Abstract
Toxic metals have disturbed the quality of freshwater ecosystems worldwide. The concentration of heavy metals was investigated in liver, gills and muscle tissues of Schizothorax niger and Cyprinus carpio captured from river Jhelum of Kashmir Himalaya. The heavy metals displayed a wide range of disparity in studied tissues, seasons, sites and species. Cu2+ exhibited the highest concentration (279.6 μg/kg) in the liver tissues of S. niger in autumn at site 2 and the lowest (53.1 μg/kg) in the gill tissues in winter at site 1. In C. carpio, the Cu2+ was recorded highest (309.4 μg/kg) in the liver tissues in autumn at site 2. The concentration of Zn2+ was found highest (575.7 μg/kg) in the liver tissues at site 2 and the lowest (65.8 μg/kg) was recorded in the muscle tissues in autumn at site 1. Zn2+ was recorded highest (416.6 μg/kg) in the liver tissues in autumn at site 3 and lowest (51.5 μg/kg) in the gills of C. carpio during winter at site 1 (control). The concentration of Pb2+ (14.42 μg/kg) and Fe2+ (323.9 μg/kg) was observed in the liver tissue and gills of S. niger at site 3. Similar levels of Pb2+ and Fe2+ were recorded in the tissues of C. carpio at different sites. Four-way ANOVA (four way) indicated a statistically significant variation (p ≤ 0.05) in heavy metals with the sites, seasons, species and organs. The study emphasises the utmost need to monitor the level of heavy metals in S. niger on a regular basis as this native fish species is showing a continuous decline in the freshwater ecosystems of Kashmir Valley.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environmental concern of pollution (Bhat et al. 2017) due to heavy metals has raised pervasive worries worldwide. The surface water is more vulnerable to pollution than ground water (Aliewi and Al-Khatib 2015) due to its easy access to anthropogenic interferences (Al-Khatib et al. 2008). As consequences of human activities, this water resource gets readily polluted with numerous chemicals (Al-Khatib et al. 2003; Anayah 2006; Shomar 2011; WHO and UNICEF 2012; Tamas et al. 2013; Shamsu-Deen 2013; Hadia-e-Fatima and Ahmed 2018). These altogether give birth to a complex chain of issues leading to the deterioration of water quality, bioaccumulation of toxic chemicals and metals, encroachment and siltation of water bodies, and affects the aesthetic importance as well (Nagaprapurna and Shashikanth 2002). Surface water is suppressed to high levels of contamination (pesticides, industrial effluents, fertilisers, heavy metals, trace elements and other chemicals) by human activities (Klecka et al. 2010; Pandey et al. 2014; Skordas et al. 2015; Yadav et al. 2016). The storage, dispersal and movement of these contaminants in the aquatic ecosystem have a substantial consequence on the chemistry, ecology and biological properties of water (Jarvie et al. 1998; Ravichandran 2003; Mahvi et al. 2005; Gantidis et al. 2007; Arain et al. 2008; Liao et al. 2008). Wastewater inputs usually contribute salts, silt, oil, nutrients, pesticides, herbicides, heavy metals and other deleterious materials to fresh water bodies (Pandey et al. 2014). The impact of waste discharge could change the chemistry of concerned water body, loss of aquatic life and uptake of contaminated water by aquatic biodiversity (Jarup 2003; Bhat et al. 2018). The problem of aquatic pollution due to heavy metals has led to extensive concerns in almost all parts of the world, and observations reported by various institutions, researchers and agencies have been shocking to environmentalists (Tiwari et al. 2015). The prominent sources of heavy metal contamination to water bodies are agricultural inputs, domestic-cum-commercial sewage, untreated or partially treated effluents from small and largescale industrial units (Morais et al. 2012), metal electroplating, manufacture and disposal of batteries (Chen et al. 2012), circuit boards, car repair, motor workshops (Mandal and Sengupta 2002; Singh et al. 2018), geological quarrying (Mehmood et al. 2017), vehicular road inputs, etc. (Querol et al. 1993; Farmaki and Thomaidis 2008; Kandarp et al. 2011; Singh and Gupta 2012; Singh and Gupta 2014). Heavy metals are the most important pollutants in the lotic water bodies because of their toxic, mutagenic and carcinogenic nature (Mahurpawar 2015). Aquatic biota can get exposed and may bioaccumulate the heavy metals from several sources like overlaying water, bed sediments, settling of dust, atmospheric aerosols, etc. (Labonne et al. 2001; Goodwin et al. 2003; Nagajyoti et al. 2010; Jaishankar et al. 2014a). Once they join the aquatic system, they may get deposited or attached to sediments and might become the new source of heavy metals into the water environs, and finally accumulate and concentrate in the various tissues of aquatic biota (Oyewo and Don-Pedro 2003). Heavy metals can participate in various biochemical processes, have optimum mobility, can influence the ecosystem by means of bioaccumulation and biomagnification phenomena (Jaishankar et al. 2014b) and are potentially dangerous for human life (Trasande et al. 2005; Kumar et al. 2010). These heavy metals can cause behavioural changes and oxidative stress in animals and humans (Mehmood et al. 2017). Heavy metals can alter the normal behavioural work of fish while injuring nervous system with enhanced lipid peroxidation (Flora et al. 2008). This depicts that presence of toxic heavy metals in elevated concentrations could be one of the risk parameters for declining the fish population in lentic and lotic water bodies (Shafiq-ur-Rehman 2003; Martin and Griswold 2009).
Fish is widely utilised as a staple food and rich supply of protein all around the world. Kashmiri people consume significant quantity of fish (Mehmood et al. 2017), and most of the fishes and fish products traded in the market are captured from the local water bodies including river Jhelum (El-Naggar et al. 2009; Malik et al. 2010; Mehmood et al. 2017). In Kashmir province of Jammu and Kashmir, Baramulla is at first rank in fish production and produces about 4714.63 tons/year (Qayoom et al. 2014) followed by the district Bandipora that adds about 3854.85 tons/year to the total fish production of the valley. However, when future fish production of the valley is taken into consideration, the statistical models have indicated that overall fish production of the valley is showing a declining trend. (Qayoom et al. 2014; Mehmood et al. 2017). There could be multiple reasons for this but the major reason can be disturbance occurred to the breeding areas of various fish species especially Schizothorax niger and Cyprinus carpio by drastic decline in water quality due to heavy metal pollution and other anthropogenic impacts (Qayoom et al. 2014; Mehmood et al. 2017). In view of all the above-mentioned facts, this study is very much vital and important to get an insight about the level of heavy metal contamination in two commercially most important edible fish species viz., Schizothorax niger and Cyprinus carpio captured from the Jhelum River of Kashmir Valley.
Materials and methods
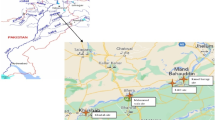
Jhelum River is culturally the prime lotic water body of Kashmir Valley; in local language, it is known as Veth. Verinag Spring in south Kashmir is considered as the main source of Jhelum. From the source, Jhelum starts its 241-km-long journey through the heart of the valley and ultimately joins Pakistan in district Baramulla of Kashmir Valley (Mehmood et al. 2017). The various tributaries and streams which join Jhelum from either banks include, Sandran, Brang, Arapat kol, Lidder, Arapal, Harwan, Sindh, Erin, Mudhumati, Pohru, Vijidakil, Vishav, Rambria, Romshi, Doodhganga, Ferozpura and Ningal. The sampling zones have been selected keeping in view the geography, settlement units, agricultural areas and commercial hubs along both banks of Jhelum River (Fig. 1). The study area has been furcated into three distinct sampling location as described in Table 1. Edible status, presence at all the selected sites of the river and decline in population (Schizothorax niger) were the criteria followed to select the test fish species for this investigation (Mehmood et al. 2017).
