Abstract
The emissions of brominated flame retardants (BFRs) from consumer products have been considered the major to the ubiquitous occurrence of contaminants in indoor environments. Direct contact with dust covering the surface of source materials in a real environment could introduce significant uncertainty. This study investigated the effects of dust coverage on the emissions of four BFRs, including 1, 2, 5, 6, 9, and 10-hexabromocyclododecane (HBCD), bis(2-ethyl-1-hexyl) tetrabromophthalate (BEHTBP), tetrabromobisphenol A (TBBPA), and hexabromobenzene (HBBZ), from decorative laminate, cotton sound insulation, PVC floor, and carpet. Direct contact with dust was confirmed to increase the total emissions by 30.8–98.1% compared with the emissions in the non-dust group. The emissions of HBCD, TBBPA, and HBBZ from cotton sound insulation were obviously enhanced by dust with smaller particles but did not linearly increase along with the dust amounts. Thus, these findings have practical implications in that the frequent removal of dust could be important to minimize the exposure risk from indoor emissions of BFRs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brominated flame retardants (BFRs) are substances used to inhibit the spread of fire in indoor products, such as plastics, textiles, construction materials, electronics and computer equipment, and other materials (Covaci et al. 2011; Daso et al. 2013; Li et al. 2015). Due to the strict prohibition or restriction on the production and use of some classical BFRs, such as polybrominated diphenyl ethers (PBDEs), novel brominated flame retardants (NBFRs) have been produced and used as PBDE replacements (Geens et al. 2010; Tokumura et al. 2017). With the increasing worldwide commercial demand for BFRs in polymeric materials in electronics, computers, and buildings, the annual production volumes for 21 NBFRs have been estimated to be ~ 100,000 tons (Covaci et al. 2011).
Recently, several alternative BFRs have received attention for their environmental impact, such as hexabromobenzene (HBBZ), 1,2-bis(2,4,6-tribromophenoxy) ethane (BTBPE), decabromodiphenyl ethane or 1,2-bis(pentabromodiphenyl) ethane (DBDPE), and bis(2-ethyl-1-hexyl) tetrabromophthalate (BEHTBP) (Covaci et al. 2011). Many of these BFRs have been considered as potential persistent, bioaccumulative, and toxic substances (Ezechiáš et al. 2014; Liu et al. 2016). For example, DBDPE has been verified to have adverse effects on thyroid hormones, neurological function, and reproductive functions (Wit et al. 2010). BTBPE and 1,2-dibromo-4-(1,2-dibromoethyl) cyclohexane (TBECH) have also shown their persistence and bioaccumulation potential (Covaci et al. 2011; Wu et al. 2011). In addition, BFRs have been frequently detected as common contaminants in indoor media (Qi et al. 2014; Stapleton et al. 2008; Takigami et al. 2009). In a nationwide investigation across China, 22 novel BFRs were detected in 81 indoor dust samples with a median concentration of 720 ng/g (Qi et al. 2014).
The levels of BFRs in indoor air have been observed to be higher than those of outdoor air, demonstrating the significance of indoor sources (Abdallah et al. 2008; Covaci et al. 2011; He et al. 2018; Law et al. 2014; Sun et al. 2016). Pollutants can enter the human body through respiratory inhalation and dust ingestion, and people spend a longer time indoors; thus, the indoor environment could be the dominant pathway for exposure (Kajiwara and Takigami 2013; Li et al. 2015). Because most BFRs (e.g., PBDEs) are directly added into materials without chemical reaction during manufacture, they may emit from the product and migrate into the environment (de Wit 2002; Tokumura et al. 2017). Moreover, growing evidence has been reported regarding the emissions of specific flame retardants from commonly used materials through chamber studies (Ghislain et al. 2016; Kajiwara and Takigami 2013; Kemmlein et al. 2003; Rauert et al. 2015). For example, tris(2-chloro-isopropyl) phosphate (TCPP) can emit from polyurethane foam with area-specific emission rates (SERs) ranging from 20 ng m−2 h−1 to 140 μg m−2 h−1 (Kemmlein et al. 2003).
