Abstract
The present study aimed to evaluate the acute and chronic toxicity of environmentally relevant concentrations of metals (Mn, Al, Fe, and Pb) in Daphnia magna and the generational transposition of reproductive and morphological damages. The effective concentration for 10% of the organisms from each metal was obtained by the acute toxicity test (96 hours); then, another five concentrations lower than this one were defined for the chronic experimentation (21 days), in which the number of neonates generated by each individual was checked daily. At the end of the exposition, the lengths and number of morphological damages were recorded in each adult daphnid. During this, the molt generated on the 14th and 21st days were collected and cultivated for posterior evaluation of the same parameters. Alterations in the reproductive performance were observed in the organisms exposed to manganese and aluminum (4.0 and 0.5 mg L−1, respectively). Organisms exposed to aluminum (0.05 mg L−1) and iron (0.27 mg L−1) showed a reduction in body length. It is also noteworthy that the molt of these adults and their respective offspring also presented reproductive alterations, especially the molt from the 14th day of lead exposure (0.02 mg L−1) and the 21st day of manganese exposure (4.0 mg L−1). Such effects allow us to conclude that environments polluted by metals can reduce the ability of the species to maintain themselves in the ecosystem. In addition, there is a need to increase the control and monitoring of metals, such as aluminum, which present risks even in low concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution from erroneous anthropogenic actions favors the presence of metals and other contaminants in water (Kassim et al. 2011). Once released, the metals can deposit in the sediment or be available in the water column (Simpson and Spadaro 2016), damaging the quality of soil, water, and all surrounding ecosystem. In addition, geogenic sources also contribute to the occurrence of these elements in groundwater and surface water (Martin et al. 2015; Winkel et al. 2008). However, they cause concern due to their persistence, toxicity, and bioaccumulation ability in living aquatic organisms (Ahmed et al. 2015; Islam et al. 2015), including plants (Salawu et al. 2018).
Among the anthropogenic polluting sources responsible for the occurrence of metals in water bodies, mining stands out (Li et al. 2015), and discharge of effluents without previous treatment (Chen et al. 2015) and the excessive use of chemical fertilizers and pesticides in agriculture (Eqani et al. 2012), and for this reason, it has been the subject of several studies that evaluate the toxicity in humans (Cerrillos et al. 2019; Deshommes et al. 2016; Myers et al. 2009), fish (Gao et al. 2019; Marins et al. 2019; Rodrigues et al. 2020; Yin et al. 2018), and rodents (Asser et al. 2019; Cobbina et al. 2015a, b; Kumar et al. 2009; Wang et al. 2018; Zhai et al. 2017), but less frequently in Daphnia sp. (Araujo et al. 2019; Cui et al. 2018; Zimmermann et al. 2017).
Consequently, the enrichment of heavy metals in wildlife through the food chain poses potential threats to humans (Lei et al. 2016). In addition, it should be noted that most current water potability systems in developing countries are insufficient for the full removal of these compounds, characterizing as an environmental and public health problem. Knowing that measuring only the total metal concentrations in water or sediment is an insufficient way to accurately assess their impact (de Miguel et al. 2005; Wu et al. 2016), to understand how these substances behave at different levels can be the best way to understand their biological risk.
Aluminum (Al), iron (Fe), manganese (Mn), and lead (Pb) are among the main environmental pollutants of freshwater (Castro et al. 2018; Dalzochio et al. 2018; Kakoi et al. 2016; Marsidi et al. 2018), and generally have their effects associated with neurotoxicity (Altenhofen et al. 2017; Guiney et al. 2017; Wang et al. 2016, 2018), with very few studies addressing reproductive aspects of toxicity caused by such substances. But some authors already consider some metals as endocrine disruptors, especially because they interfere with steroid receptors; for example, cadmium, barium, and chromium have shown estrogenic activity in estrogen receptors (Choe et al. 2003), while nickel, lead, and mercury induced the expression of genes regulated by estrogen and receptor progesterone in MCF-7 cells (Martin et al. 2003). For these reasons, some authors recognize these substances as metalloestrogens (Paschoalini et al. 2019; Safe 2003).
Some evidence indicates mechanisms of action that affect reproductive impairment through these substances on aquatic organisms, for example, in fish exposure to mercury, causing oxidative stress in the gonads and disruption of the transcription of hypothalamic-pituitary-gonadal (HPG) axis genes of zebrafish (Guchhait et al. 2018), and modulating the aromatase gene expression altering normal development of oocytes in Trichogaster fasciata (Zhang et al. 2016), or in microcrustacean exposure to cadmium, lead, and mercury, affecting the transcriptional modulation of the genes encoding antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) that are involved in cellular defense system protection against xenobiotics and consequently the induction of oxidative stress in Daphnia magna (Kim et al. 2017).
Either way, endocrine disruption can occur at different physiological levels (hormonal secretion, hormonal interaction, hormonal excretion) (Rodríguez et al. 2007), consequently causing problems for future generations. Even so, in aquatic species, there is a lack of information about the transgenerational toxicity of these metals (Mn, Al, Fe, and Pb) (Chen et al. 2017; Reis et al. 2018), that is, effects on the progeny from parents exposed at critical stages of development (Yang et al. 2013). It is noteworthy that studies that address effects on parents are important for ecotoxicology; however, the demonstrations of effects on offspring are fundamental, as they link what is commonly studied (parents) to what can represent a significant ecological effect, that is, the molts’ aptitude to sensitivity to induced stressor (Reátegui-Zirena et al. 2017).
In our study, we chose Daphnia magna as a model organism, which are freshwater zooplankton that play a key role in the food chain, because they transfer biomass and lipid-like macronutrients from primary producers to higher trophic levels (Ferain et al. 2018). Studies evaluating transgenerational effects in this species generally demonstrate Daphnia’s ability to alter even its normal biological behavior; for example, the offspring of parents exposed to predation are born with a mother-induced defense (Agrawal et al. 1999), or mothers cultivated in poor conditions can produce more resistant offspring (Mitchell and Read 2005). Other authors even report the transposition of genotoxic damage to the offspring of parents exposed to uranium (Plaire et al. 2013) and gamma radiation (Parisot et al. 2015). So, these transgenerational effects are critical for the survival of organisms under normal or unfavorable and unpredictable conditions (Plautz et al. 2013), especially because, if the stressor experienced by the offspring differs from that experienced by the parents, a new and potentially suitable phenotype can be expressed in these individuals, being the phenotypic variability increased in this generation of offspring (Bayadev 2005; Colombo et al. 2014).
Therefore, the aim of our study was to evaluate the toxicity and generational transposition of reproductive and morphological damage caused by metals, commonly found as water pollutants, in Daphnia magna after acute and chronic exposure.
Materials and methods
Metals
The metals used in the present study for acute and chronic toxicity were aluminum, lead, iron, and manganese, which have worldwide environmental occurrence (Deshommes et al. 2016; Marsidi et al. 2018; Panhwar et al. 2016). The concentrations used, as well as the reference values provided by the Brazilian legislation, can be observed in Table 1. The chemical reagents used for the exposures were aluminum chloride (LabSynth®), lead acetate (LabSynth®), ferric chloride (LabSynth®), and manganese chloride (LabSynth®). The concentrations of each experimental group were prepared from stock solutions of each metal and were confirmed using a bench spectrophotometer (HANNA®).
