Abstract
Trophic state allows for identification of problems and pressures that an ecosystem faces as well as demarcation of remedial measures. This study focuses on spatial and temporal variations in the trophic state and detection of possible causes of its divergence in Bhindawas Lake, India. The trophic state of the lake undulated between eutrophic and hyper-eutrophic state throughout the study period. Higher phosphorus concentration within the lake ecosystem is the dominant causal factor for its eutrophic state. The influence of other water quality parameters has also been analyzed using Spearman’s coefficient of correlation. Deviations between trophic state index (TSI)-chlorophyll-a (Chl-a), TSI-total phosphorus (TP), and TSI-Secchi depth (SD) pointed out that the lake is principally phosphorus limited, and its trophic status is influenced by non-algal turbidity to a large extent. Spatial analysis of trophic levels in geographic information system (GIS) helped in identification of pollution sources and chemical attributes affecting the trophic state of the lake. This study provides a rationale for further investigation of nutrient and sediment loading into the lake system and development of sustainable management and conservation strategy identifying suitable measures ascertaining the ecosystem integrity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands and lakes form an integral part of the inland freshwater resources performing a myriad of ecological functions and providing wide range of ecosystem services. They are important economic assets for irrigation, flood control, groundwater recharge, and as biodiversity hotspots. But these ecosystems have experienced significant degradation due to human activities performed in the wake of societal progress and development. Of all, deteriorating water quality in freshwater lakes is a burgeoning problem that threatens the ecosystem structure and integrity especially in developing countries like India. Nutrient loading (mainly nitrogen and phosphorus) resultant from either allochthonous or autochthonous sources has been identified as a major causal factor in widespread degradation of ecological structure and function of freshwater ecosystems. Trophic status of an aquatic ecosystem reflects the anthropogenic impact on water quality and its community structure and functioning (Cunha et al. 2013). Understanding of variations in trophic state allows for the identification of appropriate remedial measures for ecosystem restoration. Sustainable management of these ecosystems undergoing degradation requires insight into the spatial and temporal variations associated with their trophic status. Indices have been developed using one or more parameters that relate with lake productivity, often including phytoplankton biomass measured by chlorophyll-a (Chl-a), total phosphorus (TP), total nitrogen (TN), Secchi disk transparency (SD) or permutation of these (Carlson 1977; Vollenweider and Kerekes 1982; Havens 1994). The indices are numbers that can be used to classify freshwater ecosystems in different trophic states. These indices have proven to be useful for making policy and supporting lake management decisions (Knoll et al. 2015). These are imperative as eutrophication of inland freshwater ecosystem still remains one of the most globally prevalent environmental problems, despite of several decades of research and mitigation (Dodds et al. 2009; Smith and Schindler 2009). Trophic status index (TSI) of Carlson (1977) has been widely applied and is well accepted for measuring trophic state of aquatic ecosystems world over. Three different variables (CHL, TP, SD) independently estimate algal biomass. TSI allows for classification of lakes based on their trophic state and make predictive statements about the biotic and abiotic relationships existing within the ecosystem. The Carlson TSI has been previously applied for assessment of trophic status for various Indian (Sheela et al. 2011a, b; Upadhyay et al. 2012; Nibedita and Krishna 2009) as well as other important lake ecosystems of the world (Ye et al. 2007; Xu et al. 2010; Yu et al. 2011; Liu et al. 2011; Ndungu et al. 2013). Theoretically, Carlson’s TSIs yield the same value regardless of which type of measurement is used. In practice, the TSIs vary suggesting that something is different between variable relationships as originally derived for the index and the relationship between variables in the new dataset (Carlson 1991). Deviations of the indices can therefore be used to identify these conditions based on Carlson’s plotting the differences technique (Carlson and Havens 2005).
Lake trophic state assessment requires not only a large number of variables but also a spatial distribution of eutrophication levels based on each of these variables. The spatial assessment, however, of trophic state levels may become quite intricate, since the function and dynamics of each parameter may lead to different trophic state trends. There seems to exist, then, a need for appropriate methodologies and tool, such as geographic information system (GIS), capable of synthesizing spatially the trophic state trends presented by various variables.
Due to its growing eminence as an Important Bird Area, Bhindawas Lake was identified for this study. Bhindawas Lake is the largest inland man-made freshwater lake in the state of Haryana, India. The lake harbors unique floral and avifaunal biodiversity, which led it to being declared as a Wildlife Sanctuary in 1986 and also an Important Bird Area with IBA site code IN-HR-02. It is also identified with the National Plan for Conservation of Aquatic Ecosystems (NPCA) of Ministry of Environment, Forest, and Climate Change (MOEF). Inspite of its protected status, Bhindawas Lake faces severe problem of siltation, nutrient loading from nearby agricultural fields and eutrophication. Sustainable management of the lake and its development as a major ecotourism site requires for reliable data and information regarding the conditions prevailing within the lake.
Specifically, the objectives of the study were to (i) assess the water quality in relation to physico-chemical parameters and nutrients in Bhindawas Lake; (ii) evaluate spatio-temporal dynamics associated with trophic state of Bhindawas Lake; and (iii) identify possible causes of deviations in trophic state of the lake, as proposed by Carlson and Havens (2005).
