Abstract
The distribution of 10 macronutrients and trace metals in the arable soils of Isfahan Province, their phytoavailability, and associated health risks were investigated; 134 plant and 114 soil samples (from 114 crop fields) were collected and analyzed at harvesting time. Calculation of the soil pollution index (SPI) revealed that arable soil polluted by metals was more severe in the north and southwest of the study area. The results of cluster analysis indicated that Pb, Zn, and Cu share a similar origin from industries and traffic. The concentrations of macronutrients and trace metals in the sampled crops were found in the order of K > Ca > S > Mg > P and Fe > Mn > Zn > Cu > Pb, respectively, whereas calculation of the bioconcentration factor (BCF) indicated that the accumulation of the investigated elements in crops was generally in the order of S ≈ K > P > Mg > Ca and Zn > Cu > Mn > Pb > Fe, respectively. Thus, various parameters including crop species and the physical, chemical, and biological properties of soil also affected the bioavailability of the elements besides the total element contents in soil. Daily intake (DI) values of elements were lower than the recommended daily intake (RDI) levels in rice grains except for Fe and Mn, but for wheat grains, all elements displayed DI values higher than the RDI. Moreover, based on the hazard index (HI) values, inhabitants are experiencing a significant potential health risk solely due to the consumption of wheat and rice grains (particularly wheat grains). Mn health quotient (HQ) also indicated a high risk of Mn absorption for crop consumer inhabitants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Humans require a number of mineral nutrients known to play key roles in maintaining human health (Gupta and Gupta 2014). Hence, the quality of human life depends in part on the chemical composition of the consumed food (Kabata-Pendias and Mukherjee 2007). In trace amounts, most heavy metals are necessary in living organisms. However, excessive metal concentrations in polluted soils can result in soil quality degradation, crop yield reduction, and poor quality of agricultural products (McGrath 1998). Considering the nonbiodegradability and the persistent nature of heavy metals in soil, they may enter the human body via dust inhalation, direct soil ingestion, or consumption of food crops. Compared with inhalation and dermal contact exposure, food consumption has been identified as the major pathway (Zheng et al. 2007). In agricultural soils, the presence of metals is of increasing concern because they have the potential to be accumulated in less soluble forms, transferred into soil solution, and subsequently deteriorate the groundwater and crop quality (Kelepertzis 2014). Increasing soil heavy metal concentrations may result in increased uptake by plants and potentially cause serious health problems such as stomachache, renal dysfunction, pulmonary emphysema, and the notorious Itai-Itai to humans (Yeung and Hsu 2005) mostly via food chains (Jackson and Alloway 1992).
In addition to the natural weathering-pedological (geogenic) inputs, anthropogenic activities are said to be significantly responsible for elevated trace metal concentrations in soils (Devkota and Schmidst 2000; Frost and Ketchum 2000; Singh et al. 2004; Mapanda et al. 2005). Soil to plant transfer is a key process that exposes humans to heavy metals through the food chain. When a plant grows on a heavy metal-contaminated soil, the heavy metals will be first absorbed by the roots of the plant and then transferred to the aboveground organs such as the stem, leaf, and grain (Li et al. 2009). Trace metal mobility depends on soil characteristics including soil pH and texture, the type and quantity of the oxyhydroxides present, the content of organic matter, and soil main constituents such as carbonates, phosphates, and clays which are responsible for toxic metal and metalloid sorption (e.g., Holm et al. 2003; Marcussen et al. 2009) and trace metal-specific chemical form or strength of binding to the solid phase (Bermond et al. 2005).
Understanding and the prediction of the accumulation of heavy metals and metalloids in crops grown on contaminated croplands were motivated to secure crop quality and food safety (McLaughlin et al. 1999; Adamsa et al. 2004). Therefore, based on the above background, the aims of this study are to (a) evaluate the spatial variability of macronutrients and trace metals in surface soil and crops, (b) assess the transfer of elements from soil to plant, (c) determine soil factors influencing the availability of macronutrients and trace elements to crops, and (d) evaluate noncarcinogenic risk of wheat and rice grain consumptions in Isfahan Province of Iran.
Materials and methods
Study area, geological setting, and soil type
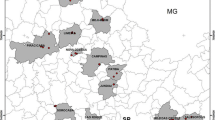
The study area is located in Isfahan Province (within 50 km from the Isfahan City center). The surface area is about 7850 km2. The geographic coordinates are 32° 11′–33° 6′ N and 51° 8′–52° 12′ E. Several populated cities including Najafabad, Shahinshahr, Mobarakeh, Khomeynishahr, and Isfahan are located in the study area (Fig. 1). Isfahan is the largest industrial city in Iran, well known for its steel mills and chemical plants. Arable lands surround different industrial centers such as steel and cement-making mills and also the famous Shahkuh Pb mine. Climate in the northern, eastern, and central parts of the study area is hot and dry and semihumid in the western parts, while toward the south, it is cold and semiarid. The mean annual precipitation is 120 mm. Annual mean temperature is 16.7 °C, ranging from a minimum of 10.6 °C in the winter to a maximum of 40.6 °C in the summer. Geologically, the study area is covered by sedimentary rocks (limestone, dolomite, evaporite, sandstone, and shale), along with Zayandehrud River alluvium. The soils in the study area are mostly composed of immature calcaric regosols, lithic teptosols, and haplic calcisols with undeveloped horizons.
Sampling and chemical analysis
Sampling was carried out in the period of April to July 2011. A total of 134 crop samples (rice and wheat grains, alfalfa, lettuce, cabbage, beet, celery, radish, coriander, basil, parsley, leek, and dill) and 114 topsoil samples (1–10 cm) were collected at harvest time. The reason for the smaller number of soil samples is that on several occasions more than one crop type was collected from the same farm. Moreover, due to water resource limitations, arable lands in the study area are confined to areas surrounding the Zayandehrud River. The sampling locations are shown in Fig. 1.
