Abstract
The bioaccumulation of several elements in “mouthless crabs” (Cardisoma crassum) and their concentrations in environmental samples were assessed in a mangrove forest within a natural protected area located in the middle of Puerto Vallarta, Mexico. The concentrations of Al, As, Ba, Cd, Cr, Cu, Fe, Ni, Pb, Sr, Si, V, and Zn in sediments, mangrove litterfall, and pore water and their bioaccumulations in the muscle and shell tissue of C. crassum were determined during rainy and dry seasons. Two different sampling sites were compared, selected according to the influence of the tide. The samples were analyzed by ICP-OES. In pore water, half of the elements of interest were below the limits of detection. Pb and Cd concentrations were only detected in the sedimentary phase. Al and Fe presented the highest concentrations at both sampling sites in the sediments and mangrove litterfall. There were no significant differences between sampling seasons in water samples (P > 0.05). Only Cu presented significant differences between sampling seasons in the mangrove litterfall samples and V in the sediment samples (P < 0.05). The sediment quality guidelines indicated that only Cd was above the threshold effect limit. As, Cd, Cr, Ni, and Pb remained below the limits of detection in muscle tissue and shell samples. The biota–sediment accumulation factors (BSAFs) determined for Cu and Zn were above 1.0, indicating the accumulation of these elements in the muscle tissue of C. crassum, while the BSAF values in the shell were above the threshold for Ba, Si, and Sr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal ecosystems play an important role by providing a variety of services for human being, including food, recreation, protection against flooding and storms, and retention of pollutants from urban runoff (MacFarlane and Burchett 2000; Brodie et al. 2012; Ghasemi et al. 2018). In addition to all of the benefits of these ecosystems, they often represent a link between urban settlements and marine ecosystems (Liquete et al. 2013).
Mangrove forests are one of the most important types of coastal ecosystems in the biosphere, as they play an essential role in primary productivity due to their unique range of natural resources (Barbier 2016). These characteristics have promoted the recognition of the ecological and economic importance of mangrove forests during the last several decades (FAO 2007a, b). Mexico is one of six countries that have the greatest mangrove forest area (5% of the world’s total mangrove area) (Spalding et al. 2010). There are four species of mangroves in Mexico: Rhizophora mangle, Laguncularia racemosa, Avicennia germinans, and Conocarpus erectus. The principal threats to mangrove habitats in Mexico are anthropogenic activities (CONABIO 2009) along with ineffective or poor management. These human actions have consequences, such as pollution (e.g., chemical runoff into waterways), aquaculture pond conversion, overexploitation, land use change, and pressure to some key species (Laegdsgaard and Johnson 2001; Romañach et al. 2018). These are the leading causes of the loss of original global mangrove cover, with a 35% decrease within the past two decades and the expectation that this rate will further accelerate (FAO 2007a, b; Waycott et al. 2009). One of the most critical strategies regarding conservation in Mexico is the designation of natural protected areas (NPAs); this is one of the most vital endeavors for preserving essential ecosystems in this country (Ortega-Rubio et al. 2015). The NPAs of mangrove forests in Mexico are usually regions with a high diversity of fauna, including abundant populations of crabs. Crab species are highly important to physical and chemical processes in mangrove sediments because of their ecological function in bioturbation activities (Theuerkauff et al. 2018). Another important aspect of these organisms in Mexico is that they are traditionally extracted from natural protected areas via the surrounding inhabitants for consumption purposes, especially during the rainy season (Galmiche-Tejeda and Solana-Villanueva 2011).
