Abstract
The present study is to evaluate trophic status and its limiting factors in the Renuka Lake, using surface water samples. The water of the lake is found to be slightly alkaline with pH 8.33–8.70 (avg. 8.61 ± 0.1). The turbidity 4.63–6.62 NTU (avg. 5.48 ± 0.63 NTU) indicated low level of clarity in the lake. The Carlson’s index based on the Secchi disc transparency (SD), chlorophyll-a (Chl-a), and total phosphorus (TP) has indicated that the Renuka Lake is turned hyper-eutrophic in status. The correlation coefficient has indicated that most parameters in the lake are contributed by different sources. Factor-1 marked 26.40% variance, which may be due to higher impact of rock weathering than the anthropogenic activities, whereas 18.56% variance shown by factor-2 may be due to natural and anthropogenic activities. However, factor-3 (14.38% variance) inferred that the higher contribution of Chl-a, pH, TDS, NH4+, NO3−, salinity, and SO42− is due to major impacts of the anthropogenic activities. The inverse distance weighting method has indicated spatial interpolation and area of influence of different parameters in the lake. The “nutrient (TP) limited large sized algae” as well as phosphorus is considered as a major limiting factor for increasing productivity and trophic state index. The present study has inferred that the nutrient enrichment should be controlled to restore the Renuka Lake, owing to its social and ecological significances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The changing scenario of water quality has become a worldwide problem. The developed countries are able to solve the problems of water pollution owing to the resources and technologies they have, but the developing and underdeveloped countries are still far behind. The Himalayan lakes have always been a major source of freshwater for drinking purposes, but the eutrophication has deteriorated the water quality of these lakes as a result of anthropogenic activities, viz. use of chemical fertilizers, waste disposal, and animal’s excreta (Dixit and Tiwari 2005; Guyuan et al. 2011; Schindler 2012; Qi and Lu 2016; Sarkar et al. 2016). The presence of varied organic and inorganic matters is known to cause nutrient enrichment in the lakes. The non-biodegradable organic matter can stay in water bodies for the long time, whereas the biodegradable compounds like glutamate and glucose are degraded by microorganism very fast (Ogawa et al. 2001). The organic material produced by the algae population directly could be biodegradable, but if converted to organic matter by microorganism, then it becomes refractory by nature (Lee et al. 2016). The use of phosphorus as a fertilizer is considered as a primary factor for increasing eutrophication in the lakes. The phosphorus is utilized by the plants in the form of adenosine tri-phosphate (ATP), and therefore acts as a primary producer.
The phosphorus on reaching the lake settles down at the bottom and gets adsorbed with the sediment, which has the ability to store and release it even after the external supply is stopped (Kowalczewska-Madura et al. 2017; Gaury et al. 2018). It has always been emphasized to control the external phosphorus supply, but its presence with the sediments was never realized, which is the main problem and essential to control the eutrophication (Qin et al. 2016). The enrichment of nutrients in lakes produces algal bloom, i.e., a worldwide problem of eutrophic lakes (Lu et al. 2016). The algal bloom generates a toxin that causes death of flora and fauna (Pathak and Pathak 2012). Besides, the cyanobacterial bloom is also known to create hazards in the lakes. It is therefore essential to control the growth of cyanobacteria during pre- and post-stage of the bloom formation (Lee et al. 2016), which is only possible by controlling the nutrient supply in the lakes.
The nutrient enrichment and autotrophic production in the lakes are generally assessed using the trophic state index (TSI). This index categorizes the lakes in different classes and based on the measurement of Secchi disc transparency (SD), total phosphorus (TP), and algal biomass in the form of chlorophyll-a (Chl-a) present in all types of green plants. It is considered that, if the TSI (Chl-a) and TSI (SD) are having good signature, then there is a possibility of light attenuation due to algal dominancy (Carlson and Robert 1991). On the other hand, if TSI (Chl-a) is < TSI (SD), then certain other factors, such as color of the water and non-algal seston reduce the water transparency (Havens and Karl 2000). It also has further been inferred that the value of TSI (Chl-a) < TSI (SD) may be due to pico-plankton in hyper-eutrophic lakes, owing to the large surface area per unit biomass of small cells, which reduce more light as compared to the micro or nano-planktons (Edmondson 1980; Carlson and Robert 1991). However, TSI (Chl-a) ≥ TSI (TP) infers that the algal growth is controlled by TP, whereas TSI (Chl-a) < TSI (TP) reveals the less algal growth in comparison to that of TP availability and contribution of other factors (Havens and Karl 2000). Several studies have been carried out using Carlson’s index for the trophic status assessment of the lakes, all over the world (Ye et al. 2007; Xu et al. 2010; Sheela et al. 2011; Upadhyay et al. 2012; Jekatierynczuk-Rudczyk et al. 2014; Ochocka and Pasztaleniec 2016; Boros et al. 2017; Liao et al. 2017; Saluja and Garg 2017).