Materials and methods
Study area
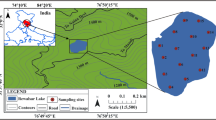
Bhindawas wildlife sanctuary, a man-made wetland designated as eco-sensitive zone by the Ministry of Environment, Forest, and Climate change, Govt. of India (The Gazette of India, 2011), is the largest wetland in state of Haryana with a periphery of 12 km (28° 28′ 00″ to 28° 36′ 00″ N; 76° 28′ 00″ to 76° 38′ 00″ E; Fig. 1). An area of 513 ha was declared a sanctuary in the year 1986 for the protection of waterfowl. “Bhindawas wildlife sanctuary” is regarded as the Keoladeo National Park of Haryana (Gupta et al. 2011). It has also been declared as an Important Bird Area, with IBA site code IN-HR-02, since it meets IBA criteria A4i, wherein more than 1% global population of bar-headed goose and greylag goose are recorded. It also meets IBA criteria A4(iii) as 20,000 birds visit the wetland during winter. It is also identified as an important wetland under NCPA of MOEF. The sanctuary is located in Jhajjar district about 80 km west of Delhi. Owing to the recurring water crisis in the Keoladeo National Park over the past few years, Bhindawas has grown importance as an alternative site for a number of migratory birds. There is an enormous concentration of migratory as well as resident waterfowl during the winter and its importance as a waterfowl habitat has been well recognized. About 287 species of birds, belonging to 18 orders and 64 families, have been recorded from the sanctuary till date (Harvey et al. 2006; Ganguli 1975). Some of the frequently observed species include dabbling ducks, plovers, and waders in shallow waters. The islands in the lake are haunts of spoonbills, geese, ducks, herons, and cormorants. The trees are favorite perches of darters, ibises, painted storks, kingfishers, and parakeets among other avifauna. Endangered species like the sarus, black-necked stork and a variety of raptors have also been observed in the lake. The freshwater swamp is fed by nearby Jawaharlal Nehru canal escape waters. Outflow is from gate numbers 1 and 2 into the drain number 8. The maximum depth of water in the sanctuary is approximately 8–10 ft (Gupta et al. 2011). The climate of the Jhajjar district can be described as tropical steppe, semi-arid, and hot which is characterized by extreme dryness of the air except during monsoon months, intensely hot summers, and cold winters with an annual precipitation of 441 mm. The area experiences climate extremes from hot summer (March-June) to cold winters (January-March) with short monsoon period (June-September) and post-monsoon (October-December). The temperature varies from a minimum of 7 °C (January) to a maximum temperature of 43 °C (May and June).
Sample collection and analytical measurements
Based on the tonal variation within the lake area as manifested on LISS IV (Resourcesat2) imagery and water depth, 18 sampling sites were selected. Water samples were collected during summer (May 2014), monsoon (September 2014), and winter (December 2014). Sampling site locations were marked and identified using a GARMIN etrex-12 channel Global Positioning System. All samples were collected, preserved, and stored for analysis as outlined in standard methods (APHA 1998). Two-liter precleaned polyethylene bottles were used for sample collection. The collected samples were transported to the laboratory in a cooler box and stored at 4 °C until processing and analysis. All the samples were analyzed in triplicates to ensure data quality.
The water samples were analyzed to determine their physical, chemical, and trophic state parameters based on standard methods as described in APHA (1998). The physical parameters analyzed included temperature, pH, SD, and dissolved oxygen (DO) using a portable infrared thermometer (OAKTON: InfraPro5), pH meter, 20-cm diameter Secchi disk, and DO analyzer (HACH-HQ20D), respectively. Basic chemical parameters, including biological oxygen demand (BOD) and chemical oxygen demand (COD), were analyzed using modified 3-day BOD and open reflux method. Trophic status indicators including nitrate-nitrogen (NO3 −), TN, phosphate-phosphorus (PO4 3−), TP, and Chl-a were analyzed using ultraviolet spectrophotometric screening method, persulfate method, stannous chloride spectrophotometric method, and H2SO4-HNO3 digestion followed by stannous chloride method and spectrophotometric determination of chlorophyll a, respectively.
Evaluation of trophic status and its deviations
Various indices have been developed for the classification of lakes and to indicate their trophic status. Of all, the most popular and widely applied indices, Carlson (1977) and OECD (1982) eutrophication status index have been applied here to quantitatively assess the trophic state of Bhindawas Lake. Carlson (1977), a well-accepted indicator of eutrophication, allows for the classification and ranking of the lakes world over. It is based on the following equations which correlate SD, TP, and algal biomass as Chl-a value.
where TSI-TP = trophic state index referenced to total phosphorus; TP = total phosphorus (μg/l); TSI-CHL = trophic state referenced to chlorophyll-a; CHL = chlorophyll-a (μg/l); TSI-SD = trophic state index referenced to Secchi depth; SD = Secchi depth (m); TSI-AVG = TSI averaged for all three parameters; ln = natural logarithm. This index classifies the lakes into four different categories, i.e., oligotrophic, mesotrophic, eutrophic, and hyper-eutrophic based on doubling of concentrations of algal biomass (Chl-a). The TSI approach has also been used as a quantitative measure for analyzing the degree of eutrophication and also nutrient limiting status and deviations among the three indices (Carlson 1991; Havens 1995; Carlson and Havens 2005).
Statistical analysis
Spatial and temporal variations within the dataset were analyzed using measures of descriptive statistics and analysis of variance (ANOVA) test. The multivariate analysis of the dataset using Spearman’s correlation coefficient enabled the identification of various sources and distinguishing the contribution of natural and anthropogenic contributions of pollutants into the lake ecosystem. Both ANOVA and correlation were performed in Statistical Package for Social Sciences 19.0 (SPSS 19.0).
Spatial interpolation using geographic information system
Since the point data collected is incapable of providing a holistic picture of the conditions prevailing within an ecosystem, the point data collected was interpolated and surface maps were generated from it in the GIS environment using ArcGIS software (ESRI 2011). Spatial interpolation algorithm applied for spatial simulation was inverse distance weighing (IDW).