Metals and metalloids represent an important pollution threat to mangrove forests because of their potential toxicity, persistence, and bioavailability (Wu et al. 2014). Some metals are frequently existent in mangrove ecosystems due to their high affinity to organic matter and fine particles that commonly compound the sediments in these coastal ecosystems (Ranjan et al. 2018). It is important to consider in metal pollution assessments all potential sources that influence the exposure of key species to these pollutants. Leaf litter, sediments, and water are sources of metal exposure for primary consumers and detritivore organisms (Falusi and Olanipekun 2007). Once metals and metalloids enter the trophic web in coastal ecosystems, they become a potential risk for human health (Ghasemi et al. 2018). Crabs are commonly chosen in bioaccumulation studies due to their role as detritivores and their long-life cycle, participation in bioturbation processes, and abundance (Kristensen 2008). The bioaccumulation processes in organisms are related to their exposure to different concentrations of pollutants in the environment and the mechanisms behind the detoxification of each species (Prowe et al. 2006). The biota–sediment accumulation factors (BSAFs) are used to confirm the elements that are accumulated in the organisms according to the concentrations of elements present in the associated sediments, and they are especially helpful when the species of interest spend most of their life cycle in contact with this component (Deforest et al. 2007; Al-Farsi et al. 2015). Research on mangroves has increased during the last decades with the purpose of encouraging their conservation and improving their management plans. However, the variability and dynamics of metals along with the inherent complexity of mangrove forests have not been fully understood. The purpose of this study was to quantify the concentrations of Al, As, Ba, Cu, Cr, Fe, Ni, Pb, Sr, Si, V, and Zn in sediments, mangrove litterfall, and pore water and analyze the bioaccumulation of the same elements in the muscle tissue and shell of the crab species Cardisoma crassum to assess the impact of surrounding urban activities on the mangrove area “Estero El Salado” (Puerto Vallarta, Mexico). Moreover, the BSAFs were determined to confirm the elements that are accumulated in the shell and muscle tissues of C. crassum crabs, which are consumed by the local human population.
Materials and methods
Study area
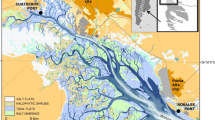
Estero El Salado is an estuary located in the state of Jalisco, which is in the city of Puerto Vallarta, Mexico. It is located on the Mexican Pacific coast and is important in the export of nutrients to Banderas Bay. This zone was declared a natural protected area by the congress of the state of Jalisco in 2000. Over the last four decades, this area has been surrounded by urban sprawl from Puerto Vallarta, which is a highly touristic site in Mexico. This has increased the vulnerability of this area due to a wide variety of urban activities. The NPA El Salado has a permanent connection with the ocean via a 20-m wide channel, which is the main input pathway for saline water. The temporal streams and surface runoff represent the freshwater input into the estuary. This area has an average annual temperature of 26–28 °C and an average precipitation of 931–1668 mm per year (García 1981).
The biodiversity of the El Salado estuary is high. It includes 100 species of birds (most of them are migratory), 30 species of reptiles, more than 10 species of mammals, and many fish species that have not been quantified yet. Most of the species of flora and fauna are endangered, and they are protected by the official Mexican norms (NOMS in Spanish). There are 125.6 ha of mangrove forest in this area, which is represented by three species: Rhizophora mangle (red mangrove), Laguncularia racemosa (white mangrove), and Avicennia germinans (black mangrove). The mangrove zone is bordered by saltmarsh and meadow zones. Moreover, El Salado has an abundant population of “mouthless crabs” (Cardisoma crassum), which is relevant in this study because they are traditionally consumed by the surrounded inhabitants (mainly during the rainy season) (observations of the “Consejo Técnico Asesor” at the NPA El Salado). According to the conservation council of the NPA El Salado, the population of C. crassum migrates to the coast during July and August for reproductive purposes. Crabs are more active at night and feed on the fresh or dried mangrove leaves of R. mangle and A. germinans, seeds, and fruit remains (observations of the “Consejo Técnico Asesor” at the NPA El Salado).
