Abstract
In the present study, the water quality status of the Sadar block in Pratapgarh district of Uttar Pradesh, India, was calculated by Water Pollution Index (WPI) using 15 groundwater quality parameters (pH, turbidity, EC (electric conductivity), TDS (total dissolved solids), salinity, total hardness, Ca2+, K+, Mg2+, Na+, Cl−, F−, SO42−, HCO3−, and NO3−) at 40 different study locations. It is found that 10%, 25%, and 65%, of samples, fall under the good, moderately polluted, and highly polluted category, respectively. Irrigation suitability of groundwater has also been evaluated by using irrigation suitability parameters, i.e., Sodium Absorption Ratio (SAR), Residual Sodium Carbonate (RSC), Permeability Index (PI), Kelly Ratio (KR), Percentage Sodium (% Na), and Magnesium Hazards (MH). Based on the analysis, it was determined that most of the samples were suitable for irrigation in the area. Irrigation practices are found to be unsuitable for Kelly’s ratio of 37.5%, MH 60%, and RSC 55% samples. WPI and Pearson’s correlation matrix (PCA) have suggested a significant linear relationship among the water quality parameters based on the test results. The Gibbs plot indicates that the majority of samples fall under the “evaporation dominance” category while the rest fall under the “rock weathering dominance” suggesting that the complex geochemical mechanisms are active in the study area. This study would be very helpful to government authorities and it would aid them to suggest alternate drinking water resources in affected areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water quality is one of the major challenges in the world. It has a great impact on domestic and agricultural production and the standard of living for humans. Consumption of contaminated water leads to many health-related problems, i.e., dental fluorosis due to the presence of excess fluoride (Kumar, 2017), arthritis, high blood pressure caused by the presence of salinity in the drinking water (Vineis et al., 2011) (methemoglobinemia), blue baby syndrome (Sadeq et al., 2008) caused by the presence of excess nitrate, and hyperkeratosis, melanosis vascular diseases, and lung diseases due to arsenic (Tsuji et al., 2014). In India, it remains an urgent need to have safe drinking water as its 30% urban and 90% rural populations rely entirely on surface and groundwater that has not been treated (Singh et al., 2014). In India, groundwater sources provide more than 80% of the domestic water requirements, and about 50% of its industrial, municipal, and irrigation water supplies (Tiwari et al., 2018). Water quality is usually poor in waterlogged areas and shows the trend of increasing salinity, sulfate, carbonate, and bicarbonate ions in groundwater (Kumar & Saxena, 2011). Waterlogging is a result of paleochannels and oxbow lakes caused by the Sai river in the study area. Defining water quality requires monitoring and analysis of several physicochemical parameters (pH, EC, TDS, total hardness, total cation, and total anions) and identifying contaminant sources. Many of the Central Gangetic Plains face problems related to groundwater quality and water scarcity. In addition, water quality is also deteriorating due to pollution and over-exploitation of its resources through various anthropogenic activities. The current study focuses on the Pratapgarh district of Uttar Pradesh, a part of the Central Gangetic Plain. The groundwater in this region is mainly being utilized for drinking and irrigation purposes. There is a growing concern about the deterioration of groundwater quality due to geogenic and anthropogenic activities in this region. The Pratapgarh district covers an area of 3730 km2, mostly in the eastern portion of the Central Gangetic Plain of Uttar Pradesh. Kunda, Kalganj, Pratapgarh, Patti, and Raniganj are the five tehsils that make up the district, which is further divided into 17 development blocks. There has been an investigation conducted of the region spread over 193 km2 to better understand the problems faced by residents due to poor quality water consumption. The groundwater availability in the region is 4759.23 ha-m, and a groundwater draft of 5184.92 ha-m is marked under the overexploited category (CGWB, 2019–20). According to the previous study, different areas (Sadar, Baba Belkhernath, Mandhata, Sandva Chandrika) of Pratapgarh have severe problems with groundwater depletion due to over-exploitation. The water tables are deepening in the central Ganga plain due to overexploitation of water resources; it is caused by changes in land use and land cover patterns (Ahmad & Nigar, 2020). In the future, only deeper groundwater characterized by high levels of Na, Cl, and TDS will be available for drinking and irrigation. Several health-related problems, i.e., dental fluorosis, gastric problems, high blood pressure, arthritis, and so on, are evident in the region (Tiwari et al., 2018), which are largely caused by drinking bad quality water regularly. Many residents also reported difficulty in cooking, washing clothes, and daily use requirements, with the regular water they are getting in the region from other sources, i.e., packaged drinking water, a government-run water body (Jal Nigam Pratapgarh, available at a few locations in the block), and deep tube wells (depths greater than 200 feet). Sai river also passes through the block and many residents in the vicinity utilize its water for purposes other than drinking, during the rainy season. There are various methods used for the assessment of groundwater quality, including the National Sanitation Foundation Water Quality Index (Effendi, 2016; Lumb et al., 2011), the Water Quality Index of the Canadian Council of Ministers of the Environment (CCMEWQI), and the weighted arithmetic WQI method for the Oregon Water Quality Index (OWQI) (Singh et al., 2021; Tyagi et al., 2020). Other important methods are the Multi-Criteria Decision-Making (MCDM) Method (Akhtar et al., 2021), the Stream Health Index (SHI), a weighted WQI calculated by the Bureau of Indian Standards, and the landfill water pollution index (Singh et al., 2021). The WQI method has the disadvantage of using ideal values, especially for pH, where even a small change in pH results in significantly different groundwater quality. Hence, an integrated methodology (the Pollution Index of groundwater PIG) (Adimalla & Qian, 2019; Shukla & Saxena, 2021) and entropy weighted water quality index (Adimalla & Qian, 2019) can be used for the calculation of the groundwater quality index.