The presence of BFRs in indoor dust may be due to three hypothesized pathways: (1) subsequent deposition of volatile gasses emitted from treated materials (Rauert et al. 2014); (2) physical transfer from materials by abrasion, followed by migration directly to dust (Wagner et al. 2013); and (3) direct transfer via contact with the treated material to dust (Takigami et al. 2008). A growing body of information has demonstrated that semi-volatile organic compounds (SVOCs) can migrate to dust by volatilization with subsequent distribution or direct transfer from raw materials covered with dust (Clausen et al. 2004; He et al. 2016). However, when the surface of a BFR-treated product was covered with dust, the effect of dust on volatilization and direct transfer was unclear.
Thus, it is significant to obtain a better understanding of the direct contact between dust and material surface by observing the potential enhanced/suppressed volatilization, transfer, and total emissions from the main source products. Accordingly, this study presents the effect of dust coverage on the emissions of four BFRs from four materials used in common consumer products by chamber experiments. In particular, the effects of dust amount and dust particle size were evaluated.
Material and methods
Chemicals and sample materials
The standards of 1,2,5,6,9,10-hexabromocyclododecane (HBCD), tetrabromobisphenol A (TBBPA), HBBZ, and BEHTBP were purchased from Wellington Laboratories Inc. (Guelph ON, Canada). Isotope-labeled standards of 13C-BDE-209 and 13C-CB-141 were obtained from Cambridge Isotope Laboratories (Andover, MA, USA). All organic solvents used were of HPLC grade (Tedia Company, Shanghai, China). Polyurethane foam (PUF) plugs were purchased from Tisch Environmental, Inc. (Cleves, USA), and cleaned through Soxhlet extraction with acetone for 24 h, and the solvent was completely removed by a vacuum drying system before use. The consumer materials surveyed as targets, including cotton sound insulation, PVC floor, carpet, and decorative laminate, were directly purchased from their respective manufacturers.
Chamber experiments design
The emission measurements were carried out in a batch of glass test chambers designed based upon published methods with some modifications (Rauert and Harrad 2015). The procedure and chamber dimensions were described in our previous study (Sun et al. 2018a). In brief, a round glass chamber (total chamber volume of 328 cm3) was utilized with the air inflow into the chamber set at 100 mL/min. A sample platform with a diameter of 3 cm was constructed inside the chamber. Each material, with an area of 28.26 cm2, was transferred onto the platform for the emission test. PUF plugs were placed on the outlet air vent for the purpose of collecting the compounds in both the gas and suspended particulate phases. The chambers were maintained at room temperature (20 °C) after being sealed with Teflon tape.
The comparison between the dust-covered group (with 100-mg dust) and the non-dust group was conducted with a test duration of 7 days with the four materials. The dust was liberally sprinkled onto the surface of the materials in the dust-covered groups. To evaluate the effect of covered dust amounts, the experiment was conducted on cotton sound insulation with dust amounts of 100 mg, 200 mg, 400 mg, and 800 mg. In addition, the test on cotton sound insulation with 100 mg was also designed to estimate the effect of particle sizes by using 100 mesh, 80 mesh, and 50 mesh dust. The PUF and dust samples were collected and analyzed separately.
Chemical analysis
The method of treating PUF plugs and dust samples used herein has been reported previously (Sun et al. 2016). Briefly, prior to extraction, a certain amount of 13C-BDE-209 as a surrogate standard was spiked in the PUF/dust sample to monitor the recovery of the BFRs during treatment and analysis. The samples were Soxhlet extracted for 48 h using 140 mL of DCM and concentrated to 5 mL using a rotary evaporator. To collect the compounds attached on the inner wall of the chamber, the inner wall was washed with a solvent, and the rinse was combined with the extract. The extracts were subsequently concentrated to 2 mL under a gentle nitrogen stream with a solvent exchange to n-hexane, blown with a gentle stream of high-purity nitrogen to 1.0 mL, combined with the internal standard of 13C-CB-141 and maintained at − 20 °C before instrumental analysis. Based on previous work, the derivatization procedure was used to analyze TBBPA (Sun et al. 2018b).