Collection and analysis of samples
Samples of mangrove litterfall (n = 24), pore water (n = 12), sediments (n = 24), and organisms of the species C. crassum (n = 24) were collected at two sampling sites (A and B). The sampling site in zone A is strongly influenced by the tide, and the sampling site in zone B is weakly influenced by the tide. The samples were collected during the rainy and dry seasons (the months of June and September, respectively). A 30-cm sediment core sampler was used to collect sediments and superficial mangrove litterfall samples with similar characteristics (e.g., low degradation, color, and size), and clean bottles were used to obtain the water samples. The organisms were collected using fishing nets and a cooler with ice. The organisms were collected according to the ethical specifications and recommendations of the Mexican official norm (NOM-126-ECOL-2000). All samples were transported to the laboratory in ice to preserve the samples (temperature of approximately 0 °C). The mangrove litterfall samples were cleaned with deionized water, and the samples were dried in a stove at 45 °C until they reached a constant weight (MacFarlane 2002). The sediment samples were also dried and then sieved (63-μm mesh plastic sieve) to separate the fine particles in the samples. The water samples were pretreated with nitric acid (HNO3) before transportation, and they were filtrated in a laboratory. Finally, the organisms were cleaned and dissected to obtain samples of the shell and muscle tissues. Muscle and shell samples were dried at 40 °C, and both types of samples were triturated in a porcelain mortar, homogenized, and stored in plastic bags in a dry and cool place (FAO/WHO 1984).
Sediment and water samples were digested using the 3051A and 3015A (US EPA) methods, respectively. For water samples, 5 mL of concentrated HNO3 and 1 mL HCl (38%) were added to 45-mL samples at a 130 °C ramp temperature for approximately 30 min. Sediments were treated by using 5-g samples and adding a mixture of 3 mL HNO3 and 10 mL HCl Instra (ASTM 1981).
The digestion method for the muscle tissue of organisms was first validated by comparing three digestion methods. Better recovery percentages (i.e., acceptable percentages between 80 and 120%) were obtained via a digestion method with 3 mL of HNO3 and HCl (Instra quality). DOLT-4 (NRC, Canada) certified reference material (CRM) was used during the quality control procedures. Digestion was performed using a microwave digestion system.
Shell samples were digested by using 0.5-g samples and adding 5 mL of H2O2 (30%) and 5 mL of concentrated HNO3. Sample digestion was performed on a hot plate (60–75 °C) for approximately 1 h.
The digestion of mangrove litterfall was also validated by comparing three analytical methods to obtain better recovery percentages: the mixtures of 3 mL of HNO3, 10 mL of HCl, and 1 mL of H2O2. The CRM used was 1573a tomato leaves (NIST, USA). Digestion was performed on a hot plate for approximately 12 h at 110 °C.
After digestion, all samples were filtered with Whatman filter paper no. 2 and diluted to a specified mark in 50-mL class A volumetric flasks (water samples were filtered in 100-mL volumetric flasks). To validate the analytical methods, the recovery percentages, linearity, limits of quantification (LOQs), and limits of detection (LODs) were calculated.
All samples were analyzed by Thermo Scientific iCAP™ 6000 inductively coupled plasma optical emission spectrometry (ICP-OES). The elements Al, As, Ba, Cu, Cr, Fe, Pb, Ni, Sr, Si, V, and Zn were determined in all samples. Pore water samples were analyzed using a low-concentration calibration curve via axial viewing. Sediments, mangrove litterfall, and tissue samples were analyzed using a low-concentration curve, and a higher concentration calibration curve was used for the elements Al, Fe, and Zn. The samples were analyzed at three repetitions for each sample. Multielemental standard solutions and CRMs were also included in the analysis.
Bioaccumulation factors
The bioaccumulation of the elements of interest in C. crassum was calculated. Bioaccumulation was determined by the proportion in which the total metal concentration was present in the organism in relation to a component of the ecosystem; in this case, it was the associated sediment. The biota–sediment accumulation factors (BSAFs) were calculated using the following formula (Falusi and Olanipekun 2007; Huang et al. 2008):
where CBiota and CSediment represent the mean concentrations of a metal (total form) in the organism and associated sediment, respectively.