This study aims to conduct a complete evaluation of groundwater quality in the Sadar block of Pratapgarh district of the U.P, where the majority of the study locations are rural. During the field investigations, it was discovered that individuals are unaware of the water quality requirements and compliance standards with the water they consume. This research generates a WPI report for the Sadar block of Pratapgarh district, assessing the quality of groundwater for drinking and irrigation purposes. Continuous assessment of various groundwater contaminants removal provides chances for safe drinking water, and this might be achieved by integrating traditional knowledge with recent innovative technologies to develop future groundwater security.
Study area
Location, climate, and demography
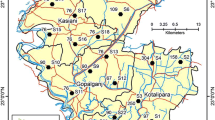
In the middle of the west Ganga plain, the study area Sadar block of Pratapgarh district lies between 25°48' N and 26° N latitudes and 81°52' E and 82°3' E longitudes (Fig. 1). It covers 193 km2, of which 61% is arable. Only 67% of this cultivable land is irrigated, while the rest is heavily dependent on monsoon rain (Mishra, 2018). According to the financial year 2019, the rural population of Sadar block is 209,727 (CGWB, 2019–20). Most of the area’s climate is tropical, with cold winters and warm summers. During January, the minimum and maximum temperatures range from 7.5 to 24.1 °C, respectively, making it the coldest month. The hottest month is May, with a daily maximum temperature of 42.5 °C and a minimum temperature of 26.4 °C. Approximately 90% of rainfall occurs from June to September, with an average annual rainfall of 1180 cm, a potential evapotranspiration rate of about 1400 mm, and a monthly relative humidity rate of 55.96% (Singh et al., 2016).
Geology, hydrology, and drainage features
The entire block is covered with unconsolidated Quaternary alluvium deposits, consisting mostly of fine to very fine sand Kanker intermixed along with varying amounts of calcareous nodules (Tiwari et al., 2018). In the study area 52 m below ground level (m bgl) depth, the quality of water is saline to very saline. The total groundwater availability in the block is 4759.23 ha-m, with the major sources of water for drinking and irrigation purposes being deep wells, tube wells, hand pumps, and river water. The village has 133 sources of drinking water, including wells, tube wells, hand pumps, and taps. The perennial river Sai, which runs through the heart of Pratapgarh district from west to east, flanks the block Sadar (Chilbila).
In the Jaunpur district, this river meets the Gomti river downstream and irrigates parts of the northern and southern Pratapgarh district. The district of Pratapgarh is dominated by agriculture and is the top fruit producer. It is one of the biggest cultivators of Aonla, owing to its plasticity due to growth in sandy, clayey, and loamy soil types in arid and semiarid climates, both deciduous and deep-rooted (Gantait et al., 2021). In salt-affected regions, Emblica officinalis has also proven to be a promising crop for commercial cultivation. In India, this tree covers as many as a million hectares of saline wastelands and ravine land (Gantait et al., 2021) and is an extremely valuable ingredient of various Ayurvedic medicines and an extremely rich source of Vitamin C in India.
Material and methods
Sample collection and analysis
Forty samples of groundwater (tube wells, hand pumps, dug well) were collected from some of the major locations during the pre-monsoon (Feb 2021) and post-monsoon (Jul 2021) seasons. To ensure accuracy and full confidence in the results, all the sampling bottles and glasses were soaked in a 5% nitric acid solution overnight, then rinsed 2–3 times with deionized water before sampling. Pre-washed 1-L polyethylene bottles with narrow mouths were used to collect the samples and GPS devices were used to determine the locations of all the 40 sampling points. These samples were immediately preserved and stored at 4 °C temperature, until analyzed (to prevent chemical reactions within the water) and transported to the laboratory of Shri Ramswaroop Memorial University, Barabanki, India, for further analysis. Physicochemical analysis of the samples was then performed. The pH, EC, TDS, and salinity properties of water can be measured directly in the field from hand pumps, dug wells, and tube wells, with a portable multi-parameter meter.
We used the titrimetric method (APHA, 2012) to analyze the carbonate, bicarbonate, and total hardness of the samples after the sample collection. In addition, the major cations potassium (K+), calcium (Ca2+), magnesium (Mg2+), and sodium (Na+) were analyzed by the titrimetric method (APHA, 2012), after calibrating the flame photometer.
Using the silver nitrate solution, the concentration of chloride (Cl−) was measured. Thereafter, ion-sensitive electrodes were used to measure fluoride (F−) and nitrate (NO3−), and sulfate (SO42−) was analyzed using an ultraviolet (UV) spectrophotometer as described in the American Public Health Association’s standard methods for the examination of water and wastewater (APHA, 2012). Table 1 illustrates the statistical analysis of each parameter.