An Agilent 7890A GC-5975C mass spectrometer with negative chemical ionization (GC–NCI-MS) in the selected ion-monitoring (SIM) mode was used for the target substance analysis. Helium (purity > 99.999%) was employed as the carrier gas at a flow rate of 1.0 mL min−1. Methane (purity > 99.995%) was used as the chemical ionization moderating gas. The injector and transfer line temperatures were set at 250 °C and 280 °C, respectively. Separation was carried out on a DB-5MS capillary column (15 m × 0.25 μm × 0.10 μm film thickness, J&W Scientific, USA) with the temperature program being increased from 110 °C (held 1 min), ramped at 5 °C min−1 to 220 °C, and ramped at 20 °C min−1 to 310 °C (held 15 min). The injection volume was 2 μL with a splitless mode.
Quality assurance/quality control
The controls and three replicates were included in each emission test. Contamination that arises from solvents and glassware was evaluated by the analysis of procedural blanks for extraction. Foil paper with a few punctures was used in the device top to avoid the potential deposition of contaminants from indoor air. Additionally, the dust covering on material surfaces was confirmed with the absence of targeted compounds. The breakthrough and sampling efficiency of the PUF plugs and tracing of the distribution of compounds in the chamber wall and PUF plugs were validated in a chamber experiment in our previous study, which showed that the breakthrough did not occur and the sampling efficiency was acceptable (Sun et al. 2018a). The target recovery ranged from 85.0–106%.
Data analysis
Emission amount was calculated using the combined value of BFRs adsorbed to the PUF, chamber walls, and dust. Means and standard deviations were calculated in triplicate. The data were subjected to statistical analysis by using Origin Pro (v.8.0; OriginLab, Northampton, MA).
Results and discussion
Influence of dust coverage on BFR emissions
The BFR amounts trapped by PUF represent the direct volatilized mass, and the amounts extracted from dust represent direct migration from materials to dust. A summary of the target BFRs from materials with and without dust covering is presented in Table 1. The emissions of all four BFRs were detected from all four materials. Among them, the cotton sound insulation was detected three BFRs. The emission potential of these BFRs was confirmed to be positively correlated to their initial contents in the manufacturing process of materials (Sun et al. 2018a). The total emitted amounts of the four selected BFRs in each material measured in the control group are as follows: cotton sound insulation > carpet > decorative laminate > PVC floor. Among the four targeted BFRs, HBCD was the most abundant compound detected from all materials in this study. HBCD was detected with the highest emission in cotton sound insulation with a mean amount of 97.4 ng in controls (193 pg h−1 g−1), followed by carpet, PVC floor, and decorative laminate, which is mainly because HBCD as an additive flame retardant could migrate to indoor dust more easily (Abdallah et al. 2008). As shown in Table 1, it is obvious that the amounts of emitted target BFRs in the dust-covered group (PUF + dust) were higher than all of those in the control group (without dust coverage), with elevated rates ranging from 30.8 to 98.1%. The highest elevated rate was observed in the emission of HBCD from carpet, while that of BEHTBP from carpet was found to be the lowest. In addition, this enhanced effect of dust coverage on emissions was compound specific. For example, the emission of HBCD was elevated 98.1% in the carpet but only 47.8% in the decorative laminate.
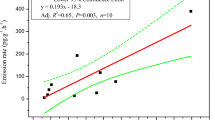
Our study demonstrated an elevated total emission of HBBZ, TBBPA, HBCD, and BEHTBP from consumer products, including the portion of the volatile substances from source to PUF and the direct transfer to dust covering the surface. Because the emitted amounts in PUF from the dust-covered group were comparable to the amounts in the control group, the dust did not significantly affect the direct volatilization to air, and their substantial elevations may be attributed to direct transfer from source to dust. In addition, a considerable amount of substances adsorbed with dust were detected in dust samples from dust-covered groups. The detected amounts in dust samples were observed to be positively correlated with the total emissions in dust-covered groups (Fig. 1, R2 = 0.94, p = 0.001), which may indicate the contribution of dust to the elevated emissions of dust covered materials.