Sediment quality guidelines
Sediment quality was determined using the effect range low (ERL) and effect range median (ERM) guidance values proposed by the National Oceanic and Atmospheric Administration (NOAA; Long and Morgan 1990) and the threshold effects limit (TEL) and probable effects limit (PEL) used by the Canadian sediment quality guidelines for the protection of aquatic life by the Canadian Council of Ministers of the Environment (CCME 2002).
Data analysis
A one-way analysis of variance (ANOVA) was performed to determine significant differences among sampling seasons in all of the environmental samples (i.e., water, sediment, and mangrove litterfall samples). Descriptive statistics and Pearson’s correlation test were also calculated for all elements within each type of sample, with a statistical significance level of α = 0.05. Finally, a cluster analysis based on single-linkage Euclidean distances was performed to obtain a better understanding of the association patterns among types of samples. The statistical analysis was performed using the software Statistica for Windows (Statsoft Inc., USA, 2015; version 13.0).
Results and discussion
Sediments
Table 1 presents the comparison of As, Cd, Cu, Pb, and Zn with the guidance values of ERL, ERM, TEL, and PEL. All of the elements did not exceed the ERL and ERM values, except for Cd, which slightly exceeded the ERL value during the dry season. Moreover, Cd was the only element that exceeded the TEL value during both sampling seasons. The relationships of Cu–Cd and Pb–Zn had the highest significant positive correlations (0.858 and 0.841, respectively; P < 0.05), while the relationships of Sr–Ba and Zn–Ba had significant negative correlations (− 0.831 and − 0.700, respectively; P < 0.05). A significant positive correlation represents a close association between the paired elements, which suggest that those elements may have common pathways. On the other hand, elements with high significant negative correlations could have an opposite distribution pattern in the sediment stratum. Concentrations found in sediment samples for each sampling season are presented in Table 2. In the sediment samples, As, Cr, and Ni concentrations were below the LOD, but the elements of Pb and Cd were only detected in this component. The descending order of concentrations for the elements found in the sediment samples was Al > Fe > Ba > Si > Sr > Zn > Pb > Cu > Cd at sampling site A, while at site B, the order was Al > Fe > Sr > Si > Zn > Ba > Cu > Pb > Cd.
The concentrations at sampling site B were slightly higher to those at site A for all elements analyzed (Table 2). V was the only element that presented significant differences between the sampling seasons (F = 4.98; P < 0.05).
Mangrove litterfall
Cr, Cd, Pb, and Ni were below the LODs in both sampling seasons and at both sites. The concentration order at site A was Fe > Al > Si > Sr > Ba > Zn > Cu > As > V, while the concentration order at site B was Fe > Al > Si > Sr > Zn > Ba > V > Cu. As was only detected at site A. Table 2 presents the concentrations of elements determined in mangrove litterfall at both sampling sites during the dry and rainy seasons. Only Cu (F = 7.67, P < 0.05) and V (P < 0.05) presented significant differences between sampling seasons. High concentrations of elements Fe and Al were found compared to the rest of the elements presented, which was consistent with the sediment samples. The highest positive significant correlations were obtained for Al–Fe and Al–V (0.877 and 0.853, respectively; P < 0.05), and there was a high negative significant correlation obtained for the pair V–Sr (− 0.803, P < 0.05).
Pore water samples
In the pore water samples, the elements As, Ba, Cd, Cr, Ni, Pb, and Zn were below the LOD, and Al and Cu were slightly above the LOQ. The order of concentrations was Si > Sr > V > Fe > Al > Cu at both sampling sites. There were no significant differences between the sampling seasons in the water samples (P > 0.05). The concentrations of elements determined in the water samples are presented in Table 2. There was only one positive significant correlation, which was in the relation of Cu–V (0.690, P < 0.05).
Tissues of Cardisoma crassum
Concentrations in muscle tissue
The elements As, Cd, Cr, Pb, and Ni were below the LOD. The concentrations found for each sampling season and sampling site are presented in Table 3.