Accuracy of analytical data
Using the analytical data, we assessed the accuracy of ionic measurements by:
-
(a)
Estimating the percentage error of ion balance (APHA, 2012, Shukla & Saxena, 2020; Chen et al., 2017).
-
(b)
Calculating the EC and TDS ratios for accuracy and precision for the analytical data.
In this case, anions and cations were mentioned in meq/L, and E is the error balance. The ideal value of percentage error of ion balance should be in the range of ± 5% for ideal water quality analysis. It was found that the groundwater samples exhibited a good charge balance within the range of ± 5%, except for five samples where the “E” value was 8.2, − 74, − 8, − 72, and 1, which was acceptable. The TDS/EC ratio (Shukla & Saxena, 2021) was within the range of 0.56 to 0.8, indicating high precision for the analytical data (APHA, 2012).
Water pollution index (WPI)
In the present study, we used the WPI proposed by Hossain and Patra (2020), which is one of the best methods based upon the difference between observed concentrations (Ci) and a standardized value (Si) divided by respective Si, to determine the increase or decrease in the pollution load (PLi). In the case of pH, the minimum Si limit is 6.5 for pH < 7, and the maximum Si limit is 8.5 for pH > 7 (Wang et al., 2021). The small variation in pH results in a significantly different (acidic or alkaline) groundwater quality.
The pollution load of groundwater can be calculated by the water pollution index (WPI), where 15 water quality parameters are used, pH, turbidity, EC (electric conductivity), TDS (total dissolved solids), salinity, total hardness, Ca2+, K+, Mg2+, Na+, Cl−, F−, SO42−, HCO3−, and NO3−.
For all the abovementioned parameters (other than pH), pollution load, PL, is calculated as:
where “i” represents different parameters of water quality, “Ci” represents the observed concentrations of the parameter, and “Si” represents the maximum value.
In the case of pH, the value of PLi computed from Eq. (2) is considered problematic when pH is less than or greater than 7; hence, pollution load for different pH scales is calculated as:
where the minimum Si limit is 6.5 when pH < 7, and the maximum Si limit is 8.5 when pH > 7.
Thereafter, the Water Pollution Index (WPI), with n number of parameters, can be calculated by taking an average of all pollution loads.
Irrigation suitability
A variety of ratios and parameters are used for agricultural water suitability, Sodium Absorbance Ratio (SAR), Residual Sodium Carbonate (RSC), sodium percentage (Na%), Magnesium Hazard (MH), Kelly Ratio (KR), and Permeability Index (PI). These indicators are explained as follows:
Matrix pearson’s correlation
The 15 physicochemical parameters have been used for Pearson’s correlation study. The degree of linear correlation between two parameters is represented by the correlation coefficient “r” (Kothari et al., 2021). The correlation coefficient “r” varies from − 1 (negative correlation) to + 1 (positive correlation). If the values of “r” are zero between the parameters, then there exists no correlation (Singh et al., 2021).
Chloro-alkaline indices
The values of CAI 1 and CAI 2 in (meq/L) are given by Schoeller (1977), and the values of these indices can be positive or negative. Chloro-alkaline indices (CAIs) estimate ion-exchange reaction between groundwater and aquifer materials. The negative or positive values of chloro-alkaline indices depend on whether the exchange of Na+ and K+ is from water, with Mg2+ and Ca2+ in rock/soil or vice versa. A positive CAI value indicates hardening (direct ion exchange). If the process of reverse ion exchange is occurring, the CAI value will be negative (Sunkari et al., 2021). The CAI value is close to zero when there is no ion exchange in the aquifer materials. The CAI 1 and CAI 2 can be calculated by the following equations:
Result and discussion
Tables 2 and 3 summarize the results of various physicochemical analyses of groundwater samples.
pH
The pH is one of the important physical parameters of groundwater. The acceptable limit of pH for drinking water is 6.5 to 8.4 (WHO, 11), and the range of pH values in the sample varies from 6.7 to 7.9 with a mean value of 7.26. According to World Health Organization (BIS, 2012), the groundwater in the study area is slightly alkaline.
Turbidity
Turbidity describes the optical properties of liquids and solids. The amount of light emitted is used to calculate the count of suspended particles in the water. The turbidity in the samples ranges from 0.9 to 122 NTU with a mean value of 10.1455 NTU, and this range is above the recommended level of 5 NTU.
Total dissolved solids (TDS)
The minimum and maximum TDS values in the sample ranged from 232 to 3040 mg/L with a mean value of 1020 mg/L. As per Indian standards (BIS, 2012), a water sample containing more than 500 mg/L of TDS is not suitable for drinking purposes. The elevated values of TDS in the sample are due to various types of minerals and organic matter dissolved in the water. Various salts, i.e., sodium, potassium, calcium, magnesium, bicarbonates, carbonates, chlorides, organic matter, phosphate, and other particles, might get dissolved in water. These parameters affect the osmoregulation of freshwater organics due to an increase in water density (Kumar, 2017; Saleem et al., 2018).