Each soil sample was prepared by first randomly collecting several subsamples at each sampling site, followed by thoroughly mixing the subsamples to form a composite sample of approximately 1 kg using the quartile method. Collected samples were sealed in clean polyethylene bags and transported to the laboratory. The soil samples were air dried at room temperature, part of each sample was sieved through a 0.63 μm screen for the determination of macronutrients and trace metals, and for the rest of the physicochemical parameters, the soil was sieved to 2 mm. The total concentrations of macronutrients Ca, Mg, P, K, and S and trace metals Cu, Zn, Pb, Fe, and Mn were measured following aqua regia digestion for low to ultralow determinations by using inductively coupled plasma mass spectrometry (ICP-MS) method in a certified commercial Canadian laboratory (Acme Analytical Laboratories, Ltd.).
Soil pH and electrical conductivity (EC) were measured in aqueous suspensions (1:2.5 and 1:5 soil/water ratios, respectively). Soil organic matter was determined following potassium dichromate wet combustion procedure. Cation exchange capacity (CEC) of soil was measured using 0.1 M NaCl according to the ion retention method of Schofield (1949). CaCO3 was determined by the titrimetric method in which two equivalents of acid are assumed to react with 1 mol of CaCO3 (Ryan et al. 2001). Soil particle size distribution (sand, silt, and clay content) was determined using the hydrometer method.
Crop samples (rice and wheat grains and some green vegetables) were collected along with growing media soils. The distribution of the samples reflects the dominant crop type at each sampling site and the fact that no farming is practiced in the eastern and northeastern parts. After sample collection, the food crops were immediately transported to the laboratory and cleaned with deionized water. Crop samples were air dried, and their edible parts were ground using a precleaned steel grinder and passed through a 250 μm sieve. Each fine crop powder was then stored in a polythene zip bag. The samples were measured for their Ca, Mg, P, K, S, Cu, Zn, Pb, Fe, and Mn contents using 1 g aliquots digested in aqua regia and then analyzed by ICP-MS in the Acme Analytical Laboratories, Ltd.
Data analysis
Soil pollution index (SPI i )
SPI i was calculated at each sampling station and then interpolated to better visualize pollution sources (Lee et al. 2006):
where i = the sampling locations, j = the enriched trace metals, MC i = the metal concentrations at the sampling location, TC j = the target concentrations of jth metals that are highly enriched, and N = the number of enriched trace metals.
Bioconcentration factor (BCF)
Bioconcentration factor is a parameter used to describe the transfer of trace elements from soil to plant. It was calculated as the ratio between the concentration of elements in the crops and that in the corresponding soil (all based on dry weight) for each crop separately (Liu et al. 2006).
where C plant is the concentration of the element in the plant, and C soil is its concentration in the soil.
Daily intake (DI i ) of elements
The DI i of elements was calculated based on the equation presented by Jung et al. (2005) as follows:
where C i is the concentration of the i element in crop (mg kg−1), and Q is the daily consumption of crop in the area (kg day−1).
Risk of individual trace metals
A potential noncancer risk for individual trace metals is expressed as the hazard quotient (HQ) (EC 2002) and is calculated as follows:
where chronic daily intake (CDI) exposure is expressed as mass of a substance contacted per unit body weight per unit time, averaged over a long period of time (lifetime in this study); RfDo is the oral reference dose (mg kg−1 day−1). Units of CDI and RfDo are the same (US EPA 1989). CF is the median concentration of elements in plant (mg kg−1); IR is the ingestion rate of plant (kg person−1 day−1); EF is the exposure frequency (365 days year−1); ED is the exposure duration (70 years for adults and 6 years for children in this study), equivalent to the average lifetime (Bennett et al. 1999); BW is the average body weight (61.6 kg for adults) (Yuan et al. 2007) and 18.7 kg for 0–6 year old children is presumed (Xu 2001); and AT is the average exposure time for noncarcinogenic effects (ED × 365 days year−1) (Huang et al. 2008).
If the CDI exceeds the threshold (i.e., if HQ exceeds unity), potential noncancer effects may be a concern. In addition, to determine the noncancer hazard for all exposure routes and pathways, the HI which consists of the sum of the HQ determined for individual pollutants was calculated (Bermudez et al. 2011). The RfDo values are 4.0 × 10−2, 7.0 × 10−1, 1.4 × 10−2, and 3.0 × 10−1 mg kg−1 day−1 for Cu, Fe, Mn, and Zn, respectively (US EPA 2010). Since US EPA is yet to establish an RfDo for Pb, the RfDo for Pb in this study was taken as 4.0 × 10−3 mg kg−1 day−1 calculated from the tolerable weekly Pb intake limit (25 μg kg−1 body weights) recommended by the FAO/WHO for adults (FAO/WHO 1984; Ostapczuk et al. 1987).
Statistical analysis
The data is statistically analyzed using Statistical Package for the Social Sciences (SPSS), version 19. In order to better describe and interpret the results of analysis and also to classify objects into categories or clusters based on their nearness or similarity, cluster analysis was employed. Hierarchical cluster analysis was used to assess relationships between variables and possible patterns in the distribution of the measured data. Also, principal component analysis (PCA) was employed to the data set with the aim to identify associations and common origin among elements (Lu et al. 2012). Moreover, Pearson’s correlation coefficients between soil physicochemical parameters and elements in crops were calculated to evaluate the possible influence of these parameters on the bioavailability of elements.
Geographic information system (GIS)
In order to display the spatial distribution of the elements in soil, spatial interpolation technique was used. There are several spatial interpolation techniques, including inverse distance weighting (IDW), radial basic functions, local polynomial interpolation, etc. Compared with the others, IDW is the simplest and most practical interpolation method (Wang 2006). Using elemental concentrations and soil physicochemical parameters as variables, in this study, IDW was applied to create a model for presenting spatial patterns in the area.