The order of concentrations in the muscle samples was Zn > Si > Cu > Sr > Fe > Al > V > Ba at sampling site A; there were few differences at site B, but the concentrations of Zn, Si, and Cu were also remarkably high. Using only data from sampling site A, the elements of Al (F = 38.92; P < 0.05), Fe (F = 28.29; P < 0.05), Si (F = 50.68; P < 0.05), and Sr (F = 46.80; P < 0.05) presented significant differences between sampling seasons. The concentrations of Cu, Fe, Si, and Zn were high in the muscle tissue of C. crassum, and they are mostly essential elements and have important roles in the growth and physiological functions of crabs (Falusi and Olanipekun 2007). Moreover, Cu and Zn have been found in high concentrations in several studies of metals and trace elements for different crustacean species (Gbaruko and Friday 2007; Falusi and Olanipekun 2007; Kamaruzzaman et al. 2012).
The concentrations of Cr, Ni, and Pb were below the LOD; however, Ba, Sr, and V, which do not have specific roles in the enzymatic processes of crabs, were detected in all the organisms.
The concentrations of V have been determined in some crustacean species (Ikemoto et al. 2008; Iwuoha and Onojake 2016). The concentrations of V are more likely to be related to anthropogenic activities in the study area (e.g., steel processes and the combustion of fossil fuels). Most elements detected during the analysis are not regulated by any international guidelines and do not have maximum permitted limits. Nevertheless, there are some regulations for Cu and Zn in crabmeat, such as the concentration ranges specified by the FAO in 1984 (20–70 mg kg−1 for Cu and 40–150 mg kg−1 for Zn). Moreover, Malaysia has a maximum permitted limit of 30 mg kg−1 for Cu, and the same limit has been established in Brazilian regulations (Neves et al. 2011), while Zn has a maximum permitted limit of 100 mg kg−1 in Malaysian regulations (1985).
Concentrations in shells
The concentrations found in shells are presented in Table 4. The elements below the LOD were the same as in the muscle tissue samples. The sequence of concentrations found at both sampling sites was Sr > Ba > Si > Al > Fe > V > Zn > Cu, with remarkably high concentrations of Sr (approximately one order of magnitude higher than the concentrations found in the muscle tissue samples). Ba, Si, and Sr presented the highest concentrations in the shells of C. crassum. In the shell, there were significant differences between the sampling seasons for most of the elements analyzed: Al (F = 21.68; P < 0.05), Ba (F = 5.33; P < 0.05), Cu (F = 6.58; P < 0.05), Fe (F = 35.04; P < 0.05), Sr (F = 13.27; P < 0.05), V (F = 114.2; P < 0.05), and Zn (F = 9.03; P < 0.05). Si was the only element that did not present significant differences between sampling seasons.
It has been observed that C. crassum organisms molt during the rainy season (the observations of management workers at the Estero El Salado estuary). Therefore, metal concentrations were expected to be higher during the dry season due to the decrease in metal concentrations during the molting process (Engel and Brouwer 1987; Bergey and Weis 2007). This effect was only observed for the elements Al, Cu, Fe, and V.
The analysis of shell samples is useful to have a better understanding of the capacity of this structure as a potential biosorbent. The shells of some crab species have shown efficiency as a biosorbent, specifically when removing dissolved As, Cd, and Cu from contaminated water (Aris et al. 2014); however, in this study, the concentrations determined for Cu in the shells were low, and the As and Cd concentrations were below the LOD.
Bioaccumulation
In this study, the bioaccumulation factors were calculated by using the concentrations of elements analyzed in the muscle tissues and shells of C. crassum, which was related to the concentrations of the same elements in the associated sediment, by considering that the organisms of this species spend most of their biological cycle on land due to their roles as detritivores. The BSAFs calculated for muscle tissues and shells are presented in Fig. 1.
The accumulation of elements is confirmed when the BSAFs exceed the unit value (Vrhovnik et al. 2013; Jitar et al. 2015).