IDW is a simple local interpolation method most commonly used. It is usually implemented in the form of a moving window to define zone of influence. In vector-based processing, a circular moving window is often used. This method is based on the assumption that the influence of each input point on the interpolated value at the center of the window is inversely proportional to a power (p) of its distance from the center as expressed in the following formula (Burrough and McDonnell 1998).
where z(u 0) is the estimated value at an unsampled point, z(u i ) are the data points, d ij is the distance between each data point to the point at an unsampled location, and p is a parameter. Typically, p = 2; in other words, the weights are usually inversely proportional to the squared distance between the data point and the point at the unsampled location. The quality of the resulting surface as indicated by the fitting between the surface and the input points is dependent on the density, distribution, and accuracy of input points (Lo and Yeung 2002).
Results and discussion
Seasonal variations of water quality
Water temperature
Temperature greatly influences the biological activity and growth of aquatic organisms within a lake ecosystem. Higher temperatures normally increase biological activity. Temperature in the entire lake was higher during summer and lower during winter season. During summer, it varied from 26 to 34.6 °C; from 19.1 to 32.3 °C during monsoon, and from 13 to 18.6 °C during winter season, demonstrating a seasonal cycle (ANOVA, p < 0.05).
pH
Biological activities of aquatic micro flora are expressively influenced by the pH of the water. The principle ion regulating pH in natural waters is carbonate, comprising CO2, CO3 2−, and HCO3. All samples were found to have pH values in the slight basic range (6.82–9.65) without any significant variation between the seasons.
SD
Secchi disk transparency is basically a function of reflection of light from the surface of the disk and, therefore, is affected by the absorption characteristics of the water and dissolved and particulate matter contained in the water (Wetzel 2001). It is the simplest index for determination of euphotic zone within the lake and acts as a proxy for suspended material. In general, SD shows both spatial and temporal variation in the lake (Fig. 2). SD was minimum during summer and maximum during winter season. Similar trend was observed in Akkulam-Veli Lake by Sheela et al. (2011b). In accordance with the OECD (1982) standards, a lake with a Secchi disk transparency within the range of 1.5–0.7 m is classified as hyper-eutrophic. During this study, SD was recorded between 0.19 and 1.35 m with an average value of 0.47 m signifying the prevalence of hyper-eutrophic conditions in Bhindawas Lake ecosystem, signifying a low euphotic zone.
Dissolved oxygen
The amount of oxygen dissolved in water is essential for respiratory metabolism of most aquatic organisms and affects the solubility and availability of many nutrients and, therefore, determine the productivity of aquatic ecosystems (Wetzel 2001). The oxygen dynamics in inland waters are governed by a balance between the inputs from the atmosphere, photosynthetic activity, and microbial decomposition of organic matter. Dissolved oxygen concentration varied from highest value of 11.76 mg/l in winters to a lowest of 0.81 mg/l in summer. Highest average value of 8.55 mg/l was observed in winter. DO levels within the lake followed a seasonal cycle (ANOVA, p < 0.05). Lower DO during summer might be due to the removal of free oxygen through degradation of organic matter and respiration by bacteria. An increase in the temperature during summer was accompanied by a decrease in DO (avg. DO = 4.19 mg/l). This is explained by the dependence of the solubility of DO on water temperature, which rises in summer. In addition to this, lower wind speeds in summer also cause the DO to be lower. Temperature and precipitation are the main drivers of increased DO levels during monsoon and winter.
Oxygen demand
BOD concentration in the lake varied between 0 and 18.47 mg/l with an average value of 2.59 mg/l. BOD values were highest during monsoon (avg. = 4.89 mg/l). This might be due to input of organic wastes and enhanced bacterial activity (Prasannakumari et al. 2003). Adakole (2000) categorized surface waters based on BOD levels into unpolluted (BOD <1.00 mg/l), moderately polluted (2–9 mg/l), and heavily polluted (BOD >10 mg/l). In the present study, the BOD values indicate moderately polluted state of the lake. COD concentration ranged from 8 to 144 mg/l. Higher average values of COD (56.89 mg/l) were observed during summer due to decreased levels of dissolved oxygen. Higher rates of organic matter decomposition act as a stimulus to rapid uptake of DO (along with above discussed factors, i.e., temperature and wind speed).
Nitrate-N
Nitrate is the most readily utilized form of nitrogen. Nitrate-N is the common form of inorganic nitrogen entering the lakes from drainage basin. It is formed as a result of aerobic oxidation of organic nitrogenous matter. The concentration of nitrate-nitrogen varied from 0.047 to 5.38 mg/l with an average concentration of 0.65 mg/l during the study period. The seasonal variation was usually attributed to the increasing assimilation by algae (ANOVA, p < 0.05). According to Vollenweider (1970), lakes having inorganic nitrogen concentration in the range of 500–1500 μg/l are considered to be eutrophic. Bhindawas Lake with an average nitrate concentration of 650 μg/l is thus a eutrophic ecosystem.
TN
Total nitrogen concentration recorded in the lake ranged between 0.35 and 18.9 mg/l with an average of 5.03 mg/l. The average concentration of TN was found to be minimum during summer which corroborates with large absorption of lot of nutrients especially N and P by algae for their rapid growth during summer (ANOVA, p < 0.05). Total nitrogen levels were higher during monsoon which could indicate allochthonous sources of nitrogen contributed by the runoff from the drainage area.