Electric conductivity (EC)
The minimum and maximum values of EC in the test samples varied from 446 to 5257 µS/cm. The permissible limit of EC in drinking water is 750 µS/cm with a mean value of 1938 µS/cm. The higher value of electric conductivity in water samples may be due to the presence of inorganic acids, bases, and salts. Various health-related issues, like the sour and unsuitable taste, arthritis, gastral and high blood pressure, hypertension, miscarriage among pregnant women, skin diseases, acute respiratory infection, renal failure, and pulmonary edema (Vineis et al., 2011), are caused due to ingesting salty water containing a large number of dissolved minerals. The most affected area which was severely affected by TDS and EC is PG33 (3045 mg/L and 5257 µS/cm) which indicates a high pollution load at this sampling site.
Total hardness (TH)
The total hardness (TH) of the analyzed groundwater varies from 160 to 1600 mg/L with a mean value of 593 mg/L, indicating moderately hard to very hard types of groundwater recorded in sample PG33 (Mohanganj village, Sadar block). The maximum permissible limit of hardness in groundwater is 500 mg/L. Intake of very hard water for a long time may result in an elevated occurrence of anencephaly, urolithiasis, prenatal mortality, a few forms of cancer, and cardiovascular diseases (Durvey et al., 1991; Tiwari et al., 2018).
Major anionic chemistry
The study area Sadar (Chilbila, Mohanganj) is flanked by the perennial river Sai; the water with long residence time led to leaching of the soil horizon and increases the major ionic concentration of Na, K, Cl, and HCO3 (Tiwari et al., 2017, 2018). The order of the anion abundance in groundwater was found to be HCO3− > Cl− > SO4− > NO3− > F−.
Bicarbonate (HCO3.−)
The concentration of bicarbonate varied from a minimum of 18 mg/L to a maximum value of 760 mg/L with a mean value of 274 mg/ L, which is mainly derived from the soil zone by carbon dioxide (CO2), dissolution of carbonates, and silicates minerals (Tiwari et al., 2018). The results from ion chemistry show that HCO3− is the leading anion in the study area associated with kankar or caliche formation. Bicarbonate levels may be higher due to the decomposition of rocks during heavy rainfall and groundwater movement (Mishra et al., 2016; Misra & Mishra, 2006).
Chloride (Cl.−)
The high chloride concentration of 35 mg/L to 1488 mg/L with a mean value of 608 mg/L suggests that the study area has been adversely affected by groundwater salinity. Chloride is an inorganic anion of great importance, and its presence in groundwater is most often caused by improper hygiene practices, methods of irrigation, improper disposal of untreated sewage, and linkage of the septic tank to underground water (Shukla & Saxena, 2020). People who are not accustomed to drinking water that contains high levels of Cl− will notice a salty taste and a laxative effect (Singh et al., 2008).
Sulfate (SO4.−)
According to WHO guidelines, the maximum permissible value of sulfate in water is 250 mg/L. The concentration of sulfate in the test sample in the study area varies between 28 and 2660 mg/L, with a mean value of 421 mg/L. Groundwater generally contains sulfate due to mineral deposition in the rock and untreated sewage discharge. In addition to pyrite, gypsum and anhydrite are also able to form sulfate in water due to oxidative weathering of sulfide minerals (Han et al., 2013). Drinking water with high sulfate levels can cause a bad taste and respiratory disorder (Rao, 1993).
Nitrate (NO3.−)
The concentration of NO3− in groundwater samples varies from 0.9 to 54 mg/L, with a mean value of 25.2 mg/L. According to WHO guidelines, the acceptable range of nitrate in drinking water is 45 mg/L. The high concentration of NO3− in village Sitkaha Mohanganj may be due to excessive application of nitrogenous fertilizer in the intensive cropping system (> 250%) and higher net irrigated area (Tiwari et al., 2017).
Fluoride (F.−)
The values of fluoride in the samples vary from 0.2 to 3.4 mg/L, with a mean value of 1.265 mg/L. The maximum concentration of F– was found at site PG33 (3.20 mg/L), where the geogenic sources could be the possible reason behind the increased level of fluoride. According to WHO guidelines, the acceptable range of fluoride is 1.5 mg/L in drinking water. Drinking water with F– a level greater than 1.5 mg/L may cause dental fluorosis (Gaikwad et al., 2020). During the public interaction in the study area, fluorosis was found to be a problem in several residents.
Major cationic chemistry
The order of cation abundance in groundwater was found to be Mg2+ > Na+ > Ca2+ > K+
Magnesium (Mg.2+)
The minimum and maximum values of magnesium vary from 11.6 to 116 mg/L, with a mean value of 45.6 mg/L. The elevated level of magnesium in drinking water causes heart ailments, high blood pressure, and even causes death (Singh et al., 2021).
Sodium (Na.2+)
The sodium concentration in groundwater is 8 mg/L to 192 mg/L, with a mean value of 64 mg/L, which suggests that the study area has been adversely affected by groundwater salinity. An excess of potassium can cause congenital diseases, kidney problems, and neurological issues (Adimalla & Qian, 2019; Shukla & Saxena, 2021) .
Calcium (Ca.2+)
The minimum and maximum values of calcium in groundwater were found between 12 and 201 mg/L with a mean value of 46 mg/L. The desirable limit of calcium in groundwater is 75 mg/ L. The calcium and magnesium ions are mainly released in water due to the weathering of silicate minerals (Tiwari et al., 2018).