The Renuka Lake is declared as a Ramsar site since 2005 and some researchers have studied this lake in the past. Das and Kaur (2001) in an earlier study observed the sedimentation rate and carbonate weathering as a dominant source of major ions. The moderate weathering of the surrounding rocks and the presence of rare earth elements were observed by Das et al. (2008). This lake has not yet been monitored in terms of trophic status. Therefore, the present study is to evaluate the trophic status of the Renuka Lake using Carlson’s index and those factors which are responsible for promoting the algal growth in the lake. The Renuka Lake has lots of societal and ecological significances and needs mitigation measures for preservation and further development.
Methodology
Study site
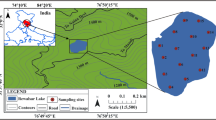
The Renuka Lake is situated in the Lesser Himalayan region (north-west Himalaya) at 77° 27′ 30″ E longitude, and 30° 36′ 36″ N latitude in the Sirmaur district of Himachal Pradesh, India (Fig. 1). It has been formed by the natural processes and has width ~ 204 m, depth ~ 13 m, and length ~ 1706.7 m, with catchment area ~ 254.3 ha. The annual rainfall in the region is around 150 to 199.9 cm (Das and Kaur 2001). The geology of the region includes Lesser Himalayan rocks, i.e., carbonaceous shale, purpled dolomitic limestone, quartzite, siltstone, calcareous, pyrites, carbonate, sericite, intra-bedded red shale of tertiary period, and slate (Das et al. 2008). The monsoon rain is the prime source of water in the lake besides the seepage of underground water. The water from the lake continues to flow outside through its outlet and via Parshuram Tal to the low-lying region.
Sample collection and preparation
The water samples were collected from 15 different locations of the Renuka Lake, using polyethylene bottles of 2 L in size (Fig. 1). The samples were taken to the laboratory using ice bin and kept at 4 °C. To avoid direct exposure of the sunlight, the sampling bottles were covered with black paper sheets. The next step was the preparation of water samples. The saturated magnesium carbonate (MgCO3) solution was prepared by dissolving its 1 g powder in 100 ml of Milli-Q (MQ) water and an acetone solution was prepared by dissolving acetone (HPLC grade) and MQ water in the ratio of 9:1. A 0.1 N HCL (analytical reagent grade) solution was also made for the acidification of chlorophyll-a samples during analysis. Thereafter, 750 ml water sample from each bottle was filtered with Whatman GF/C glass fiber filter paper of 47 mm diameter (Rahmati et al. 2011), and during or just before the end of the filtering process, 2–3 ml of MgCO3 solution was added to avoid degradation of chlorophyll. The filter paper containing filtrate was folded and wrapped in aluminum foil and again kept at 4 °C till further processing (Axler and Owen 1994; APHA 2012).
Thereafter, one filter paper containing filtrate was taken and chopped in a tissue grinder and 2–3 ml of acetone solution was added. The content was then grinded at 500 rpm/min for 2–3 min and inverted into a 15-ml centrifuge tube. The grinder was rinsed with acetone solution to remove the entire chlorophyll content to the centrifuge tube. A total volume of 10 ml in the centrifuge tube was adjusted by adding further acetone aqueous solution. Then, the tube was centrifuged at 3000 rpm for 20 min and the supernatant was taken in a clean tube for chlorophyll-a analysis (Elmaci et al. 2009; APHA 2012). Similar process was followed for all the samples. The lake samples for the analysis of total phosphorus were prepared according to the persulfate digestion method (APHA 2012). In a 50 ml lake sample, 0.05 ml of phenolphthalein indicator was added. The color was changed to a slight pink, which was discharged by adding few drops of H2SO4 solution (30%). Then, 1 ml of H2SO4 and 0.4 g of ammonium persulfate [(NH4)2S2O8] powder were added in the sample and boiled for 30 min or till the volume reduced to 10 ml.