Model organism
The organisms used were originated from Daphnia magna clones grown at the Feevale University Ecotoxicology laboratory under standard conditions for approximately 3 years. The cultivated water used comes from reverse osmosis and then reconstituted with salts, as recommended by NBR 12.713 (ABNT 2016), with pH and hardness adjusted between 7.0 and 8.0 and between 175 and 225 mg L−1 CaCO3, respectively, and previous aeration of at least 12 h. The clones used as reproductive matrices were kept in batches of up to 25 adults per liter, in a 1000 mL container, with diffused light (16 h photoperiod of light) and temperature ranging from 18 to 22 °C in a modified BOD incubator. For food, the green alga Desmodesmus subspicatus Chodat, 1942 was used, providing the amount of approximately 106 cells mL−1 per adult organism every 48 h. For acute and chronic toxicity tests, neonates aged less than 24 h old were obtained from females with an average of 20 days old.
Acute toxicity assay
For acute toxicity assay, Daphnia magna neonates were exposed (n = 20 per group, 4 replicates with 5 organisms in 50 mL) to dilutions prepared with different concentrations of metals to be tested for 48 h. During this period, the organisms were kept at 18 to 22 °C without food (ABNT 2016). At the end of the exposure, the number of immobile organisms was observed, aiming to calculate the effective concentration for 10% of the organisms (EC10), that is, the concentration responsible for the acute effect in 10% of the animals. This concentration was used as the maximum concentration in the chronic toxicity assay, aiming to maintain an adequate number of organisms throughout the 21 days of exposure.
Chronic toxicity assay
From the EC10 of each metal of the organisms, lower concentrations were established for the chronic test. Briefly, 10 female neonates were exposed at these concentrations for 21 days, and in this trial each neonate was kept alone in 50 mL beakers. Renewal of the dilutions from chronic experiments was performed every 48 h. During the experiment, each individual was daily observed, recording the date of first hatching and the daily number of newborns generated per female. At the end of the experiment, images of each female were captured by optical microscopy (× 40 magnification) (Olympus IX73) in order to verify possible morphological changes (carapace damage, shortening of the digestive tract, and caudal spine shortening) and also to measure the body length with the aid of ImageJ® software. For the data on morphological damages, the percentage of changes cited in each evaluated group was calculated, for later statistical evaluation. During the 21 days of experiments, the molts generated on the 14th and 21st days were collected and kept in the laboratory, in standard culture medium, but not exposed to metals, also for 21 days, to record the number of offspring generated by this brood and also to verify the possible morphological alterations and their respective lengths (same protocols previously cited). The feeding of the organisms used in the chronic experiment, as well as their respective generations that were collected, occurred with the Desmodesmus subspicatus algae every 48 h, as already described.
Data analysis
From the results obtained after the mortality and immobility readings in the acute toxicity assay, the EC10 was calculated with the aid of the ICPIN software by the linear interpolation method. In the chronic toxicity assay, data obtained through the registration of neonates generated by female and body length were tested for data normality through the Kolmogorov-Smirnov test and subsequently submitted to parametric (one-way ANOVA and Tukey) and non-parametric (Kruskal-Wallis and Dunn) tests when appropriate, with the aid of GraphPad Prism 6 software. Results were considered statistically significant when p < 0.05.
Results
Acute toxicity assay
When conducting the acute experiment with aluminum, the concentrations used did not cause any kind of lethality or immobility to the organisms during 48 h of the test (in order to confirm this result, the acute experiment was repeated), allowing the use of the same concentrations for the chronic experiment. In contrast, the other acute tests allowed us to establish the EC10 for manganese, iron, and lead, as can be seen in Table 2.
Chronic toxicity assay: reproductive and length parameters
Over the 21 days of chronic experimentation with each metal, the number of neonates generated per female was recorded, as can be seen in Fig. 1(A1–A4), and details the cumulative number of offspring produced at different concentrations of each experiment. Microcrustaceans exposed to manganese 4.0 mg L−1 and aluminum 0.05 mg L−1 showed a significant reduction in the cumulative number of generated neonates compared to the control group (p = 0.02 and p = 0.005, respectively). Organisms exposed to iron and lead showed no significant changes in relation to control (p = 0.27 and p = 0.28, respectively). During chronic assay with aluminum, 100% lethality of organisms exposed to the highest concentrations occurred after 72 h (0.15 and 0.20 mg L−1) and 06 days (0.1 mg L−1), remaining only the organisms exposed to 0.05 and 0.025 mg L−1.
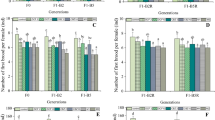
(A1–A4) Cumulative number of newborns generated by females during the 21-day chronic toxicity assay. Asterisks represent a statistical difference in relation to the control group. (B1–B4) Length measured at the end of 21 days of exposure to different metals (Data are expressed as mean and standard deviation and asterisks indicate significant difference from control, p < 0.05)
At the end of the exposure period, each D. magna was measured (Fig. 1(B1–B4)), and it was observed that organisms exposed to aluminum 0.05 mg L−1 and iron 0.27 mg L−1 showed a significant reduction in the corporal length in relation to the control group (p = 0.0002 and p = 0.0003, respectively).
Transgenerational evaluate
The molts that were generated during the chronic experiment, on the 14th and 21st day, by females exposed to different metals were cultured in the laboratory under the same conditions for 21 days. For these organisms, the accumulated offspring per female was also recorded and can be observed in Fig. 2.
In the organisms collected on the 14th day of the chronic assay, a significant difference in D. magna exposed to iron 0.03 mg L−1 and lead 0.02 mg L−1 in relation to the control group (p = 0.009 and p = 0.008) was observed, presenting a reduction and increase, respectively, in the cumulated offspring. In contrast, for organisms collected on the 21st day of the chronic assay, only D. magna exposed to manganese 4.0 mg L−1 differed from control (p < 0.0001), showing an increase in the number of accumulated offspring.
Regarding the measured body length (Fig. 3), neonates collected on the 14th day of exposure of mothers exposed to manganese 0.5 mg L−1 (presenting a reduction in the body length), aluminum 0.025 mg L−1 (increase in length), iron (0.27 and 0.03 mg L−1) (reduction in length), and lead 0.02 mg L−1 (increase in length) differed significantly from control after 21 days of observation (p = 0.01, 0.0009, 0.0004, 0.0009, and 0.016, respectively). For newborns collected on the 21st day, maternal exposure to manganese 2.0 mg L−1 (showing increase in length), 1.0 mg L−1, and 0.25 mg L−1 (reduction in length), all iron concentrations presenting an increase in the body length (except 0.03 mg L−1), and all lead concentrations, with many variations in the body length, resulted in significant differences in the size of the generated newborns, even after 21 days of observation, indicating that prolonged exposure may result in further damage to consecutive offspring.
A comparative table (Table 3) between the concentration established by the current Brazilian legislation and the minimum effect concentration, i.e., the lowest concentration used in the experiment that resulted in some change in the mothers or offspring of the respective mothers, is presented. Briefly, it can be observed that the full chronic exposure of mothers (21 days) to the lowest concentrations of metals used resulted in the largest morphological effects of body length observed in offspring (except for aluminum), confirming that prolonged exposure to metals, even at concentrations lower than those established by law, may result in generational transposition of damage. The exception is aluminum, which proved to be more toxic than the other metals studied and caused lethality in the organisms at the beginning of the chronic experiment (72 h) even in low concentrations.