Potassium (K.+)
Potassium is also one of the most important cationic parameters. The concentration of potassium in the sample varies from 1.2 to 289 mg/L, with a mean value of 13.6 mg/L in the study area. Potassium plays an important role in osmoregulation, but its exceeded level may have a bad impact on human health. According to Misra and Mishra (2006), the ions of Na+, K+, Cl−, and HCO3− are released directly into the soil through phosphate fertilizers (an important source of contamination of agricultural soil).
Water Pollution Index (WPI)
Water Pollution Index (WPI) is an indexing parameter of water quality. In the study area, it was found that the values of WPI vary from 0.6 to 7.8. Out of 40 groundwater samples, only 10% of samples were categorized as “good” quality water at sites PG1, PG3, PG21, and PG22, where the range varies from 0.5 to 0.75 (Table 4). In the area, 25% of samples were “moderately” polluted at sites PG2, PG4, PG5, PG11, PG16, PG17, PG18, PG19, PG20, and PG40, where the WPI varies from 0.75 to 1 (Table 4). Although the water at these moderately polluted sites was not suitable for drinking purposes, it can be used for cleaning and other such purposes. Sixty-five percent of the samples were classified as “highly polluted” at sites PG7, PG8, PG13, PG26, and PG33. The value of WPI at these sites was found to be greater than 1 and hence the water was not fit for drinking purposes. At site PG33, Mohanganj Sitkaha village, all the parameters were found to be more than the permissible limits, and an alarmingly high WPI value of 7.8 was noted. Samples with WPI > 1 (Hossain & Patra, 2020) are categorized as “highly polluted” and cannot be used for drinking, domestic purposes, and cattle feeding purposes. The correlation matrix verifies that WPI shows a good correlation with turbidity, salinity, EC, TH, TDS, F–, Ca2+, and Mg2+ (r = 0.81 to 0.88), suggesting the importance of these parameters in shaping the drinking water quality. Notably, the percentage of samples in “excellent” categories is not identified in the region. The WPI values may be classified, based on the “n” number of parameters into four different categories as mentioned in Table 4, and the water pollution index of these sample sites is graphically represented (Fig. 2).
Pearson’s correlation matrix
Fifteen physicochemical parameters have been used for the study of Pearson’s correlation. A strong correlation exists between TDS and EC, salinity, calcium, magnesium, and bicarbonate (r = 0.96), which indicates that the aquifer system is undergoing oxidation/reduction or positive direct ion exchange. TDS, salinity, chloride, and sulfate are all negatively correlated with pH. The fluoride has a good (r = 0.7–0.8) correlation with sulfate and bicarbonate, whereas hardness has a good correlation with calcium and magnesium. Having a strong or good correlation suggests that they are derived from the same source and exhibit similar geochemical behavior during different processes. The majority of these ions are derived from human activities, i.e., municipal sewage discharge, septic tanks, and the use of fertilizer (Singh et al., 2021). Potassium shows weak correlation (r = < 0.5) with all parameters. Major physicochemical analysis results of groundwater in the study area are expressed in Table 5.
Chloro-alkaline indices
The range of CAI 1 values in the study area varies from −0.82 to 39 meq/L with a mean value of 16.82 meq/L and CAI 2 values vary from 1.2 to 52 meq/L with a mean value of 21.50 meq/L (Table 1). In this process, Mg2+ and Ca2+ ions are released from aquifer materials. In the study area, 90% of samples show direction exchange. In this region, only 10% of samples (PG1, PG3, and PG18) show softening process due to the release of sodium and potassium ions from aquifer materials. The results of these processes are shown in Fig. 3.
Gibbs plot
Gibb’s plots are commonly used to recognize the composition of water with their respective aquifer attributes, evaporation dominance, which might be due to anthropogenic activities, and rock weathering dominance (mainly geogenic activities). When the sample falls between rock dominance and evaporation dominance zones, there will be the dominance of precipitation for groundwater. The Gibbs diagram represents the (Cl− + NO3−) / (Cl− + NO3− + HCO3−) and (Na+ + K+)/ (Na+ + K+ + Ca2+) ratio with reference to TDS. Figure 4 shows that 80% of samples fall under the evaporation dominance zone and the remaining fall under rock weathering dominance. There is no precipitation dominance in any of these samples. The physicochemical data on Gibbs’s plot shows the evaporation and rock weathering as a major motive power, mainly controlled by complex geochemical mechanisms in the study area. A similar analysis was conducted in a few other recent and previous studies (Baloch et al., 2021; Rashid et al., 2020).
Irrigation suitability of groundwater
Sodium absorbance ratio (SAR)
The sodium absorption ratio is an indexing parameter of water irrigation. In the study area, values of sodium absorption ratio vary from 1.3 to 22.8 (Table 6). Seventy percent of samples are in excellent categories, 25% samples are in good categories, and the remaining 5% are in doubtful categories. According to sodium absorption ratio, none of the samples fall into unfit categories. Ninety-five percent of samples are suitable for irrigation and only 12% of samples are unsuitable for irrigation purposes. In the groundwater, there is an increase in sodium absorption ratio values due to the combined effects of evaporation and calcite precipitation (Marghade et al., 2021).