The sample was kept for cooling, and thereafter, its final volume was increased to 30 ml. The sample was kept in autoclave for 30 min, followed by the addition of 0.05 ml phenolphthalein indicator after cooling and few drops of NaOH. Then, the final volume of sample was made 100 ml using distilled water. All the remaining samples were prepared by following the above steps. The samples to be analyzed using the instrument “Ion Chromatography” were double filtered; first, with filter paper of 0.45 μm pore size and 47 mm diameter (HVLP04700) and again with filter paper having 0.22 μm pore size and 25 mm diameter (GVWP02500; Gaury et al. 2018).
Sample analysis
The samples for determination of “Chlorophyll-a” and total phosphorus were analyzed using UV-VIS spectrophotometer in the GB Pant National Institute of Himalayan Environment and Sustainable Development, Mohal Kullu, Himachal Pradesh, India. The spectrophotometer was standardized using chlorophyll-a standard (C5753-Spinach) and then the lake sample was taken in 1-cm-sized cuvette and absorbance was noted at 750 and 664 nm. That sample was acidified with 0.1 N HCl solution and with a gap of 90 s; the absorbance was again noted at 750 and 665 nm. All the samples were analyzed through a similar procedure. The lake samples for determination of the total phosphorus were analyzed according to the ascorbic acid method (Elmaci et al. 2009; APHA 2012). Twenty grams of ammonium molybdate ((NH4)6Mo7O24.4H2O) and 1.37 g of antimony potassium tartrate (K(SbO)C4H4O6.1/2H2O) were separately added in two different volumetric flasks containing distilled water and then diluted to 500 ml. A 5 N H2SO4 solution was made using distilled water.
A solution of ascorbic acid (C6H8O6) was prepared by adding 1.76 g ascorbic acid to 100 ml distilled water. The above-prepared reagents were mixed in a volumetric flask for the preparation of a combined reagent as follows: 15 ml ammonium molybdate solution, 5 ml antimony potassium tartrate, 50 ml H2SO4 solution, and 30 ml ascorbic acid solution. The standard solution was prepared using 219.5 g anhydrous potassium di-hydrogen phosphate (KH2PO4) dissolved in 1 liter (L) distilled water. Fifty milliliters of this standard solution was further diluted to 1 L, and 1.0 ml of that solution was equal to the 2.50 μg P (APHA 2012). Then, 50 ml of the above-prepared lake sample was taken in a titration flask and 0.05 ml of phenolphthalein indicator was added. The color of the sample turned to a slight pink, and to discharge that, 2 drops of a 5 N H2SO4 solution were added. Then, 8 ml of combined reagent was added to the sample to be analyzed and the absorbance was observed at 880 nm using UV-VIS spectrophotometer. The concentration of the total phosphorus from the readings of the spectrophotometer was calculated (APHA 2012) as:
The parameters like pH, TDS, EC, salinity, and dissolved oxygen (DO) were analyzed with the help of a water analysis kit, whereas the turbidity was noticed using the turbidity meter. The ionic species like NH4+, NO3−, and SO42− were analyzed using the instrument ion chromatography (Metrohm 883 Basic IC Plus) in the Department of Environmental Sciences, Central University of Himachal Pradesh, India, according to the detailed methodology as given by Gaury et al. (2018). Secchi disc transparency was measured using a circular iron disc of 20 cm diameter. The disc attached to a rope was lowered in the lake water and the difference of disappearance and reappearance of the disc was known using a measuring tape.
Statistical analysis
The chlorophyll-a was calculated using the following formula:
where 664b and 665a are the absorbance of spectrophotometer, before and after acidification of the samples.
V1 and V2 are the volume of the extract and sample filtered, respectively.
L is the width of the cuvette or light path length.
Trophic state index (TSI)
The trophic state index was calculated using the Carlson’s index (Carlson and Robert 1977) as:
where TP is the total phosphorus (μg/L), Chl-a denotes the chlorophyll-a (μg/L), and SD is the Secchi disc transparency (m).
The spatial interpolation of the parameters was known through the inverse distance weighting (IDW) method using the GIS platform and the factor analysis using statistical software.