Fishes were captured from all the sampling zones during four different seasons from June 2014 to March 2015. Fish samples were collected on seasonal basis viz., summer (June to August), autumn (September to November), winter (December to February) and spring (March to May), and the sampling has been performed in the first month of each season. The sampling has been achieved between 10:30 a.m. to 03:00 p.m. (Mehmood et al. 2017). Fishes were captured from each sampling location with the help of local fishermen by using net and boat. Three replicates were captured for each fish species at each sampling site. Total fish length (cm) and weight (g) has been noted on spot (Fernandesa et al. 2007). Approximately same-sized fishes were captured in order to avoid the variability in heavy metal (Mekkawy et al. 2008) content due to weight and length of the fishes (Tables 2 and 3). Fishes were dissected by a sterile surgical blade for the extraction of gills, liver and muscle tissues (Khaled et al. 2010). Fish organs were placed in neat and clean polythene bags and were properly labelled (Youssef and Said 2011), preserved in ice box and then transported to the laboratory (Omar 2013). The exact 5 g wet weight of muscle tissue and 1 g wet weight each in case of liver and gills were taken and were oven dried to achieve a constant weight at a temperature of 105 °C (Turkmen et al. 2008). Further, 50-ml conical flasks were used to contain the tissue samples (Sharaf and Shehata 2015). Thereafter, 10 ml of concentrated nitric acid was added slowly to conical flasks containing the samples (2 ml of concentrated perchloric acid was added to the flasks containing gill tissues). All the flasks containing fish tissue samples were covered with aluminium foils and then heated at 200 °C with the help of a hot plate for a duration of 3 hours, until a small volume of solution remained at the bottom of flask (Turoczy et al. 2001). A solution of 2 ml 1 N HNO3 was added to the flask and was again evaporated on the hot plate (Qadir and Malik 2011). By repeating the same digestion procedure multiple times, whole organic matter in the sample got digested completely (Youssef and Said 2011). After cooling, 2.5 ml of 1 N HNO3 was transferred in the conical flask containing digested residue and was finally brought up to the volume of 50 ml with distilled water by a standard volumetric flask (Yadav et al. 2016). The samples were filtered by a 0.4-mm Whatman filter paper before sending for instrumental analysis (Alam et al. 2002). All processed samples were analysed for the heavy metals viz., Cu2+, Pb2+, Zn2+ and Fe using ICP-OES (Varian Vista MPX) at Research Centre for Residue and Quality Analysis (RCRQA), Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir (Shalimar campus) Srinagar, Jammu and Kashmir, India (Mehmood et al. 2017).
Statistical analysis
Four-way analysis of variance (ANOVA) was applied to the data to assess the statistically significant variation in heavy metals content among all sites, species and organs, and one-way (ANOVA) was adopted to get a comparative outlook of heavy metals with respect to species in a single organ. Statistical analysis indicated the significant values for the processed data (p ≤ 0.05). All data sets were subjected and analysed for homogeneity of variances test and normality test. The data was transformed which were not found normally distributed (Fig. 2). Statistical examination was carried out with the help of SPSS 18.0 for Windows (Table 4).
Results
The concentration of the Cu2+ exhibited an extensive range of variation between various tissues, seasons, sites and species. The highest level in case of S. niger was observed in the liver tissue in autumn season with a value of 279.6 μg/kg collected from site 2 of the study area, and the lowest was recorded in the gill tissue in winter season with a value of 53.1 μg/kg collected from site 1. The highest concentration of Cu2+ in Cyprinus carpio was recorded in the liver tissue in autumn season with a value of 309.4 μg/kg collected from site 2 of the river, and the lowest was found in gill tissue in spring season with a value of 52.5 μg/kg collected from site 1.The maximum concentration of Zn in S. niger was found in the liver tissue in autumn season with a value of 575.7 μg/kg collected from site 2 of the study area, and the lowest was recorded in the muscle tissue in autumn season with a value of 65.8 μg/kg collected from site 1. The highest concentration of Zn2+ in C. carpio was recorded in the liver tissue in autumn season with a value of 416.6 μg/kg collected from site 3 of the study area, and the lowest was recorded in the gill tissue in winter season with a value of 51.5 μg/kg collected from site 1. The highest of Pb concentration in S. niger was recorded in the liver tissue in winter season with a value of 14.42 μg/kg collected from site 3 of the study area, and the lowest was found in the gill tissue in autumn season with a value of 5.16 μg/kg collected from site 1. The maximum concentration of Pb2+ in C. carpio was recorded in the liver tissue in winter season with a value of 13.51 μg/kg collected from site 2 of the river, and the minimum was found in the gill tissue in winter season with a value of 5.15 μg/kg collected from site 1. The highest concentration of Fe in S. niger was recorded in the gill tissue in spring season with a value of 323.9 μg/kg collected from site 3 of the river, and the lowest was also found in the muscle tissue in autumn season with a value of 52.58 μg/kg collected from site 1. The highest concentration of Fe2+ in C. carpio was recorded in the liver tissue in summer season with a value of 491.7 μg/kg collected from site 3 of the study area, and the lowest was found in the gill tissue in autumn season with a value of 62.01 μg/kg collected from site 1.
As is evident from Fig. 3, the site wise comparison for the heavy metal accumulation followed the following patterns;
Metal | Schizothorax niger | Cyprinus carpio |
|---|---|---|
Cu2+ | Site 2 > site 3 > site1 | Site 2 > site 3 > site1 |
Zn2+ | Site 2 > site 3 > site1 | Site 3 > site 2 > site1 |
Pb2+ | Site 3 > site 2 > site1 | Site 3 > site 1 > site2 |
Fe2+ | Site 3 > site 2 > site1 | Site 3 > site 2 > site1 |
It can clearly be observed in Fig. 4 that the metals preferred a certain tissue (liver) for maximum accumulation and exhibited a comparable difference between the tissues. The accumulation pattern for all the metals in both species followed the sequence as:
Metal | Schizothorax niger | Cyprinus carpio |
|---|---|---|
Cu2+ | Liver > gills > muscle | Liver > gills > muscle |
Zn2+ | Liver > gills > muscle | Liver > gills > muscle |
Pb2+ | Liver > gills > muscle | Liver > muscle > gills |
Fe2+ | Liver > muscle > gills | Liver > gills > muscle |
The combo plot (Fig. 5) for seasonal comparison of the metal accumulation revealed that all the metals significantly varied in their accumulation with respect to seasons. Cyprinus carpio showed maximum accumulation in summer season for all heavy metals except for Pb2+ while Schizothorax niger showed more accumulation in winter season as compared to other seasons.