Results and discussion
Results of soil analysis
Chemical characteristics of soil samples
The summary statistics for each soil parameter and concentrations of the investigated elements are presented in Table 1. The soil pH ranged from 7.42 to 8.62, suggesting slightly to strongly alkaline soil. Ryan et al. (2001) suggested that soils with pH ranging from 7.5 to 8.4 usually have free carbonate and excellent infiltration and percolation of water resulting from high Ca saturation of clays. They also proposed that soil pH values higher than 8.5 indicate sodic soil with poor physical conditions, low infiltration and percolation, and possible root deterioration and organic matter dissolution. EC was variable (261 to 13,890 μS/cm) averaging 2267.35 μS/cm. Based on HACH Company (1993), 64, 27, 8, and 1 % of soil samples in the area bear very low, low, medium, and high hazard crop growth, respectively. Soil calcium carbonate contents ranged from 21.48 to 45.40 % with a mean value of 34.29 %, indicating that almost 84 % of the samples should be classified as very strongly calcareous (Avery 1980). Organic matter content of soil varied from 0.97 to 6.62 % (mean 2.87 %) revealing that 58 % of the samples have medium levels of organic matter (OM %) (Metson 1961). In general, the measured CEC was variable (1.63–34.26 meq/100 g), averaging 17.96 meq/100 g.
Major and trace metals
The ranges of the concentrations of macronutrients and trace metals in the soil samples were as follows: Fe 1.82–3.56 %, Ca 1.47–15.54 %, Mg 1.15–12.56 %, K 0.26–1.87 %, P 510–3750 mg kg−1, S 100–10,600 mg kg−1, Cu 0.82–97.80 mg kg−1, Pb 12.62–362.97 mg kg−1, Zn 21.53–963.40 mg kg−1, and Mn 15.60–796.00 mg kg−1. The ranges were above the soil world average for Cu, Pb, Zn, Mn, Ca, P, S, and Mg and below the world average for K and Fe. An efficient method for the evaluation of soil quality is to compare the data with guidelines published by environmental bureaus. Figure 2 illustrates a comparison between concentrations of trace metals in soils of the study area and guideline values for agricultural use. There are no published guidelines by environmental agencies for Fe and Mn in agricultural soils; however, as shown in Fig. 2, the determined Cu, Pb, and Zn contents in agricultural soils of the study area are higher than those in the published guidelines, especially in Shahinshahr, Zarrinshahr, Talkhuncheh, and Ghahderijan cities. These cities are close to some big industries such as petrochemical complexes, steel mill, and industrial towns which can cause soil contamination in these areas.
Soil pollution index was calculated for the enriched trace metals in the area, i.e., Cu, Pb, Mn, and Zn. In general, the range of SPI spanned from 0.37 to 8.31, with a mean value of 1.40. The relatively highly enriched areas are located close to inhabited districts in Isfahan, Shahinshahr, and Zarrinshahr cities, probably reflecting rapid population growth and urban development. Industries such as the Zobe-ahan steel mill and Shahinshahr petrochemical complex along with municipal waste landfills and irrigation of farmlands with treated wastewater also contribute to the observed soil pollution. The Shahinshahr freeway as a transit road was found to be a hotspot for Cu, Pb, Mn, and Zn concentrations, probably resulting from high traffic load. The spatial distributions of the studied macronutrients and trace metals along with the calculated SPI in the area are shown in Figs. 3 and 4, respectively. The figures indicate that arable soil pollution and trace metal enrichment are more severe in the northern and southern parts of the study area.
Numerous studies have been reported on metal contamination in agricultural soils around the world, the data of which can be used for comparison with the results of the present study. Table 2 compares the concentrations of trace metals in agricultural soils sampled in Isfahan with those reported from other areas in the world. The mean concentration of Cu is higher in agricultural soils of the study area than that in Zagreb and Alicante, Thiva, Huizhou, and Dehui and lower than that in Argolida, Dhaka, and Yangtze. Also, when compared with Argolida and Alicante agricultural soils, the study area soils are richer in Fe. Manganese levels in the study area of agricultural soils are higher than those in Dhaka and Alicante and lower than those in Thiva and Argolida, whereas the mean concentration of this metal is similar to those obtained in Zagreb. Moreover, Pb and Zn concentrations are obviously richer in agricultural soils of Isfahan Province than all areas mentioned in Table 2 (except Huizhou, Dehui, and Yangtze for Pb).
Relationship between soil parameters
The soil parameters, i.e., macronutrients (Ca, K, Mg, P, S), trace metals (Cu, Pb, Zn), along with Fe, Mn, pH, EC, CEC, CaCO3, organic matter, and clay, silt, and sand contents, were used for cluster analysis (CA). The normality of all elements and parameters were checked using the Kolmogorov-Smirnov normality test (p > 0.01) and, all data were log transformed prior to CA because the normality test indicated that the data are not normally distributed. In CA, the data was standardized to Z score and then classified using Ward’s method. The distance measured used in CA is the squared Euclidean distance. Ward’s method uses an analysis of variance approach to evaluate the distance between clusters (Kim et al. 2005). The result of CA is illustrated in the dendrogram (Fig. 5). The relationship between Pb, Zn, and Cu indicates that the metals originate from two major sources, i.e., industry and traffic. The correlation between Cu and OM is probably the result of the stabilizing effects induced by the organic matter through adsorption and complexation with insoluble humic substances. Adriano (1986) also showed that Cu forms the most stable complexes with organic matter. On the other hand, the association of Cu, Pb, and Zn with CEC, clay, silt, and OM supports the assumption that these metals have anthropogenic sources in the area and had been adsorbed by clay and silt particles and organic matter (especially for Cu). Fe, Mn, and Mg are strongly correlated and fall within one group/cluster. This is because metals with an ionic potential slightly lower than that required to form M–O bonds, namely Mg2+, Fe2+, Mn2+, Li+, and Na+, are strongly hydrated elements, and hydration significantly affects the chemical properties of an ion and its capacity to be adsorbed or enter into the crystal structure of a mineral (Bjørlykke 2010). On the other hand, as with iron, manganese geochemistry in sedimentary environments is largely governed by redox reactions. However, Mn behavior in low redox potential (Eh) is controlled by carbonate minerals, contrary to Fe, which is controlled more by the sulfides (Maynard 1983).