Phosphate
A large proportion of phosphorus within freshwater ecosystems exists as organic phosphates and as cellular constituents in the biota and absorbed into inorganic and dead particulate organic materials. According to Niswander and Mitsch (1995), addition of phosphate to water brings about eutrophication by increase in oxygen demand and increase in production of growth factors for algae thus resulting in increased algal growth. During monsoon and winter season, phosphate concentration in the lake varied from 0.013 to 0.324 and 0.014 to 0.036 mg/l. The highest average phosphate concentration (avg. = 0.056 mg/l) observed during monsoon may be due to release of phosphates from the sediments into water or due to the loads carried by the surface runoff. Lower values during summer (avg. = 0.029 mg/l) indicate its utilization by the phytoplankton community within the lake. An average concentration of 30 μg/l is sufficient to cause algal blooms (Sawyer et al. 1945). Phosphate content within the lake well exceeds this critical limit of algal blooms within most parts of the lake.
TP
Phosphorus is considered the most important element for determining biological productivity in many freshwater systems (Scholten et al. 2005). Higher values of TP during summer are a result of lower water levels in the lake (Fig. 2). Levels of microbial activity rise at higher temperature, so the release of phosphorus from sediment is quite dependent on temperature. Increase in phosphorus concentration in turn leads to increased production of phytoplankton, even algal bloom in lakes and reservoirs (Watson et al. 1997). This acts as an input for higher organic matter load in the lake (or COD) due to large amount of dissolved organic matter and decomposition of phytoplankton. Vollenweider (1970) and Wetzel (1975) recognized thresholds for hyper-eutrophication to be equivalent to 100 μg/l. Trophic state classification using OECD (1982) criterion defined a limit for TP concentration as 35 μg/l for demarcating eutrophic state. In Bhindawas Lake, TP concentration was almost always higher than the prescribed eutrophication limits.
Chlorophyll-a
Chlorophyll-a concentration is useful for evaluating the concentrations of phytoplankton biomass (Harper 2010). Chlorophyll-a was estimated in the range of 1.13–217.8 μg/l with an average concentration of 28.07 μg/l. Chl-a values followed a seasonal pattern with higher concentrations during summer (ANOVA, p < 0.05). Concentration of Chlorophyll-a was highest during summer due to high phytoplankton activities and lower water levels in the lake system. Other studies have also noted an inverse relationship between Chl-a and water levels (Wang et al. 2012). Chl-a concentrations were lowest in winter, which can be explained by low primary productivity during this period. The temperature, photosynthetically active radiation (PAR), nutrient concentration, and hydraulic retention time, all of which influence phytoplankton growth, reach their suboptimum levels in winter (Hoffman 1988). OECD (1982) identified 8 μg/l of chlorophyll-a as the threshold for eutrophic state within an aquatic ecosystem, and in this study, almost all of the samples were found to have chlorophyll-a content exceeding the prescribed limit.
TN/TP ratio
Both nitrogen and phosphorus are often identified as major contributing factors for eutrophication in surface waters. Nitrogen to phosphorus ratios have been used to indicate the limiting nutrient and degree of nutrient limitation (Havens and Walker 2002). From theoretical perspective, if the TN/TP ratio is less than 7, N is the limiting nutrient to phytoplankton growth and if greater than 10, phosphorus is the limiting factor (Meybeck and Helmer 1989). Generally, freshwater ecosystems, like lakes and rivers, tend to be phosphorus-limited (Schindler 1977) and marine waters, N-limited (Howarth et al. 1996). Bhindawas Lake showed a wide range in the TN/TP ratio and did not indicate any seasonal pattern. The average TN/TP ratio in Bhindawas Lake was much higher than the critical ratio, thus proving that TP is the main limiting factor that results in eutrophication. In eutrophication studies, phosphorus is regarded as the crucial factor for controlling and management of Lake eutrophication and reduction of phosphorus input is critical to reduce eutrophication (Schindler et al. 2008).
Multivariate correlation analysis
The water quality data was analyzed for multivariate correlations among the variables for all the three seasons using Spearman’s rho correlation (Table 1). Secchi disk transparency (SD) showed strong positive correlations with T (r = 0.486, p < 0.01) and TP (r = 0.367, p < 0.01). SD is a key environmental factor affecting chlorophyll-a concentration because it determines euphotic zone and consequently leads to variations in productivity, thus supporting a strong correlation (r = 0.336, p < 0.05) between SD and phytoplankton abundance (Chl-a). SD shows strong negative correlations with TN. DO and pH exhibited a strong positive correlation (r = 0.631, p < 0.01) indicating photosynthetic formation of DO. During summer, the productivity is quite high and majority of the lake area gets covered by floating island of macrophytes which lead to rapid release of carbon dioxide and, thus, rise in the pH of lake water.
During the study period, it was found that an inverse relationship existed between the temperature and dissolved oxygen concentration within the lake (r = −0.653, p < 0.001). Dissolved oxygen concentration reduced with increase in temperatures during summer, and the concentration increased with decrease in temperature during winters.