Metal | Schizothorax niger | Cyprinus carpio |
|---|---|---|
Cu2+ | Autumn> spring> summer > winter | Summer > autumn > spring > winter |
Zn2+ | Winter> spring> summer> autumn | Summer > autumn > spring > winter |
Pb2+ | Winter> spring> autumn> summer | Autumn > winter > spring > summer |
Fe2+ | Summer> spring> winter> autumn | Summer > autumn > spring > winter |
Discussion
Acquaintance of heavy metal bioaccumulation in fish tissues plays a very important role for the proper management and sustainable consumption (Tunca et al. 2013). Numerous studies are available which provide the significant information about heavy metal analysis in fish tissues and have reported that the quantum of heavy metal in fishes vary considerably among various tissues and species (Javed 2005; Chattopadhyay et al. 2002; Papagiannis et al. 2004; Naggar et al. 2018). Fluctuation in bioaccumulation of heavy metals may be due to physico-chemical properties of water from which fishes were sampled, ecological preferences, age, metabolic rate, feeding ground of fishes, sampling season, etc. (Fernandesa et al. 2007; Mekkawy et al. 2008; Khaled et al. 2010; Youssef and Said 2011; Omar 2013; Sharaf and Shehata 2015). In the riverine system, fishes occupy the topmost trophic level of the food web and have accordingly more tendency to be affected by heavy metal exposure from water and sediments (Mansour and Sidky 2002; Mehmood et al. 2017). Therefore, bioaccumulation and biomagnification of heavy metals in fishes has been used extensively as a potential indicator of heavy metal pollution of aquatic environs (Javed 2005; Tawari-Fufeyin and Ekaye 2007; Karadede-Akin and Unlu 2007; Naggar et al. 2018) that could be a useful means to get an insight into the biochemical disturbance of fish (Dural et al. 2011; Youssef and Said 2011). Bioaccumulation is the capability of an organism to accumulate an element or a chemical compound from food source or any other inorganic source to a level exceeding its surrounding environmental level (Bryan and Langston 1992; Mendil and Uluözlu 2007). Process of heavy metal bioaccumulation is the consequential process of many interactions within different tissues of an organism (Goodwin et al. 2003; Mekkawy et al. 2008; Khaled et al. 2010; Nagajyoti et al. 2010; Jaishankar et al. 2014a). Uptake, toxicity and distribution of heavy metal in aquatic biota are influenced by several physico-chemical factors like pH, alkalinity, hardness of water, temperature, dissolved oxygen, etc. (Pandey et al. 2014; Skordas et al. 2015; Yadav et al. 2016). Oxidation states of heavy metals play a very crucial role in their toxic activity, which in turn depends on their nature, concentration and chemical forms (Bryan et al. 1995; Mehmood et al. 2017).
Cu2+ constitutes as an essential and integral part of many enzymes and has a pivotal role in haemoglobin production (Yacoub 2007; Matasin et al. 2011), but can lead to detrimental health effects if over-consumed (Czédli et al. 2012). The Cu2+ revealed a wide range of variation in tissues, seasons, sites and species (Table 5). Significant concentration of Cu2+ in fish organs could be due to raw sewage discharged from industries, built-up and unplanned urbanisation (Shaw and Handy 1976; Antal et al. 2013). Furthermore, this may be attributed to enhanced complexity in the synthesis of metal-binding proteins (Yacoub 2007). Cu2+ exposure to fish can be directly from the surrounding water through gills (WHO 1994). Consequence of high levels of Cu2+ in fish tissues are not well investigated; however, there are studies available which confirm that high concentrations of Cu2+ in fish can be toxic and dangerous (Woodward et al. 1996; Tapia et al. 2012). Cu2+ can actively combine with other chemicals and elements like ammonia (NH3, Hg and Zn2+) to produce synergistic detrimental effects on fish (Yacoub 2007; Qadir and Malik 2011). The Cu2+ in gill tissues was observed slightly lower than that of liver. Cu2+ has lower binding affinity on the gill surface (Antal et al. 2013; Malik et al. 2014). Gills and associated proteins are directly in contact with water and have the tendency to accumulate heavy metals due to adsorption (Mutlu et al. 2012; Zhuang et al. 2013). Bioaccumulation affinity of some organs for heavy metals were found slightly lesser due to extreme secretion of mucous and clogging of gills (Widianarko et al. 2000; Mehmood et al. 2017). Muscle tissue was observed to accumulate least quantity of Cu2+ and might have reached to it via blood circulation (Woodward et al. 1996; Malik et al. 2014). The weak binding potential of Cu2+ with proteins may be attributed to its least accumulation in muscle tissue (Matasin et al. 2011; Mutlu et al. 2012). Moreover, the weak binding capacity of Cu with muscle proteins has a significant role in the environment as muscle tissue are consumed as food worldwide (Yacoub 2007; Tapia et al. 2012; Pandey et al. 2014).
Zn is an essential micronutrient and is one of the most common contaminants in aquatic environment (Romanenko et al. 1986). The Zn2+ levels depicted a significant variation between tissues, seasons, sites and species (Table 6). Sewage disposal and geological quarrying are the major sources of Zn2+ pollution to surface water bodies (Spry et al. 1988; Stonard et al. 1976). Zn exposure to fishes takes place directly from water, especially through gills and mucous (Sultana and Rao 1998; El-Naggar et al. 2009). The elevated levels of Zn2+ in fish liver in both species could be due to its role in activating several liver (Yamamoto et al. 1977; Vinikour et al. 1980; Yacoub 2007). Zn2+ was bioaccumulated in almost all tissues of the fish samples in all seasons and sampling sites.
The Pb2+ content showed a discrete fluctuation between different organs, seasons, sites and species (Table 7). Lead is non-essential trace metal and eminent quanta in aquatic organisms can occur in areas situated very close to anthropogenic sources (Ryan et al. 2000; Tuzen 2009; Mohammadi et al. 2011). It is toxic and dangerous even at lower concentrations (Kojadinovic et al. 2007) and has no known role to play in biochemical processes (Karadede and Ünlü 2000; El-Naggar et al. 2009; Malik et al. 2010). Pb2+ has been reported to supress the impulse conductivity by impeding the activities of monoamine oxidase enzyme (Dhaneesh et al. 2012) and acetylcholine esterase (Altindag and Yigit 2005; Agarwal et al. 2007), and renders the tissue with pathological changes (Bustamante et al. 2003; Dural et al. 2011; Ali et al. 2011). The significant quantum of Pb2+ could be attributed to industrial effluents, agricultural inputs (Amundsen et al. 1997) and household sewage (Gorur et al. 2012) in the study area. The recorded level of Pb2+ could be due to sufficient Pb2+ content in water and sediment (Tayel et al. 2008) of the river.
Fe is an important and most abundant element, unparalleled by any other metal in the geosphere (Aucoin et al. 1999; Abdullah et al. 2007; El-Naggar et al. 2009). The significant levels of Fe2+ content in fish liver tissue may be attributed to increased concentration of total dissolved Fe2+ in Jhelum water that consequently lead to increase in its overall availability in river ecosystem (Koli et al. 1977; Basa et al. 2003) and finally accelerate Fe2+ uptake by aquatic organisms (Adami et al. 2002; Rajotte and Couture 2002; Tayel et al. 2008). Yacoub (2007) observed bioaccumulation of Fe2+ ligand protein (Hemosidrin) diffused in liver tissue of fish exposed to very high Fe2+ levels (Table 8).