The relationship between K, P, and S in the soil samples is probably related to the application of ammonium sulfate, potash, and phosphate fertilizers in the croplands of Isfahan Province. Ca, CaCO3, pH, and sand are strongly correlated, indicating that the high pH of the soil is the result of the presence of calcium carbonate and also local lithological characteristics.
The principal component analysis aims to explain most of the data variance while reducing the number of variables to a few uncorrelated components and is an effective method to determine human impacts on a spatial scale. In this study, PCA was conducted using factor extraction with an eigenvalue >1 after varimax rotation with Kaiser normalization. To eliminate the potential influences of positive skewness in the distributions of the parameters (Table 1), a logarithmic transformation was applied. The results of PCA for heavy metal contents were presented in Table 3. Three principal components with eigenvalues higher than 1 explaining more than 76 % variance of the data were extracted. PC1 explained 38.73 % of the total variance and comprises Mn, Fe, Mg, and EC. PC2 explained 60.90 % of the total variance and loadings on Cu, Pb, Zn, and OM. The third component (PC3) included P and S. Potassium and clay (%) did not demonstrate a clear association with any of the components, but higher loading plots were observed for PC3 (0.59) and PC2 (0.50), respectively. The results of PCA confirmed the results obtained by CA. In general, all parameters in the study area except pH, CaCO3, Ca, and sand were affected by anthropogenic activities to some extent, but their potential sources, which are mentioned above, are different.
Nutrients and trace metals in crops
Table 4 summarizes the elemental concentrations in the agricultural crops (rice and wheat grains, alfalfa, lettuce, cabbage, beet, celery, radish, coriander, basil, parsley, leek, and dill) collected from where the agricultural soil samples were also collected. The overall concentrations of macronutrients and trace metals in the crops displayed the following decreasing order: K > Ca > S > Mg > P and Fe > Mn > Zn > Cu > Pb.
The mean concentration of Cu in selected crops varied between 2.61 and 49.02 mg kg−1 (Table 4). The lowest Cu concentration (1.17 mg kg−1) occurred in rice grains and the highest (441 mg kg−1) in lettuce. The overall mean concentration of Pb varied between 0.05 and 2.56 mg kg−1, with the lowest and highest concentrations being in rice grains (0.01 mg kg−1) and cabbage (15.25 mg kg−1), respectively. The mean concentration of Zn varied from 24.03 to 63.77 mg kg−1 and for Mn from 14.65 to 75.83 mg kg−1 (Table 4). The lowest and highest concentrations of Zn also occurred in rice grains (12.80 mg kg−1) and alfalfa (123 mg kg−1) and for Mn in rice grains (9 mg kg−1) and cabbage (267 mg kg−1), respectively. Similarly, the mean concentrations of Fe varied between 23.33 and 667.06 mg kg−1, with the lowest and highest concentrations (10 and 6440 mg kg−1) being in rice grains. The mean concentrations of Ca varied from 0.23 to 26.86 g kg−1, with the lowest and highest concentrations being in rice grains, celery, and beet (0.1 g kg−1) and in rice grains and cabbage (56.50 g kg−1) respectively. For P and Mg, the mean concentrations ranged from 2.79 to 5.51 g kg−1 and 0.97 to 9.23 g kg−1, respectively. The lowest and highest P concentrations occurred in cabbage (1.70 g kg−1) and basil (4.64 g kg−1) and for Mg in cabbage (0.55 and 17.22 g kg−1). In addition, the mean concentrations of K and S in selected crops varied from 2.63 to 51.15 g kg−1 and 1.63 to 10.50 g kg−1, respectively (Table 4). Also, the lowest and highest concentrations of K and S were observed in rice grains and cabbage (1.40 g kg−1) and leek (66.70 g kg−1) and in rice grains (0.7 g kg−1) and cabbage (29.70 g kg−1), respectively (Table 4).
Table 5 shows a comparison of the elemental concentrations of wheat and rice grains (as the dominant consumed crops in the area) in this study with data from other studies around the world and guidelines for trace metal concentrations in crops or foodstuffs. Compared with available data presented in Table 5, the concentrations of Cu, Pb, Fe, and all five macronutrients were higher in wheat grain samples of the study area. Pb and Mn concentrations in wheat grains of Isfahan Province were lower than those for Argentinean pampas and Figgia, respectively. Based on the table, rice grains cultivated in Pakistan had lower Cu, Zn, Fe, and Mn concentrations than Isfahan. Also, Zn, Mn, Mg, and K concentrations in Thailand rice grains were lower than those for Isfahan Province. Besides, Pb concentrations in rice samples of the area were higher than those in Macedonia and Changshu (China). Rice grains in Macedonia also had higher Cu and Zn concentrations. Moreover, compared with Japan, rice samples in the study area had higher Ca, Mg, and K concentrations and lower P contents. According to Chinese standards, 32.08 and 22.38 % of crop samples exceeded tolerance limits for Cu (mostly in wheat, alfalfa, and vegetables) and Zn (particularly in vegetables), respectively. In addition, 52.98 % of the samples exceeded the maximum levels for Pb (except in celery) when compared with the European Commission standards.
Knowing the overall elemental contamination in agricultural crops (Table 4), CA with Ward’s method was adopted to divide the agricultural crops into several groups as shown in the dendrogram in Fig. 6. Various clusters were formed between different selected crops; the crops in each group were of similar nature (Khan et al. 2013). Moreover, on the basis of elemental concentrations, some food crops showed strong significant correlations by forming primary groups/clusters (Fig. 6).