Morphological analysis
Despite reproductive and size changes, no major morphological changes were observed in the mothers of the chronic experiment and their respective molts (14th and 21st) over the 21 days of observation. Figure 4 exemplifies the changes that were sought, namely caudal spine shortening, digestive tract shortening, and carapace deformations. It is noteworthy that the differences were sought between the groups but only within the same experimental period (mothers exposed to 21 days, neonates collected on the 14th day, or neonates collected on the 21st day), and are shown in the same graph as an illustration. The p values of statistical comparisons can be seen in the Table 4.
(A1–A4) Graphs of means demonstrating that there was no significant difference in morphological analysis between groups from the same experimental period (mothers exposed to 21 days, offspring collected on the 14th day, or offspring collected on the 21st day) for manganese (A1), aluminum (A2), iron (A3), or lead (A4). (B1–B4) Daphnia magna with normal morphology (B1); caudal spine shortening, represented by red arrow (B2); digestive tract shortening, represented by black arrow (B3); and carapace deformation, shown by blue arrow (B4)
Discussion
Changes that organisms show after prolonged exposure to different contaminants can vary from resistance/tolerance to increased sensitivity, both leading to problems in the management of the species (Kimberly and Salice 2015), because under stressful conditions the organisms generally use different strategies that increase survival and reproductive success, changing mainly their reproduction and growth rates (Guan and Wang 2006). In the metallic environments, these compensations serve to keep functions at a basic level; that is, the organisms injured by the stressor dedicate energy to improve the effects of stressors and, as a result, have less energy available for growth and reproduction (Kimberly and Salice 2015). However, as previously mentioned, organisms can also increase their tolerance to the environment, by increasing the expression of the metallothionein protein, for example, which is known to increase the resistance of different animal species to metals (Beg et al. 2015; Amiard et al. 2006; Wang and Rainbow 2010).
Interferences caused by heavy metals in the reproductive system of microcrustaceans are reported by some authors; Pérez and Hoang (2018) report alterations in the embryonic development of D. magna after cadmium exposure and cadmium and zinc mixture exposure. Differing from our findings, Araujo et al. (2019) report reduced reproductive performance of D. magna exposed to lead nitrate; however, the same authors describe a higher sensitivity of D. similis compared to D. magna for lead ecotoxicological evaluation, which may justify the non-reproducibility of data obtained from different studies with D. magna. Regaldo et al. (2014) also report reproductive changes in D. magna after exposure to copper, lead, and chromium. However, to date, we are unaware of studies reporting exposure to iron, aluminum, and manganese. In addition, no previous study has chronicled offspring generated by the organisms studied, as presented here. And, although no significant differences were observed in the morphological analysis, the mothers’ exposure to metals resulted in reproductive and body length changes in the organisms as well as their respective offspring, indicating that the damage caused was transferred to subsequent generations, even when the concentrations used were lower than those established by law.
The alterations found in our study for the length of daphnids, in real environmental situations, could bring problems for the species, since species with reduced size end up becoming more vulnerable to predators (Lynch 1980). Therefore, we can affirm that exposures to low concentrations of Al and Fe (0.27 and 0.05 mg L−1 respectively) during the growth of daphnids would result in a greater exposure to predators in the environment. However, it has already been described that reproduction occurs regardless of this growth inhibition or not, but with the brood quality altered (Skinner 1985; LeBlanc and Mclachlan 2009). There are reports that daphnids in the presence of a stressor produce fewer eggs, but of larger size, rich in lipids, which may even be more resistant (Knops et al. 2001). The alterations in growth rate can also be affected by changes in the intestinal microbiota of D. magna (Akbar et al. 2020), and it is worth mentioning that, although there is still no correlation in this species, in others there are already reports of changes in the intestinal microbiota caused by manganese (Ding et al. 2020), iron (Fang et al. 2018), lead (Xing et al. 2019), and also aluminum (Alexandrov et al. 2020). So, we can assume that all changes in size induced by direct or mother exposure to metals can be considered toxicological responses of the species.
Manganese and iron can be classified as essential metals, which in low concentrations are essential for life, whereas aluminum and lead are considered non-essential, which have no known function in the body (Chang et al. 1996; Gebara et al. 2020; WHO/FAO/IAEA 1996). This fact may justify our finding of greater “tolerance” of daphnids to manganese and iron, since they endured exposures to higher concentrations in comparison to aluminum and lead, and it also goes according to the literature (Okamoto et al. 2014). Especially, since non-essential metals can cause toxicity even at low concentrations (Menon et al. 2016). For crustaceans, it is already known that there are several possible destinations for a metal: binding to metallothionein, transport to mitochondria, accumulation by lysosomes, transfer to the endoplasmic reticulum, or discharge back into the blood (Ahearn and Zhuang 1996; Ahearn et al. 2004), and the same is described for different animals (Banday et al. 2020; Frank et al. 2008; Monteiro et al. 2019), but all can assist in the imbalance of metallic homeostasis, and manifest through DNA damage, gene expression, and increased oxidative stress (Menon et al. 2016).
Anyway, metals are potentially toxic to the DNA of many organisms and are capable of inducing the production of reactive oxygen species (ROS) that can overwhelm the antioxidant defenses of the cells and result as a mechanism for oxidative stress, especially in long-term exposure (Ercal et al. 2001; Kim et al. 2017). Therefore, as already mentioned, the fact that progenies from newborns of mothers exposed for 21 days to Pb and Fe were able to normalize the production of neonates that had been deregulated in progeny of molt from mothers exposed for 14 days to these substances may be associated with the increased antioxidant defense system that activates detoxification mechanisms (Guan and Wang 2004), suggesting an adaptive response of this species against toxicity (Kim et al. 2017; Massarin et al. 2010). These results demonstrated that, when returning to the clean culture medium, D. magna is able to regulate its reproduction pattern, but even if the defense system acts, repairing the damage, other effects may increase (Ercal et al. 2001), as the repair process is subject to errors, corroborating the increase in body length changes of offspring of the newborns that were collected on the 21st day of exposure of mothers to metals.
Such adaptations to changes in the environment occur due to maternal effects, whereby mothers transmit information about environmental variability to the next generation, thus influencing their children’s adaptive phenotypic responses or not (Enserink et al. 1993; Krylov and Osipova 2019; Mousseau and Fox 1998). In other words, Marshall and Uller (2007) state that maternal effects occur when the mother’s phenotype or the environment she experiences influences her children’s phenotype, in addition to the direct effect of the transmitted genes. The environments to which mothers are exposed can lead to either an increase or decrease in offspring, as well as changes in the size of their children (Lampert 1993), corroborating with our study, where the exposure of mothers to different metals resulted in different responses observed in the molts and their respective offspring.
In other aquatic organisms such as fish, metals in general are associated with numerous neurological, hepatic, renal, intestinal, and even genetic changes (Altenhofen et al. 2017; Coppo et al. 2018; Rodrigues et al. 2018, 2020; Wang et al. 2016); however, few studies address reproductive changes caused by these substances, as can be seen in a recent review (Rodrigues et al. 2019). Gárriz et al. (2018) report morphological effects on the testis of Odontesthes bonariensis treated with cadmium, copper, and chromium, such as testicular fibrosis and pyknotic cells. With the metals worked in this article, studies are further restricted; Kida et al. (2016) report increased levels of testosterone and 17β estradiol in Astyanax altiparanae species sharply exposed to aluminum and manganese, respectively (0.5 mg L−1).