In the muscle samples, the BSAFs calculated for Cu and Zn were notably above the unit value. Cu had mean BSAF values of 5.70 and 3.27 at sites A and B, and the values calculated for Zn at both sampling sites were 9.37 and 6.29, respectively. The elements Si and Ba were also above the unit value, but the values obtained for Si were slightly above this limit, and Ba only exceeded the unit at site B, with a value of 3.13.
In the shell samples, the elements of Ba and Sr had mean BSAF values above 1 at both sampling sites. The BSAF values calculated for Ba and Sr at site A were 7.84 and 14.83 and 2.9 and 6.07 at site B, respectively. The Si BSAF values were slightly above the unit value at both sites, which was consistent with the BSAF values for muscle. V only exceeded the unit value at sampling site B, with a value of 2.73.
Most of the elements remained below the unit value, indicating that C. crassum is not an effective accumulator of these elements. The elements that presented high BSAF values also showed significant correlations in the sediments (Sr–Ba = − 0.831 and Zn–Ba = − 0.700; P < 0.05), which indicated the influence of sediments on the accumulation of these elements in the organisms.
According to the BSAF values obtained, C. crassum seemed to be an effective accumulator of Cu and Zn in the muscle tissue, which has been observed in different species by other authors (Gammon et al. 2009; Shi et al. 2015). These elements are well regulated by the organisms; they have defined enzymatic functions in crustaceans, and they are found in muscle tissue at similar proportions (Depledge and Rainbow 1990; Wu and Chen 2004). Also, it is well known that the intake of Cu and Zn in crustaceans (and most crab species) is high due to the use of these elements for the synthesis of hemocyanin, which is a copper-containing respiratory protein (Depledge and Rainbow 1990). Moreover, these elements are essential trace elements in human nutrition, which are important to the proper functioning of organs and metabolic processes. Therefore, the accumulation of these elements in the muscle tissue of C. crassum does not represent a relevant risk to human health.
On the other hand, the shell tissue of C. crassum clearly accumulates Ba and Sr. These elements, along with Ca, have been involved in several studies on marine organisms (Gillikin et al. 2006). Moreover, many authors have pointed out the biochemical similarities between Sr and Ca. The association of Ca–Sr in calcium carbonate structures for different fish species has been observed in several studies. Sr concentrations have been determined in the dorsal spines and operculum of fish, and they have also been found in the structures of corals (Weber 1973; Pollard and Kingsford 1999). It has been suggested that aquatic organisms might assimilate Sr when Ca concentrations are low (Pollard and Kingsford 1999). Ca was not an element of interest in this study, but the concentrations of Ca were determined in the shells to evaluate its correlation with Sr. The correlation between Ca and Sr in the shell samples of C. crassum was significantly high, with a value of 0.887 (P < 0.05).
Cluster analysis
The cluster analysis is presented in Fig. 2 based on the single linkage Euclidean distance. The cluster analysis includes the 13 elements analyzed in all types of samples collected at the two sampling sites during the dry and rainy season. The clustering patterns indicated the grouping of each type of sample based on the different sampling sites and seasons. The organism samples were grouped along the left side, while sediment samples were group along the right side, which was probably due to their higher concentrations. The cluster analysis showed that the metal concentrations at site B during the dry season grouped the rest of the concentrations in each type of sample, while the concentrations of the sediment samples grouped the other types of samples. These patterns confirm the influence of sediments on metal concentrations in other components of mangrove ecosystems. Moreover, mangrove litterfall concentrations at site A during the rainy season appeared separately along the right side from groups with different sample matrices, which is probably due to the high variability in the concentrations within this type of sample.
