Abstract
In this study, we evaluate the geochemistry of macro- (Fe, P, Ca, S, K, Na, Mg) and micronutrients (Mo, Cu, Pb, Zn, Co, Mn, Cd, Sr, Cr, Hg, Se), along with possible health risks of heavy metals contamination in agricultural soils and vegetables of the Mahshahr industrial port in Iran. Calculation of geochemical coefficients revealed the low pollution load of Mahshahr agricultural soils. Most of the investigated elements exhibited lower concentrations in soil than international standards. Element concentrations in plant samples were far below the permissible values set by environmental agencies. Based on permissible values, there was deficiency of several soil elements, including Cu, in vegetables because they are mostly present in the soil residual phase. An exception was Mn, which is the most mobile element in soil. The transfer factor (TF) of elements showed the following trend: K > Na > P > S > Mo > Hg > Se > Zn > Cd > Cu > Mg > Mn > Ca > Cr > Co ≈ Fe = Pb. There was high transfer of major elements from soil to plants, and lower values for micronutrients and heavy metals. The calculated daily intake (DIM) and health risk index (HRI) for ten plant species for adults were < 1, while the HRI was larger than 1 for Mn via radish consumption. According to geochemical investigations and statistical tests such as principal component analysis, Kruskal–Wallis, and correlation coefficient calculations, plant species play the most important role in elemental uptake by plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants and especially vegetables are extremely important for the human diet as they contain vitamins, proteins, and essential metals such as zinc, iron, and other nutrients. They can also help the body during the digestion process (Khan et al., 2008, 2013; Jan et al., 2010; Dong et al., 2011; Hu et al., 2013; Alimohammadi et al., 2020). Humans’ life quality depends on composition of consumed food (Kabata-Pendias & Mukherjee, 2007). In needful amounts, some potentially toxic elements (PTEs, e.g., Zn, Mo, Cu, Ni, Se, Mn) are crucial for organisms to live as they contribute to bodily functions. However, excessive accumulation of such elements may cause several diseases (Nagajyoti et al., 2010; Adamo et al., 2014; Qureshi et al., 2016). For instance, high Ni ingestion can cause oxidative stress, skin rashes, fatigue, dizziness, neurotoxicity, and cancer (Ismail et al., 2019; Khan et al., 2010, 2013). On the other side, the deficiency of some metals can affect normal body functions. For example, Zn deficiency can lead to diarrhea, depression, immune dysfunction, and anorexia (Muhammad et al., 2011).
In recent years, the concentration of heavy metals has created a great public concern mostly due to their non-biodegradable nature, transfer from soil to the food chain, and long biological half-lives in the environment and human body (Chabukdhara & Nema, 2012; Esmaeili et al., 2014; Khan et al., 2016; Rastegari Mehr et al., 2016; Wang et al., 2015).
Beyond geogenic sources of heavy metals in soil, human activities such as irrigation with sewage effluent, petrochemical activities, use of fertilizers, pesticides, and livestock manures are considered as the most important anthropogenic sources (Nicholson et al., 2003; Liu et al., 2005; Liu et al., 2009; Luo et al., 2009; Jiao et al., 2012; Daldoul et al., 2015). Soil to plant transfer factor (TF) demonstrates metal mobility in soil with implications for human exposure to heavy metals through the food chain (Kachenko & Singh, 2006; Liu et al., 2009; Adamo et al., 2014). Free metal ions in soil solutions are the major determinants of bioavailability (Degryse et al., 2009; Daldoul et al., 2015). The mobility of heavy metals depends on different parameters including pH, organic matter (OM), soil texture, cation exchange capacity (CEC), and soil’s main constituents (e.g., phosphate, carbonate, clays) that impact their ability to adsorb metal ions (Adamo et al., 2014; Alloway, 1990; Ettler et al., 2007; Gupta & Sinha, 2006; Holm et al., 2003; Marcussen et al., 2009).
The main objective of this study is to evaluate the concentration of selected macro/micronutrients and heavy metals in vegetables and to determine their sources, transferability from soil to plants, and potential health risks for consumers. The study area is an industrial city with petrochemical complexes, in which vegetables are consumed as part of the daily diet of inhabitants, which is an ideal natural laboratory for such an investigation.
Materials and methods
Study area
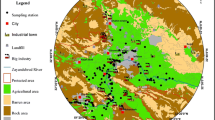
Mahshahr, also as (Bandar-e Mahshahr), is located in Khouzestan province (southwest Iran). The surface area is about 1908 km2. The geographic coordinates are 48´ 55º—49´ 22º E and 30´ 14º—30´ 56º N (Fig. 1). Mahshahr is famous for its petrochemical complexes and oil industries, which are mostly located in the suburbia. This area has an arid climate with an annual average temperature of 25.5 ˚C, (ranging from a minimum of 8.3 ˚C in January to a maximum of 45.6 ˚C in July). The average annual rainfall accumulation is 213.4 mm (Khuzestan Meteorological Organization, 2016). The soil type constitutes entisols and aridisols. The dominant wind direction is northwesterly (Rastegari Mehr et al., 2016).
The Maroon river circles the city of Mahshahr. According to geological reports, the study area is covered with alluvial deposits and quaternary sediments (Moore & Keshavarzi, 2014), leading to fertile soil suitable for farming. Therefore, vegetable cultivation is mostly practiced by people living in rural parts of this area. Hence, a large number of small farms are located on both sides of the river.