Higher temperatures decrease dissolution rate of oxygen into water further affected by the lower wind speed. Chl-a, on the other hand, was positively correlated with the temperature (r = 0.660, p < 0.01) as suitable water temperature can promote the growth of algae. Chloride also showed strong negative correlation with temperature (r = −0.584, p < 0.01), COD(r = −0.321, p < 0.05), TP(r = −0.312, p < 0.01), Chl-a (r = −0.470, p < 0.01), and NO3 − (r = −0.427, p < 0.01), further indicating drainage from nearby land is the source of nutrients that are coming to the lake. Strong correlation between NO3 − and DO (r = −0.468, p < 0.01) indicates the dependence of nitrification on oxygen supply. Positive correlation of BOD with phosphate (r = 0.348, p < 0.05) indicates it being a source of organic load in the lake ecosystem. COD exhibits strong positive correlation with TP (r = 0.415, p < 0.01) as both the parameters can have anthropogenic controls and, therefore, are critical in lowering and controlling eutrophication within the lake ecosystem. COD also shows xnegative correlations with DO (r = −0.285, p < 0.05) and chloride (r = −0.321, p < 0.05). Nitrate showed significant positive correlation with Chl-a (r = 0.545, p < 0.01).
Assessment of trophic status of Bhindawas Lake
Eutrophication has emerged as a major worldwide problem in the recent years. Majority of the lakes researched either are eutrophied or are on the verge of eutrophication highlighting the impact of anthropogenic forcing. In the present study, Carlson TSI was calculated with the seasonal averages of TP, SD, and Chl-a for the entire lake during the study period (2014) as shown in Fig. 3. Trophic status of Bhindawas Lake when compared with the trophic state of some of the major lakes of the world necessitates the immediate need of eutrophication control (Table 2). The calculated TSI values were compared with the Carlson’s trophic state classification criteria (Table 3). According to Carlson TSI, the Bhindawas Lake was hyper-eutrophic during summer and monsoon (73.9 and 71.2, respectively) whereas eutrophic in winters (64.6).
Trophic state index based on TP
The variation in the TSI-TP, as shown in the Fig. 3, indicates temporal variation with highest average values during summer. During summer, the entire lake is in hyper-eutrophic state with an average value of 77.5. During monsoon and post-monsoon, the entire lake falls in trophic class of hyper-eutrophy (TSI-TP = 73 and TSI-TP = 70.4, respectively). The trophic status of the lake based on TP lowers down due to precipitation, monsoonal discharge from the canal, and mixing during monsoon and winter season.
Trophic state index based on SD
In general, TSI-SD indicates both spatial and temporal variations (Fig. 3). Average TSI-SD values display hyper-eutrophic levels during summer (TSI-SD = 70.44) and eutrophic levels during monsoon season (TSI-SD = 69.6). The lower TSI-SD value during monsoon might be attributable to the dilution of lake water due to monsoon rains, discharge from the canal and drain. While during winter season, average TSI-SD value (TSI-SD = 76.03) shows even higher values than summer indicating hyper-eutrophic conditions in the lake probably due to high wind action and mixing of inorganic suspended particles.
Trophic state index based on CHL
TSI-CHL shows distinctive pattern of spatial and temporal variation throughout the year. During summer, the average TSI-CHL of Bhindawas Lake was 73.86. As per Carlson’s trophic state classification, the lake falls under hyper-eutrophic state. During monsoon season also, the lake falls under hyper-eutrophic state which might be attributed to the additional nutrient flows brought in with the surface runoff. Whereas, during winter season, the TSI-CHL shows a steep fall (avg. = 47.47). Lower ambient temperatures and lower productivity corroborate lower values during winters.
Spatial variation of TSI in Bhindawas Lake
A GIS-based method is utilized for lake assessment in order to study the spatial distribution of TSI based on three variables, SD, CHL, and TP, using IDW interpolation. Thematic maps indicating spatial distribution of TSI based on three variables, i.e., SD, CHL, and TP, are shown in Fig. 4. Overlay analysis was performed in GIS to develop a spatial map illustrating the average trophic status within the lake for each season.
Figure 4a shows trophic level distribution map based on Chl-a. The summer dataset has brought out that based on Chl-a, the entire lake largely is hyper-eutrophic indicating high algal biomass production within the lake. TSI-CHL is highest during summer season which might be attributed to the existence of favorable environmental as well as nutritional factors. TSI-CHL for monsoon season highlights the impact of discharge from the source canal as well as drain no. 8. Since drain no. 8 is responsible for addition of higher organic load to enter the lake from the eastern zone, it shows hyper-eutrophic conditions as compared to eutrophic conditions dominating in the western zone. TSI-CHL was minimum during winter indicating the onset of unfavorable conditions in terms of temperature, light penetration, transparency, etc., highlighting the antagonistic effect of sediment mixing and higher suspended solids concentrations on algal productivity.
Trophic level distribution map derived using seasonal SD data shows that a large western section of the lake is at extreme hyper-eutrophic level during summer (Fig. 4a). Higher temperatures during the summer season might cause high evaporation rates leading to lower water levels. The decreased water level, however, may also cause a concentration effect for the present algae and therefore, lower transparency in lake waters during summer. The TSI values experience a fall during monsoon season. At this time, the rainfall period leads to clearer water, thereby promoting light penetration in the water column of the lake. During winter season, the TSI values spike again with extreme hyper-eutrophic levels covering the entire western part of the lake. This was due to sediment resuspension and high suspended solid concentration. The variability of SD during seasons essentially depends on periods of high runoff from the catchment basin, temperature, and wind regime, as well as conditions favouring development of lake microorganisms. Secchi depth is affected not only by the autochthonous lake inputs but also very much by allochthonous flows and inputs.
Interpolation results of TSI-TP (Fig. 4b) indicate that the lake condition is much more serious with respect to total phosphorus. Except for certain southeastern region, majority of the lake is at hyper-eutrophic level with TSI-TP >> 70, emphasizing that there is severe impact of TP on lake’s trophic status. Major sources probably responsible for high phosphorus levels and its spatial distribution within the lake could be of indigenous and exogenous origin. Indigenous sources include internal loading and release from the sediment during rainfall or wind events, whereas exogenous sources include runoff from catchment dominated by agricultural land use and inflow from the source canal or nearby drain.