Results and discussion
The results of the water sample analysis for different parameters indicated that the Renuka Lake is hyper-eutrophic in status. The pH values 8.33–8.70 (avg. 8.61 ± 0.1) have indicated the water of the lake as slightly alkaline. The pH is generally influenced by the presence of CO3− and HCO3− as well as CO2 in the aquatic system and plays an important role for various lake processes. Electric conductivity (EC) is the sum of the ionic species in the water body and was found to be in the range from 431 to 483 μS/cm (avg. 455.13 ± 13.6 μS/cm). Salinity represents the total salt concentration and was observed in the range of 216–243 mg L−1 (avg. 227.7 ± 7.4 mg L−1), whereas the TDS was noticed between 280 and 312 mg L−1 (avg. 299.6 ± 9.1 mg L−1). The SO42− in the lake was found to be 155–168 mg L−1 (avg. 159.8 ± 3.3 mg L−1), and on the other hand, turbidity was observed between 4.63–6.62 NTU (avg. 5.48 ± 0.63 NTU).
The SO42− in the lake may be derived from the dissolution of the dolomite and gypsum minerals and the nitrogenous fertilizers (Alexakis et al. 2012; Gaury et al. 2018). The NH4+ was noticed in the range of 8.23–10.2 mg L−1 (avg. 9.29 ± 0.55 mg L−1), and on the other hand, NO3− was found from 1.26 to 3.25 mg L−1 (avg. 2.66 ± 0.5 mg L−1). These are the resultants of the nitrogenous fertilizers and organic materials (Sheela et al. 2011), and may be due to process of ammonification and nitrification. The nitrogen present in the lake first converts to ammonia and then to ammonium; however, the degradation of the plants and animal remains also produces ammonium in the water. The ammonia also converts to nitrite and then nitrate (Ward 2008; Yuzawa et al. 2012). The nitrogenous compounds indicate the nutrient availability in the Renuka Lake, but less observed in Rewalsar, Bhimtal, Nainital, Sattal, and Naukuchiatal lakes during other studies (Chakrapani 2002; Gaury et al. 2018).
Dissolved oxygen (DO) in the Renuka Lake was found to have a slight difference of occurrence, i.e., from 5.31 to 6.98 mg L−1 (avg. 6.12 ± 0.5 mg L−1). The DO is utilized by microorganism and considered as a parameter to indicate the nutrient status of the water bodies (Wetzel 2001) and its variation depends on the decomposition of organic matter by microorganism, atmospheric inputs, and photosynthetic activities (Saluja and Garg 2017). Most of the Himalayan lakes are facing a problem of low dissolved oxygen and are anoxic (Singh et al. 2008). The chlorophyll-a in the Renuka Lake was observed to be 39.11 ± 24.26 mg L−1, whereas the total phosphorus (TP) in the lake was found to be in the range of 0.02–0.28 mg L−1 (avg. 0.11 ± 0.09 mg L−1). The Rewalsar Lake (Himachal Pradesh) in an earlier study by Jindal et al. (2014) had indicated the average Chl-a concentration as 26.43 mg L−1, although the Chl-a content observed from the Renuka Lake is more than that of the observed from the Akkulam, Veli, and Mansi Ganga lakes (Sharma et al. 2010; Sheela et al. 2011). The Secchi disc transparency (SD) indicates the amount of light penetration and suspended solid particles in the water. The SD in the Renuka Lake was monitored in the range of 0.99–1.14 m (avg. 1.06 ± 0.04 m), which is more than the Palik Lake, Serbia (0.27 m; Grzetic and Camprag 2010), and Manchar Lake, Pakistan (0.90 m; Jafri et al. 2006), but less as compared to the El Carancho (1.31 m) and La Amarga Lake (1.54 m), Argentina (Echaniz and Vignatti 2013). The average value of trophic state index (TSI) for the Renuka Lake was observed in the range of 71.26–85.85 (avg. 79.16 ± 4.83; Table 1), which indicated the lake hyper-eutrophic according to the criteria of classification (Table 2). It has been inferred by the OECD (1982) standard that the lake having SD values in the range of 0.7–1.5 m is considered as hyper-eutrophic and the SD value for the Renuka Lake (0.9–1.14 m) is also lying in the similar range, and the lake is thus hyper-eutrophic.