Sampling
In February 2016, a total of 72 crop samples including spinach (Spinacia oleracea), parsley (Petroselinum crispum), alfalfa (Medicago sativa), coriander (Coriandrum sativum), lettuce (Lactuca sativa), red and white radish (Raphanus sativus), dill (Anethum graveolens), leek (Allium ampeloprasum), garden cress (Lepidium sativum), and 26 associated top-soil samples (0–10 cm) were collected randomly at harvest time. The sampling locations are shown in Fig. 1. The sampling sites were selected based on land use patterns, field observations, cultivated farms, economic activity, the extent of fertile area, and plant species. Also, ten vegetable species samples were chosen depending on their consumption in the resident’s diet, harvesting rate, availability, affinity to accumulate heavy metals, and uniform dispersion in the area. Since each small farmland contains various vegetables, one soil sample is collected for available crop samples in each farm. A summary of crop types from which soil samples were collected is provided in Table S1.
At each sampling site, soil samples were prepared first by randomly collecting at least four subsamples. The subsamples were then mixed to form a composite sample of 1 kg using the quartile method. The samples were transferred to sealed polyethylene bags and to the laboratory. In the laboratory, soil samples were air-dried at room temperature and then sieved through a 63-µm screen mesh to determine macronutrients and trace metals, as metals concentrate more in smaller particles (Eby, 2016; Yao et al., 2015; Rastegari Mehr et al., 2016).
Fresh vegetable samples were collected in clean polyethylene bags along with growing soils and immediately transported to the laboratory. Samples were then thoroughly washed with tap water to remove dust particles, the edible part of each vegetable was air dried and subsequently ground using a pre-cleaned steel grinder. Finally, each crop powder was stored in a polyethylene zipped bag for analysis.
Both vegetables and corresponding soil samples were collected from the same agricultural fields. The samples were analyzed for the total concentration of macronutrients (Ca, P, Na, K, S and Mg) and trace metals (Mo, Cu, Pb, Zn, Ni, Co, Mn, Fe, Cd, Cr, Hg and Se) following aqua regia digestion (1:1:1 HNO3: HCl: H2O) using inductively coupled plasma mass spectrometry (ICP-MS) at Acme Analytical Laboratories, Ltd (Vancouver, Canada) (AQ250 code designed for soil, and VQ110 for vegetable samples). Soil pH and electrical conductivity (EC) were measured in aqueous suspensions (1:1 soil/water ratios) (Ryan et al., 2007). Cation exchange capacity (CEC) of soil was determined using 0.1 M NaCl according to the ion retention method of Schofield (1950). Soil organic matter and CaCO3 were measured using the muffle furnace method (Heiri et al., 2001). Sequential extraction is a widely used method for assessing the bioavailability of toxic metals in soil (Adamo et al., 2014). Since most of the area’s soil is high in carbonate, following the recommendations of Sulkowski and Hirner (2006), the BCR method of extraction was used in this study.
Data analysis
Enrichment factor
The most effective way to estimate metal pollution in the environment (Mohiuddin et al., 2011) and their source (Kamani et al., 2015) is to calculate enrichment factor using the following equation:
where CAl and Cx are the concentration of the reference element and selected element of interest, respectively, in true samples and background samples. Enrichment factor (EF) ˂ 3 indicates minor enrichment, 3–5 is moderate enrichment, 5–25 is severe enrichment, 25–50 is very severe enrichment, and ˃ 50 is extremely severe enrichment (Acevedo – Figueroa et al., 2006).
Transfer factor
The transfer factor is an index to describe the potential transfer of trace elements from soil to plants. It quantifies the ratio of metal concentration between plants and associated soil:
where C-plant and C-soil are the concentration of the elements in the plant and in the corresponding soil (mg kg−1, day weight), respectively (Khan et al., 2010; Lui et al., 2006; Cui et al., 2004; Adamo et al., 2014).
Daily intake of metals
The average daily intake of metals (DIM) was quantitatively evaluated by the following equation (Jan et al., 2010; Khan et al., 2008, 2010):
where C-metal and C-factor are metal concentration in plants (mg/kg) and the conversion factor (fresh crops into dry weight = 0.085), respectively (Jan et al., 2010). C-food intake is the average daily intake of all vegetable species, which is 0.232 and 0.345 kg/ person-day for children and adults, respectively (Khan et al., 2008, 2010; Rehman et al., 2019; Guadie et al, 2021). The average body weight for a child and an adult is considered to be 32.7 kg and 73 kg, respectively (Jan et al., 2010).
Health risk index
This index is a measure that indicates how much of daily consumption of these vegetables is dangerous for inhabitants:
DIM is daily intake of metals for an individual with characteristics described above. RFD (reference oral dose) is the tolerable daily intake of the contaminant. RFD values for Cu, Pb, Zn, Ni, Mn, Cr, Cd, and Hg are 0.04, 0.0035, 0.30, 0.02, 0.033, 1.5, 0.001, and 0.003, respectively (WHO, 1982, 1999; USEPA, 2006). A health risk less than 1 is assumed to be safe, while health risk index (HRI) ˃ 1 indicates adverse health effects (Khan et al., 2013).
Quality control and quality assurance
The quality control (QC) testing for elemental analysis relied on blank control samples, sample duplicates, and standard certified reference materials (STD OREAS45EA, STD DS-10 for soil and STD V16, STD CDV-1 for vegetable). According to Table 1, analytical results of the quality control samples indicated a satisfactory performance of element quantification within the range of certified values (94–120% recovery) for the studied elements in soils except for Hg (76.19%). Also, it is shown in Table 2 that the vegetable recovery percent varied from 88.7% for Ni to 105.6% for Fe, which is within the specified limits.
Statistical analysis
Data were statistically analyzed using SPSS software (version 21). To reduce a set of original variables, PCA was employed as a multivariate analytical tool (Golobocanin et al., 2004). Cluster analysis was used to classify the object into categories. Lastly, to determine crop species effects on element adsorption, the Kruskal–Wallis test was used.