Elements in the soil-plant system
In order to understand the accumulation difference for the analyzed elements in crops, bioconcentration factor was determined for all sampled species in this study. Figure 7a, b shows the calculated bioconcentration factors for macronutrients and trace metals in the collected crops, respectively. In general, the values for macronutrients (particularly P, S, and K) were much higher than those for trace metals. The accumulation trend for macronutrients and trace metals is S ≈ K > P > Mg > Ca and Zn > Cu > Mn > Pb > Fe. However, different crops display different BCF values, indicating that element availability to the crop’s edible parts besides the total metal content of the soils is affected by plant species. Moreover, based on the study of Zhao et al. (2009), the physical, chemical, and biological properties of soil also affect the bioavailability of the elements. Soil pH is the most important factor that controls the uptake of elements (Jung and Thornton 1997; Basta et al. 2005). Therefore, the alkaline nature of the soil in Isfahan Province was probably the main reason for the observed high BCF for S, P, and K. It must be pointed out that plants exude many low molecular organic acids, which can influence the speciation of elements in soil solutions and the uptake of elements by plants. The rhizosphere is a small but important subsystem of the pedosphere, and the properties of the rhizosphere are largely different from those of bulk soils (Kraffczyk et al. 1984; Curl and Truelove 1986). Soil-plant interactions in the rhizosphere determine metal speciation, transformation, uptake, and accumulation in plants, which in turn determines the overall metal bioavailability (Wenzel et al. 1999).
Sulfate and phosphate (SO4 2−, H2PO4 −, or HPO4 2−) are generally the dominant species of sulfur and phosphorous in vast ranges of pH and Eh (Brookins 1988). As in the case for phosphate, sulfate anions are also adsorbed by aluminum and iron oxides at low pH. Phosphate is even more strongly adsorbed than sulfate, but it is usually present at such low levels that it does not compete with sulfate for adsorption sites (Eby 2004). Potassium uptake is related to the uptake of bicarbonate ions present in the alkaline solution; the additional potassium ions accumulated through accompanying bicarbonate anions taken up (Hurd 1958). Moreover, increasing concentrations of NaNO2 have resulted in decreased K+ uptake through the root system. The inhibitory effect of NO2 − is strongly influenced by the H+ concentration of the outer medium. pH decrease in external solution has apparently led to increased inhibitory effect of NO2 − on both ion uptake and the growth of seedlings (Zsoldos et al. 1994).
Figures 8 and 9 depict the relations between soil and crops for selected macronutrients and trace metals, respectively. Only soil P contents were positively correlated with those in crops. For most of the investigated elements, there was no significant correlation between the concentrations of elements in soil and those in crops. As already noticed by Zhao et al. (2009), it may be concluded that the total element content in soil is not solely responsible for the observed concentrations in crops. For further evaluation, Pearson’s correlation coefficients were calculated between soil physicochemical parameters and elemental concentration in crops after normalizing the data (Table 6). There were no obvious correlations between most of the parameters, likely due to the complicated conditions and various parameters affecting the transfer of elements from soil to plant such as soil total element concentrations and plant species as already mentioned. There are clear correlations between soil pH and concentrations of P, S, K, Ca, and Mg in crop samples. Ca and Mg are the major components of calcite and dolomite which can increase soil pH. Also, the positive correlations between S, K, and P levels in crops with soil pH are due to the fact that these nutrients are more bioavailable in alkaline soils than acidic soils. Soil OM contents showed slightly negative correlations with almost all element concentrations in crops, indicating that OM may decrease the mobility and bioavailability of the elements in soil. Further, there are negative correlations between soil EC and elemental concentrations in crops which could indicate that soil salinity may inhibit the transfer of nutrients and trace metals from soil to plant.
Health risks in the consumption of crops
To assess the human health risks of metals, it is essential to estimate the level of exposure by quantifying exposure routes. Therefore, to evaluate the potential human health risks in the area, both the DI and HQ were calculated. Because rice and wheat are the dominant cultivated and consumed crops in the area, DI and HQ were only calculated for these two agricultural products.
Per capita consumption of wheat and rice in Iran on the basis of an unpublished regional dietary intake survey is 194 and 40 kg hab−1 year−1, respectively. Tables 7 and 8 display estimated DI for macronutrients and trace metals through consumption of rice and wheat grains. The results were compared with the recommended daily intake (RDI) level as suggested by the NIH or HHS (HHS 2010).
Daily intake of the investigated elements was lower than the RDI levels for rice grains, except for Fe and Mn. However, for wheat grains, all elements had higher DI values than the recommended daily intake levels. It may be suggested that macronutrient deficiency should be compensated by consuming foods rich in nutrients. Moreover, all investigated elements had high DI rates in wheat grains and, hence, could pose a serious threat to human health in time.
Although there are numerous pathways of human exposure to trace metals, wheat and rice consumption is the major pathway. Risk to human health from the intake of metal-contaminated food crops was characterized through calculating the health quotient. Results for individual elements are shown in Table 9. HQ for the majority of the elements was lower than unity, with an exception for Cu (in wheat grains) and Mn (in rice and wheat grains). A high dose of Mn and Cu is reported to cause mental diseases such as Alzheimer’s and manganism (Dieter et al. 2005). The HQ of trace metals resulting from rice and wheat consumption displays the following decreasing trend: Mn > Zn > Cu > Fe > Pb and Mn > Cu > Zn > Fe > Pb, respectively.
The overall potential for noncarcinogenic effects of elements can be estimated by the HI. When the hazard index exceeds unity, there should be concern for potential health effects (Bermudez et al. 2011). In this study, both rice and wheat, the most widely used crops in Iran, had HI values greater than unity, suggesting that inhabitants are experiencing a significant potential health risk solely from the consumption of wheat and rice grains (particularly wheat grains) (Fig. 10).