More recently, evidence has shown that direct transfer is a primary and efficient route for pollutants to migrate from source material to dust covering the surface of consumer products (Sukiene et al. 2017). With regard to BFRs, especially the less volatile BDE-209, the direct contact between dust and material surfaces has been confirmed as a potentially important pathway (Rauert and Harrad 2015). Takigami et al. (2008) reported that the levels of BDE-209 in dust sampled from the back casings of TVs were significantly higher than those in the surrounding floor dust (Takigami et al. 2008). The concentrations of eight phthalates in dust directly in contact with the source material surfaces were up to three orders of magnitude higher than those of indoor dust without direct source contact (Sukiene et al. 2017). A small-scale field study also concluded that direct transfer of phthalates from materials into dust greatly increased the amounts in the covering dust and thus may cause serious human exposure (Sukiene et al. 2017). The direct transfer of HBCDs (Rauert et al. 2016), polychlorinated biphenyls (PCBs) (Sukiene et al. 2017), di(ethyl-hexyl) phthalate (DEHP) (Schripp et al. 2010), and PBDEs (Rauert and Harrad 2015) from indoor sources to house dust has also been investigated in previous chamber experiments. Among them, a dust layer covering on DEHP-treated PVC flooring resulted in a 300% increase in total emissions and an elevated amount of DEHP being sorbed to dust (Schripp et al. 2010). These findings are also consistent with the report of phthalates from PVC polymer by a small chamber experiment (Schripp et al. 2010).
Effects of dust amount and particle size on BFR emissions
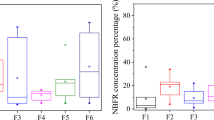
The cotton sound insulation emitted three BFRs and was chosen as the model material for the evaluation of the effect of different dust amounts and particle sizes. As shown in Fig. 2, the emissions of each BFR from cotton sound insulation were promoted by covering the surface with scattered dust. However, the elevated emissions did not linearly increase with the variation in dust amount, indicating that the different amounts of dust may not be a dominant factor in predicting the elevation of emissions. Among the three BFRs, HBBZ showed the highest elevated rate (94.2%), while HBCD had the lowest rate (12.1%). The emissions of TBBPA and HBCD from the dust-covered group were not significantly increased when the dust amount was higher than 200 mg, which may be attributed to the relatively thick dust layer. One possible reason for this nonlinear correlation may be that the different amounts of dust changed only the thickness on the surface of the material, not the surface area, where adsorption by dust via capillary forces and direct contact with the covered dust occurred.
The effect of different sizes of dust particles was also investigated with cotton sound insulation. As shown in Fig. 3, the total emissions of all three BFRs were obviously increased by dust with smaller particle sizes. For example, the total HBBZ emission in the 100 mesh dust-covered group was 1.7 times higher than that in the 50 mesh group. The amounts of BFRs in dust were significantly positively increased, but the amounts trapped by PUF were consistent. Thus, these results revealed the role of dust in the enhanced emission from those BFR-treated materials with regard to different dust particle sizes. The adsorbability, permeability, and compactness on the surface of the material may have been changed by different dust sizes (He et al. 2018). Direct contact at the interface of dust and material surface partially allows dust particles to serve as a sorbent accumulating the emitted pollutants directly from the material (Clausen et al. 2004). In this scenario, adsorption by dust will be less affected by the volatility of the chemicals but affected more by its availability at the dust-material interface (Rauert et al. 2016). Studies have illustrated that the particle size of sorbents plays an important role in sorption kinetics of organic pollutants (Choi et al. 2013). A smaller particle size of dust provides a greater specific surface area in this study. Finer sorbent particles also offer shorter intraparticle diffusion lengths of hydrophobic organic pollutants and thus decrease the required equilibrium time. Thus, the properties of dust-covered materials, such as the dust covered area, porosity roughness, dust particle size, and material thickness, can impact their sorption potential (Zhang et al. 2002).
The positive impact of direct contact between material and dust coverage in the processes affecting the migration of BFRs is not yet completely understood. Schripp et al. (2010) suggested that SVOC transfer due to the contact between dust and gaseous pollutants exists in the boundary layer of the source, i.e., the air interface directly above the surface of the material (Schripp et al. 2010). Theoretically, chemicals with relatively lower vapor pressures will be less abundant in the boundary layer, leading to lower mass transfer. However, our results with the four targeted compounds were not completely consistent with this hypothesis. Another alternative explanation given by Clausen et al. (2004) is that direct transfer may occur as a subsequent result after direct contact between the materials and dust particles (Clausen et al. 2004). The chemical levels in the air of the boundary layer may be much higher than that in well-mixed air, and the transportation of chemicals in that layer is limited by the direct adsorption to the dust (Clausen et al. 2004). In this scenario, the role of the boundary layer is replaced by the dust particles, and the influence of the vapor pressure of the chemicals is minimized.