Results and discussion
Physicochemical characteristics of soil samples
The results of each soil parameter and summary statistics of major and trace element concentrations of the investigated agricultural soils are presented in Table 1. According to the Harmonized World Soil Database (2008), the soil samples showed neutral to slightly alkaline properties with an average pH of 7.36 (range: 6.95 to 7.76). Since the Canadian Council of Ministers for the Environment (CCME, 1991) has estimated agricultural soils pH values to range between 6 and 8, the study area has natural agricultural soil. According to Metson, (1961) classification, 46% and 54% of soil samples in the study area exhibited low to medium levels of cation exchange capacity, respectively. Also, soil calcium carbonate estimation indicated that 96% of the soil samples can be classified as highly calcareous (FAO et al., 2008).
The mineralogical composition of soil samples showed that Calcite and Dolomite cover most of the study area (Rastegari Mehr et al., 2016). Moreover, the organic matter content of soil revealed that all samples contain very high levels of organic matter.
Metals content in soil
The determined Fe content in agricultural soil samples of Mahshahr city (in Albo-ghobeish, Makser and Bonivar village) and Cr content were higher than those in published guidelines by the EPA. Also P and Ni concentration in the study area were higher than the maximum allowable concentration published by CCME (2007).
The EF index did not show extreme enrichment for the studied elements (except for Se and Sb at two stations with extensive fertilizer use) in the study area (Fig. 2). Also according to Kabata–Pendias and Mukherjee (2007), Se is naturally high in carbonate and organic soils. Areas with soil pollution index (SPI) > 1 probably became polluted by use of organic and chemical fertilizers and irrigation with polluted water or sewage.
Sequential extraction indicated that Al, As, Cd, Cr, Cu, Fe, Pb, and Zn mostly occur in residual phase with low bioavailability. Mn was present in the exchangeable phase. This metal has high bioavailability for plants while it can be harmful for vegetables and human.
Table S2 compares the concentration of trace metals in agricultural soils in Mahshahr with those reported from other areas of the world. The mean concentration of Ni is higher in agricultural soils of Mahshahr city than in Aswan (Darwish & Pollmann, 2015), Dehui (Sun et al., 2013), Taiyuan (Yong et al., 2015), Yongtze (Xu et al., 2014), Shandong province (in China) (Ping et al., 2011), and Dainyor (Khan et al., 2010). In contrast, Ni values in Mahshahr were lower than those reported for Isfahan (Keshavarzi et al., 2015), Ahvaz (Bahrami et al., 2019), and peloponnese (Kelepertzis, 2014). Also, the mean concentrations of Ni, Se, and Hg in the study area are higher than those in Samo River Basin in Italy (Adamo et al., 2014). Cd levels in Mahshahr exceed those in Ahvaz, Taiyuan, and Yongtze and lower in Alicante (Mico et al., 2006). The total metal concentration measured in Isfahan is higher than in our study area except for Se.
The soil macronutrients (Al, S, K, Na, Mg, Ca, P, and Fe), trace metals (Cu, Pb, Zn, Mo, Ni, Co, Mn, As, Sr, Cd, Sb, Hg, Se, and V), and pH, EC, CEC, OM, clay, silt and sand contents were used for cluster analysis (CA). To evaluate the clusters’ distances, clusters were classified by Ward’s method and the data were standardized to Z-scores. The CA dendrogram is shown in Figure S1. Ca was carried out based on sampling stations. The stations were divided into two main branches. The first branch included lower content of anthropogenic elements (Pb, Cu, Cd, As, Cr, and Co), macronutrients (Na, Fe, and k), and higher content of Ca and Sr. Nearly similar contents of some elements such as Ca and As are the reason of dissociation of other stations in the second main branch.
PCA
PCA was applied to obtain more information on metal origins (Table S3). The PCA gave four components accounting for more than 75% of the cumulative variance. PC1 explained 36.35% of the total variance and comprises Ni, Co, Fe, As, Cr, Mg, Al, and V. Considering the results of geochemical factors, the above mentioned metals are mostly of crustal origin. Also, Na and K displayed high factor loading in PC1. The second factor that accounts for 21.12% of the total variance is represented by Mo, Cu, Zn, P, Hg, Se, and K. Compared with the first factor elements, these components are affected by pollution sources including fertilizers, pesticides, and irrigation with polluted water. The high loading factor of CEC and silt in this factor reflects the fact that silt plays the most important role in cation exchange capacity. The presence of P and K indicates that potassium and phosphate fertilizers are the most influential anthropogenic sources of these components. Factor 3 (Na, S, EC, and Na) and 4 (Mn, Pb, Sb, and Cd) account for 10.32% and 7.46% of the total variance, respectively. Halite (NaCl) in the study area (Rastegari Mehr et al., 2016) and accompaniment of Na and EC in third factor is consistent with the high salinity in the study area.
Metal content in crops
Elemental concentrations were calculated for agricultural crops (dill, lettuce, garden cress, leek, coriander, parsley, alfalfa, spinach, and red and white radish). The overall concentrations of macronutrients and trace metals in the crops displayed the following decreasing order: K > Sr > Ca > Na > S > P > Mn > Zn > Mg > Cu > Mo > Fe > Cr > Ni > Se > Cd > Co > Pb > Hg.
The lowest and highest Fe concentrations occurred in white radish glands (0.01 g kg−1) and red radish leaves (0.04 g kg−1), respectively. According to world standards, Fe concentrations in all vegetable samples do not exceed the normal range. However, concentrations of Fe in two samples of alfalfa exceeded the maximum allowable concentration of 0.25 g kg−1 (Vitosh et al., 1994).