Spatial variation revealed no constant increase of heavy metal content at a specific location except site 2, which depicted more content of heavy metals as compared to other two sites. Remarkable variations were observed among seasons, sites and species for all tested heavy metals. However, it has been observed that, different organs showed different heavy metal bioaccumulation patterns. Fish samples from the same species collected from different sites and seasons also depicted a significant variation in metal bioaccumulation (ANOVA; p < 0.001, Table 4). Current investigation recorded the lowest concentration of heavy metals in muscle tissue and the maximum content of heavy metals in liver tissue. The maximum bioaccumulation of heavy metals in the liver tissue may be due to its role in body metabolism (Malik et al. 2010; Zhao et al. 2012). Remarkable amount of heavy metals in liver tissues could be attributed to organ binding specific proteins such as metallothioneins (MT) (Yacoub 2007; Gorur et al. 2012) which act as storing units for heavy metals (Roesijadi 1996; Amiard et al. 2006). Similarly, remarkable Fe2+ bioaccumulation in liver may be attributed to the role of liver in the synthesis of blood cells and haemoglobin (Aucoin et al. 1999; Amiard et al. 2006; Abdullah et al. 2007). Elevated levels of Pb2+ and Cu2+ could be attributed to the ability of these heavy metals to replace the MT-combined essential trace elements in the hepatic tissue (Qadir and Malik 2011; Mehmood et al. 2017). The fishes bioaccumulated some heavy metals in their gills, as this tissue contacts the water directly (Qadir and Malik 2011; Antal et al. 2013; Malik et al. 2014) and their large surface area facilitates rapid exposure of heavy metals inside the fishes (Yacoub 2007; Matasin et al. 2011; Dhaneesh et al. 2012). Therefore, it can be understood that overlaying water containing heavy metals enter mainly through gills into the fish body (Moore and Ramamoorthy 1984; Mekkawy et al. 2008; Khaled et al. 2010; Youssef and Said 2011; Omar 2013; Sharaf and Shehata 2015).
Significant seasonal effect on the heavy metal uptake of fish species was also recorded which might reflect the variation in metabolic activity of the species (Sultana and Rao 1998). The highest levels of heavy metals were found during summer season of the year due to the elevated anthropogenic pressure of industrial, commercial, domestic and agricultural practices along the river (Saad et al. 1985) followed by autumn season is mainly due to decrease in overall water discharge in the river (Mzimela et al. 2003) which in turn concentrate the heavy metals in the river (Long et al. 1995), and least content of heavy metals and anthropogenic inputs have been recorded in spring season as the discharge of the river in this season increases drastically (Hamza-Chffai et al. 1996; Karadede and Ünlü 2000; Kucuksezgin et al. 2001). Seasonal heavy metal variation has been well studied by researchers all around the world (Foster et al. 2000; Kargin et al. 2001; Eastwood and Couture 2002; Canli and Atli 2003). It has been reported that due to different seasonal growth rate, varying reproductive cycle, water salinity fluctuations and change in temperature are season dependent. Larson et al. (1985) also reported that fish physiological parameters are affected by seasonal variations.
Fishes are mostly in continuous movement and seldom stay at a single place. Heavy metal bioaccumulation in fish tissues provides an index of contaminated aquatic environment of the area (Qadir and Malik 2011). In the present study, spatial distribution of heavy metal bioaccumulation in fish tissues showed significant concentration of Cu2+, Zn2+, Pb2+ and Fe2+ at site 2 of the study area. This phenomenon can be attributed mainly with commercialisation, urbanisation, industrialisation and other anthropogenic activities in Srinagar City and its adjoining areas (Khaled et al. 2010). Least content of heavy metals has been observed at site 1 of the study area. The reason might be that site 1 is located near to the source of Jhelum River, i.e. Verinag, which is a least polluted site for being at high altitude with least anthropogenic pressure (Amiard et al. 2006; Abdullah et al. 2007). Muscle tissue is an inactive destination for heavy metal bioaccumulation (Elnabris et al. 2013). But in polluted aquatic systems, significant quantum of heavy metals can be found in muscles as can lead to adverse health effects (Rajotte and Couture 2002; Tayel et al. 2008). To get an insight into public health risk in our study, heavy metal levels were compared with the maximum permissible limits for human consumption (Skordas et al. 2015; Yadav et al. 2016). The tested heavy metal contents were well below the maximum permissible limit for human consumption suggested by FAO and WHO (Asgedom et al. 2012).
Field and laboratory results from many studies showed that heavy metal bioaccumulation depends on water contamination level and duration of exposure (Jeffree et al. 2006). Some other significant parameters include salinity (Jeffree et al. 2006), pH (Quan et al. 2007), hardness (Singh et al. 2007), temperature (Has-Schon et al. 2007), etc. Furthermore, ecological needs, fish age and size (Rurangwa et al. 1998), fish life cycle (Basa et al. 2003), fish feeding grounds (Kime et al. 1996) and the capturing season also affect the observed results significantly (Yadav et al. 2016). Low heavy metal concentration may not show any apparent detrimental effect on fish (Gorur et al. 2012) but may lead to decrease in fish fertility (Tuzen 2009; Mohammadi et al. 2011), which in turn may lead to decline in population of some sensitive fishes like Schzothoraz niger, etc. (Mehmood et al. 2017; Krishnani et al. 2003; Burger and Gochfeld 2005). Reproduction effects like sperm or gamete formation gets badly affected even by traces of heavy metals in water (Omar 2013; Sharaf and Shehata 2015). Effect of heavy metals on fish reproduction is a complicated process regulating multiple factors and slight heavy metal concentration could affect this pathway at any stage (Youssef and Said 2011; Dural et al. 2011). On conclusion, it can be suggested that decline of Schizothorax population in Kashmir waters can be due to several reasons, but the presence of significant amount of heavy metals inside the tissues might be one of the potential reasons (Rurangwa et al. 1998; Krishnani et al. 2003; Yousuf et al. 2013; Mehmood et al. 2017).
Conclusion
Quantum of heavy metals in three studied locations were found below the permissible limit set by international authorities. The findings also revealed that heavy metal bioaccumulation significantly varied between sites, seasons, species and organs depending on various factors like geographical location, climate, genetic tendency, feeding behaviour, swimming patterns, etc. leading to fluctuation in heavy metal accumulation between the two fish species and samples within the same species. Health risk date indicated that fishes are safe for human consumption. However, heavy metal content in Schizothorax niger species must be monitored on a regular basis in Jhelum River since population of this fish is showing a continuous declining trend in Kashmir waters.
References
Abdullah, S., Javed, M., & Javed, A. (2007). Studies on acute toxicity of metals to the fish (Labeo rohita). International Journal of Agricultural Biology, 9, 333–337.
Adami, G. M., Barbieri, P., Fabiani, M., Piselli, S., Predonzani, S., & Reisenhofer, E. (2002). Level of cadmium and zinc in hepatopancreas of reared Mytilus galloprovincialis from the Gulf of Trieste (Italy). Chemosphere, 48, 671–677.
Agarwal, R., Kumar, R., & Behari, J. R. (2007). Mercury and lead content in fish species from the river Gomti, Lucknow, India, as biomarkers of contamination. Bulletin of Environmental Contamination and Toxicology, 78, 118–122.
Alam, M. G. M., Tanaka, A., Allinson, G., Laurenson, L. J. B., Stagnitti, F., & Snow, E. T. (2002). A comparison of trace element concentrations in cultured and wild carp (Cyprinus carpio) of Lake Kasumigaura, Japan. Ecotoxicology and Environmental Safety, 53, 348–354.
Ali, A. A., Elazein, E. M., & Alian, M. A. (2011). Investigation of heavy metals pollution in water, sediment and fish at Red Sea- Jeddah coast- KSA at two different locations. Journal of Applied Environmental and Biological Science, 1(12), 630–637.