Environmental implications
People in urbanized regions spend 80% of their time in indoor conditions where they are continuously exposed to dust and volatile pollutants; thus, indoor air quality is critical to human health (Johnson-Restrepo and Kannan 2009). Indoor exposures of gaseous and particle phases via inhalation and ingestion have been considered to be the major exposure routes to BFRs as along with dietary intake (Allen et al. 2007). Dust has been reported to substantially increase human exposure to various chemicals, such as flame retardants and phthalates (Blanchard et al. 2014; He et al. 2016; Trudel et al. 2011). In addition, the BFRs were ubiquitously detected in indoor air and indoor dust, which were generated by the emissions from various consumer products assembled from BFR-treated materials (Ezechiáš et al. 2014). In particular, dust could be an effective transfer and storage medium for low-volatile BFRs released from consumer products (Weschler et al. 2008). In this study, the dust also displayed an enhanced effect on total emission from indoor sources, mainly because dust acts as a lipophilic sorbent through direct contact.
Therefore, assessments of human exposure to indoor pollutants should consider whether a large area of material surface of a source of the targeted pollutants is in direct contact with dust, since this may lead to enhanced human exposure. Because the total surface area covered by dust in a real environment is significantly larger than that in experimental conditions, the direct transfer may generate a considerably enhanced effect on the total level of BFRs in an indoor environment. Therefore, the surface of the source material covered by dust is important for studies on substance emission. Since the dust accumulating the BFRs from source materials could be a sink of pollutants, slow re-emission to indoor air in the gas phase and re-suspension of the dust in the particle phase in air may lead to greater exposure through inhalation as a result of dust coverage. Thus, frequent cleaning activities for dust removal, such as wiping, vacuuming, mopping, and carpet dedusting, could also be important to minimize the influence of dust on the risk of emission exposure in indoor environments.
References
Abdallah, A. E., Harrad, S., & Covaci, A. (2008). Hexabromocyclododecanes and tetrabromobisphenol-A in indoor air and dust in Birmingham, UK: Implications for human exposure. Environmental Science & Technology, 42(18), 6855–6861.
Allen, J. G., Mcclean, M. D., Stapleton, H. M., Nelson, J. W., & Webster, T. F. (2007). Personal exposure to polybrominated diphenyl ethers (PBDEs) in residential indoor air. Environmental Science & Technology, 41(13), 4574–4579.
Blanchard, O., Glorennec, P., Mercier, F., Bonvallot, N., Chevrier, C., Ramalho, O., Mandin, C., & Bot, B. L. (2014). Semivolatile organic compounds in indoor air and settled dust in 30 French dwellings. Environmental Science & Technology, 48(7), 3959–3969.
Choi, Y., Cho, Y. M., Gala, W. R., & Luthy, R. G. (2013). Measurement and modeling of activated carbon performance for the sequestration of parent- and alkylated-polycyclic aromatic hydrocarbons in petroleum-impacted sediments. Environmental Science & Technology, 47(2), 1024–1032.
Clausen, P. A., Hansen, V., Gunnarsen, L., Afshari, A., & Wolkoff, P. (2004). Emission of di-2-ethylhexyl phthalate from PVC flooring into air and uptake in dust: Emission and sorption experiments in FLEC and CLIMPAQ. Environmental Science & Technology, 38(9), 2531–2537.
Covaci, A., Harrad, S., Abdallah, M. A., Ali, N., Law, R. J., Herzke, D., & de Wit, C. A. (2011). Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour. Environment International, 37(2), 532–556.
Daso, A. P., Fatoki, O. S., Odendaal, J. P., & Olujimi, O. O. (2013). Polybrominated diphenyl ethers (PBDEs) and 2,2′,4,4′,5,5′-hexabromobiphenyl (BB-153) in landfill leachate in Cape Town, South Africa. Environmental Monitoring and Assessment, 185(1), 431–439.
de Wit, C. A. (2002). An overview of brominated flame retardants in the environment. Chemosphere, 46(5), 583–624.