The lowest and highest concentrations of Mg were detected in dill (0.19 g kg−1) and spinach (1.08 g kg−1), respectively. Some alfalfa samples showed lower concentrations than the Mg deficiency limit. Among the analyzed vegetable samples, dill and radish glands displayed lower Mg deficiency (Table 2), which probably reflects the low ability of alfalfa and dill in absorbing Mg. Mg has high ambulation and can easily transfer from old parts of a plant to newer parts (Vitosh et al., 1994), which is why there can be higher Mg concentration in leaves compared to a plant’s root. In addition, spinach showed high concentration of Mg as compared to other selected vegetables in this study. The mean concentration of Na in selected crops was 1.10 g kg−1. The lowest and highest concentration of Na occurred in alfalfa (0.21 g kg−1) and white radish glands (4.50 g kg−1).
The mean concentration of K is 5.49 g kg−1 with the lowest and highest concentration being 2.28 g kg−1 (red radish leaves) and 9.94 g kg−1 (lettuce), respectively. Potassium concentration in different species and also in a single species was unstable, so no relationship was found. The mean concentration of S and P in selected crops is 0.64 g kg−1 and 0.51 g kg−1, respectively. Also, the lowest and highest concentrations of S occur in lettuce (0.29 g kg−1) and garden cress (1.29 g kg−1), respectively, while the lowest and highest levels of P are in alfalfa (0.25 g kg−1) and garden cress (1 g kg−1), respectively. Radish (leaves and roots) and leek are good sources of S and garden cress is a rich source for both S and P.
Calcium is a major element in crops. The mean concentration of this element in selected crops was 0.47 g kg−1 with the lowest and highest concentrations occurring in red and white radish leaves (2.70 g kg−1), respectively. It is worth to note that deficiency limit for alfalfa and vegetables is 1.76 g kg−1 and 0.25 g kg−1, respectively. With the exception of one alfalfa sample, all other samples had normal concentrations.
Mn concentration in selected crops varied between 0.11 mg kg−1 in white radish glands and 106 mg kg−1 in red radish leaves. The mean concentration is calculated to be 40.57 mg kg−1. Radish glands, coriander and some leek samples, parsley, lettuce, and spinach showed lower Mn content than the deficiency limit (30 mg kg−1) (Vitosh et al., 1994). The reason is likely the low ability of these vegetables to absorb Mn. Also, it seems that radish leaves are rich in Mn.
The mean concentration of Co in the study area is 0.21 mg kg−1, while the minimum and maximum concentrations are 0.06 mg kg−1 in coriander and 0.68 mg kg−1 in red radish leaves, respectively. The maximum allowable concentration of cobalt exceeded about four times that in radish leaves and glands. It is also about two times in spinach and garden cress and 0.5 times in parsley.
The mean concentration of Cu was measured to be 4.14 mg kg−1, varying between 1.12 mg kg−1 in white radish glands to 13.38 mg kg−1 in leek. With due attention to standards given in Table 2, crops in the study area generally showed lower Cu content than the deficiency limit.
Mo exhibited a mean content of 2.40 mg kg−1 and varied between 0.37 mg kg−1 in white radish glands and 7.49 mg kg−1 in alfalfa. According to deficiency limits and maximum allowable concentration given for Mo (Table 2), in vegetables and alfalfa, generally no Mo deficiency was found, except in alfalfa where Mo content was found to be more than the maximum allowable concentration. It seems that parsley and garden cress have more tendency towards absorbing Mo compared with spinach and radishes.
The mean concentration of Ni in the study area was measured to be 1.22 mg kg−1. The standard maximum allowable concentration is 10 mg kg−1 (SEPA, 2005 and FAO/WHO, 2011) for vegetables. Ni concentration in whole vegetables is lower than the maximum allowable concentration. Since Ni concentration in soil samples was measured to be high, it seems that some selected vegetables species do not absorb this metal.
The mean concentration of Zn in the selected crops was 40.11 mg kg−1 (Table 2). The lowest Zn concentration (16.50 mg kg−1) was observed in coriander, while the highest (106.40 mg kg−1) was in garden cress. Between the selected vegetable species, coriander and parsley absorb lower contents of Zn. However, garden cress and spinach also have a great ability to absorb this metal, resulting in Zn content above the maximum allowable concentration in some samples.
The average content of lead (0.12 mg kg−1) and cadmium (0.26 mg kg−1) in vegetables from the study area were higher than the maximum allowable concentrations of these elements (Table 2). Since lead content in soil samples is much lower than the maximum allowable concentration of this metal, plant or soil physiological factors such as soil texture can result in such a concentration. High mobility of cadmium in soil is the reason for high bioavailability of this toxic metal in crops in the study area, especially in spinach, lettuce, garden cress, and radishes.
The average concentration of Hg was 24.26 µg kg−1, with the lowest and highest concentrations being in parsley (8 µg kg−1) and lettuce (65 µg kg−1). Compared with the maximum permissible concentration (100 µg kg−1, FAO/WHO, 2011), Hg content in vegetables is lower. In addition, the mean concentrations of Se and Cr in selected crops were observed to be 0.62 mg kg−1 and 1.83 mg kg−1, respectively. Also, the lowest and highest concentrations of Se and Cr occurred in parsley (0.08 mg kg−1) and leek (3.90 mg kg−1) and in white radish glands (1.10 mg kg−1) and dill (3.70 mg kg−1), respectively (Table 2).
Soil to plant transfer factor
Soil to plant transfer factor is a key component in determining different human exposure routes to macro- and micronutrients and potential toxic elements through the food chain. Figure S2 shows the TF value for P, Na, K, S, Co, Mn, Ca, Mg, Fe, Mo, Cu, Zn, Se, Cd, Hg, Pb, Cr, and Ni for soil to edible parts of selected vegetable samples. The trend from soil to crops for macronutrients and trace metals was as follows: K > Na > P > S > Mo > Hg > Se > Zn > Cd > Cu > Mg > Mn > Ca > Cr > Co > Ni ≈ Fe = Pb.