Aliewi, A., & Al-Khatib, I. A. (2015). Hazard and risk assessment of pollution on the groundwater resources and residents’ health of Salfit District, Palestine. Journal of Hydrology: Regional Studies, 4, 472–486.
Al-Khatib, I. A., Kamal, S., Taha, B., Al Hamad, J., & Jaber, H. (2003). Water–health relationships in developing countries: a case study in Tulkarem district in Palestine. International Journal of Environmental Health Research, 13, 199–206.
Al-Khatib, A., Eshkair, A. A., & Manasreh, N. K. (2008). Factors affecting water quality in the West Bank and Gaza strip of Palestine Dirasat. Jordan Journal of Mechanical and Industrial Engineering, 35(2), 131–141.
Altindag, A., & Yigit, S. (2005). Assessment of heavy metal concentrations in the food web of lake Beysehir, Turkey. Chemosphere, 60, 552–555.
Amiard, J. C., Amiard-Triquet, C., Barka, S., Pellerin, J., & Rainbow, P. S. (2006). Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquatic Toxicology, 76, 160–202.
Amundsen, P. A., Staldvik, F. J., Lukin, A. A., Kashulin, N. A., Popova, O. A., & Reshetnikov, Y. S. (1997). Heavy metal contamination in freshwater fish from the border region between Norway and Russia. Science of Total Environment, 201, 211–224.
Anayah, F. M. Y. (2006). An assessment of the nitrate and chloride in the West Bank groundwater resources using GIS (master thesis) an-Najah National University. Palestine: Nablus.
Antal, L., Halasi-Kovacs, B., & Nagy, S. A. (2013). Changes in fish assemblage in the Hungarian section of river Szamos/Someş after a massive cyanide and heavy metal pollution. Northwestern Journal of Zoology, 9(1), 131–138.
Arain, M. B., Kazi, T. G., Jamali, M. K., Jalbani, N., Afridi, H. I., & Shah, A. (2008). Total dissolved and bioavailable elements in water and sediment samples and their accumulation in Oreochromis mossambicus of polluted Manchar Lake. Chemosphere, 70(10), 1845–1856.
Asgedom, A. G., Destal, M. B., & Gebremedhin, Y. W. (2012). Bioaccumulation of heavy metals in fishes of Hashenge Lake, Tigray, northern highlands of Ethiopia. American Journal of Chemistry, 2(6), 326–334.
Aucoin, J., Blanchand, R., & Billiot, C. (1999). Trace metals in fish and sediments from Lake boeuf, south eastern Louisiana. Microbiology and Chemistry Journal, 62, 299–360.
Basa, P., Siraj, & Usha, A. R. (2003). Cadmium induced antioxidant defense mechanism in fresh water teleost, Oreochromis mosembica (Tilapia). Ecotoxicology and Environmental Safety, 56, 218–221.
Bhat, R. A., Rehman, S., Mehmood, M. A., Dervash, M. A., Mushtaq, A., Bhat, J. I. A., & Dar, G. H. (2017). Status of nutrient load in dal Lake of Kashmir Himalaya. Journal of Pharmacognosy and Phytochemistry, 6(6), 165–169.
Bhat, R. A., Dervash, M. A., Mehmood, M. A., & Hakeem, K. R. (2018). Municipal solid waste generation and its management, a growing threat to fragile ecosystem in Kashmir Himalaya. American Journal of Environmental Sciences, 2(11), 145–167.
Bryan, G. W., & Langston, W. J. (1992). Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environmental Pollution, 76(2), 89–131.
Bryan, M., Atchison, G., & Sandheinrich, M. (1995). Effects of cadmium on the foraging behaviour and growth of juvenile bluegill, lepomis macrochirus. Canadian Journal of Fisheries and Aquatic Sciences, 52, 1630–1638.
Burger, J., & Gochfeld, M. (2005). Heavy metals in commercial fish in New Jersey. Environmental Research, 99(3), 403–412.
Bustamante, P., Bocher, P., Cheerel, Y., Miramand, P., & Caurant, F. (2003). Distribution of trace elements in the tissues of benthic and pelagic fish from the Kerguelen Islands. Science of Total Environment, 313, 25–39.
Canli, M., & Atli, G. (2003). The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn2+) levels and size of six Mediterranean fish species. Environmental Pollution, 121, 129–136.
Chattopadhyay, B., Chatterjee, A., & Mukhopadhyay, S. (2002). Bioaccumulation of metals in the East Calcutta wetland ecosystem. Aquatic Ecosystem Health & Management, 5(2), 191–202.
Chen, C. W., Chen, C. F., & Dong, C. D. (2012). Distribution and accumulation of mercury in sediments of Kaohsiung River mouth, Taiwan. APCBEE Procedia, 01, 153–158.
Czédli, H., Jolánkai, G., Hancz, C., & Nagy, S. A. (2012). PIXE monitored laboratory feeding test for bioaccumulation of copper. Journal of Environmental Science and Engineering, A1, 802–807.
Dhaneesh, K. V., Gopi, M., Ganeshamurthy, R., Kumar, T. T. A., & Balasubramanian, T. (2012). Bioaccumulation of metals on reef associated organisms of Lakshadweep archipelago. Food Chemistry, 131, 985–991.
Dural, M., Goksu, M. Z. L., & Ozak, A. A. (2011). Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chemistry, 102, 415–421.
Eastwood, S., & Couture, P. (2002). Seasonal variations in condition and liver metal concentrations of yellow perch (Perca flavescens) from a metal contaminated environment. Aquatic Toxicology, 58, 43–56.
Elnabris, K. J., Muzyed, S. K., & El-Ashgar, N. M. (2013). Heavy metal concentrations in some commercially important fishes and their contribution to heavy metals exposure in Palestinian people of Gaza strip (Palestine). Journal of the Association of Arab Universities for Basic and Applied Sciences, 13, 44–51.
El-Naggar, A., Mahmoud, S. A., & Tayel, S. I. (2009). Bioaccumulation of some heavy metals and histopathological alterations in liver of Oreochromis niloticus in relation to water quality at different localities along the River Nile, Egypt. World Journal of Fish and Marine Sciences, 1(2), 105–114.
Farmaki, E. G., & Thomaidis, N. S. (2008). Current status of the metal pollution of the environment of Greece- A review. Global NEST Journal, 10(3), 366–375.
Fernandesa, C., Fontainhas, F. A., Peixotoc, F., & Salgadod, M. A. (2007). Bioaccumulation of heavy metals in Liza saliens from the Esmoriz –Paramos coastal lagoon, Portugal. Ecotoxicology and Environmental Safety, 66(3), 426–431.
Flora, S. J. S., Mittal, M., & Mehta, A. (2008). Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian Journal of Medical Research, 128, 501–523.
Foster, E. P., Drake, D. L., & Domenico, G. (2000). Seasonal changes and tissue distribution of mercury in largemouth bass (Micropterus salmoides) from Dorena Reservoir, Oregon. Archives of Environmental Contamination and Toxicology, 38, 78–82.
Gantidis, N., Pervolarakis, M., & Fytianos, K. (2007). Assessment of the quality characteristics of two lakes (Koronia and Volvi) of N. Greece. Environmental Monitoring and Assessment, 125(1–3), 175–181.
Goodwin, T. H., Young, A. R., Holmes, M. G. R., Old, G. H., & Hewitt, N. (2003). The temporal and spatial variability of sediment transport and yields within the Bradford Beck catchment, West Yorkshire. Science of Total Environment, 314, 475–494.