Ezechiáš, M., Covino, S., & Cajthaml, T. (2014). Ecotoxicity and biodegradability of new brominated flame retardants: A review. Ecotoxicology & Environmental Safety, 110, 153–167.
Geens, T., Ali, N., Roosens, L., Neels, H., & Covaci, A. (2010). Analytical characteristics of several new brominated flame retardants. Talanta, 81(4), 1865–1869.
Ghislain, M., Beigbeder, J., Dumazert, L., Lopez-Cuesta, J.-M., Lounis, M., Leconte, S., & Desauziers, V. (2016). Determination of the volatile fraction of phosphorus flame retardants in cushioning foam of upholstered furniture: Towards respiratory exposure assessment. Environmental Monitoring and Assessment, 188(10), 576.
He, R., Li, Y., Xiang, P., Li, C., Zhou, C., Zhang, S., Cui, X., & Ma, L. Q. (2016). Organophosphorus flame retardants and phthalate esters in indoor dust from different microenvironments: Bioaccessibility and risk assessment. Chemosphere, 150, 528–535.
He, R. W., Li, Y. Z., Xiang, P., Li, C., Cui, X. Y., & Ma, L. Q. (2018). Impact of particle size on distribution and human exposure of flame retardants in indoor dust. Environmental Research, 162, 166–172.
Johnson-Restrepo, B., & Kannan, K. (2009). An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere, 76(4), 542–548.
Kajiwara, N., & Takigami, H. (2013). Emission behavior of hexabromocyclododecanes and polybrominated diphenyl ethers from flame-retardant-treated textiles. Environmental Science Processes & Impacts, 15(10), 1957–1963.
Kemmlein, S., Hahn, O., & Jann, O. (2003). Emissions of organophosphate and brominated flame retardants from selected consumer products and building materials. Atmospheric Environment, 37(39–40), 5485–5493.
Law, R. J., Covaci, A., Harrad, S., Herzke, D., Abdallah, A. E., Fernie, K., Toms, L. M. L., & Takigami, H. (2014). Levels and trends of PBDEs and HBCDs in the global environment: Status at the end of 2012. Environment International, 65(2), 147–158.
Li, P., Wu, H., Li, Q. X., Jin, J., & Wang, Y. (2015). Brominated flame retardants in food and environmental samples from a production area in China: Concentrations and human exposure assessment. Environmental Monitoring and Assessment, 187(11), 719.
Liu, X., Wen, S., Li, J. G., Zhang, L., Zhao, Y. F., & Wu, Y. N. (2016). A study on the levels of a polybrominated biphenyl in Chinese human milk samples collected in 2007 and 2011. Environmental Monitoring and Assessment, 188(9), 515.
Qi, H., Li, W. L., Liu, L. Y., Zhang, Z. F., Zhu, N. Z., Song, W. W., Ma, W. L., & Li, Y. F. (2014). Levels, distribution and human exposure of new non-BDE brominated flame retardants in the indoor dust of China. Environmental Pollution, 195, 1–8.
Rauert, C., & Harrad, S. (2015). Mass transfer of PBDEs from plastic TV casing to indoor dust via three migration pathways--A test chamber investigation. Science of the Total Environment, 536, 568–574.
Rauert, C., Lazarov, B., Harrad, S., Covaci, A., & Stranger, M. (2014). A review of chamber experiments for determining specific emission rates and investigating migration pathways of flame retardants. Atmospheric Environment, 82(82), 44–55.
Rauert, C., Harrad, S., Stranger, M., & Lazarov, B. (2015). Test chamber investigation of the volatilization from source materials of brominated flame retardants and their subsequent deposition to indoor dust. Indoor Air, 25(4), 393–404.
Rauert, C., Kuribara, I., Kataoka, T., Wada, T., Kajiwara, N., Suzuki, G., Takigami, H., & Harrad, S. (2016). Direct contact between dust and HBCD-treated fabrics is an important pathway of source-to-dust transfer. Science of the Total Environment, 545-546, 77–83.
Schripp, T., Fauck, C., & Salthammer, T. (2010). Chamber studies on mass-transfer of di(2-ethylhexyl) phthalate (DEHP) and di--butylphthalate (DnBP) from emission sources into house dust. Atmospheric Environment, 44(24), 2840–2845.