Plant species will affect element availability of the crops (Keshavarzi et al., 2015). Biological, chemical, and physical properties of soil can also affect the bioavailability of the elements (Zhao et al., 2009). But the most important factor that controls the element’s uptake is soil pH (Basta et al., 2005; Jung & Thornton, 1997). Generally, the value for macronutrients (especially P, K, Na and S) was much higher than that for trace metals. Na is more easily transported to dill, spinach, and garden cress. Also spinach and garden cress do not adsorb K and P more than other selected crops. Since S transfer factor in red radish leaves is much higher than those in glands, it is likely that S mobility in red radish caused this element to accumulate in leaves.
For whole selected vegetables except white radish, it was more difficult to transfer Fe from soil into their edible parts. The highest TF values for Mg and Ca were in white radish, while the lowest Cu content was observed in red and white radishes. Spinach, garden cress, and lettuce showed high TF value for Zn. The Co concentration in crops is highly dependent on soil parameters and absorption ability of the plant (Kabata-Pendias & Mukherjee, 2007). The maximum TF value for Pb occurs in garden cress and for Cd in spinach and garden cress. The highest Se absorption occurs in leek and the lowest in alfalfa and parsley. The results confirmed that elements’ accumulation in leafy edible part of crops is higher than root parts (Dziubanek et al., 2015; Zhuang et al., 2009).
Daily intake of metals and health risk index
Table S4 displays estimated daily intake of metals (DIM) for macronutrients and trace metals through consumption of selected vegetables. The results were compared with the oral reference doses (RFD) as suggested by FAO/WHO and USEPA/IRIS. Health risk indices were also calculated resulting from the consumption of these metals in food chain for both children and adults.
Results showed that health risk index (HRI) for the majority of metals was lower than unity, with the exception of Mn in both white and red radish leaves for children. Therefore, the consumption of these vegetables for children can cause health problem (Figure S3). A comparison between mean concentrations of heavy metals in selected vegetables in the study area with other cities around the world is given in Table S5. The mean metal concentrations in radish crops in the study area are lower than those in Beijing (Khan et al., 2008), Lahor (Mahmood & Malik, 2014), Dohuk (Sulaivany & Al-Mezori, 2007), and Isfahan (Keshavarzi et al., 2015). Also in lettuce, the mean metal concentration is lower than in Beijing and higher than Jiangsu (Cao et al., 2010). In spinach, the mean selected element concentration from Mahshahr city is higher than Beijing (Zn concentration) and Lahor (Cu and Mn concentration). Comparison between parsley and coriander in the study area with same crops in Isfahan, Titagarh (Gupta et al., 2012), and Istanbul (Osma et al., 2012) showed that, except for Cu concentration in Istanbul parsley, the selected element concentration in other cities is higher than those in Mahshahr city. The mean Cd concentration in leek in the study area was higher than the reported concentration at Dohuk, while the mean Mn concentration in garden cress sampled in Ahvaz (Bahrami et al., 2019) is higher than Mahshahr. Also, the mean Zn concentration in garden cress in Ahvaz is lower than in the study area.
To obtain more information regarding heavy metals in crop samples, PCA was performed. In this method after varimax rotation, elements were organized in four components representing more than 70% of the total variance. Factor 1 with 24.64% of the total variance included Pb, Mn, Fe, Co, and Cr. These metals include higher concentration in some special crops species, particularly radish, while Mo, Cu, and Ni in factor 2 (19.89% of the total variance) displayed lower and higher concentration in radishes and parsley, respectively. The third factor explained 15.21% of the total variance and included Zn, Cd, and Hg. These elements show the highest concentration in garden cress, spinach, and lettuce samples (Table S6).
In this study, crop species were not separated. Thus, it was not possible to separate vegetables according to their own sources. Therefore, Kruskal–Wallis non-parametic test was used for element absorption. (Table 3).
The results indicate that, except for Se and Cr, other elements (with P-value < 0.05) in crop species play an effective role in absorbing these elements (Table 3). Correlation coefficients between element concentrations in soil and crops showed that no element (except for Se) correlate with soil and crop (Table 4).
Conclusion
In the present study, we assessed the contamination and human health risks posed by heavy metals in soils and edible parts of crops in Mahshahr city of Iran. Chemical analysis of agricultural soil in the study area indicated that concentrations of Fe, Ni, and P were marginally higher than the maximum allowable concentration set by the United States Environmental Protection Agency (EPA) and the Canadian Council of Ministers of the Environment (CCME). However, the results showed that the concentration of these metals is not considerable. Based on the vegetable results, radish displayed the highest accumulation rate for Mn and Co, while high concentrations of Cd occurred in spinach, lettuce, and garden cress due to its remarkable mobility and bioavailability in soil and plants. Also, crop species play a significant role in metal absorption such as how there is high Zn accumulation in garden cress and spinach as compared with low absorption of Ni in vegetables. Mn with HRI > 1 was the most dangerous elements for children via radish consumption.
References
Acevedo-Figueroa, D., Jiménez, B. D., & Rodríguez-Sierra, C. J. (2006). Trace metals in sediments of two estuarine lagoons from Puerto Rico. Environmental Pollution, 141(2), 336–342.
Adamo, P., Iavazzo, P., Albanese, S., Agrelli, D., De Vivo, B., & Lima, A. (2014). Bioavailability and soil-to-plant transfer factors as indicators of potentially toxic element contamination in agricultural soils. Science of the Total Environment, 500, 11–22.
Alimohammadi, M., Younesian, M., Madihi-Bidgoli, S., Nabizadeh Nodehi, R., Jahed Khaniki, G. R., Hadi, M., & Ghanbari, F. (2020). Heavy metal (oid) s concentration in Tehran supermarket vegetables: Carcinogenic and non-carcinogenic health risk assessment. Toxin Reviews, 39(3), 303–310.