Spatial statistics in GIS (Fig. 5) revealed that during summer season, areas of extreme hyper-eutrophic regions cover 69.16% of the whole area of Bhindawas Lake defining the severity of the conditions. However, during monsoon, hyper-eutrophic regions reduced to 56.23% of the total area, decreasing the pressure on lake from extreme hyper-eutrophic conditions. Winter season exhibited the dominance of eutrophic regions covering 96.63% of the whole area.
Deviation in TSI based on Carlson’s 2D approach
According to Carlson (1991), subtracting TSI (CHL) from any other TSI will equal zero or nearly so, with only random variation causing deviation from zero. However, in practical situations, there are usually predictable deviations between TSI (CHL), TSI (TP), and TSI (SD) that can be used to assess the degree and type of nutrient limitation. Deviations and nutrient limitation within the lake ecosystem were analyzed based on Carlson’s two-dimensional approach. This approach identifies four different conditions based on differences between the three TSIs. If TSI (CHL) is significantly lower than TSI (TP), it indicates lower algal productivity than expected, also highlighting the fact that some factor other than phosphorus is having the controlling effect. If TSI (CHL) is lower than TSI (SD), it signifies that abiotic particles dominate turbidity and are a probable cause of light attenuation.
Figure 6 illustrates a plot of deviations between TSI (CHL)-TSI (SD) versus TSI (CHL)-TSI (TP) derived from Chl-a, SD, and TP data collected for Bhindawas Lake during the year 2014. Points lying in the first quadrant indicate the dominance of large particles such as blue green algae or where transparency is less affected by the particles. Points lying in the second quadrant indicate an increase in zooplankton grazing, thus decreasing the chlorophyll content and increasing the transparency. Points lying in third quadrant indicate the dominance of non-algal turbidity within the lake ecosystem where phosphorus is bound to non-algal particles. Points lying in the fourth quadrant indicate predominance of smaller particles or where transparency is affected by organic color. The points lying close to the diagonal indicate that TSI-TP and TSI-SD are positively correlated to each other, but TSI-CHL has only a weak correlation with TSI-SD highlighting an increase in percentage of inorganic seston.
Analysis of summer dataset revealed that some of the sampling sites had higher TSI-CHL deviation values than expected based on TSI-SD denoting the prevalence of large filamentous or colonial blue green algae which attenuate less light than an equal biomass of small algal particles (Edmondson 1980), and also, higher TSI-CHL than TSI-TP indicates that the phosphorus might be a factor limiting phytoplankton growth during this season. Also, high zooplankton grazing exists at a few sites reducing the algal biomass below levels predicted from total phosphorus. High variability among sampling sites has been observed during this season. The factors controlling such deviations could be varying macrophyte cover, water depth, and location of the sampling sites (Havens 2000). Similar observations were made during monsoon season with data points dispersed in all the four quadrants. During winter, for all sampling sites, the deviations of TSI (CHL)-TSI (SD) and TSI (CHL)-TSI (TP) showed highly negative values. These negative deviation values can also be attributed to the suboptimum levels of phytoplankton growth as a result of low ambient temperature, photosynthetically available radiation (PAR), and hydraulic retention time (Hoffman 1988) despite of high phosphorus levels. Higher divergence of TSI (CHL)-TSI (SD) could also signify the effect of non-algal turbidity on algal biomass. Non-algal turbidity could be contributed by organic and/or inorganic particles such as detrital particles, clay, etc., dominating the seston, thus limiting algal productivity by decreasing light penetration and increasing light attenuation. TSI (CHL)-TSI (TP) indicates that nutrients (i.e., phosphorus in this case) exceeded the input required for phytoplankton growth and suggesting that other factors like ambient temperature were limiting phytoplankton productivity (Carlson and Havens 2005).
Conclusions and recommendations
Present study evaluated the spatio-temporal variations of the pollution load and trophic state variables in Bhindawas Lake for the year 2014. Correlations between the water quality parameters have been established. Carlson’s trophic state index (TSI) based on total phosphorus, Secchi disk transparency, and chlorophyll-a was used to assess the trophic status of Bhindawas Lake. Deviations in TSI have been analyzed using Carlson’s 2D approach. The following conclusions have been drawn from this study:
-
I.
Bhindawas Lake is highly enriched with nutrients such as nitrogen and phosphorus. The concentration of nitrogen and phosphorus has been found to be well above the prescribed limits of eutrophic levels. TN/TP ratio also indicates that phosphorus might be the limiting factor that results in eutrophication. Perusal of the lake water quality divulges that phosphorus concentration is mainly responsible for eutrophic state and has significant impacts on lake’s water quality. Increased phosphorus loading in the lake might lead to eutrophication and increased productivity.
-
II.
Carlson trophic state index based on SD and TP were found to be consistently higher than 70 during all the three seasons signifying that Bhindawas Lake is in hyper-eutrophic. Consistent prevalence of such conditions of hyper-eutrophy may provide impetus for reduction of oxygen levels and, thus, hampering the ecosystem functioning. If hyper-eutrophic state is prevalent for longer durations, the entire lake might be engulfed by macrophyte beds leading to annihilation of lake. Comparatively lower TSI values based on Chl-a within the lake may be due to dominance of non-algal seston and prolific growth of water hyacinth in the lake.
-
III.