Correlation coefficient
The Pearson’s correlation coefficient among different parameters of the Renuka Lake is given in Table 3. The positive correlation indicates single source, while the negative correlation infers multiple sources of parameters (Gaury et al. 2018). The strong correlation among EC and salinity indicates that the salinity in the lake is strongly influenced by the electrical conductivity.
Factor analysis
The factor analysis is used to indicate the expected sources of various parameters in the Renuka Lake (Table 4). Three factors with Eigen values > 1 were identified and having 59.33% total variance sum. Factor-1 revealed 26.40% variance and the dominancy of the EC, salinity, SO42−, NO3−, TDS, and turbidity, which may be due to higher impact of rock weathering as well as the anthropogenic activities. On the other hand, factor-2 indicated 18.56% variance with dominancy of SD, pH, TDS, DO, EC, turbidity, and salinity, which indicate natural and anthropogenic impacts on the water of the lake. However, factor-3 showed 14.38% variance and higher contribution of Chl-a, pH, TDS, NH4+, NO3−, salinity, SO42−, etc., which are due to major impacts of the anthropogenic activities.
Spatial interpolation
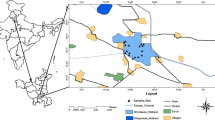
The spatial interpolation is a way to interpret the distribution and area of influence of various parameters in the lake. It is based on an assumption that each sampling point is inversely proportional to the power of distance to the center (Saluja and Garg 2017) and the closer sampling points generally have more similarities as compared to the points having large distance from each other. Instead, the density and number also impact the similarities and differences among the sampling points (Lo and Yeung 2002). The inverse distance weighting (IDW) method is used for the Renuka Lake with the help of the GIS platform, which indicated less or more spatial variations of different parameters as well as their influential zones in the Renuka Lake (Fig. 2).
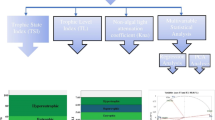
Limiting factor for the algal growth
The limiting factor related to the growth of the algae has been calculated using TSI (Chl-a), TSI (TP), and TSI (SD). There could be four limiting factors like (1) nutrient (TP)-limited small-sized algae, (2) nutrient (TP)-limited large-sized algae, (3) light-limited algae or abiotic seston, and (4) zooplankton grazing-limited large-sized algae (Carlson and Robert 1991; Havens and Karl 2000). A scatter plot of TSI (Chl-a)—TSI (SD) versus TSI (Chl-a)—TSI (TP) on the x and y axis, respectively, signifies TSI (Chl-a) > TSI (SD) and TSI (Chl-a) > TSI (TP) as well as “nutrient (TP)-limited large-sized algae” (Fig. 3). Thus, phosphorus is considered as a main limiting factor for increasing productivity in the lake and higher trophic state index; as a result, the Renuka Lake is turned hyper-eutrophic. In an earlier study, Saluja and Garg (2017) had also indicated all the four factors responsible for the trophic status (eutrophic to hyper-eutrophic) of the Bhindawas Lake of Haryana, India, whereas Jekatierynczuk-Rudczyk et al. (2014) had determined presence of nutrient as a limiting factor for the productivity in the Pogorzalek, Okragle, Udziejek, and Krajwelek lakes. The higher concentration of nutrient (TP) in the Renuka Lake is responsible for its hyper-eutrophic nature as well as degradation of the water quality, which is also inferred by Liao et al. (2017) in their study of another lake. Elmaci et al. (2009) in a previous study of Uluabat Lake, Turkey, had also indicated phosphorus as a limiting factor for the eutrophic status of the lake.
Comparison of trophic status among different lakes
The trophic status of the Renuka Lake as evaluated during the present study is compared with different lakes of the Himalayan regions (Mansar and Dal lakes) and those located in other parts of India (Mansi Ganga, Akkulam, Veli, Bhindawas, and Upper lakes) and abroad (Chaohu and Trichonis lakes), which indicated that the lakes are either turned eutrophic and hyper-eutrophic. The higher value of trophic state index (TSI) for the lakes is mainly due to mismanaged anthropogenic activities rather than the natural, and in addition, several other limiting factors. The trophic status of the Renuka Lake is similar to the Dal, Akkulam, Veli, Upper, and Bhindawas lakes, but the value of TSI is less than that of the Akkulum Lake and more than all the other. The Mansar, Mansi Ganga, Chaohu, Akkamahadevi, and Mullankere lakes are having TSI < 70 and are eutrophic in status; however, the Trichonis Lake is having least TSI (< 40) and hence oligotrophic in status (Table 5).