Alloway, B. J. (1990). Soil processes and the behaviour of metals. Heavy metals in soils., 7–28.
ATSDR. (1999). Toxicological profile for mercury. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=115&tid=24
ATSDR. (2004). Toxicological profile for cobalt. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=373&tid=64
Bahrami, S., Moore, F., & Keshavarzi, B. (2019). Evaluation, source apportionment and health risk assessment of heavy metal and polycyclic aromatic hydrocarbons in soil and vegetable of Ahvaz metropolis. Human and Ecological Risk Assessment: An International Journal, 1–30.
Basta, N. T., Ryan, J. A., & Chaney, R. L. (2005). Trace element chemistry in residual-treated soil: Key concepts and metal bioavailability. Journal of Environmental Quality, 34(1), 49–63.
Cao, H., Chen, J., Zhang, J., Zhang, H., Qiao, L., & Men, Y. (2010). Heavy metals in rice and garden vegetables and their potential health risks to inhabitants in the vicinity of an industrial zone in Jiangsu. China. Journal of Environmental Sciences, 22(11), 1792–1799.
CCME, Canadian Council of Ministers of the Environment. (2007). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health. Update 7.0, Excerpt from Publication No. 1299; ISBN 1–896997–34–1.
CCME. (1991). (1997). (1999). (2015). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health. Canadian Council of Minister of the Environment.
Chabukdhara, M., & Nema, A. K. (2012). Assessment of heavy metal contamination in Hindon River sediments: A chemometric and geochemical approach. Chemosphere, 87(8), 945–953.
CODEX, A., & INTERGOVERNMENTAL, T. F. O. (2001). Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission Twenty-Fourth Session Geneva, 2-7 July 2001. Codex.
Cui, Y. J., Zhu, Y. G., Zhai, R. H., Chen, D. Y., Huang, Y. Z., Qiu, Y., & Liang, J. Z. (2004). Transfer of metals from soil to vegetables in an area near a smelter in Nanning. China. Environment International, 30(6), 785–791.
Daldoul, G., Souissi, R., Souissi, F., Jemmali, N., & Chakroun, H. K. (2015). Assessment and mobility of heavy metals in carbonated soils contaminated by old mine tailings in North Tunisia. Journal of African Earth Sciences, 110, 150–159.
Darwish, M. A. G., & Pöllmann, H. (2015). Trace elements assessment in agricultural and desert soils of Aswan area, south Egypt: Geochemical characteristics and environmental impacts. Journal of African Earth Sciences, 112, 358–373.
Degryse, F., Smolders, E., & Parker, D. R. (2009). Partitioning of metals (Cd, Co, Cu, Ni, Pb, Zn) in soils: concepts, methodologies, prediction and applications - a review. European Journal of Soil Science, 60(4), 590–612. https://doi.org/10.1111/j.1365-2389.2009.01142.x
Dong, J., Yang, Q. W., Sun, L. N., Zeng, Q., Liu, S. J., Pan, J., & Liu, X. L. (2011). Assessing the concentration and potential dietary risk of heavy metals in vegetables at a Pb/Zn mine site. China. Environmental Earth Sciences, 64(5), 1317–1321.
Dziubanek, G., Piekut, A., Rusin, M., Baranowska, R., & Hajok, I. (2015). Contamination of food crops grown on soils with elevated heavy metals content. Ecotoxicology and Environmental Safety, 118, 183–189.
Eby, G. N. (2016). Principles of environmental geochemistry. Waveland Press.
Ettler, V., Mihaljevič, M., Šebek, O., & Grygar, T. (2007). Assessment of single extractions for the determination of mobile forms of metals in highly polluted soils and sediments—Analytical and thermodynamic approaches. Analytica Chimica Acta, 602(1), 131–140.
Esmaeili, A., Moore, F., Keshavarzi, B., Jaafarzadeh, N., & Kermani, M. (2014). A geochemical survey of heavy metals in agricultural and background soils of the Isfahan industrial zone. Iran. Catena, 121, 88–98.
FAO, IASA, ISRIC, JRC, IIASA., (2008). Food and Agriculture Organization of the United Nations (FAO), the International Institute for Applied Systems Analysis (IIASA), World Soil Information (ISRIC), Institute of Soil Science – Chinese Academy of Science (ISSCAS) or Joint Research Centre of the European Commission (JRC) concerning the legal status of any country, Harmonized World Soil Database (version 1.0). FAO, Rome, Italy and IIASA, Laxenburg, Austria.
Food and Agriculture Organization of the World Health Organization (FAO/WHO). (2011). Joint FAO/WHO Food Standards Program Codex Committee on Con-taminants in Foods. Fifth Session CF/5INF/1,21–25 March 2011.The Hague, The Netherlands.
Golobočanin, D. D., Škrbić, B. D., & Miljević, N. R. (2004). Principal component analysis for soil contamination with PAHs. Chemometrics and Intelligent Laboratory Systems, 72(2), 219–223.
Guadie, A., Yesigat, A., Gatew, S., Worku, A., Liu, W., Ajibade, F. O., & Wang, A. (2021). Evaluating the health risks of heavy metals from vegetables grown on soil irrigated with untreated and treated wastewater in Arba Minch, Ethiopia. Science of The Total Environment, 761, 143302.
Gupta, A. K., & Sinha, S. (2006). Role of Brassica juncea (L.) Czern.(var. Vaibhav) in the phytoextraction of Ni from soil amended with fly ash: Selection of extractant for metal bioavailability. Journal of Hazardous Materials, 136(2), 371–378.