Conclusions
Chemical analysis of arable soils in the study area revealed that concentrations of Cu, Pb, Zn, Mn, Ca, P, S, and Mg are higher than the world average. Also, compared with soil quality guidelines, Cu, Pb, and Zn showed higher concentrations in samples located in industrial zones such as petrochemical complexes, steel mill, and industrial townships. Cluster analysis indicated that Pb, Zn, and Cu share the same sources, i.e., industry and traffic. In addition, the application of ammonium sulfate, potash, and phosphate fertilizers is believed to be the major source of S, K, and P in croplands. The calculated soil pollution index for enriched trace metals in the soil samples (Cu, Pb, Mn, and Zn) indicates that arable soil is enriched in trace metals in the north and south of the study area. This may be related to the Shahinshahr petrochemical complex and Shahinshahr freeway in the north and the industrial plants such as the Zobe-ahan steel mill in the south. The overall order of macronutrients is K > Ca > S > Mg > P, while the order for trace metal concentrations in the crops is Fe > Mn > Zn > Cu > Pb. When compared with standards, a large proportion of the crop samples seem to be contaminated with Cu, Pb, and Zn. In this study, no direct relationship between metal concentrations in soils and crops was found, and we believe that besides the total metal content, parameters such as the physical, chemical, and biological properties of soil and plant species also play a role in the bioavailability of elements to crops. Our findings showed that the alkaline nature of soil in Isfahan Province plays a major role in the observed high BCF values for S, P, and K. Some soil parameters such as EC and OM also play a critical role in the bioavailability of elements to crops.
The results show that DI values are lower than the RDI levels in rice grains except for Fe and Mn. Therefore, nutrient deficiency (especially macronutrients) may occur in the habitants through consumption of rice grains. On the other hand, all investigated elements displayed high DI in wheat grains and not only compensate for the deficiencies in rice grains but also pose a threat to human health through excessive intake. Calculation of HQ in rice and wheat grains revealed that immediate health risk from individual trace metals is not serious except for Cu (in wheat grains) and Mn (in wheat and rice grains). However, the calculated HI for most metals is more than unity, indicating that consumers of the investigated crops may experience health risks in time.
References
Abbas, S. T., Sarfraz, M., Mehdi, S. M., Hassan, G., & Obaid-Ur, R. (2007). Trace elements accumulation in soil and rice plants irrigated with the contaminated water. Soil & Tillage Research, 94, 503–509.
Adamsa, M. L., Zhao, F. J., McGratha, S. P., Nicholsonb, F. A., & Chambersb, B. J. (2004). Predicting cadmium concentrations in wheat and barley grain using soil properties. Journal of Environmental Quality, 33, 532–541.
Adriano, D. C. (1986). Trace elements in the terrestrial environment. New York: Springer.
Antibachi, D., Kelepertzis, E., & Kelepertis, A. (2012). Heavy metals in agricultural soils of the Mouriki-Thiva area and environmental impact implications. Soil and Sediment Contamination, 21, 434–450.
Avery, B. W. (1980). Soil classification for England and Wales (higher categories). Soil Survey Technical Monograph: No. 14. Harpenden.
Basta, N. T., Ryan, J. A., & Chaney, R. L. (2005). Trace element chemistry in residual-treated soil: key concepts and metal bioavailability. Journal of Environmental Quality, 34, 49–63.
Bennett, D. H., Kastenberg, W. E., & McKone, T. E. (1999). A multimedia, multiple pathway risk assessment of atrazine: the impact of age differentiated exposure including joint uncertainty and variability. Reliability Engineering System Safety, 63, 185–98.
Bermond, A., Varrault, G., Sappin-Didier, V., & Mench, M. (2005). A kinetic approach to predict soil trace metal bioavailability: preliminary results. Plant and Soil, 275, 21–29.
Bermudez, G. M. A., Jasanc, R., Plác, R., & Pignata, M. L. (2011). Heavy metal and trace element concentrations in wheat grains: assessment of potential non-carcinogenic health hazard through their consumption. Journal of Hazardous Materials, 193, 264–271.
Bjørlykke, K. (2010). Petroleum geoscience: from sedimentary environments to rock physics. Berlin: Springer.
Bowen, H. J. M. (1982). Environmental chemistry vol. 2 (specialist periodical report). The Royal Society of Chemistry.
Brookins, D. G. (1988). Eh-pH diagrams for geochemistry. Berlin: Springer.
Cai, L., Xu, Z., Ren, M., Guo, Q., Hu, X., Hu, G., Wan, H., & Peng, P. (2012). Source identification of eight hazardous heavy metals in agricultural soils of Huizhou, Guangdong Province, China. Ecotoxicology and Environmental Safety, 78, 2–8.
CCME (2007). Canadian soil quality guidelines for the protection of environmental and human health. Canadian Council of Minister of the Environment.
Curl, E. A., & Truelove, B. (1986). The rhizosphere (p. 286). Berlin: Springer.
Devkota, B., & Schmidst, G. H. (2000). Accumulation of heavy metals in food plants and grasshoppers from Taigetos Mountains, Greece. Agriculture Ecosystem and Environment, 78, 85–91.
Dieter, H. H., Bayer, T. A., & Multhaup, G. (2005). Environmental copper and manganese in the pathophysiology of neurologic diseases (Alzheimer’s disease and manganism). Acta Hydrochemica Et Hydrobiologica, 33, 72–78.
Eby, G. N. (2004). Principles of environmental geochemistry. Brooks Cole.
European Commission (2006). Setting maximum levels for certain contaminants in foodstuffs. Commission Regulation (EC) No. 1881/2006.
European Commission (EC), Commission Regulation (2002). No. 466/2001 setting maximum levels for certain contaminants in foodstuffs.
FAO/WHO. (1984). Contaminants, Codex Alimentarius, vol. XVII (1st ed.). Rome: FAO/WHO. Codex Alimentarius Commission.