Aluminum and lead are considered metal estrogens because of their ability to interfere with the action of estrogen hormones (Safe 2003), by blocking or decreasing the binding of endogenous estrogens to their receptors and acting as agonists by interacting with ligand binding domains at estrogen receptors (Darbre 2006). In addition, the abnormal increase in the number of spermatogonia in fish may be related to constant exposure to metals capable of mimicking the action of 17β estradiol, such as lead and aluminum (Georgescu et al. 2011). In contrast, when there is an increase in the number of germ cells but a reduction in sperm, and consequently a reduction in reproductive performance, there may be disturbances in androgen production and signaling, affecting final spermatogenesis steps (Chaube et al. 2010). This type of behavior was reported by Paschoalini et al. (2019) in Prochilodus argenteus fish from a place contaminated by different heavy metals. The authors also defend the idea that metals can positively or negatively alter the reproductive success of fish. As we cannot assume that the mechanisms responsible for the alterations in the number of D. magna offspring studied are equivalent to the alterations found in fish or humans, studies that evaluate the pathways involved in the reproductive process in the species are necessary, and in order to refine all species reports of reproductive toxicity with the species.
Furthermore, it can be observed that the number of neonates generated by the mothers of the control groups of chronic exposure to metals differs from the number of neonates generated by this molt when followed for 21 days, perhaps because, although reproduction in the species occurs by parthenogenesis, as a classic study of the area (Guisande 1993) reveals, as the population density increases, there is a reduction in the sexual maturation age of the females, consequently resulting in a greater number of newborns generated during the experimental period. What we mean is that the females used for chronic exposures are the result of lots where the organisms are kept inside a beaker in a small population, and their reproductive behavior cannot be compared with the reproductive behavior of their molt, which has origin of females kept in isolation. Moreover, there are reports that the temporal social isolation, as well as agglomeration, can lead to the ephippium formation by Daphnia (Gerber et al. 2018). Although it was not observed in our study, this corroborates with data from the literature that indicates that the environmental determination of sex involves the sensory transduction of environmental stimuli, in addition to the subsequent modification of hormonal pathways, in order to influence the sex of the offspring (Camp et al. 2019; Devlin and Nagahama 2002; Korpelainen 1989).
Finally, among the tested metals, the aluminum with the highest toxicity stands out, since, when performing the acute test, as recommended by Brazilian legislation, none of the tested concentrations caused lethality or immobility to the organisms; however, 24 h after the acute experimentation period, that is, after 72 h, we obtained 100% lethality of organisms exposed to the highest concentrations (0.15 and 0.20 mg L−1). It is noteworthy that such values are only twice as high as allowed by the legislation, being well below the values found in some localities, which often exceed up to ten times higher (Bianchi et al. 2019; Dalzochio et al. 2018; Gurgel et al. 2016; Machado et al. 2017; Matos et al. 2017; Rodrigues et al. 2016; Souza et al. 2016).
The effective concentrations for 10% of the organisms obtained in the present study, for manganese, iron, and lead, are above the allowed values by the legislation, but also represent the reality of many Brazilian localities, such as Rio Grande do Sul, Sinos River, Paraná, Alto do Iguaçu River, and Minas Gerais, Pardo River, where the values of iron, manganese, and lead reach 2.0 mg L−1, 7.84 mg L−1, and 0.86 mg L−1, respectively (Alves et al. 2014; Bianchi et al. 2019; Brito et al. 2018). It is also noteworthy, as shown in Table 2, that the four metals studied caused progeny damage to mothers chronically exposed to concentrations lower than those allowed by the law, noting that acute toxicity tests do not reflect well the risk that these contaminants pose to aquatic biota.
So, the findings point to the need for studies to assess the chronic toxicity of substances, as they do come close to the conditions to which different organisms are exposed daily in the ecosystem, and as in the case of aluminum, for example, it was found that acute tests can provide mistaken answers about the actual toxicity of substances. In addition, lethality and immobility parameters commonly evaluated in acute toxicity tests do not fully reflect the effects caused by certain contaminants. The present study sets precedents for future work to evaluate whether acute exposure, as well as chronic exposure, to these and other metals is capable of interfering with the quality of generated offspring, as well as a biochemical evaluation of such organisms.
Conclusion
Brazilian and worldwide legislations establish reference limits for the occurrence of metals in water. However, watercourses that meet these limits are rare; generally, the values quantified by the studies are much higher than the allowed ones. Therefore, in our study, we evaluated the concentrations of environmental polluting metals which represent both general legislation and environmental reality. All metals (Al, Fe, Mn, and Pb) were able to cause some effect, whether in organisms chronically exposed to substances or their respective offspring. Besides, aluminum, which is strongly present in many types of soil, therefore also in surface waters, and even in drinking water in developing countries, generated the absolute lethality of organisms in less than 7 days of exposure in concentrations lower than permitted by the Brazilian legislation for surface water.
Transposing the results obtained to a systematic reality, the metals, capable of altering the reproductive normality and the morphology of the daphnids in our study, could interfere in the maintenance of the species in the ecosystem, as well as alter their intake by predators, and so on.
Our study warns the need to review global standards for testing toxicity samples with the species (Daphnia magna), since the acute test does not seem to accurately demonstrate the risk that substances offer to organisms, in addition to the need to increase the control and the monitoring of domestic and industrial waste containing such substances since they offer risks even in low concentrations. The study also sets precedents for investigating the mechanisms responsible for the generational transposition of damage caused by metals.
References
Agrawal, A. A., Laforsch, C., & Tollrin, R. (1999). Transgenerational induction of defences in animals and plants. Nature, 401, 60–63.
Ahearn, G. A., & Zhuang, Z. (1996). Cellular mechanisms of calcium transport in crustaceans. Physiological Zoology, 69, 383–402.
Ahearn, G. A., Mandal, P. K., & Mandal, A. (2004). Mechanisms of heavy-metal sequestration and detoxification in crustaceans: a review. Journal of Toxicology and Environmental Health, Part B, 174(6), 439–425.
Ahmed, M. K., Baki, M. A., Islam, M. S., Kundu, G. K., Sarkar, S. K., & Hossain, M. M. (2015). Human health risk assessment of heavy metals in tropical fish and shell fish collected from the river Buriganga, Bangladesh. Environmental Science and Pollution Research, 22(20), 15880–15890.
Akbar, S., Gu, L., Sun, Y., Zhou, Q., Zhang, L., Lyu, K., Huang, Y., & Yang, Z. (2020). Changes in the life history traits of Daphnia magna are associated with the gut microbiota composition shaped by diet and antibiotics. The Science of the Total Environment, 705, 1352827.
Alexandrov, P. N., Hill, J. M., Zhao, Y., Bond, T., Taylor, C. M., Percy, M. E., Li, W., & Lukiw, W. J. (2020). Aluminum-induced generation of lipopolysaccharide (LPS) from the human gastrointestinal (GI)-tract microbiome-resident Bacteroides fragilis. Journal of Inorganic Biochemistry, 203, 110886.