Gupta, N., Khan, D. K., & Santra, S. C. (2012). Heavy metal accumulation in vegetables grown in a long-term wastewater-irrigated agricultural land of tropical India. Environmental Monitoring and Assessment, 184(11), 6673–6682.
Heiri, O., Lotter, A. F., & Lemcke, G. (2001). Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. Journal of Paleolimnology, 25(1), 101–110.
Holm, P. E., Rootzen, H., Borggaard, O. K., & M⊘ berg, J. P., & Christensen, T. H. (2003). Correlation of cadmium distribution coefficients to soil characteristics. Journal of Environmental Quality, 32(1), 138–145.
Hu, J., Wu, F., Wu, S., Cao, Z., Lin, X., & Wong, M. H. (2013). Bioaccessibility, dietary exposure and human risk assessment of heavy metals from market vegetables in Hong Kong revealed with an in vitro gastrointestinal model. Chemosphere, 91(4), 455–461.
Ismail, A., Riaz, M., Akhtar, S., Goodwill, J. E., & Sun, J. (2019). Heavy metals in milk: Global prevalence and health risk assessment. Toxin Reviews, 38(1), 1–12.
Jan, F. A., Ishaq, M., Khan, S., Ihsanullah, I., Ahmad, I., & Shakirullah, M. (2010). A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir). Journal of Hazardous Materials, 179(1–3), 612–621.
Jiao, W., Chen, W., Chang, A. C., & Page, A. L. (2012). Environmental risks of trace elements associated with long-term phosphate fertilizers applications: A review. Environmental Pollution, 168, 44–53.
Joint FAO/WHO Expert Committee on Food Additives. (1999). Toxicological evaluation of certain food additives. ILSI Press International.
Jung, M. C., & Thornton, I. (1997). Environmental contamination and seasonal variation of metals in soils, plants and waters in the paddy fields around a Pb/Zn mine in Korea. Science of the Total Environment, 198(2), 105–121.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. Springer Science & Business Media.
Kachenko, A. G., & Singh, B. (2006). Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water, Air, and Soil Pollution, 169(1–4), 101–123.
Kamani, H., Ashrafi, S. D., Isazadeh, S., Jaafari, J., Hoseini, M., Mostafapour, F. K., & Mahvi, A. H. (2015). Heavy metal contamination in street dusts with various land uses in Zahedan, Iran. Bulletin of environmental contamination and toxicology, 94(3), 382–386.
Kelepertzis, E. (2014). Accumulation of heavy metals in agricultural soils of Mediterranean: Insights from Argolida basin Peloponnese Greece. Geoderma, 221–222, 82–90. https://doi.org/10.1016/j.geoderma.2014.01.007
Keshavarzi, B., Moore, F., Ansari, M., Mehr, M. R., Kaabi, H., & Kermani, M. (2015). Macronutrients and trace metals in soil and food crops of Isfahan Province. Iran. Environmental Monitoring and Assessment, 187(1), 4113.
Khan, A., Khan, S., Alam, M., Khan, M. A., Aamir, M., Qamar, Z., & Perveen, S. (2016). Toxic metal interactions affect the bioaccumulation and dietary intake of macro-and micro-nutrients. Chemosphere, 146, 121–128.
Khan, K., Lu, Y., Khan, H., Ishtiaq, M., Khan, S., Waqas, M., & Wang, T. (2013). Heavy metals in agricultural soils and crops and their health risks in Swat District, northern Pakistan. Food and Chemical Toxicology, 58, 449–458.
Khan, S., Cao, Q., Zheng, Y. M., Huang, Y. Z., & Zhu, Y. G. (2008). Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing. China. Environmental Pollution, 152(3), 686–692.
Khan, S., Rehman, S., Khan, A. Z., Khan, M. A., & Shah, M. T. (2010). Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicology and Environmental Safety, 73(7), 1820–1827.
Khuzestan Meteorological Organization. (2016). Iran Meteorological Organization, Ministry of Roads and Urban Development. http//:37.32.40.3/.
Liu, Z., He, X., Chen, W., Yuan, F., Yan, K., & Tao, D. (2009). Accumulation and tolerance characteristics of cadmium in a potential hyperaccumulator—Lonicera japonica Thunb. Journal of Hazardous Materials, 169(1–3), 170–175.
Liu, H., Probst, A., & Liao, B. (2005). Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Science of the Total Environment, 339(1–3), 153–166.
Lui, W. X., Li, H. H., Li, S. R., & Wang, Y. W. (2006). Heavy metal accumulation of edible vegetables cultivated in agricultural soil in the suburb of Zhengzhou City, People’s Republic of China. Bulletin of Environmental Contamination and Toxicology, 76(1), 163–170.
Luo, L., Ma, Y., Zhang, S., Wei, D., & Zhu, Y. G. (2009). An inventory of trace element inputs to agricultural soils in China. Journal of Environmental Management, 90(8), 2524–2530.
Mahmood, A., & Malik, R. N. (2014). Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore. Pakistan. Arabian Journal of Chemistry, 7(1), 91–99.
Marcussen, H., Holm, P. E., Strobel, B. W., & Hansen, H. C. B. (2009). Nickel sorption to goethite and montmorillonite in presence of citrate. Environmental Science & Technology, 43(4), 1122–1127.
Metson, A. J. (1961). NZCSIR Soils Bull.
Micó, C., Recatalá, L., Peris, M., & Sánchez, J. (2006). Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere, 65(5), 863–872.
Mohiuddin, K. M., Ogawa, Y. Z. H. M., Zakir, H. M., Otomo, K., & Shikazono, N. (2011). Heavy metals contamination in water and sediments of an urban river in a developing country. International Journal of Environmental Science & Technology, 8(4), 723–736.
Moore, F., & Keshavarzi, B. (2014). Medical geology of Khuzestan Province (Phase 1). Iran Environmental Protection Agency (EPA), Khuzestan province: Internal Report.