Ficco, D. B. M., Riefolo, C., Nicastro, G., De Simone, V., Di Gesu, A. M., Beleggia, R., Platani, C., Cattivelli, L., & De Vita, P. (2009). Phytate and mineral elements concentration in a collection of Italian durum wheat cultivars. Field Crops Research, 111, 235–242.
Frost, H. L., & Ketchum, L. H. (2000). Trace metal concentration in durum wheat from application of sewage sludge and commercial fertilizer. Advances in Environmental Research, 4, 347–355.
Gupta, U. C., & Gupta, S. C. (2014). Sources and deficiency diseases of mineral nutrients in human health and nutrition: a review. Pedosphere, 24(1), 13–38.
HACH Company. (1993). Soil and irrigation water interpretation manual. Loveland: HACH Co.
Hang, X., Wang, H., Zhou, J., Maa, C., Du, C., & Chen, X. (2009). Risk assessment of potentially toxic element pollution in soils and rice (Oryza sativa) in a typical area of the Yangtze River Delta. Environmental Pollution, 157, 2542–2549.
HHS. (2010). Dietary guidelines for Americans. Washington: United States Department of Agriculture/United States Department of Health and Human Services.
Holm, P. E., Rootzén, H., Borggaard, O. K., Møberg, J. P., & Christensen, T. H. (2003). Correlation of cadmium distribution coefficients to soil characteristics. Journal of Environmental Quality, 32, 138–145.
Huang, M., Zhou, S., Sun, B., & Zhao, O. (2008). Heavy metals in wheat grain: assessment of potential health risk for inhabitants in Kunshan, China. Science of the Total Environment, 405, 54–61.
Hurd, R. G. (1958). The effect of pH and bicarbonate ions on the uptake of salts by disks of red beet. Journal of Experimental Botany. doi:10.1093/jxb/9.2.159.
Jackson, A. P., & Alloway, B. J. (1992). The transfer of cadmium from agricultural soils to the human food chain. In D. C. Adriano (Ed.), Biogeochemistry of trace metals (pp. 109–158). Boca Raton: Lewis.
Jung, M. C., & Thornton, I. (1997). Environmental contamination and seasonal variation of metals in soils, plants and waters in the paddy fields around a Pb–Zn mine in Korea. Science of the Total Environment, 198, 105–121.
Jung, M. C., Yun, S. T., Lee, J. S., & Lee, J. U. (2005). Baseline study on essential and trace elements in polished rice from South Korea. Environmental Geochemistry and Health, 27, 455–464.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. Berlin: Springer.
Kelepertzis, E. (2014). Accumulation of heavy metals in agricultural soils of Mediterranean: insights from Argolida basin, Peloponnese, Greece. Geoderma, 221–222, 82–90.
Khan, K., Lu, Y., Khan, H., Ishtiaq, M., Khan, S., Waqas, M., Wei, L., & Wang, T. (2013). Heavy metals in agricultural soils and crops and their health risks in Swat District, northern Pakistan. Food and Chemical Toxicology, 58, 449–458.
Kim, J. H., Kim, R. H., Lee, J., Cheong, T. J., Yum, B. W., & Chang, H. W. (2005). Multivariate statistical analysis to identify the major factors governing groundwater quality in the coastal area of Kimje, South Korea. Hydrological Processes, 19, 1261–1276.
Kraffczyk, I., Trolldenir, G., & Beringer, H. (1984). Soluble root exudates of maize: influence of potassium supply and rhizosphere microorganism. Soil Biology and Biochemistry, 16, 316–322.
Lavado, R. S., Porcelli, C. A., & Alvarez, R. (2001). Nutrient and heavy metal concentration and distribution in corn, soybean, and wheat as affected by different tillage systems in the Argentine Pampas. Soil and Tillage Research, 62, 55–60.
Lee, C. S., Li, X. D., Shi, W. Z., Cheung, S. C., & Thornton, I. (2006). Metal contamination in urban, suburban and country park soils of Hong Kong: a study based on GIS and multivariate statistics. Science of the Total Environment, 356, 45–61.
Li, P., Wang, X., Zhang, T., Zhou, D., & He, Y. (2009). Distribution and accumulation of copper and cadmium in soil–rice system as affected by soil amendments. Water, Air, and Soil Pollution. doi:10.1007/s11270-008-9755-3.
Liu, W., Li, X., Li, H. H., Sr., & Wang, Y. W. (2006). Heavy metal accumulation of edible vegetables cultivated in agricultural soil in the suburb of Zhengzhou City, People’s Republic of China. Bulletin of Environmental Contamination and Toxicology. doi:10.1007/s00128-006-0981-3.
Lu, A., Wang, J., Qin, X., Wang, K., Han, P., & Zhang, S. (2012). Multivariate and geostatistical analyses of the spatial distribution and origin of heavy metals in the agricultural soils in Shunyi, Beijing, China. Science of the Total Environment, 425, 66–74.
Mapanda, F., Mangwayana, E. N., Nyamangara, J., & Giller, K. E. (2005). The effect of long term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agricultural Ecosystem and Environment, 107, 151–165.
Marcussen, H., Holm, P. E., Strobel, B. W., & Hansen, H. C. B. (2009). Nickel sorption to goethite and montmorillonite in presence of citrate. Environmental Science & Technology, 43(4), 1122–1127.
Maynard, J. B. (1983). Geochemistry of sedimentary ore deposits. New York: Springer.
McGrath, S. P. (1998). Phytoextraction for soil remediation. In Brooks R (ed) Plants that hyperaccumulate heavy metals their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining. New York, pp: 261–287.
McLaughlin, M. J., Parker, D. R., & Clarke, J. M. (1999). Metals and micronutrients—food safety issues. Field Crop Research, 60, 143–163.
Metson, A. J. (1961). Methods of chemical analysis for soil survey samples, New Zealand, DSIR, Soil Bur. Bull., 12., New Zealand: GVT Printer Wellington
Micó, C., Peris, M., Sánchez, J., & Recatalá, L. (2006). Heavy metal content of agricultural soils in a Mediterranean semiarid area: the Segura River Valley (Alicante, Spain). Spanish Journal of Agricultural Research, 4(4), 363–372.