Conclusions
An estuary has maritime and continental influences, which is clearly reflected in most of the elements determined in all of the environmental samples analyzed. Nevertheless, elements such as Pb and Cr have been widely released into the environment due to anthropogenic activities near the study area. The presence of these elements is relevant in natural protected areas due to their high toxicity, particularly in mangrove ecosystems with vulnerable species, such as migratory birds. However, in this study, Pb and Cd concentrations were only detected in the sedimentary phase, which reflects the importance of the stratum as a trap for pollutants. Cd was the only element that exceeded the threshold effect limit (TEL) during both sampling seasons according to the NOAA and CCME sediment quality guidelines. Al and Fe presented the highest concentrations at both sampling sites in the sediments and mangrove litterfall, which shows that these ecosystem components are not very affected by the influence of tide. This finding is supported by the results obtained from the pore water sample analysis, which showed no significant differences (P < 0.05) in this type of sample between the sampling seasons, and half of the elements analyzed remained below the LODs.
According to the high concentrations and stability (i.e., the presence of elements during both sampling seasons at both sites) of the elements determined in the sediments, this component is the principal source for the exposure of C. crassum to metals and metalloids in the study area, while mangrove litterfall and pore water were less relevant sources. This fact is clearly reflected in the cluster analysis, which confirms the influence of sediments on metal concentrations in other components of the mangrove ecosystem. The BSAFs calculated for Cu and Zn were above the unit value in the muscle samples, which indicates that C. crassum is a good bioaccumulator of these elements in muscle tissue. Cu and Zn are essential elements in human nutrition and do not represent a risk to human health. Ba, Si, and Sr showed bioaccumulation in the shells of C. crassum, and the concentrations of Sr in the shell tissue were remarkably high, with BSAF values of 14.83 and 6.04 at sampling sites A and B, respectively.
References
Al-Farsi, A. H., Sulaiman, H., & Al-Reasi, H. A. (2015). Metal transfer from marine coastal sediment to food chain: evaluating Strombus (Conomurex) persicus for monitoring metal bioaccumulation. Procedia Environmental Sciences, 28, 37–44.
Aris, A. Z., Ismail, F. A., & Praveena, H. Y. (2014). An experimental and modelling study of selected heavy metals removal from aqueous solution using Scylla serrata as biosorbent. Pertanika, Journal of Science and Technology, 22(2), 553–566.
ASTM D 3974. (1981). Standard practices for extraction of trace elements from sediments (pp. 393–395). West Conshohocken: ASTM International.
Barbier, E. B. (2016). The protective service of mangrove ecosystems: a review of valuation methods. Marine Pollution Bulletin, 109(2), 676–681.
Bergey, I., & Weis, J. (2007). Molting as a mechanism of depuration of metals in the fiddler crab, Uca pugnax. Marine Environmental Research, 64(5), 556–562.
Brodie, J. E., Kroon, F. J., Schaffelke, B., Wolanski, E. C., Lewis, S. E., Devlin, M. J., Bohnet, I. C., Bainbridge, Z. T., Waterhouse, J., & Davis, A. M. (2012). Terrestrial pollutant runoff to the Great Barrier Reef: an update of issues, priorities and management responses. Marine Pollution Bulletin, 65(4–9), 81–100.
Canadian Council of Ministers of the Environment (2002) Canadian sediment quality guidelines for the protection of aquatic life. Available at: http://www.ccme.ca/assets/pdf/e1_06. Accessed 20 Dec 2017.
CONABIO National Commission for the Knowledge and Use of Biodiversity (2009) Mangroves of Mexico: Extension and distribution, 2, 99.
Deforest, D. K., Brix, K. V., & Adams, W. J. (2007). Assessing metal bioaccumulation in aquatic environments: the inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquatic Toxicology, 84, 236–246.
Depledge, M. H., & Rainbow, P. S. (1990). Models of regulation and accumulation of trace metals in marine invertebrates. Comparative Biochemistry and Physiology, 97(1), 1–7.
Engel, D. W., & Brouwer, M. (1987). Metal regulation and molting in the blue crab, Callinectes sapidus: metallothionein function in metal metabolism. The Biological Bulletin, 173(1), 239–251.
Falusi BA, Olanipekun EO (2007) Bioconcentration factors of heavy metals in tropical crab (Carcinus sp.) from river Aponwe, Ado-Ekiti, Nigeria. World Bank assisted National Agricultural Research Project (NARP)—University of Port Harcourt, 11, 51–54.