Residual sodium carbonate (RSC)
The residual sodium carbonate is an indexing parameter of water irrigation, depending on bicarbonates, calcium, and magnesium. The range of RSC values in the study area varies from 0.4 to 14 (Table 6). Fifteen percent of samples are of good categories, 22% samples are in doubtful categories, and 55% samples are unfit for irrigation practices. Calcium and magnesium ions have less soluble content than carbonate and bicarbonate ions, as indicated by the positive values of RSC. By accumulating sodium carbonate in the soil, high RSC water might make it sterile (Marghade et al., 2021).
Sodium percentage (Na %)
It is an irrigation indexing parameter used for the irrigation suitability of water. In the study area, the range of Na% varies from 0.1 to 2.1 (Table 6). Only 37.5% of samples are of excellent categories, 37.5% of samples are good categories, 32.5% are of permissible categories, 22.5% are in doubtful categories, and no samples are identified in unfit categories for irrigation purposes in this region. In Fig. 5 Wilcox plot (Wilcox, 1955) between soluble sodium percentage and electric conductivity suggested that groundwater quality of the study area is in excellent to good and good to permissible categories, but some samples are also coming in doubtful to unsuitable categories. High sodium percentage (Na %) in the soil impacts the porosity of the soil texture, resulting in depletion of drainage capacity.
Magnesium hazard (MH)
The range of magnesium hazards varies from 36 to 69.6 (Table 6). In this region, due to the increased level of MH, only 40% of samples are in suitable categories and the other 60% are not suitable for irrigation purposes. A high percentage of magnesium ions in soil reduces the infiltration capacity and crop yield of soil, which may also lead to damage to the crop.
Kelly’s ratio
In the study area, Kelly’s ratio varies from 0.1 to 2.1 (Table 6). In this region, 63% of samples are suitable and fit for irrigation purposes, and the remaining 38% are in unsuitable categories that might be fit for irrigation due to sodium enhancement.
Permeability index (PI)
The value of the permeability index varies from 20 to 169 (Table 6). Doneen (1964) proposed three-class indexing for evaluating the suitability of water for irrigation. In this region, 40% of samples are of class first categories which are more suitable for groundwater, 57.5% in class second categories, and the remaining 2.5% samples are in class third categories which are considered less suitable for irrigation.
Conclusion
WPI and irrigation suitability indices were applied to evaluate the groundwater quality for drinking and irrigation purposes in the study area. The maximum water WPI was reported to be 7.8 from the sample collected at the Mohanganj Sadar block (site PG33). A total of 25% of samples were in the poor category and 65% of samples were in the very poor category according to WPI. All result analysis suggests that 65% of water is not suitable for drinking purposes due to the occurrence of higher concentrations of TDS, EC, calcium, and chloride in the groundwater of the area. The crop growth in the region will be adversely affected by higher irrigation indices. Geogenic and agricultural activities may be responsible for the contamination. However, no major industrial activity was seen in the Sadar block of Pratapgarh district that could cause water contamination. The ratio plot of (Cl− + NO3−) / (Cl− + NO3 − + HCO3−) and (Na+ + K+)/ (Na+ + K+ + Ca2+) with reference to TDS suggests evaporation and rock weathering dominant process that may be active in the study area. Using the chloro-alkaline index, it was found that 80% of the samples are under the influence of the hardening process.
Based on these findings, it appears that the rural and urban populations living in the study area are at risk for potential health problems from drinking the contaminated groundwater. For the implementation of effective water management programs, it is necessary to determine the geogenic and anthropogenic sources of water contamination. A groundwater contamination study would assist in spreading awareness about anthropogenic interventions. Moreover, it will raise awareness of the harmful effects of contaminated water among residents and medical professionals in rural areas. Thereafter, there is an urgent need to take some remedial measures for water pollution control and to improve the quality of water in the study area.
Data availability
Data shall be made available on request.
References
Adimalla, N., & Qian, H. (2019). Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicology and Environmental Safety, 176, 153–161. https://doi.org/10.1016/j.ecoenv.2019.03.066
Akhtar, N., Ishak, M. I. S., Ahmad, M. I., Umar, K., Md Yusuff, M. S., Anees, M. T., Qadir, A., & Ali Almanasir, Y. K. (2021). Modification of the Water Quality Index (WQI) process for simple calculation using the Multi-Criteria Decision-Making (MCDM) method: A review. Water, 13(7), 905. https://doi.org/10.3390/w13070905
Ahmad, S., & Nigar, M. S. (2020). Hydrochemical characteristics, groundwater quality and sources of solute in the Ramganga Aquifer. Central Ganga Plain, Bareilly District, Uttar Pradesh Journal Geological Society of India, 95, 616–625. https://doi.org/10.1007/s12594-020-1488-y
APHA. (2012). Standard methods for the examination of water and wastewater, American Public Health Association, Washington, DC, USA, 22nd edn.