Ogiyama, S., Tagami, K., & Uchida, S. (2008). The concentration and distribution of essential elements in brown rice associated with the polishing rate: use of ICP-AES and micro-PIXE. Nuclear Instruments and Methods in Physics Research B, 266, 3625–3632.
Ostapczuk, P., Valenta, P., Rutzel, H., & Nurnberg, H. W. (1987). Application of differential pulse anodic stripping voltammetry to the determination of heavy metals in environmental samples. Science of the Total Environment, 60, 1–16.
Parengam, M., Judprasong, K., Srianujata, S., Jittinandana, S., Laoharojanaphand, S., & Busamongko, A. (2010). Study of nutrients and toxic minerals in rice and legumes by instrumental neutron activation analysis and graphite furnace atomic absorption spectrophotometry. Journal of Food Composition and Analysis, 23, 340–345.
Rahman, S. H., Khanam, D., Adyel, T. M., Shahidul Islam, M., Aminul Ahsan, M., & Ahedul Akbor, M. (2012). Assessment of heavy metal contamination of agricultural soil around Dhaka Export Processing Zone (DEPZ), Bangladesh: implication of seasonal variation and indices. Applied Sciences, 2, 584–601. doi:10.3390/app2030584.
Romic, M., & Romic, D. (2003). Heavy metals distribution in agricultural topsoils in urban area. Environmental Geology, 43, 795–805.
Ryan, J., Estefan, G., & Rashid, A. (2001). Soil and plant analysis lab manual. International Center for Agricultural Research in the Dry land Areas (ICARDA) 2nd ed.
Schofield, R. K. (1949). Effect of pH on electric charges carried by clay particles. Journal of Soil Science. doi:10.1111/j.1365-2389.1950.tb00713.x.
Shtangeeva, I., Steinnes, E., & Lierhagen, S. (2011). Macronutrients and trace elements in rye and wheat: similarities and differences in uptake and relationships between elements. Environmental and Experimental Botany, 70, 259–265.
Singh, K. P., Mohon, D., Sinha, S., & Dalwani, R. (2004). Impact assessment of treated/untreated wastewater toxicants discharge by sewage treatment plants on health, agricultural, and environmental quality in wastewater disposal area. Chemosphere, 55, 227–255.
Smuc, N. R., Dolenec, T., Serafimovski, T., Tasev, G., Dolenec, M., & Vrhovnik, P. (2012). Heavy metal characteristics in Kočani Field plant system (Republic of Macedonia). Environmental Geochemistry and Health, 34, 513–526. doi:10.1007/s10653-011-9439-6.
Sun, C., Liu, J., Wang, Y., Sun, L., & Yu, H. (2013). Multivariate and geostatistical analyses of the spatial distribution and sources of heavy metals in agricultural soil in Dehui, Northeast China. Chemosphere, 92, 517–523.
US EPA (United States Environmental Protection Agency) (1989). Risk assessment guidance for superfund. Human health evaluation manual (part A). Interim final, vol. I, Washington (DC): United States Environmental Protection Agency; EPA/540/1-89/002
US EPA (United States Environmental Protection Agency) (2010). Region 9 preliminary remediation goals, http://www.epa.gov/ region9/superfund/prg/index.html.
Wang, F. H. (2006). Quantitative methods and applications in GIS[M]. New York: Taylor & Francis.
Wenzel, W. W., Adriano, D. C., Salt, D., & Smith, R. (1999). Phytoremediation: a plant–microbe-based remediation system. In D. C. Adriano, J. M. Bollag, W. T. Frankenberger, & R. C. Sims (Eds.), Bioremediation of contaminated soils (pp. 457–508). Madison: ASA. Monog no. 37.
Xu, L. (2001). Investigation of the body development of 1526 preschoolers in Suzhou City. Acta Academy Med Suzhou, 21(1), 106–7.
Xu, X., Zhao, Y., Zhao, X., Wang, Y., & Deng, W. (2014). Sources of heavy metal pollution in agricultural soils of a rapidly industrializing area in the Yangtze Delta of China. Ecotoxicology and Environmental Safety, 108, 161–167.
Yeung, A. T., & Hsu, C. N. (2005). Electrokinetic remediation of cadmium contaminated clay. Journal of Environmental Engineering, 131(2), 298. doi:10.1061/(ASCE)0733–937.
Yuan, B. J., Pan, X. Q., Dai, Y., & Shi, X. M. (2007). Changes in the dietary structure and nutrition status of the inhabitants in Jiangsu province. Acta Nutrimenta Sinica, 29(6), 569–72.
Zhao, K., Zhang, W., Zhou, L., Liu, X., Xu, J., & Huang, P. (2009). Modeling transfer of heavy metals in soil–rice system and their risk assessment in paddy fields. Environmental Earth Sciences, 59, 519–527.
Zheng, N., Wang, Q. C., Zhang, X. W., Zheng, D. M., Zhang, Z. S., & Zhang, S. Q. (2007). Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Science of the Total Environment, 387, 96–104.
Zsoldos, F., Vashegyi, A., & Pécsváradi, A. (1994). Effects of pH and nitrite on potassium uptake and growth of rice seedlings. Journal of Plant Physiology, 144(3), 358–361.
Acknowledgments
The authors would like to acknowledge the help of Shiraz University Research Committee and Isfahan Department of Environment Protection for financial support. Thanks are also extended to the Shiraz University Medical Geology Research Centre and the Centre of Excellence for Environmental Geology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keshavarzi, B., Moore, F., Ansari, M. et al. Macronutrients and trace metals in soil and food crops of Isfahan Province, Iran. Environ Monit Assess 187, 4113 (2015). https://doi.org/10.1007/s10661-014-4113-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4113-y