Gorur, F. K., Keser, R., Akcay, N., & Dizman, S. (2012). Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea Region of Turkey. Chemosphere, 87, 356–361.
Hadia-e-Fatima, & Ahmed, A. (2018). Heavy metal pollution – A mini review. Journal of Bacteriology & Mycology, 6(3), 179–181.
Hamza-Chffai, A., Romeo, M., & Elabed, A. (1996). Heavy metals in different fishes from the middle eastern coast of Tunisia. Bulletin of Environmental Contamination and Toxicology, 56, 766–773.
Has-Schon, E., Bogut, I., Kralik, G., Bogut, S., Horvatic, J., & Cacic, I. (2007). Heavy metal concentration in fish tissues inhabiting waters of “busko blato” reservoir (Bosnia and Herzegovina). Environmental Monitoring & Assessment, 54(1), 75–83.
Jaishankar, M., Mathew, B. B., Shah, M. S., & Gowda, K. R. S. (2014a). Biosorption of few heavy metal ions using agricultural wastes. Journal of Environment Pollution and Human Health, 2(1), 1–6.
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014b). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60–72.
Jarup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68, 167–182.
Jarvie, H. P., Whitton, B. A., & Neal, C. (1998). Nitrogen and phosphorus in east coast British rivers: Speciation, sources and biological significance. Science of the Total Environment, 210-211, 179–189.
Javed, M. (2005). Heavy metal contamination of freshwater fish and bed sediments in the river Ravi stretch and related tributaries. Pakistan Journal of Biological Sciences, 8(10), 1337–1341.
Jeffree, R. A., Warnau, M., Oberhansli, F., & Teyssie, J. L. (2006). Bioaccumulation of heavy metals and radio nuclides from seawater by encased embryos of the spotted dogfish scyliorhinus canicula. Marine Pollution Bulletin, 52(10), 1278–1286.
Kandarp, K., Shivpuri, B., Lokeshappa, D., Kulkarni, A., & Dikshit, A. K. (2011). Metal leaching potential in coal fly ash. American Journal of Environmental Engineering, 1(1), 21–27.
Karadede, H., & Ünlü, E. (2000). Concentrations of some heavy metals in water, sediment and fish species from the Atatürk Dam Lake (Euphrates), Turkey. Chemosphere, 41, 1371–1376.
Karadede-Akin, H., & Unlu, E. (2007). Heavy metal concentrations in water, sediment, fish and some benthic organisms from Tigris River, Turkey. Environmental Monitoring & Assessment, 131(1–3), 323–337.
Kargin, F., Donmez, A., & Cogun, H. Y. (2001). Distribution of heavy metals in different tissues of the shrimp Penaeus semiculazus and Metapenaeus monocerus from the Iskenderun gulf Turkey: Seasonal variations. Bulletin of Environmental Contamination and Toxicology, 66, 102–109.
Khaled, A., El-Nemr, A., El-Sikaily, A., & El-Sarraf, W. M. (2010). Assessment of heavy metals in the sediments of the Mediterranean coast of Egypt. Egyptian Journal of Aquatic Research, 36, 43–53.
Kime, D. E., Ebrahimi, M., Nysten, K., Roelants, I., Moore, H. D. M., & Ollevier, F. (1996). Use of computer assisted sperm analysis (casa) for monitoring the effects of pollution on sperm quality of fish; application to effects of heavy metals. Aquatic Toxicology, 36(1), 223–237.
Klecka, G., Persoon, C., & Currie, R. (2010). Chemicals of emerging concern in the Great Lakes Basin: an analysis of environmental exposures. Reviews of Environmental Contamination and Toxicology, 207, 1–93.
Kojadinovic, J., Potier, M., Le Corre, M., Cosson, R. P., & Bustamante, P. (2007). Bioaccumulation of trace elements in pelagic fish from the western Indian Ocean. Environmental Pollution, 146, 548–566.
Koli, A. K., Williams, W. R., McClary, E. B., Wright, E. L., & Burrell, T. M. (1977). Mercury levels in freshwater fish of the state of South Carolina. Bulletin of Environmental Contamination and Toxicology, 17, 82–89.
Krishnani, K. K., Azad, I. S., Kailasam, M., Thirunavukkarasu, A. R., Gupta, B. P., Joseph, K. O., & Muralidhar, M. (2003). Acute toxicity of some heavy metals to lates calcarifer fry with a note on its histopathological manifestations. Journal of Environmental Science and Health, Part C, 38(4), 645–655.
Kucuksezgin, F., Altay, O., Uluturhan, E., & Kontas, A. (2001). Trace metal and organochlorine residue levels in red mullet (Mullus barbatus) from the eastern Aegean, Turkey. Water Research, 35, 2327–2332.
Kumar, P., Kumar, S., & Agarawal, A. (2010). Impact of industrial effluents on water quality of Behgul River at Bareilly. Advances in Biological Research, 1(2), 127–130.
Labonne, M., Othman, D. B., & Luck, J. M. (2001). Lead isotopes in muscles as tracers of metal sources and water movements in a lagoon (Thau Basin, S. France). Chemical Geology, 181, 181–191.
Larson, A., Haux, C., & Sjobeck, M. (1985). Fish physiology and metal pollution: results and experiences from laboratory and field studies. Ecotoxicology and Environmental Safety, 9, 250–281.
Liao, S., Gau, H., Lai, W., Chen, J., & Lee, C. (2008). Identification of pollution of Tapeng lagoon from neighbouring rivers using multivariate statistical method. Journal of Environmental Management, 88(2), 286–292.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 18–97.
Mahurpawar, M. (2015). Effects of heavy metals on human health. International Journal of Research-Granthaalayah, Social Issues and Environmental Problems, 2015, 1–7.
Mahvi, A. H., Nouri, J., Babaei, A. A., & Nabizadeh, R. (2005). Agricultural activities impact on groundwater nitrate pollution. International journal of Environmental Science and Technology, 2(1), 41–47.
Malik, N., Biswas, A. K., Qureshi, T. A., Borana, K., & Virha, R. (2010). Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environmental Monitoring & Assessment, 160(4), 267–276.
Malik, R. N., Hashmi, M. Z., & Huma, Y. (2014). Heavy metal accumulation in edible fish species from Rawal reservoir, Pakistan. Environmental Science and Pollution Research, 21, 1188–1196.
Mandal, A., & Sengupta, D. (2002). Characterisation of coal and fly ash from coal fired thermal power plant at Kolaghat possible environmental hazards. Indian Journal of Environmental Protection, 22(8), 885–891.
Mansour, S., & Sidky, M. (2002). Heavy metals contaminating water and fish from Fayoum governorate, Egypt. Food Chemistry, 78(1), 15–22.
Martin, S., & Griswold, W. (2009). Human health effects of heavy metals. Environmental Science and Technology Briefs for Citizens, 15, 1–6.
Matasin, Z., Orescanin, V., Jukic, V. V., Nejedli, S., Matasin, M., & Gajger, I. T. (2011). Heavy metals in mud, water and cultivated grass carp (Ctenopharyngodon idella) and bighead carp (Hypophthalmichtys molitrix) from Croatia. Journal of Animal and Veterinary Advances, 10(8), 1069–1072.
Mehmood, M. A., Shafiq-ur-Rehman, Ganie, S. A., Rashid, A., & Bhat, R. A. (2017). Seasonal and spatial variation in bioaccumulation of heavy metals in two commercial fish species from river Jhelum of Kashmir valley. International Journal of Current Advanced Research, 06(07), 4650–4658.