Conclusion
The Himalayan lakes at present are facing an acute problem of survival due to high pressure of anthropogenic activities. The present study has indicated that the Renuka Lake is hyper-eutrophic in status due to nutrient enrichment. The turbidity level indicated low clarity in the water and the correlation coefficient has indicated the multiple sources of all the parameters. Factor-1 (26.40% variance) indicated higher impact of rock weathering than anthropogenic activities in the Renuka Lake, whereas factor-2 (18.56% variance) indicated the similar impacts of the natural and anthropogenic factors. However, factor-3 (14.38% variance) inferred that the higher contribution of Chl-a, pH, TDS, NH4+, NO3−, salinity, SO42−, etc. is due to greater impact of the anthropogenic activities. The spatial interpolation has indicated that the parameters are randomly distributed in the lake. The “nutrient limited large sized algae” as well as the phosphorus is considered as the main factor for increasing productivity in the Renuka Lake and trophic state index (79.16). The present study has concluded that the nutrient enrichment in the lake must be reduced to save this lake from further degradation. It is therefore inferred to take mitigation measures to restore the Renuka Lake from hyper-eutrophic to oligotrophic status, as the lake has lots of societal and ecological significances.
References
Alexakis, D., Gotsis, D., & Giakoumakis, S. (2012). Assessment of drainage water quality in pre-and post-irrigation seasons for supplemental irrigation use. Environmental Monitoring and Assessment, 184(8), 5051–5063.
APHA. (2012). Standard method for the examination of water and wastewater (22nd ed.). Washington DC: American Public Health Association ISBN- 9780875530139.
Axler, R. P., & Owen, C. J. (1994). Measuring chlorophyll and phaeophytin: whom should you believe? Lake and Reservoir Management, 8(2), 143–151.
Barki, D. N., & Singa, P. (2014). Assessment of trophic state of lakes in terms of Carlson’s trophic state index. International Journal of Innovative Research In Science, Engineering and Technology, 3(7), 14297–14302.
Boros, E., Jurecska, L., Tatar, E., Voros, L., & Kolpakova, M. (2017). Chemical composition and trophic state of shallow saline steppe lakes in central Asia (North Kazakhstan). Environmental Monitoring and Assessment, 189(11), 546.
Carlson, & Robert, E. (1977). A trophic state index for lakes. Limnology and Oceanography, 22(2), 361–369.
Carlson, & Robert, E. (1991). Expanding the trophic state concept to identify non-nutrient limited lakes and reservoirs. Enhancing the states’s lake management programs. pp. 59–71.
Chakrapani, G. J. (2002). Water and sediment geochemistry of major Kumaun Himalayan lakes, India. Environmental Geology, 43, 99–107.
Das, B. K., & Kaur, P. (2001). Major ion chemistry of Renuka Lake and weathering processes, Sirmaur District, Himachal Pradesh, India. Environmental Geology, 40, 908–917.
Das, B. K., Gaye, B., & Kaur, P. (2008). Geochemistry of Renuka Lake and wetland sediments, lesser Himalaya (India): Implication for source-area weathering, provenance and tectonic setting. Environmental Geology, 54, 147–163.
Dixit, S., & Tiwari, S. (2005). Nutrient overloading of freshwater lake in Bhopal, India, Earth day, 21, ISSN-1076-7975.
Echaniz, S. A., & Vignatti, A. M. (2013). Trophic status of shallow lakes of La Pampa (Argentina) and its relation with the land use in the basin and nutrient internal load. Journal of Environmental Protection, 4(11), 51–60.
Edmondson, W. T. (1980). Secchi disk and chlorophyll. Limnology and Oceanography, 25(2), 378–379.
Elmaci, A., Ozengin, N., Teksoy, A., Topac, F. O., & Baskaya, H. S. (2009). Evaluation of trophic state of lake Uluabat, Turkey. Journal of Environmental Biology, 30(5), 757–760.
Gaury, P. K., Meena, N. K., & Mahajan, A. K. (2018). Hydrochemistry and water quality of Rewalsar Lake of lesser Himalaya, Himachal Pradesh, India. Environmental Monitoring and Assessment, 190(2), 84.