Stapleton, H. M., Allen, J. G., Kelly, S. M., Konstantinov, A., Klosterhaus, S., Watkins, D., Mcclean, M. D., & Webster, T. F. (2008). Alternate and new brominated flame retardants detected in U.S. house dust. Environmental Science & Technology, 42(24), 9455–9456.
Sukiene, V., Goetz, N. V., Gerecke, A. C., Bakker, M. I., Delmaar, C. J. E., & Hungerbühler, K. (2017). Direct and air-mediated transfer of labeled SVOCs from indoor sources to dust. Environmental Science & Technology, 51(6), 3269–3277.
Sun, J., Wang, Q., Zhuang, S., & Zhang, A. (2016). Occurrence of polybrominated diphenyl ethers in indoor air and dust in Hangzhou, China: Level, role of electric appliances, and human exposure. Environmental Pollution, 218, 942–949.
Sun, J., Chen, Q., Han, Y., Zhou, H., & Zhang, A. (2018a). Emissions of selected brominated flame retardants from consumer materials: The effects of content, temperature, and timescale. Environmental Science & Pollution Research, 25(24), 24201–24209.
Sun, J., Xu, Y., Zhou, H., Zhang, A., & Qi, H. (2018b). Levels, occurrence and human exposure to novel brominated flame retardants (NBFRs) and dechlorane plus (DP) in dust from different indoor environments in Hangzhou, China. Science of the Total Environment, 631-632, 1212–1220.
Takigami, H., Suzuki, G., Hirai, Y., & Sakai, S. I. (2008). Transfer of brominated flame retardants from components into dust inside television cabinets. Chemosphere, 73(2), 161–169.
Takigami, H., Suzuki, G., Hirai, Y., & Sakai, S. (2009). Brominated flame retardants and other polyhalogenated compounds in indoor air and dust from two houses in Japan. Chemosphere, 76(2), 270–277.
Tokumura, M., Hatayama, R., Tatsu, K., Naito, T., Takeda, T., Raknuzzaman, M., Al-Mamun, M. H., & Masunaga, S. (2017). Organophosphate flame retardants in the indoor air and dust in cars in Japan. Environmental Monitoring and Assessment, 189(2), 48.
Trudel, D., Scheringer, M., Goetz, N. V., & Hungerbühler, K. (2011). Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environmental Science & Technology, 45(6), 2391–2397.
Wagner, J., Ghosal, S., Whitehead, T., & Metayer, C. (2013). Morphology, spatial distribution, and concentration of flame retardants in consumer products and environmental dusts using scanning electron microscopy and Raman micro-spectroscopy. Environment International, 59(3), 16–26.
Weschler, C. J., Salthammer, T., & Fromme, H. (2008). Partitioning of phthalates among the gas phase, airborne particles and settled dust in indoor environments. Atmospheric Environment, 42(7), 1449–1460.
Wit, C. A. D., Herzke, D., Vorkamp, K., Muir, D., & Wit, C. D. (2010). Brominated flame retardants in the Arctic environment — Trends and new candidates. Science of the Total Environment, 408(15), 2885–2918.
Wu, J. P., Guan, Y. T., Zhang, Y., Luo, X. J., Zhi, H., Chen, S. J., & Mai, B. X. (2011). Several current-use, non-PBDE brominated flame retardants are highly bioaccumulative: Evidence from field determined bioaccumulation factors. Environment International, 37(1), 210–215.
Zhang, J., Zhang, J. Q., Chen, X., & Yang (2002). A critical review on studies of volatile organic compound (VOC) sorption by building materials (RP-1097). ASHRAE Transactions, 108(1), 162–174.
Funding
This study was supported by the National Natural Science Foundation of China (21577127, 21307111), the Natural Science Foundation of Zhejiang Province (LY17B070006, LY13B070009), and the College Student Science and Technology Innovation Program of Zhejiang Province (Xinmiao Talents Program).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qian, Z., Xu, Y., Zheng, C. et al. Enhanced emissions of brominated flame retardants from indoor sources by direct contact with dust. Environ Monit Assess 191, 170 (2019). https://doi.org/10.1007/s10661-019-7303-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7303-9