Baloch, M. Y. J., Zhang, W., Chai, J., Li, S., Alqurashi, M., Rehman, G., Tariq, A., Talpur, S. A., Iqbal, J., Munir, M., et al. (2021). Shallow groundwater quality assessment and its suitability analysis for drinking and irrigation purposes. Water, 13, 3361. https://doi.org/10.3390/w13233361
BIS, Bureau of Indian Standards. (2012). Drinking water-specification, Second Revision, 2012. IS10500. Retrieved December 10, 2019, from https://cgwb.gov.in/Documents/WQ-standards.pdf
Central Ground Water Board (CGWB). (2019-20). http://cgwb.gov.in/wqreports.html
Chen, J., Wu, H., Qian, H., & Gao, Y. (2017). Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of northwest China. Export Health, 9, (3),183–195. https://doi.org/10.1007/s12403-016-0231-9
Doneen, L. D. (1964). Notes on Water Quality in Agriculture. Published as a Water Science and Engineering. Department of Water Sciences and Engineering, University of California, Davis. Paper 4001.
Durvey, V. S., Sharma, L. L., Saini, V. P., & Sharma, B. K. (1991). Handbook on the methodology of water quality assessment. Rajasthan Agricultural University.
Effendi, H. (2016). River water quality preliminary rapid assessment using Pollution Index. Procedia Environmental Sciences, 33, 562–567. https://doi.org/10.1016/j.proenv.2016.03.108
Gaikwad, S. K., Kadam, A. K., Ramgir, R. R., Kashikar, A. S., Wagh, V. M., Kandekar, A. M., Gaikwad, S. P., Madale, R. B., Pawar, N. J., & Kamble, K. D. (2020). Assessment of the groundwater geochemistry from a part of the west coast of India using statistical methods and Water Quality Index. HydroResearch, 3, 48–60. https://doi.org/10.1016/j.hydres.2020.04.001
Gantait, S., Mahanta, M., Bera, S., & Verma, S. K. (2021). Advances in biotechnology of Emblica Officinalis Gaertn. syn. Phyllanthus Emblica L.: A nutraceuticals-rich fruit tree with multifaceted ethnomedicinal uses. 3 Biotech, 11:62. https://doi.org/10.1007/s13205-020-02615-5
Han, Y., Wang, G., Cravotta, C. A., Hu, W., Bian, Y., Zhang, Z., & Liu, Y. (2013). Hydrogeochemical evolution of ordovician limestone groundwater in Yanzhou. North China Hydrol Process, 27(16), 2247–2257.
Hossain, M., & Patra, P. K. (2020). Water Pollution Index – A new integrated approach to rank water quality. Ecological Indicators, 117, 106668. https://doi.org/10.1016/j.ecolind.2020.106668
Kothari, V., Vij, S., Sharma, S., & Gupta, N. (2021). Correlation of various water quality parameters and water quality index of districts of Uttarakhand. Environmental and Sustainability Indicators, 9, 100093. https://doi.org/10.1016/j.indic.2020.100093
Kumar, A. (2017). Evaluation of water quality available for direct use and in beverages in Agra (India). Journal of Applied Chemistry, 6(6), 1229–1233. https://doi.org/10.1016/B978-0-12-815272-0.00006-4
Kumar, S., & Saxena, A. (2011). Chemical weathering of the Indo- Gangetic alluvium with special reference to release of fluoride in the groundwater, Unnao district. Uttar Pradesh Journal of the Geological Society of India, 77, 459–477. https://doi.org/10.1007/s12594-011-0048-x
Lumb, A., Sharma, T. C., & Bibeault, J. F. (2011). A review of genesis and evolution of Water Quality Index (WQI) and some future directions. Water Quality, Exposure, and Health, 3(1), 11–24. https://doi.org/10.1007/s12403-011-0040-0
Marghade, D., Malpe, D. B., Duraisamy, K. et al. (2021). Hydrogeochemical evaluation, suitability, and health risk assessment of groundwater in the watershed of Godavari basin, Maharashtra, Central India. Environmental Science and Pollution Research, 28, 18471–18494. https://doi.org/10.1007/s11356-020-10032-7
Mishra, A. (2018). Correlates of rural development: A case study of Sadar development block of Pratapgarh District in Uttar Pradesh. India Environmental & Socio-Economic Studies, 6(3), 19–23. https://doi.org/10.2478/environ-2018-0019
Mishra, S., Kumar, A., & Shukla, P. (2016). Study of water quality in Hindon River using pollution index and environmetrics. India Desalination and Water Treatment, 57(41), 19121–19130. https://doi.org/10.1080/19443994.2015.1098570
Misra, A. K., & Mishra, A. (2006). Groundwater quality monitoring in shallow and deep aquifers in Saidabad Tahsil Area, Mathura District. India Environmental Monitoring and Assessment, 117(1–3), 345–355. https://doi.org/10.1007/s10661-006-0993-9
Rao, N. S. (1993). Environmental impact of industrial effluents in groundwater regions of Visakhapatnam industrial complex. Indian Journal of Geology, 65, 35–43.
Rashid, A., Farooqi, A., Gao, X., Zahir, S., Noor, S., & Khattak, J. A. (2020). Geochemical modeling, source apportionment, health risk exposure and control of higher fluoride in groundwater of sub-district Dargai. Pakistan. Chemosphere, 243, 125409.