Altenhofen, S., Wiprich, M. T., Nery, L. R., Leite, C. E., Vianna, M. R. M. R., & Bonan, C. D. (2017). Manganese (II) chloride alters behavioral and neurochemical parameters in larvae and adult zebrafish. Aquatic Toxicology, 182, 172–183.
Alves, R. I. S., Sampaio, C. F., Nadal, M., Schuhmacher, M., Domingo, J. L., & Segura-Muñoz, S. I. (2014). Metal concentrations in surface water and sediments from Pardo River, Brazil: Human health risks. Environmental Research, 133, 149–155.
Amiard, J. C., Amiard-Triquet, C., Barka, S., Pellerin, J., & Rainbow, P. S. (2006). Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquatic Toxicology, 76, 160–202.
Araujo, G. S., Abessa, D. M. S., Soares, A. M. V. M., & Loureiro, S. (2019). Multi-generational exposure to Pb in two monophyletic Daphnia species: Individual, functional and population related endpoints. Ecotoxicology and Environmental Safety, 30, 77–85.
Asser, A., Köks, S., Soomets, U., Terasmaa, A., Sauk, M., Eltermaa, S., Piip, P., Ubhayasekera, K., Bergquist, J., & Taba, P. (2019). Acute effects of methcathinone and manganese in mice: a dose response study. Heliyon, 5(9), e02475.
Associação Brasileira de Normas Técnicas. ABNT. (2016). NBR 12713: ecotoxicologia aquática: toxicidade aguda: método de ensaio com Daphnia spp (Crustacea, Cladocera). Rio de Janeiro.
Banday, U. Z., Swaleh, S. B., & Usmani, N. (2020). Heavy metal toxicity has an immunomodulatory effect on metallothionein and glutathione peroxidase gene expression in Cyprinus carpio inhabiting a wetland lake and a culture pond. Chemosphere, 225, 126311.
Bayadev, A. V. (2005). Stress-induced variation in evolution: from behavioral plasticity to genetic assimilation. Proceedings of the Royal Society - Biological Sciences, 272(1566), 877–886.
Beg, U. M., Al-Jandal, N., Al-Subiai, S., Karam, Q., Husain, S., Butt, S. A., Al-Hasan, E., Al-Dufaileej, E., & Al-Husaini, M. (2015). Metallothionein, oxidative stress and trace metals in gills and liver of demersal and pelagic fish species from Kuwaits’ marine area. Marine Pollution Bulletin, 100(2), 662–672.
Bianchi, E., Dalzochio, T., Simões, L. A. R., Rodrigues, G. Z. P., Silva, C. E. M., Gehlen, G., Nascimento, C. A., Spilki, F. R., Ziulkoski, A. L., & Silva, L. B. (2019). Water quality monitoring of the Sinos River basin, southern Brazil, using physicochemical and microbiological analysis and biomarkers in laboratory-exposed fish. Ecohydrology and Hydrobiology, 19(3), 328–338.
BRASIL. Conselho Nacional do Meio Ambiente – CONAMA (2005). Resolução CONAMA n° 357, de 17 de março de 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de efluentes, e dá outras providencias. Diário Oficial da República Federativa do Brasil, Brasilia, 18 mar. pp. 58–63. Disponível em: <http://www.mma.gov.br/port/conama/res/res05/res35705.pdf>
Brito, I. A., Garcia, J. R. E., Salaroli, A. B., Figueira, R. C. L., Martins, C. C., Neto, A. C., Gusso-Choueri, P. K., Choureri, R. B., Araujo, S. B. L., & Ribeiro, C. A. O. (2018). Embryo toxicity assay in the fish species Rhamdia quelen (Teleostei, Heptaridae) to assess water quality in the Upper Iguaçu basin (Parana, Brazil). Chemosphere, 208, 207–218.
Camp, A. A., Haeba, M. H., & LeBlanc, G. A. (2019). Complementary roles of photoperiod and temperature in environmental sex determination in Daphnia spp. Journal of Experimental Biology, 222(Pt 4):jeb195289. https://doi.org/10.1242/jeb.195289.
Castro, L. N., Rendina, A. E., & Orgeira, M. J. (2018). Assessment of toxic metal contamination using a regional lithogenic geochemical background, Pampean area river basin, Argentina. Sci. Total Environ, 627, 125–133.
Cerrillos, L., Fernández, R., Machado, M. J., Morillas, I., Dahiri, B., Paz, S., Gonzalles-Weller, D., Gutiérrez, A., Rubio, C., Hardisson, A., Moreno, I., & Fernández-Palacín, A. (2019). Placental levels of metals and associated factors in urban and sub-urban areas of Seville (Spain). Journal of Trace Elements in Medicine and Biology, 54, 21–26.
Chang, L. W., Magos, L., & Suzuki, T. (1996). Toxicology of metals. Boca Raton: CRC Press.
Chaube, R., Mishra, S., & Rahil Singh, K. (2010). In vitro effects of lead nitrate on steroid profiles in the postvitellogenic ovary of the catfish Heteropneustes fossilis. Toxicology In Vitro, 24(7), 1899–1904.
Chen, H., Teng, Y., Lu, S., Wang, Y., & Wang, J. (2015). Contamination features and health risk of soil heavy metals in China. The Science of the Total Environment, 512, 143–153.
Chen, L., Wang, X., Zhang, X., Lam, P. K. S., Guo, Y., Lam, J. C. W., & Zhou, B. (2017). Transgenerational endocrine disruption and neurotoxicity in zebrafish larvae after parental exposure to binary mixtures of decabromodiphenyl ether (BDE-209) and lead. Environmental Pollution, 230, 96–106.
Choe, S. Y., Kim, S. J., Kim, H. G., Lee, J. H., Choi, Y., Lee, H., & Kim, H. (2003). Evaluation of estrogenicity of major heavy metals. The Science of the Total Environment, 312(1–2), 15–21.
Cobbina, S. J., Chen, Y., Zhou, Z., Wu, X., Feng, W., Wang, W., Li, Q., Zhao, T., Mao, G., Wu, X., & Yang, L. (2015a). Interaction of four low dose toxic metals with essential metals in brain, liver and kidneys of mice on sub-chronic exposure. Environmental Toxicology and Pharmacology, 39(1), 280–291.
Cobbina, S. J., Chen, Y., Zhou, Z., Wu, X., Zhao, T., Zhang, Z., Feng, W., Wang, W., Li, Q., Wu, X., & Yang, L. (2015b). Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. Journal of Hazardous Materials, 294, 109–120.
Colombo, V., Pettigrove, V. J., Golding, L. A., & Hoffmann, A. A. (2014). Transgenerational effects of parental nutritional status on offspring development time, survival, fecundity, and sensitivity to zinc in Chironomus tepperi midges. Ecotoxicology and Environmental Safety, 132, 366–371.
Coppo, G. C., Passos, L. S., Lopes, T. O. M., Pereira, T. M., Merçon, J., Cabral, D. S., Barbosa, B. V., Caetano, L. S., Kampke, E. H., & Chippari-Gomes, A. A. (2018). Genotoxic, biochemical and bioconcentration effects of manganese on Oreochromis niloticus (Cichlidae). Ecotoxicology, 27, 1150.
Cui, R., Kwark, J. I., & An, Y. (2018). Comparative study of the sensitivity of Daphnia galeata and Daphnia magna to heavy metals. Ecotoxicology and Environmental Safety, 162, 63–70.