Graphical representation of the deviation of TSI-CHL and TSI-TP indicates that within the lake, TP might predominantly be a factor limiting phytoplankton growth. Higher divergence of TSI-CHL and TSI-SD signifies the effect of non-algal turbidity on algal biomass. These findings suggest that further investigation of nutrient and sediment loading into the lake ecosystem is required.
-
IV.
Trophic level distribution maps helped in identification of factors that have the maximum influence on the trophic status of the lake. These factors include pollution sources and chemical attributes in the lake. Pollution sources comprise nutrient inflows, distance from inflow entrance, mixing, etc. Demarcation of these pollution sources may have important implications for suitable lake management and eutrophication control strategies within the lake ecosystem.
-
V.
Conservation and management plans of Bhindawas Lake must take cognizance of drainage basin as the nodal point. Anthropogenic nutrient inputs into the lake must be managed with high precedence. Ecological processes within the lake can be effectively controlled by employing eco-technologies or biomanipulation for upgradation of water quality and to control eutrophication. Internal phosphorus loading from the sediments must be reduced and internal cycling of phosphorus prevented for maintenance of ecosystem integrity of the Bhindawas Lake ecosystem.
References
Adakole, J.A. (2000). The effect of domestic, agricultural and industrial effluents on the water quality and biota of Bindare stream, Zaria, Nigeria. Ph.D. Thesis, Department of Biological Sciences, Ahmadu Bello University, Zaria, Nigeria, 256 pp.
APHA (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington, DC: APHA, AWWA, WEF.
Burrough, P. A., & McDonnell, R. A. (1998). Creating continuous surfaces from point data. Principles of geographic information system. Oxford: Oxford University Press.
Carlson, R. E. (1977). A trophic state index for lakes. American Society of Limnology and Oceanography , 22 (2), 361–369.Lawrence, Kan
Carlson, R.E. (1991). Expanding the trophic state concept to identify non-nutrient limited lakes and reservoirs. Enhancing the States Lake Management Programs, 59–71.
Carlson, R. E., & Havens, K. E. (2005). Simple graphical methods for interpretation of relationships between trophic state variables. Lakes and Reservoirs: Research and Management, 21, 107–118.
Cunha, D. G. F., do Carmo Calijuri, M., & Lamparelli, M. C. (2013). A trophic state index for tropical/subtropical reservoirs (TSItsr). Ecological Engineering, 60, 126–134.
Dodds, W. K., Bouska, W. W., Eitzmann, J. L., Pilger, T. J., Pitts, K. L., Riley, A. J.,... & Thornbrugh, D. J. (2009). Eutrophication of US freshwaters: analysis of potential economic damages. Environmental Science & Technology, 43(1), 12–19.
Edmondson, W. T. (1980). Secchi disk and chlorophyll. Limnology and Oceanography, 25, 378–379.
ESRI (2011). ArcGIS Desktop. Release 10. Redlands: Environmental Systems Research Institute.
Ganguli, U. (1975). A guide to the birds of the Delhi area. New Delhi: Indian Council of Agricultural Research.
Gupta, R. C., Parasher, M., & Kaushik, T. K. (2011). An enquiry into the avian biodiversity of Bhindawas bird sanctuary in Jhajjar District in Haryana state in India. Journal of Experimental Zoology, 14(2), 457–465.
Harper, H. H. (2010). Evaluation of surface water quality characteristics in Casselberry Lakes. Florida: Final Report, City of Casselberry Public Works Department.
Harvey, B., Devasar, N., & Grewal, B. (2006). Atlas of birds of Delhi and Haryana. New Delhi: Rupa& Co.
Havens, K. E. (1994). Seasonal and spatial variation in nutrient limitation in a shallow sub-tropical lake (Lake Okeechobee, Florida) as evidenced by trophic state index deviations. Archiv für Hydrobiologie, 131(1), 39–53.
Havens, K. E. (1995). Secondary nitrogen limitation in a subtropical lake impacted by non-point source agricultural pollution. Environment Pollution., 89, 241–246.
Havens, K. E. (2000). Using trophic state index (TSI) values to draw inferences regarding phytoplankton limiting factors and seston composition from routine water quality monitoring data. Korean Journal of Limnology, 33(3), 187–196.
Havens, K. E., & Walker Jr., W. W. (2002). Development of a total phosphorus concentration goal in the TMDL process for Lake Okeechobee, Florida (USA). Lake and Reservoir Management, 18(3), 227–238.
Hoffman, E. E. (1988). Plankton dynamics on the outer southern US continental shelf 3. A coupled physical-biological model. Journal of Marine Research, 46, 919–946.
Howarth, R. W., Billen, G., Swaney, D., Townsend, A., Joworski, N., Lajtha, K., Downing, J. A., Elmgren, R., Caraco, N., Jordan, T., Berendse, F., Freney, J., Kudeyarov, V., Murdoch, P., & Zhu, Z.-l. (1996). Regional nitrogen budgets and riverine inputs of N and P for the drainages to the North Atlantic Ocean: natural and human influences. Biogeochemistry, 35, 75–139.
Knoll, L. B., Hagenbuch, E. J., Stevens, M. H., Vanni, M. J., Renwick, W. H., Denlinger, J. C.,... & Gonzalez, M. J. (2015). Predicting eutrophication status in reservoirs at large spatial scales using landscape and morphometric variables. Inland Waters, 5(3), 203–214.
Liu, X., Lu, X., & Chen, Y. (2011). The effects of temperature and nutrient ratios on Microcyctis blooms in Lake Taihu, China: an 11-year investigation. Harmful Algae, 10(3), 337–343.
Lo, C. P., & Yeung, A. K. W. (2000). Concepts and techniques of geographic information systems (2nd ed.). John Wiley and Sons.