Sadeq, M., Moe, C. L., Attarassi, B., Cherkaoui, I., Elaouad, R., & Idrissi, L. (2008). Drinking water nitrate and prevalence of methemoglobinemia among infants and children aged 1–7 years in Moroccan areas. International Journal of Hygiene and Environmental Health, 211, 546–554.
Saleem, M., Hussain, A., Mahmood, G., & Waseem, M. (2018). Hydrogeochemical assessment of groundwater in shallow aquifer of greater Noida region, Uttar Pradesh (U.P), India. Applied Water Science, 8, 186. https://doi.org/10.1007/s13201-018-0828-1
Schoeller, H. (1977). Geochemistry of groundwater. In Groundwater studies an international guide for research and practice Ch. 15. UNESCO, Paris, pp.1–8.
Shukla, S., & Saxena, A. (2020). Water Quality Index assessment of groundwater in the Central Ganga Plain concerning Raebareli District, Uttar Pradesh, India. Current Science, 119(8), 1308. https://doi.org/10.18520/cs/v119/i8/1308-1315
Shukla, S., & Saxena, A. (2021). Appraisal of groundwater quality with human health risk assessment in parts of Indo-Gangetic Alluvial Plain, North India. Archives of Environmental Contamination and Toxicology, 80(1), 55–73. https://doi.org/10.1007/s00244-020-00771-6
Singh, A. K., Mondal, G. C., Kumar, S., Singh, T. B., Tewary, B. K., & Sinha, A. (2008). Major ion chemistry, weathering processes and water quality assessment in the upper catchment of Damodar River basin, India. Environmental Geology, 54, 745–758.
Singh, P., Saxena, A., Khan, R., & Shukla, S. (2021). Groundwater Quality Index assessment at Raebareli district: A part of Indo‐Gangetic Alluvium, India. Environmental Quality Management, tqem.21801. https://doi.org/10.1002/tqem.21801
Singh, P., Tiwari, A., & Singh, P. (2014). Hydrochemical characteristic and quality assessment of groundwater of Ranchi Township Area, Jharkhand, India. Current World Environment, 9(3), 804–813. https://doi.org/10.12944/CWE.9.3.30.
Singh, S., Rakshit, A., & Sivaranjani, S. (2016). Assessment of Water Quality Index of Pratapgarh district of Uttar Pradesh, India. Journal of Indian Water Resources Society, 36, 1.
Sunkari, E. D., Abu, M., & Zango, M. S. (2021). Geochemical evolution and tracing of groundwater salinization using different ionic ratios, multivariate statistical and geochemical modeling approaches in a typical semi-arid basin. J. Contam. Journal of Contaminant Hydrolog., 236, 103742. https://doi.org/10.1016/jconhyd.2020.103742
Tiwari, A. K., Singh, A. K., & Mahato, M. K. (2018). Assessment of groundwater quality of Pratapgarh district in India for suitability of drinking purpose using water quality index (WQI) and GIS technique. Sustainable Water Resources Management, 4, (3), 601–616. https://doi.org/10.1007/s40899-017-0144-1
Tiwari, A. K., Singh, A. K., Singh, A. K., & Singh, M. P. (2017). Hydrogeochemical analysis and evaluation of surface water quality of Pratapgarh district, Uttar Pradesh. India. Applied Water Science, 7(4), 1609–1623. https://doi.org/10.1007/s13201-015-0313-z
Tsuji, J. S., Perez, V., Garry, M. R., & Alexander, D. D. (2014). Association of low-level arsenic exposure in drinking water with cardiovascular disease: A systematic review and risk assessment. Toxicology, 323, 78–94.
Tyagi, S., Sharma, B., Singh, P., & Dobhal, R. (2020). Water quality assessment in terms of Water Quality Index. American Journal of Water Resources, 1(3), 34–38. https://doi.org/10.12691/ajwr-1-3-3
Vineis, P., Chan, Q., & Khan, A. (2011). Climate change impacts water salinity and health. Epidemiology and Global Health, 1, 5–10. https://doi.org/10.1016/j.proeps.2013.03.189
Wang, B., Wang, Y., & Wang, S. (2021). Improved Water Pollution Index for determining spatiotemporal water quality dynamics: A case study in the Erdao Songhua River Basin. China. Ecological Indicators, 129, 107931. https://doi.org/10.1016/j.ecolind.2021.107931
WHO. (2011). World health organization. (WHO), guidelines for drinking water quality, recommendation. Retrieved December 10, 2019, from, 978 (92)4:154815 1.
Wilcox, L. V. (1955). Classification and Use of Irrigation Waters (Arc 969). US Department of Agriculture.
Acknowledgements
The authors are thankful to Dr. A. K. Singh, Vice-Chancellor, Shri Ramswaroop Memorial University, for providing the laboratory facilities required for the conduction of this study. Our families are to be highly appreciated for being a constant motivation behind the conduction of this study.
Author information
Authors and Affiliations
Contributions
SM conducted the investigation, developed the suitable methodology and approach, and was involved with the writing (original draft) of the manuscript. AS provided supervision in the presented work and helped in the review and editing of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maurya, S., Saxena, A. Spatiotemporal assessment of groundwater quality in the Central Ganga Plain, India, using multivariate statistical tools. Environ Monit Assess 194, 865 (2022). https://doi.org/10.1007/s10661-022-10442-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10442-9