Grzetic, I., & Camprag, N. (2010). The evolution of the trophic state of the Palić Lake (Serbia). Journal of the Serbian Chemical Society, 75(5), 717–732.
Guyuan, L., Faping, B., Xiaoyi, X., Jia, C., & Weiqun, S. (2011). Seasonal variation of dissolved inorganic nutrients transported to the Linjiang bay of the three gorges reservoir China. Environmental Monitoring and Assessment, 73, 55–64.
Havens, & Karl, E. (2000). Using trophic state index (TSI) values to draw inferences regarding phytoplankton limiting factors and seston composition from routine water quality monitoring data. Korean Journal of Limnology, 33, 187–196.
Jafri, S. I. H., Mahar, M. A., Baloch, W. A., & Narejo, N. T. (2006). Trophic State Index, morphoedaphic index and fish yield prediction in a sub-tropical lake, Manchar (Sindh), Pakistan. Bangladesh Journal of Fisheries Research, 10(2), 131–137.
Jekatierynczuk-Rudczyk, E., Zielinski, P., Grabowska, M., Ejsmont-Karabin, J., Karpowicz, M., & Więcko, A. (2014). The trophic status of Suwałki Landscape Park lakes based on selected parameters (NE Poland). Environmental Monitoring and Assessment, 186(8), 5101–5121.
Jindal, R., Thakur, R. K., Singh, U. B., & Ahluwalia, A. S. (2014). Phytoplankton dynamics and species diversity in a shallow eutrophic, natural mid-altitude lake in Himachal Pradesh (India): role of physicochemical factors. Chemistry and Ecology, 30(4), 328–338.
Kehayias, G., & Doulka, E. (2014). Trophic state evaluation of a large Mediterranean lake utilizing abiotic and biotic elements. Journal of Environmental Protection, 5(1), 17.
Kowalczewska-Madura, K., Dondajewksa, R., Goldyn, R., & Podsiadlowski, S. (2017). The influence of restoration measures on phosphorus internal loading from the sediments of a hyper-eutrophic lake. Environmental Science and Pollution Research, 24, 14417–14429.
Lee, Y., Lee, B., Hur, J., Min, J. O., Ha, S. Y., Ra, K., Kim, K. T., & Shin, K. H. (2016). Biodegradability of algal-derived organic matter in a large artificial lake by using stable isotope tracers. Environmental Science and Pollution Research, 23(9), 8358–8366.
Liao, M., Ge, Y., & Ya, G. (2017). Eutrophication in Poyang Lake (eastern China) over the last 300 years in response to changes in climate and lake biomass. PLoS One, 12(1), e0169319.
Lo, C. P., & Yeung, A. K. W. (2002). Concepts and techniques of geographic information systems (p. 350). Upper Saddle River: Prentice-Hall.
Lu, Y., Wang, J., Zhang, X., & Kong, F. (2016). Inhibition of the growth of cyanobacteria during the recruitment stage in Lake Taihu. Environmental Science and Pollution Research, 23(6), 5830–5838.
Ochocka, A., & Pasztaleniec, A. (2016). Sensitivity of plankton indices to lake trophic conditions. Environmental Monitoring and Assessment, 188(11), 622.
OECD. (1982). Eutrophication of waters: monitoring, assessment and control. Paris: Technical Report, Environmental Directorate, OECD, pp 147.
Ogawa, H., Amagai, Y., Koike, I., Kaiser, K., & Benner, R. (2001). Production of refractory dissolved organic matter by bacteria. Science, 292(5518), 917–920.
Pathak, H., & Pathak, D. (2012). Eutrophication: impact of excess nutrient status in lake water ecosystem. Journal of Environmental & Analytical Toxicology, 2(5), 148–156. https://doi.org/10.4172/2161-0525.1000148.
Qi, H., & Lu, J. (2016). Water age prediction and its potential impacts on water quality using a hydrodynamic model for Poyang Lake, China. Environmental Science and Pollution Research, 23(13), 13327–13341.
Qin, L., Zeng, Q., Zhang, W., Li, X., Steinman, A. D., & Du, X. (2016). Estimating internal P loading in a deep water reservoir of northern China using three different methods. Environmental Science and Pollution Research, 23(18), 18512–18523.