Dalzochio, T., Rodrigues, G. Z. P., Simões, L. A. R., Souza, M. S., Petry, I. E., Andriguetti, N. B., Silva, G. J. H., Silva, L. B., & Gehlen, G. (2018). In situ monitoring of the Sinos River, southern Brazil: water quality parameters, biomarkers, and metal bioaccumulation in fish. Environemental Science and Pollution Research, 25, 9485–9500.
Darbre, P. D. (2006). Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. Journal of Applied Toxicology, 26, 191–197.
De Miguel, E., Charlesworth, S., Ordóñez, A., & Seijas, E. (2005). Geochemical fingerprints and controls in the sediments of an urban river: River Manzanares, Madrid (Spain). Sci. Total Environ, 340(1–3), 137–148.
Deshommes, E., Andrews, R. C., Gagnon, G., Mccluskey, T., Mcllwain, B., Doré, E., Nour, S., & Prévost, M. (2016). Evaluation of exposure to lead from drinking water in large buildings. Water Research, 99, 46–55.
Devlin, R. H., & Nagahama, Y. (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture, 208(3–4), 191–364.
Ding, Z., Chen, X., Kong, Y., Shao, X., Zhang, Y., & Ye, J. (2020). Dietary manganese requirement and its effects on antioxidant enzyme activities, intestinal morphology and microbiota in oriental river prawn Macrobrachium nipponense (De Haan). Aquaculture, 516, 734622.
Enserink, L., de la Haye, M., & Maas, H. (1993). Reproductive strategy of Daphnia magna: implications for chronic toxicity tests. Aquatic Toxicology, 25, 111–123.
Eqani, S. A. M. A. S., Malik, R. N., Alamdar, A., & Faheem, H. (2012). Status of organochlorine contaminants in the different environmental compartments of Pakistan: a review on occurrence and levels. Bulletin of Environmental Contamination and Toxicology, 88, 303–310.
Ercal, N., Orhan-Gurer, H., & Aykin-Burns, N. (2001). Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Current Topics in Medicinal Chemistry, 1(6), 529–539.
Fang, S., Zhuo, Z., Yu, X., Wang, H., & Feng, J. (2018). Oral administration of liquid iron preparation containing excess iron induces intestine and liver injury, impairs intestinal barrier function and alters the gut microbiota in rats. Journal of Trace Elements in Medicine and Biology, 47, 12–20.
Ferain, A., Saeyer, N. D., Larondelle, Y., Rees, J. F., Debier, C., & Schamphelaere, K. A. C. D. (2018). Body lipid composition modulates acute cadmium toxicity in Daphnia magna adults and juveniles. Chemosphere, 205, 328–338.
Frank, S. N., Singer, C., & Sures, B. (2008). Metallothionein (MT) response after chronic palladium exposure in the zebra mussel, Dreissena polymorpha. Environmental Research, 108(3), 309–314.
Gao, Y., Zhang, Y., Feng, J., & Zhu, L. (2019). Toxicokinetic−toxicodynamic modeling of cadmium and lead toxicity to larvae and adult zebrafish. Environmental Pollution, 251, 221–229.
Gárriz, A., Del Fresno, P. S., Carriquiribor, P., & Miranda, L. A. (2018). Effects of heavy metals identified in Chascomús shallow lake on the endocrine-reproductive axis of pejerrey fish (Odontesthes bonariensis). General and Comparative Endocrinology, 273, 152–162.
Gebara, R. C., Alho, L. O. G., Rocha, G. S., Mansano, S. A., & Melão, M. G. G. (2020). Zinc and aluminum mixtures have synergic effects to the algae Raphidocelis subcapitata at environmental concentrations. Chemosphere, 242, 125231.
Georgescu, B., Georgescu, C., Dãrãban, S., Bouaru, A., & Pascalau, S. (2011). Heavy metals acting as endocrine disrupters. Scientific Papers: Animal Science and Biotechnologies, 44, 89–93.
Gerber, N., Kokko, H., Ebert, D., & Booksmythe, I. (2018). Daphnia invest in sexual reproduction when its relative costs are reduced. Proceedings of the Royal Society B: Biological Sciences, 285(1871), 20172176.
Guan, R., & Wang, W. X. (2004). Cd an Zn uptake kinetics in Daphnia magna in relation to Cd exposure history. Environmental Science and Technology, 38, 6051–6058.
Guan, R., & Wang, W. X. (2006). Comparison between two clones of Daphnia magna: effects of multigenerational cadmium exposure on toxicity, individual fitness, and biokinetics. Aquatic Toxicology, 76(3–4), 217–229.
Guchhait, R., Chatterjee, A., Gupta, S., Debnath, M., Mukherjee, D., & Pramanick, K. (2018). Molecular mechanism of mercury-induced reproductive impairments in Banded Gourami, Trichogaster Fasciata. General and Comparative Endocrinology, 255, 40–48.
Guiney, S. J., Adlard, P. A., Bush, A. I., Finkelstein, D. I., & Ayton, S. (2017). Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochemistry International, 104, 34–48.
Guisande, C. (1993). Reproductive strategy as population density varies in Daphnia magna (Cladocera). Freshwater Biology, 29(3), 463–467. https://doi.org/10.1111/j.1365-2427.1993.tb00780.x.
Gurgel, P. M., Navoni, J. A., Ferreira, D. M., & Amaral, V. S. (2016). Ecotoxicological water assessment of an estuarine river from the Brazilian northeast, potentially affected by industrial wastewater discharge. Sci. Total Environ, 572, 324–332.
Islam, M. S., Ahmed, M. K., Habibullah-Al-Mamum, M., & Hoque, M. F. (2015). Preliminary assessment of heavy metal contamination in surface sediments from a river in Bangladesh. Environmental Earth Sciences, 73, 1837–1848.
Kakoi, B., Kaluli, J. W., Ndiba, P., & Thiong’o, G. (2016). Banana pith as a natural coagulant for polluted river water. Ecological Engineering, 95, 699–705.
Kassim, A., Rezayi, M., Ahmadzadeh, S., Rounaghi, G., Mohajeri, M., Yusof, N. A., Tee, T. W., Heng, L. Y., & Abdullah, A. H. (2011). A novel ion–selective polymeric membrane sensor for determining thallium (I) with high selectivity. IOP Conference Series: Materials Science and Engineering, 17(1), 1–7.
Kida, B. M. S., Abdalla, R. P., & Moreira, R. G. (2016). Effects of acidic water, aluminum, and manganese on testicular steroidogenesis in Astyanax altiparanae. Fish Physiology and Biochemistry, 42(5), 1347–1356.
Kim, H., Yim, B., Bae, C., & Lee, Y. M. (2017). Acute toxicity and antioxidant responses in the water flea Daphnia magna to xenobiotics (cadmium, lead, mercury, bisphenol A, and 4-nonylphenol). Toxicology and Environmental Health Sciences, 9, 41–49.
Kimberly, D. A., & Salice, C. J. (2015). Multigenerational contaminant exposures produce non-monotonic, transgenerational responses in Daphnia magna. Environmental Pollution, 207, 176–182.
Knops, M., Altenburger, R., & Segner, H. (2001). Alterations of physiological energetics, growth and reproduction of Daphnia magna under toxicant stress. Aquatic Toxicology, 53, 79–90.