Muhammad, S., Shah, M. T., & Khan, S. (2011). Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchemical Journal, 98(2), 334–343.
Nagajyoti, P. C., Lee, K. D., & Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: A review. Environmental Chemistry Letters, 8(3), 199–216.
Nicholson, F. A., Smith, S. R., Alloway, B. J., Carlton-Smith, C., & Chambers, B. J. (2003). An inventory of heavy metals inputs to agricultural soils in England and Wales. Science of the Total Environment, 311(1–3), 205–219.
Osma, E., Serin, M., Leblebici, Z., & Aksoy, A. (2012). Heavy metals accumulation in some vegetables and soils in Istanbul. Ekoloji, 21(82), 1–8.
Ping, L. I. U., Zhao, H. J., Wang, L. L., Liu, Z. H., Wei, J. L., Wang, Y. Q., & Zhang, Y. F. (2011). Analysis of heavy metal sources for vegetable soils from Shandong Province. China. Agricultural Sciences in China, 10(1), 109–119.
Qureshi, A. S., Hussain, M. I., Ismail, S., & Khan, Q. M. (2016). Evaluating heavy metal accumulation and potential health risks in vegetables irrigated with treated wastewater. Chemosphere, 163, 54–61.
Rastegari Mehr, M., Keshavarzi, B., Moore, F., Sacchi, E., Lahijanzadeh, A. R., Eydivand, S., & Rostami, S. (2016). Contamination level and human health hazard assessment of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in street dust deposited in Mahshahr, southwest of Iran. Human and Ecological Risk Assessment: An International Journal, 22(8), 1726–1748.
Ryan, J., Estefan, G., & Rashid, A. (2007). Soil and plant analysis laboratory manual. ICARDA.
Schofield, R. K. (1950). Effect of pH on electric charges carried by clay particles. Journal of Soil Science, 1(1), 1–8.
SEPA. (2005). The limits of pollutants in food. State Environmental Protection Administration, China. GB2762e2005.
Sulaivany, R. O. H., & Al-Mezori, H. A. M. (2007). Heavy metals concentration in selected vegetables grown in Dohuk City, Kurdistan region, Iraq. WIT Transactions on the Built Environment, 94.
Sulkowski, M., & Hirner, A. V. (2006). Element fractionation by sequential extraction in a soil with high carbonate content. Applied Geochemistry, 21(1), 16–28.
Sun, C., Liu, J., Wang, Y., Sun, L., & Yu, H. (2013). Multivariate and geostatistical analyses of the spatial distribution and sources of heavy metals in agricultural soil in Dehui. Northeast China. Chemosphere, 92(5), 517–523.
United State, Environmental Protection Agency, December. (2006). Region 9, Preliminary remediation goal. http://www.epa.gov/region09/waste/sfind/prg.2002.
ur Rehman, K., Bukhari, S. M., Andleeb, S., Mahmood, A., Erinle, K. O., Naeem, M. M., & Imran, Q. (2019). Ecological risk assessment of heavy metals in vegetables irrigated with groundwater and wastewater: the particular case of Sahiwal district in Pakistan. Agricultural Water Management, 226, 105816.
US-EPA, United States, Environmental Protection Agency, August. (2012). Summary of maximum allowable concentrations of chemical constituents in uncontaminated soil used as fill material at regulated fill operations (35 Ill. Adm. Code 1100.Subpart F), https://www.epa.state.il.us/land/ccdd/new-max-allowable-concentrations-table.pdf.
Vitosh, M. L., Warncke, D. D., & Lucas, R. E. (1994). Secondary and Micronutrients for vegetables and field crops. Department of Crop and Soil Sciences, Michigan State University Extension, E-486.
Wang, Y., Yang, L., Kong, L., Liu, E., Wang, L., & Zhu, J. (2015). Spatial distribution, ecological risk assessment and source identification for heavy metals in surface sediments from Dongping Lake, Shandong, East China. CATENA, 125, 200–205.
World Health Organization (WHO). (1982). Toxicological Evaluation of Certain Food Additives, Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additive Series No. 683, World Health Organization, Geneva.
Xu, X., Zhao, Y., Zhao, X., Wang, Y., & Deng, W. (2014). Sources of heavy metal pollution in agricultural soils of a rapidly industrializing area in the Yangtze Delta of China. Ecotoxicology and Environmental Safety, 108, 161–167.
Yao, Q., Wang, X., Jian, H., Chen, H., & Yu, Z. (2015). Characterization of the particle size fraction associated with heavy metals in suspended sediments of the Yellow River. International Journal of Environmental Research and Public Health, 12(6), 6725–6744.
Yong, L. I. U., Huifeng, W. A. N. G., Xiaoting, L. I., & Jinchang, L. I. (2015). Heavy metal contamination of agricultural soils in Taiyuan. China. Pedosphere, 25(6), 901–909.
Zhao, K., Zhang, W., Zhou, L., Liu, X., Xu, J., & Huang, P. (2009). Modeling transfer of heavy metals in soil–rice system and their risk assessment in paddy fields. Environmental Earth Sciences, 59(3), 519–527.
Zhuang, P., McBride, M. B., Xia, H., Li, N., & Li, Z. (2009). Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Science of the Total Environment, 407(5), 1551–1561.
Acknowledgements
The authors would like to acknowledge the help of the Bandar Imam Petrochemical Complex, the Khuzestan environmental protection office, the Research Committee, and Medical Geology Center of Shiraz University for logistic help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi, S., Keshavarzi, B., Moore, F. et al. Macronutrients, trace metals and health risk assessment in agricultural soil and edible plants of Mahshahr City, Iran. Environ Monit Assess 194, 131 (2022). https://doi.org/10.1007/s10661-021-09646-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09646-2