Rahmati, R., Pourgholam, R., Najafpour, S. H., & Doustdar, M. (2011). Trophic status of a shallow lake (north of Iran) based on the water quality and the phytoplankton community. World Applied Sciences Journal, 14, 112–120.
Rai, S. P., Kumar, V., Singh, O., Kumar, B., & Jain, A. (2001). Limnological Study of the Mansar lake of District Udhampur, J&K, Final Project Report, NIH WHRC, Jammu cant.
Saluja, R., & Garg, J. K. (2017). Trophic state assessment of Bhindawas Lake, Haryana, India. Environmental Monitoring and Assessment, 189(1), 32.
Sarkar, S., Prakasam, M., Banerji, U. S., Bhushan, R., Gaury, P. K., & Meena, N. K. (2016). Rapid sedimentation history of Rewalsar Lake, Lesser Himalaya, India during the last fifty years - estimated using 137Cs and 210Pb dating techniques: a comparative study with other North-Western Himalayan lakes. Himalayan Geology, 37(1), 1–7.
Schindler, D. W. (2012). The dilemma of controlling cultural eutrophication of lake. Proceedings of the Royal Society B. https://doi.org/10.1097/rspb.1032.
Sharma, M. P., Kumar, A., & Rajvanshi, S. (2010). Assessment of trophic state of lakes: a case of Mansi Ganga Lake in India. Hydro Nepal: Journal of Water, Energy and Environment, 6, 65–72.
Sheela, A. M., Letha, J., & Joseph, S. (2011). Environmental status of a tropical lake system. Environmental Monitoring and Assessment, 180(1–4), 427–449.
Singh, O., Rai, S. P., Kumar, V., Sharma, M. K. & Choubey, V. K. (2008). Water quality and eutrophication status of some lakes of the western Himalayan region (India). Proceeding of Taal- 2007: the 12th world lake conference, pp. 286–291.
Upadhyay, R., Pandey, A. K., Upadhyay, S. K., Bassin, J. K., & Misra, S. M. (2012). Limnochemistry and nutrient dynamics in Upper Lake, Bhopal, India. Environmental Monitoring and Assessment, 184(11), 7065–7077.
Ward, B. B. (2008). Nitrification in marine systems. Nitrogen in the marine environment, 2, pp. 199–261.
Wetzel, R. G. (2001). Limnology: lake and river ecosystems. Gulf professional publishing.
Xu, Y., Cai, Q., Han, X., Shao, M., & Liu, R. (2010). Factors regulating trophic status in a large subtropical reservoir, China. Environmental Monitoring and Assessment, 169(1–4), 237–248.
Ye, L., Han, X. Q., Xu, Y. Y., & Cai, Q. H. (2007). Spatial analysis for spring bloom and nutrient limitation in Xiangxi Bay of Three Gorges Reservoir. Environmental Monitoring and Assessment, 127(1–3), 135–145.
Yu, H., Xi, B., Jiang, J., Heaphy, M. J., Wang, H., & Li, D. (2011). Environmental heterogeneity analysis, assessment of trophic state and source identification in Chaohu Lake, China. Environmental Science and Pollution Research, 18(8), 1333–1342.
Yuzawa, H., Mori, T., Itoh, H., & Yoshida, H. (2012). Reaction mechanism of ammonia decomposition to nitrogen and hydrogen over metal loaded titanium oxide photocatalyst. The Journal of Physical Chemistry C, 116(6), 4126–4136.
Acknowledgements
The authors thank the Hon’ble Vice Chancellor, Central University of Himachal Pradesh (CUHP) for his support and the In-charge, GB Pant National Institute of Himalayan Environment and Sustainable Development, Mohal Kullu, Himachal Pradesh, India, for undertaking the lab analysis. The authors acknowledge the Director, Wadia Institute of Himalayan Geology, Dheradun, India. Dr. Khem Raj Sharma, Assistant Professor, Department of English and European Languages, CUHP, is highly acknowledged for editing the manuscript. The authors are also thankful to the administration of the Renuka Lake for providing boat facility during collection of the water samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, P., Mahajan, A.K. & Meena, N.K. Evaluation of trophic status and its limiting factors in the Renuka Lake of Lesser Himalaya, India. Environ Monit Assess 191, 105 (2019). https://doi.org/10.1007/s10661-019-7247-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7247-0