Lo, C. P. & Yeung, A. K. W. (2002). Concepts and techniques of geographic information systems (pp. 350). Upper Saddle River: Prentice-Hall.
Meybeck, M., & Helmer, R. (1989). The quality of rivers: from pristine stage to global pollution. Paleogeography, Paleoclimatology, Paleoecology (Global and Planetary Change), 1, 283–309.
Ndungu, J., Augustijn, D. C. M., Hulscher, S. J. M. H., Kitaka, N., & Mathooko, J. (2013). Spatio-temporal variations in the trophic status of Lake Naivasha, Kenya. Lakes and Reservoirs: Research and Management, 18, 317–328.
Nibedita, K., & Krishna, G. B. (2009). Temporal, spatial and depth variation of nutrients and chlorophyll content in an urban wetland. Asian Journal of Water, Environment and Pollution, 2, 43–55.
Niswander, S. F., & Mitsch, W. J. (1995). Functional analysis of a two year old created in stream wetland: hydrology, phosphorus retention and vegetation survival and growth. Wetlands, 15(3), 212–215.
OECD (1982). Eutrophication of waters: monitoring, assessment and control. Paris: Technical Report, Environmental Directorate, OECD 147pp.
Prasannakumari, A. A., Ganagadevi, T., & Sukeshkumar, C. P. (2003). Surface water quality of river Neyyar-Thiruvananthapuram, Kerala, India. Pollution Research., 22(4), 515–525.
Sawyer, C.N., Lackey, J.B., & Lenz, R.T. (1945). Report of the Governor’s Committee, Madison, Wis (Two volumes). An investigation of the odor nuisance occurring in the Madison Lakes-Monona, Waubesa and Kegonsa.
Schindler, D. W. (1977). Evolution of phosphorus limitation in lakes. Science, 195, 260–262.
Schindler, D.W., Hacky, R.E., Findlay, D.L., Stainton, M.P., Parker, B.R., Paterson, M.J,..., & Kasian, S.E.M. (2008). Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proceedings of the National Academy of Sciences, 105(32), 11254–11258.
Scholten, M. C. T. H., Foekema, E. M., Van Dokkum, H. P., Kaag, N. H. B. M., & Jak, R. G. (2005). Eutrophication management and ecotoxicology (p. 122). Berlin: Springer.
Sheela, A. M., Letha, J., Joseph, S., Rmachandran, K. K., & Sanalkumar, S. P. (2011a). Trophic state index of a lake system using IRS (P6-LISS III) satellite imagery. Environmental Monitoring and Assessment, 177(1–40), 575–592.
Sheela, A. M., Letha, J., & Joseph, S. (2011b). Environmental status of a tropical lake system. Environmental Monitoring and Assessment., 180, 427–449.
Smith, V. H., & Schindler, D. W. (2009). Eutrophication science: where do we go from here? Trends in Ecology & Evolution, 24(4), 201–207.
Upadhyay, R., Pandey, A. K., Upadhyay, S. K., Bassin, J. K., & Misra, S. M. (2012). Limnochemistry and nutrient dynamics in Upper Lake, Bhopal, India. Environmental Monitoring and Assessment, 184, 7.65–7077.
Vollenweider, R. A. (1970). Scientific fundamentals of the eutrophication of lakes and flowing water in particular reference to nitrogen and phosphorus as factors in eutrophication. Paris: OECD.
Vollenweider, R. A., & Kerekes, J. (1982). Eutrophication of waters. Monitoring, assessment and control (p. 156). Paris: Organization for Economic Co-Operation and Development (OECD).
Wang, F., Wang, X., Zhao, Y., & Yang, Z. (2012). Long-term changes of water level associated with chlorophyll a concentration in Lake Baiyangdian, North China. Procedia Environmental Sciences, 13, 1227–1237.
Watson, S. B., McCauley, E., & Downing, J. A. (1997). Patterns in phytoplankton taxonomic composition across temperate lakes of different nutrient status. Limnology and Oceanography, 42(3), 487–495.
Wetzel, R. G. (1975). Limnology . Philadelphia: Saunders Company.743pp
Wetzel, A. R. (2001). Limnology (Third ed.). USA: Academic.
Xu, Y., Cai, Q., Han, X., Shao, M., & Liu, R. (2010). Factors regulating trophic status in a large subtropical reservoir, China. Environmental Monitoring and Assessment, 169, 237–248.
Ye, L., Han, X. Q., Xu, Y. Y., & Cai, Q. H. (2007). Spatial analysis for spring bloom and nutrient limitation in Xiangxi Bay of Three Gorges Reservoir. Environmental Monitoring and Assessment, 127(1–3), 135–145.
Yu, H., Xi, B., Jiang, J., Heaphy, M. J., Wang, H., & Li, D. (2011). Environmental heterogeneity analysis, assessment of trophic state and source identification in Chaohu Lake, China. Environmental Science and Pollution Research, 18(8), 1333–1342.
Acknowledgements
First author thankfully acknowledges INSPIRE Division, Department of Science and Technology, Ministry of Science and Technology, and Govt. of India for INSPIRE Fellowship. The authors also express gratitude to Dr. Amrinder Kaur, Additional PCCF cum Chief Wildlife Warden, Haryana Forest Department; and Mr. Jai Bhagwan, wildlife inspector, and field staff at Bhindawas Lake for cooperation and necessary support during sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saluja, R., Garg, J.K. Trophic state assessment of Bhindawas Lake, Haryana, India. Environ Monit Assess 189, 32 (2017). https://doi.org/10.1007/s10661-016-5735-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5735-z