Korpelainen, H. (1989). Sex ratio of the cyclic parthenogen Daphnia magna in a variable environment. Journal of Zoological Systematics and Evolutionary Research, 27, 310–316. https://doi.org/10.1111/j.1439-0469.1989.tb00353.x.
Krylov, V. V., & Osipova, E. A. (2019). The response of Daphnia magna Straus to long-term exposure to simulated geomagnetic storms. Life Sciences and Space Research, 21, 83–88.
Kumar, B. K., Rao, Y. P., Noble, T., Weddington, K., McDowell, V. P., Rajanna, S., & Bettaiya, R. (2009). Lead-induced alteration of apoptotic proteins in different regions of adult rat brain. Toxicology Letters, 184, 56–60.
Lampert, W. (1993). Phenotypic plasticity of the size at first reproduction in Daphnia: the importance of maternal size. Ecological Society of America. https://doi.org/10.2307/1940074.
LeBlanc, G. A., & McLachlan, J. B. (2009). Molt-independent growth inhibition of Daphnia magna by a vertebrate antiandrogen. Environmental Toxicology and Chemistry, 18, 1450–1455.
Lei, K., Giubilato, E., Critto, A., Pan, H., & Lin, C. (2016). Contamination and human health risk of lead in soils around lead/zinc smelting areas in China. Environemental Science and Pollution Research, 23, 13128–13136.
Li, Z., Guo, Q., Li, Z., Fan, G., Xiong, D. B., Su, Y., Zhang, J., & Zhang, D. (2015). Enhanced mechanical properties of graphene (reduced graphene oxide)/ aluminum composites with a bioinspired nanolaminated structure. Nano Letters, 15, 8077–8083.
Lynch, M. (1980). The evolution of cladoceran life histories. The Quarterly Review of Biology, 55(1), 23–42.
Machado, C. S., Fregonesi, B. M., Alves, R. I. S., Tonani, K. A. A., Sierra, J., Martinis, B. S., Celere, B. S., Mari, M., Schuhmacher, M., Nadal, M., Domingo, J. L., & Segura-Muñoz, S. (2017). Health risks of environmental exposure to metals and herbicides in the Pardo River, Brazil. Environemental Science and Pollution Research, 24, 20160–20172.
Marins, K., Lazzarotto, L. M. V., Boschetti, G., Bertoncello, K. T., Sachett, A., Schindler, M. S. Z., Chitolina, R., Regginato, A., Zanatta, A. P., Siebel, A. M., Magro, J. D., & Zanatta, L. (2019). Iron and manganese present in underground water promote biochemical, genotoxic, and behavioral alterations in zebrafish (Danio rerio). Environmental Science and Pollution Research International, 26, 23555–53570.
Marshall, D. J., & Uller, T. (2007). When is a maternal effect adaptive? Synthesising Ecology, 116, 1957–1963.
Marsidi, N., Hasan, H. A., & Abdulah, S. R. S. (2018). A review of biological aerated filters for iron and manganese ions removal in water treatment. Journal of Water Process Engineering, 23, 1–12.
Martin, M. B., Reiter, R., Pham, T., Avellanet, Y. R., Camara, J., Lahm, M., Pentecost, E., Pratap, E., Gilmore, B. A., Divekar, S., Dagata, R. S., Bull, J. L., & Stoica, A. (2003). Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology, 144(6), 2425–2436.
Martin, J. A. R., Arana, C. D., Ramos-Miras, J. J., Gil, C., & Boluda, R. (2015). Impact of 70 years urban growth associated with heavy metal pollution. Environmental Pollution, 196, 156–163.
Massarin, S., Alonzo, F., Garcia-Sanchez, L., Gilbin, R., Garnier-Laplace, J., & Poggiale, J. G. (2010). Effects of chronic uranium exposure on life history and physiology of Daphnia magna over three successive generations. Aquatic Toxicology, 99, 309–319.
Matos, L. A., Cunha, A. C. S., Sousa, A. A., Maranhão, J. P. R., Santos, N. R. S., Gonçalves, M. M. C., Dantas, S. M. M. M., Sousa, J. M. C. S., Peron, A. P., Silva, F. C. C., Alencar, M. V. O. B., Islam, T., Aguiar, R. P. S., Melo-Calvancante, A. A., Bonecker, C. C., & Junior, H. F. J. (2017). The influence of heavy metals on toxicogenetic damage in a Brazilian tropical river. Chemosphere, 185, 852–859.
Menon, A. V., Chang, J., & Kim, J. (2016). Mechanisms of divalent metal toxicity in affective disorders. Toxicology, 339, 58–72.
Mitchell, S. E., & Read, A. F. (2005). Poor maternal environment enhances offspring disease resistance in an invertebrate. Proceedings of the Royal Society B: Biological Sciences, 272(1581), 2601–2607.
Monteiro, F., Lemos, L. S., Moura, J. F., Rocha, R. C. C., Moreira, I., Beneditto, A. D., Kehrig, H. A., Bordon, I. C. A. C., Siciliano, S., Pierre, T. D. S., & Hauser-Daves, R. A. (2019). Subcellular metal distributions and metallothionein associations in rough-toothed dolphins (Steno bredanensis) from southeastern Brazil. Marine Pollution Bulletin, 146, 263–273.
Mousseau, T. A., & Fox, C. W. (1998). Maternal effects as adaptations. New York: Oxford University Press.
Myers, J. E., Fine, J., Ormond-Brown, D., Fry, J., Thomson, A., & Thompson, M. L. (2009). Estimating the prevalence of clinical manganism using a cascaded screening process in a South African manganese smelter. Neurotoxicology, 30(6), 934–940.
Okamoto, A., Yamamuro, M., & Tatarazako, N. (2014). Acute toxicity of 50 metals to Daphnia magna. Journal of Applied Toxicology, 35(7), 824–830.
Panhwar, A. H., Kazi, T. G., Naeemullah, K., Afridi, H. I., Shah, F., Arain, M. B., & Arain, S. A. (2016). Evaluated the adverse effects of cadmium and aluminum via drinking water to kidney disease patients: application of a novel solid phase microextraction method. Environmental Toxicology and Pharmacology, 43, 242–247.
Parisot, F., Bourdineaud, J. P., Plaire, D., Adam-Guillermin, C., & Alonzo, F. (2015). DNA alterations and effects on growth and reproduction in Daphnia magna during chronic exposure to gamma radiation over three successive generations. Aquatic Toxicology, 163, 27–36.
Paschoalini, A. L., Savassi, L. A., Arantes, F. P., Rizzo, E., & Bazzoli, N. (2019). Heavy metals accumulation and endocrine disruption in Prochilodus argenteus from a polluted neotropical river. Ecotoxicology and Environmental Safety, 169, 539–550.
Pérez, E., & Hoang, T. C. (2018). Responses of Daphnia magna to chronic exposure of cadmium and nickel mixtures. Chemosphere, 208, 991–1001.
Plaire, D., Bourdineaud, J. P., Alonzo, A., Camilleri, V., Garcia-Sanchez, L., Adam-Guillermin, C., & Alonzo, F. (2013). Transmission of DNA damage and increasing reprotoxic effects over two generations of Daphnia magna exposed to uranium. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 158, 231–243.