Mekkawy, I. A. A., Mahmoud, M., & Ibrahim, A. T. A. (2008). Heavy metal distribution and their corresponding damage effect in some organs of Bagrus bajad (Forsskal, 1775) from three localities at Assiut, Egypt. Egyptian German Society of Zoology, 54, 119–137.
Mendil, D., & Uluözlu, O. D. (2007). Determination of trace metal levels in sediment and five fish species from lakes in Tokat, Turkey. Food Chemistry, 101(2), 739–745.
Mohammadi, M., Askarysary, A., & Khodadadi, M. (2011). Determination of heavy metals in two barbs, Barbus grypus and Barbus xanthopterus in Karoon and Dez Rivers, Khoozestan, Iran. Bulletin of Environmental Contamination and Toxicology, 87, 158–162.
Moore, J. W., & Ramamoorthy, S. (1984). Heavy metals in natural waters: applied monitoring and impact assessment (p. 841). New York: Springer-Verlag.
Morais, S., Costa, F. G., & Pereira, M. L. (2012). Heavy metals and human health. In J. Oosthuizen (Ed.), Environmental health – emerging issues and practice (pp. 227–246). London: IntechOpen Limited.
Mutlu, C., Türkmen, A., Türkmen, M., Tepe, Y., & Ateş, A. (2012). Comparison of the heavy metal concentrations in Atlantic horse mackerel, Trachurus trachurus, from coastal waters of Turkey. Fresenius Environmental Bulletin, 21, 304–307.
Mzimela, H. M., Wepener, V., & Cyprus, D. P. (2003). Seasonal variation of selected metals in sediments, water and tissues of the groovy mullet, Liza dumerili (Mugilidae) from the Mhlathuze estuary, South Africa. Baseline/Marine Pollution Bulletin, 46, 659–676.
Nagajyoti, P. C., Lee, K. D., & Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: a review. Environmental Chemistry Letters, 8(3), 199–216.
Nagaprapurna, & Shashikanth, K. (2002). Pollution level in Hussain Sagar lake of Hyderabad- A case study. Pollution Research, 21(2), 187–190.
Naggar, Y. A., Khalil, M. S., & Ghorabm, M. A. (2018). Environmental pollution by heavy metals in the aquatic ecosystems of Egypt. Open Access Journal of Toxicology, 03(01), 01–09.
Omar, H. E. D. M. (2013). Seasonal variation of heavy metals accumulation in muscles of the African catfish Clarias gariepinus and in River Nile water and sediments at Assiut governorate, Egypt. Journal of Biology and Earth Sciences, 03, 236–248.
Oyewo, E. O., & Don-Pedro, K. N. (2003). Lethal and sub lethal effects of copper to the African catfish (Clariasgarienpnus). West African Journal of Applied Ecology, 4, 115–123.
Pandey, P. K., Kass, P. H., Soupir, M. L., Biswas, S., & Singh, V. P. (2014). Contamination of water resources by pathogenic bacteria. ABA Express, 4, 51. https://doi.org/10.1186/s13568-014-0051-x.
Papagiannis, I., Kagalou, I., Leonardos, J., Petridis, D., & Kalfakakou, V. (2004). Copper and zinc in four freshwater fish species from Lake Pamvotis (Greece). Environment International, 30(3), 357–362.
Qadir, A., & Malik, R. N. (2011). Heavy metals in eight edible fish species from two polluted tributaries (Aik and Palkhu) of the river Chenab, Pakistan. Biological Trace Element Research, 143, 1524–1540.
Qayoom, I., Wali, A., Balkhi, M. H., & Bhat, B. A. (2014). Utilization of water resources and fishing patterns in Kashmir valley –a case study. Journal of Chemical, Biological and Physical Sciences, 4(2), 1233–1239.
Quan, W. M., Han, J. D., Shen, A. L., Ping, X. Y., Qian, P. L., Li, C. J., & Shi, L. Y. (2007). Uptake and distribution of N, P and heavy metals in three dominant salt marsh macrophytes from Yangtze River estuary, China. Marine Environmental Research, 64(1), 21–37.
Querol, X., Pares, J. M., Plana, F., Fernandez-Turiel, J. L., & Lopez-Solar, A. (1993). Fly ash content and distribution in lake sediments around a large power station: Inferences from magnetic susceptibility analysis. Environmental Geochemistry & Health, 15, 09–18.
Rajotte, J. W., & Couture, P. (2002). Effects of environmental metal contamination on the condition, swimming performance and tissue metabolic capacities of wild yellow perch (Perca flavescens). Canadian Journal of Fisheries and Aquatic Sciences, 59, 1296–1304.
Ravichandran, S. (2003). Hydrological influences on the water quality trends in Tamiraparani basin, South India. Environmental Monitoring and Assessment, 87(3), 293–309.
Roesijadi, G. (1996). Metallothionein and its role in toxic metal regulation. Comparative Biochemistry and Physiology, 13(2), 117–123.
Romanenko, V.D., Malyzheva, T. D. amp; Yevtushenko, Y. U. N. (1986). The role of various organs in regulating zinc metabolism in fish. Hydrobiology Journal, 21(3), 7–12.
Rurangwa, E., Roelants, I., Huyskens, G., Ebrahimi, M., Kime, D. E., & Ollevier, F. (1998). The minimum acceptable spermatozoa to egg ratio for artificial insemination and the effects of heavy metal pollutants on sperm motility and fertilization ability in the African catfish (Clarias gariepinus, Burchell 1822). Journal of Fish Biology, 53, 402–413.
Ryan, P. B., Huet, N., & Maclntosh, D. (2000). Longitudinal investigation of exposure to arsenic, cadmium and lead in drinking water. Environmental Health Perspectives, 108, 731–735.
Saad, M. A. H., McComas, S. R., & Eisenreich, S. J. (1985). Metals and chlorinated hydrocarbons in surficial sediments of three Nile delta lakes. Water Air Soil Pollution, 24, 27–39.
Shafiq-ur-Rehman. (2003). Lead-exposed increase in movement behaviour and brain lipid peroxidation in fish. Journal of Environmental Science and Health Part A, 32(4), 631–643.
Shamsu-Deen, Z. (2013). Assessment of the impact of water supply and sanitation on health: a study in the Savelegu/Nantong district of the northern region, Ghana. Environmental Earth Sciences, 3(3), 1–8.
Sharaf, H. M., & Shehata, A. M. (2015). Heavy metals and hydrocarbon concentrations in water, sediments and tissue of Cyclope neritea from two sites in Suez Canal, Egypt and histopathological effects. Journal of Environmental Health Science and Engineering, 13, 14–20.
Shaw, B. J., & Handy, D. (1976). Dietary copper exposure and recovery in Nile tilapia. Aquatic Toxicology, 76, 111–121.
Shomar, B. (2011). Groundwater contaminations and health perspectives in developing world case study: Gaza strip. Environmental Geochemistry and Health, 33, 189–202.
Singh, R. K., & Gupta, N. C. (2012). Leaching characteristics of trace elements in coal fly ash and ash disposal system of thermal power plants. Energy Sources Part A Recovery Utilisation and Environmental Effects, 34, 602–608.
Singh, R. K., & Gupta, N. C. (2014). Value added utilization of fly ash prospective and sustainable solutions. International Journal Applied Sciences and Engineering Research, 03, 01–16.
