrycentre.org/sea/Products/AFDbases/WD/
IUCN (2007) 2007 IUCN red list of threatened species. International Union for Conservation of Nature, Gland, Switzerland
Johns AD (1992) Vertebrate responses to selective logging: implications for the design of logging systems. Phil Trans R Soc B 335:437–442
Johns AG (1997) Timber production and biodiversity conservation in tropical rain forests. Cambridge University Press, Cambridge
Kettle C (2010) Ecological considerations for restoration of lowland dipterocarp forests in Southeast Asia. Biodivers Conserv. doi:10.1007/s10531-009-9772-6
Kitayama K, Aiba S-I (2002) Ecosystem structure and productivity of tropical rain forests along altitudinal gradients with contrasting soil phosphorous pools on Mount Kinabalu, Borneo. J Ecol 90:37–51
Köhler P (1998) Parameter research for the tropical rain forest growth model FORMIX4. Report P9801. Centre for Environmental Systems Research, University of Kassel, Germany
Lambert FR (1992) The consequences of selective logging for Bornean lowland forest birds. Phil Trans R Soc B 335:443–457
Laurance WF (2007) Have we overstated the tropical biodiversity crisis? Trends Ecol Evol 22:65–70
Lawton JH, Bignell DE, Bolton B, Bloemers GF, Eggleton P, Hammond PM et al (1998) Biodiversity inventories, indicator taxa and effects of habitat modification in tropical forest. Nature 391:72–76
Lewis SL, Lopez-Gonzalez G, Sonke B, Affum-Bafoe K, Baker TR et al (2009) Increasing carbon storage in intact African tropical forests. Nature 457:1003-U3
Magintan D (2000) Diversity, abundance and distribution of ground herbs in primary and selectively logged forest of Danum Valley (North Eastern Borneo). MSc Thesis, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia
Marsh CW, Greer AG (1992) Forest land-use in Sabah, Malaysia: an introduction to Danum Valley. Phil Trans R Soc B 335:331–339
Martin-Smith KM (1998) Effects of disturbance caused by selective timber extraction on fish communities in Sabah, Malaysia. Environ Biol Fish 53:155–167
Meijaard E, Sheil D (2007) A logged forest in Borneo is better than none at all. Nature 446:974
Meijaard E, Sheil D (2008) The persistence and conservation of Borneo’s mammals in lowland rain forests managed for timber: observations, overviews and opportunities. Ecol Res 23:21–34
Meijaard E, Shiel D, Nasi R, Augeri D, Rosenbaum B et al (2005) Life after logging. Reconciling wildlife conservation and production forestry in Indonesian Borneo. Centre for International Forestry Research, Jakarta
Miles L, Kapos V (2008) Reducing greenhouse gas emission from deforestation and forest degradation: global land-use implications. Science 320:1454–1455
Moura Costa P, Karolus A (1992) Innoprize Corporation Sdn Bhd Timber Extraction Volumes (1970–1991) Ulu Segama Forest Reserve. Innoprise, Kota Kinabalu, Malaysia
Nakicenovic N, Alcamo J, Davis G, De Vries B, Fenhann J, Gaffin S et al (2000) IPCC special report on emissions scenarios. Cambridge University Press, Cambridge
Neeff T, von Luepke H, Schoene D (2006) Choosing a forest definition for the clean development mechanism. Forests and Climate Change Working Paper 4. FAO, Rome
Osunkoya OO, Sheng TK, Mahmund N, Damit N (2007) Variation in wood density, wood water content, stem growth and mortality among twenty-seven tree species in a tropical rainforest on Borneo Island. Aust Ecol 32:191–201
Paoli GD, Curran LM, Slik JWF (2008) Soil nutrients affect spatial patterns of aboveground biomass and emergent tree density in southwestern Borneo. Oecologia 155:287–299
Phillips OL, Lewis SL, Baker TR, Chao K, Higuchi N (2008) The changing Amazon forest. Phil Trans R Soc Lond B 363:1819–1827
Pinard MA, Cropper WP (2000) Simulated effects of logging on carbon storage in dipterocarp forest. J Appl Ecol 37:267–283
Pinard MA, Putz FE (1996) Retaining forest biomass by reducing logging damage. Biotropica 28:278–295