Plautz, S. C., Funkhouser, M. A., & Salice, C. J. (2013). New insights into parental effects and toxicity: mate availability and diet in the parental environment affect offspring responses to contaminants. Environmental Pollution, 180, 47–47.
Reátegui-Zirena, E. G., Fidder, B. N., Olson, A. D., Dawson, D. E., Bilbo, T. R., & Salice, C. J. (2017). Transgenerational endpoints provide increased sensitivity and insight into multigenerational responses of Lymnaea stagnalis exposed to cadmium. Environmental Pollution, 224, 572–580.
Regaldo, L., Ulises, R., Susana, G., Horacio, T., & Gagneten, A. M. (2014). Effect of metals on Daphnia magna and cladocerans representatives of the Argentinian Fluvial Littoral. Journal of Environmental Biology, 35(4), 689–697.
Reis, P., Pereira, R., Carvalho, F. P., Oliveira, J., Malta, M., Mendo, S., & Lourenço, J. (2018). Life history traits and genotoxic effects on Daphnia magna exposed to waterborne uranium and to a uranium mine effluent - a transgenerational study. Aquatic Toxicology, 202, 16–25.
Rodríguez, E. M., Medesani, D. A., & Fingerman, M. (2007). Endocrine disruption in crustaceans due to pollutants: A review. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 146(4), 661–671
Rodrigues, G. Z. P., Dalzochio, T., & Gehlen, G. (2016). Uso do bioensaio com Allium cepa L. e análises físico-químicas e microbiológicas para avaliação da qualidade do Rio da Ilha, RS. Acta Toxicol. Argent, 2, 97–104.
Rodrigues, G. Z. P., Souza, M. S., Silva, A. H., Zwetsch, B. G., & Gehlen, G. (2018). Evaluation of intestinal histological damage in zebrafish exposed to environmentally relevant concentrations of manganese. Ciência e Natura, 40, 52.
Rodrigues, G. Z. P., Machado, A. B., & Gehlen, G. (2019). Influência de metais no comportamento reprodutivo de peixes, revisão bibliográfica. Revista GEAMA, 5, 4–13.
Rodrigues, G. Z. P., Staudt, L. B. M., Moreira, M. G., Dos Santos, T. G., Souza, M. S., Lúcio, C. J., Panizzon, J., Kayser, J. M., Simões, L. A. R., Ziulkoski, A. L., Bonan, C. D., De Oliveira, D. L., & Gehlen, G. (2020). Histopathological, genotoxic, and behavioral damages induced by manganese (II) in adult zebrafish. Chemosphere, 244, 125550.
Safe, S. (2003). Cadmium’s disguise dupes the estrogen receptor. Nature Medicine, 9, 1000–1001.
Salawu, M. O., Sunday, E. T., & Oloyede, H. O. B. (2018). Bioaccumulative activity of Ludwigia peploides on heavy metals-contaminated water. Environmental Technology and Innovation, 10, 324–334.
Simpson, S. L., & Spadaro, D. A. (2016). Bioavailability and chronic toxicity of metal sulfide minerals to benthic marine invertebrates: implications for deep sea exploration, mining and tailings disposal. Environmental Science & Technology, 50, 4061–4070.
Skinner, D. M. (1985). Molting and regeneration. Integument, Pigments and Hormonal Processes. https://doi.org/10.1016/B978-0-12-106409-9.50013-0.
Souza, M. S., Rodrigues, G. Z. P., Dalzochio, T., Goldoni, A., Simões, L. A. R., Gehlen, G., & Basso, L. S. (2016). Avaliação da qualidade da água do Rio dos Sinos (Brasil) por meio do teste de micronúcleos em Cyprinus carpio e de análises físico-químicas e microbiológicas. Acta Toxicológica Argentina, 24, 193–199.
Wang, W. X., & Rainbow, P. S. (2010). Significance of metallothioneins in metal accumulation kinetics in marine animals. Comparative Biochemistry and Physiology. C, 152(1), 1–8.
Wang, P., Du, Z., Gao, S., Zhang, X., & Giesy, J. P. (2016). Impairment of reproduction of adult zebrafish (Danio rerio) by binary mixtures of environmentally relevant concentrations of triclocarban and inorganic mercury. Ecotoxicology and Environmental Safety, 134, 124–132.
Wang, X., Zeng, Y. X., Zhao, H., Cao, J., & Jiang, W. (2018). Effects of chlorogenic acid against aluminium neurotoxicity in ICR mice through chelation and antioxidant actions. Journal of Functional Foods, 40, 365–376.
WHO/FAO/IAEA. (1996). Trace elements in human nutrition and health. Geneva, Switzerland: World Health Organization.
Winkel, L., Berg, M., Amini, M., Hug, S. J., & Johnson, A. C. (2008). Predicting groundwater arsenic contamination in Southeast Asia from surface parameters. Nature Geoscience, 1, 536–542.
Wu, Q., Zhou, H., Tham, N. F. Y., Tian, Y., Tan, Y., Zhou, S., Li, Q., Chen, Y., & Leung, Y. S. (2016). Contamination, toxicity and speciation of heavy metals in an industrialized urban river: implications for the dispersal of heavy metals. Marine Pollution Bulletin, 104, 153–161.
Xing, S., Huang, C., Mi, J., Wu, Y., & Liao, X. (2019). Bacillus coagulans R11 maintained intestinal villus health and decreased intestinal injury in lead-exposed mice by regulating the intestinal microbiota and influenced the function of faecal microRNAs. Environmental Pollution, 255, 113139.
Yang, Z., Chen, Y. X. X., Zhang, J., Wang, R., & Yin, D. (2013). Transgenerational effects of heavy metals on L3 larva of Caenorhabditis elegans with greater behavior and growth inhibitions in the progeny. Ecotoxicology and Environmental Safety, 88, 178–184.
Yin, J., Wang, A., Li, W., Shi, R., Jin, H., & Wei, J. (2018). Time-response characteristic and potential biomarker identification of heavy metal induced toxicity in zebrafish. Fish & Shellfish Immunology, 72, 309–317.
Zhai, Q., Li, T., Yu, L., Xiao, Y., Feng, S., Wu, J., Zhao, J., Zhang, H., & Chen, W. (2017). Effects of subchronic oral toxic metal exposure on the intestinal microbiota of mice. Scientific Bulletin, 62, 831–840.
Zimmermann, S., Wolff, C., & Sures, B. (2017). Toxicity of platinum, palladium and rhodium to Daphnia magna in single and binary metal exposure experiments. Environmental Pollution, 224, 368–376.
Acknowledgments
Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul – FAPERGS and Feevale University through the granting of doctorate and undergraduate scholarships.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - finance code 001.
Author information
Authors and Affiliations
Contributions
Gabriela Zimmermann Prado Rodrigues: conceptualization, methodology, formal analysis, data curation, resources, writing - original draft, writing - review and editing, visualization. Mariana Finkler: methodology. Ana Leticia Hilario Garcia: methodology, data curation, writing - original draft, writing - review and editing. Günther Gehlen: conceptualization, writing - review and editing, resources, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodrigues, G.Z.P., Finkler, M., Garcia, A.L.H. et al. Evaluation of transgenerational effects caused by metals as environmental pollutants in Daphnia magna. Environ Monit Assess 192, 755 (2020). https://doi.org/10.1007/s10661-020-08713-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08713-4