Singh, R. K., Chavan, S. L., & Sapkale, P. H. (2007). Heavy metal concentrations in water, sediments and body tissues of red worm (tubifex spp.) collected from natural habitats in Mumbai, India. Environmental Monitoring & Assessment, 129(1–3), 471–481.
Singh, D. V., Bhat, J. I. A., Bhat, R. A., Dervash, M. A., & Ganei, S. A. (2018). Vehicular stress a cause for heavy metal accumulation and change in physico-chemical characteristics of roadside soils in Pahalgam. Environmental Monitoring & Assessment, 190(6), 353. https://doi.org/10.1007/s10661-018-6731-2.
Skordas, E., Kelepertzis, E., Kosmidis, D., Panagiotaki, P., & Vafidis, D. (2015). Assessment of nutrients and heavy metals in the surface sediments of the artificially lake water reservoir Karla, Thessaly, Greece. Environmental Earth Sciences, 73, 4483–4493.
Spry, D. J., Hodson, P. V. & Wood, C. M. (1988). Relative contributions of dietary and waterborne zinc in the rainbow trout, Salmo gairdneri. Canadian Journal of Fishries and Aquatic Sciences, 45, 32–41.
Stonard, M. & Webb, M. (1976). Influence of dietary cadmium on the distribution of the essential metal copper, zinc and iron in tissues of rat. Chemico Biological Interactions. 15, 349–363.
Sultana, R., & Rao, D. P. (1998). Bioaccumulation patterns of zinc, copper, lead and cadmium in grey mullet Mugil cephalus from harbour waters of Visakhapatnam, India. Bulletin of Environmental Contamination and Toxicology, 60, 949–955.
Tamas, A., Meyer, J., & Mosler, H. (2013). Predictors of treated and untreated water consumption in rural Bolivia. Journal of Applied Social Psychology, 43, 1394–1407.
Tapia, J., Vargas-Chacoff, L., Bertrán, C., Peña-Cortés, F., Hauenstein, E., Schlatter, R., Jiménez, C., & Tapia, C. (2012). Heavy metals in the liver and muscle of Micropogonias manni fish from Budi Lake, Araucania region, Chile: Potential risk for humans. Environmental Monitoring & Assessment, 184(5), 3141–3151.
Tawari-Fufeyin, P., & Ekaye, S. (2007). Fish species diversity as indicator of pollution in Ikpoba River, Benin city, Nigeria. Reviews in Fish Biology and Fisheries, 17, 21–30.
Tayel, S., Yacoub, A. M., & Mahmoud, S. (2008). Histopathological and haematological responses to freshwater pollution in the Nile catfish Clarias gariepinus. Journal of Egyptian Academic Society for Environmental development, 9, 43–60.
Tiwari, M. K., Bajpai, S., Dewangan, U. K., & Tamrakar, R. K. (2015). Assessment of heavy metal concentrations in surface water sources in an industrial region of central India. International Journal of Modern Science, 01, 9–14.
Trasande, L., Landrigan, P. J., & Schechter, C. (2005). Public health and economic consequences of methyl mercury toxicity to the developing brain. Environmental Health Perspectives, 113(5), 590–596.
Tunca, E., Ucuncu, E., Ozkan, A. D., Ulger, Z. E., & Tekinay, T. (2013). Tissue distribution and correlation profiles if heavy metal accumulation in the freshwater cray fish Astacus leptodactylus. Archives of Environmental Contamination and Toxicology, 64, 676–691.
Turkmen, A., Tepe, Y., & Turkmen, M. (2008). Metal levels in tissues of the European anchovy, Engraulis encrasicolus L., 1758, and Picarel, Spicara smaris L., 1758, from black Marmara and Aegean seas. Bulletin of Environmental Contamination and Toxicology, 80, 521–525.
Turoczy, N. J., Mitchell, B. D., Levings, A. H., & Rajendram, V. S. (2001). Cadmium, copper, mercury, and zinc concentrations in tissues of the king crab (Pseudocarcinus gigas) from southeast Australian waters. Environment International, 27, 327–334.
Tuzen, M. (2009). Toxic and essential trace elemental contents in fish species from the Black Sea, Turkey. Food Chemistry and Toxicology, 47(8), 1785–1790.
Vinikour, W.S., Goldstein, R. M. & Anderson, R.V. (1980). Bioconcentration pattern of zinc, copper, cadmium and lead in selected fish species from the Fox River, Illinois. Bulletin of Environmental Contamination and Toxicology, 24, 727–734.
Widianarko, B., Van Gestel, C., Verweij, R., & VanStraalen, N. (2000). Associations between trace metals in sediment, water, and guppy, Poecilia reticulata (Peters), from urban streams of Semarang, Indonesia. Ecotoxicology and Environmental Safety, 46(1), 101–107.
Woodward, L., Mulvey, M., & Newman, M. (1996). Mercury contamination and population-level responses in Chironomids: can Allozyme polymorphism indicate exposure? Environmental Toxicology and Chemistry, 15(8), 1309–1316.
World Health Organization (WHO). (1994). Environmental health criteria 160-ultraviolet radiation, Published under the Joint Sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization.
World Health Organization (WHO) and UNICEF. (2012). Rapid assessment of drinking-water quality, ahandbook for implementation. Loughborough University Institutional Repository. http://www.wssinfo.org/fileadmin/user_upload/resources/RADWQHandbookv1final.pdf. Accessed 01 July 2014.
Yacoub, A. (2007). Study on some heavy metals accumulated in some organs of three river Nile fishes from Cairo and Kalubia governorates. African Journal of Biological Sciences, 03, 9–21.
Yadav, A., Sahu, P. K., Chakradhari, S., Rajhans, K. P., Ramteke, S., Dahariya, N. S., Agnihotri, G., & Patel, K. S. (2016). Urban pond water contamination in India. Journal of Environmental Protection, 07, 52–59.
Yamamoto, Y., Ishii, T. & Ikeda, S. (1977). Studies on copper metabolism in fishes. II. The site of copper accumulation in the tissues of carp. Bulletin of the Japanese Society for the Science of Fish, 43, 1327–1332.
Youssef, D. H., & Said, G. F. (2011). Assessment of some heavy metals in surface sediments of the Aqaba Gulf, Egypt. Environmental Monitoring and Assessment, 180(1–4), 229–242.
Yousuf, R., Mir, S. H., Tanveer, S., Darzi, M. M., & Mir, M. S. (2013). Metals and histopathological alterations in the liver of Schizothorax niger, Heckel from the Dal Lake of Kashmir Valley. International Journal of Plant and Animal Sciences, 1(5), 58–63.
Zhao, S., Feng, C., Quan, W., Chen, X., Niu, J., & Shen, Z. (2012). Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River estuary, China. Marine Pollution Bulletin, 64, 1163–1171.
Zhuang, P., Li, Z., McBride, M. B., Wang, G., & Zou, B. (2013). Concentrations of heavy metals in fish from a mine affected area and potential health risk. Fresenius Environmental Bulletin, 22, 2402–2408.
Acknowledgments
The corresponding author is highly thankful to the Department of Science and Technology and the Ministry of Science and Technology for providing INSPIRE fellowship for doctoral studies (Regd. No. IF130729).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehmood, M.A., Qadri, H., Bhat, R.A. et al. Heavy metal contamination in two commercial fish species of a trans-Himalayan freshwater ecosystem. Environ Monit Assess 191, 104 (2019). https://doi.org/10.1007/s10661-019-7245-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7245-2