Putz FE, Redford KH (2009) Dangers of carbon-based conservation. Glob Environ Change. doi:10.1016/jgloenvcha.2009.07.005
Putz FE, Sirot LK, Pinard MA (2001) Tropical forest management and wildlife: silvicultural effects on forest structure, fruit production, and locomotion of arboreal animals. In: Fimbel RA, Grajal A, Robinson G (eds) The cutting edge: conserving wildlife in logged tropical forests. Columbia University Press, New York, USA, pp 11–34
Putz FE, Zuidema P, Pinard MA, Boot RGA, Sayer JA, Shiel D, Sist P, Elias, Vanclay JK (2008) Improved tropical forest management for carbon retention. PLoS Biol 6:1368–1369
Sasaki N, Putz FE (2009). Critical need for new definitions of “forest” and “forest degradation” in global climate change agreements. Conserv Lett. doi:10.1111/j.1755-263x.2009.00067.x
Sodhi NS, Koh LP, Brook BW, Ng PKL (2004) Southeast Asian biodiversity: an impending disaster. Trends Ecol Evol 19:654–660
Sodhi NS, Lee TM, Koh LP, Brook BW (2009) A meta-analysis of the impact of anthropogenic forest disturbance on Southeast Asia’s biotas. Biotropica 41:103–109
Solow AR (1993) A simple test for change in community structure. J Anim Ecol 62:191–193
Suzuki E (1999) Diversity in specific gravity and water content of wood among Bornean tropical rainforest trees. Ecol Res 14:211–224
Venter O, Meijaard E, Possingham H, Dennis R, Sheil D, Wich S, Hovani L, Wilson K (2009) Carbon payments as a safeguard for threatened tropical mammals. Conserv Lett 2:123–129
Walsh RDP, Newbery DM (1999) The ecoclimatology of Danum, Sabah, in the context of the world’s rainforest regions, with particular reference to dry periods and their impact. Phil Trans R Soc Lond B 354:1869–1883
Whitmore TC (1984) Tropical rain forests of the Far East, 2nd edn. Clarendon Press, Oxford
Wilcove DS, Koh LP (2010). Addressing the threats to biodiversity from oil palm agriculture. Biodivers Conserv. doi:10.1007/s10531-009-9760-x
Willott SJ (1999) The effects of selective logging on the distribution of moths in a Bornean rainforest. Phil Trans R Soc B 354:1783–1790
Wong A (2006) The impact of forestry practices on frog communities in Sabah, Malaysia. MSc Thesis, Universiti Putra Malaysia, Kuala Lumpur, Malaysia
Woodruff D (2010) Biogeography and conservation in Southeast Asia: how 2.7 million years of repeated environmental fluctuations affect today’s patterns and the future of the remaining refugial-phase biodiversity. Biodivers Conserv. doi:10.1007/s10531-010-9783-3
Yoneda T, Yoda K, Kira T (1977) Accumulation and decomposition of big wood timber in Pasoh Forest, West Malaysia. Jpn J Ecol 27:53–60
Acknowledgments
We thank staff at the Danum Valley Field Centre for their assistance, especially Bernadus Bala Ola for help identifying seedlings, Alexander Karolus and Shaidih Samat for assistance with fieldwork, and Glen Reynolds for logistical support. Leopold Madani and Diwol Sundaling at the Forest Research Centre Sabah also assisted with seedling identification. Mike Gray helped collect and identify ants, and Aoife Delaney assisted with the seedling survey. We thank Yayasan Sabah, the Danum Valley Management Committee, the State Secretary, Sabah Chief Minister’s Department, and the Economic Planning Unit of the Prime Minister’s Department, Kuala Lumpur for permission to conduct research at Danum Valley. This study is part of the Royal Society’s Southeast Asia Rain Forest Research Programme (Project No. RS235) and was supported by a University of Leeds Earth and Biosphere Institute studentship. SLL was supported by a Royal Society University Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berry, N.J., Phillips, O.L., Lewis, S.L. et al. The high value of logged tropical forests: lessons from northern Borneo. Biodivers Conserv 19, 985–997 (2010). https://doi.org/10.1007/s10531-010-9779-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-010-9779-z