FAO/WHO. (1984). List of maximum levels recommended for contaminants by the Joint FAO/WHO Codex Alimentarius Commission. Second Series (Vol. 3, pp. 1–4). Rome: CAC/FAL.
Food and Agriculture Organization of the United Nations (2007a) The world’s mangroves 1980–2005. Rome, Italy, Nations Forestry Paper 153.
Food and Agriculture Organization of the United Nations (2007b) Mangroves of North America and Central America 1980–2005. Forest Resources Assessment Programme, Working Paper 137.
Galmiche-Tejeda A, Solana-Villanueva N (2011) The context of vulnerability of coastal populations and the designed values to mangroves in Tabasco, Mexico. Secretaria de Recursos Naturales y Protección Ambiental. Colegio de Postgraduados Campus Tabasco. Villahermosa, Tabasco, México, p. 140.
Gammon, M., Turner, A., & Brown, M. T. (2009). Accumulation of Cu and Zn in discarded antifouling paint particles by the marine gastropod, Littorina littorea. Estuarine, Coastal and Shelf Science, 84(4), 447–452.
García E (1981) Modifications to the climate system classification of Köppen. Geographic Institute, National Autonomous University of Mexico, p. 252.
Gbaruko, B. C., & Friday, O. U. (2007). Bioaccumulation of heavy metal in some fauna and flora. International Journal of Environmental Science Technology, 4(2), 197–202.
Ghasemi, S., Moghaddam, S. S., Rahimi, A., Damalas, C. A., & Naji, A. (2018). Ecological risk assessment of coastal ecosystems: the case of mangrove forests in Hormozgan Province, Iran. Chemosphere, 191, 417–426.
Gillikin, D. P., Dehairs, F., Lorrain, A., Steenmans, D., Baeyens, W., & Lucé, A. (2006). Barium uptake into shell of the common mussel (Mytilus edulis) and the potential of estuarine paleo-chemistry reconstruction. Geochimica et Cosmochimica Acta, 70(2), 395–407.
Huang, P. C., Tien, C. J., Sun, Y. M., Hsieh, C. Y., & Lee, C. C. (2008). Occurrence of phthalates in sediment and biota: relationship to aquatic factors and the biota-sediment accumulation factor. Chemosphere, 73(4), 539–544.
Ikemoto, T., Tu, N. P. C., Okuda, N., Iwata, A., Omori, K., Tanabe, S., Tuyen, B. C., & Takeuchi, I. (2008). Biomagnification of trace elements in the aquatic food web in the Mekong Delta, South Vietnam using stable carbon and nitrogen isotope analysis. Archives of Environmental Contamination and Toxicology, 54(3), 504–515.
Iwuoha GN, Onojake MC (2016) Bioaccumulation of heavy metals in crustaceans from Oron river channel, Osung area Oron-city, Akwa-Ibom, Nigeria. Journal of Chemical Society of Nigeria 41(1).
Jitar, O., Teodosiu, C., Oros, A., Plavan, G., & Nicoara, M. N. (2015). Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. Biotechnology, 32(3), 369–378.
Kamaruzzaman, B. Y., Akbar, J. B., Maryam, B. Z., Jalal, K. C. A., & Shahbuddin, S. (2012). Bioaccumulation of heavy metals (Cd, Pb, Cu y Zn) in Scylla serrata (Forsskal, 1775), collected from Sungai Penor, Pahang, Malaysia. Journal of Tropical Agriculture Science, 35(1), 183–190.
Kristensen, E. (2008). Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. Journal of Sea Research, 59, 30–43.
Laegdsgaard, P., & Johnson, C. (2001). Why do juvenile fish utilise mangrove habitats? Journal of Experimental Marine Biology and Ecology, 257(2), 229–253.
Liquete, C., Piroddi, C., Drakou, E. G., Gurney, L., Katsanevakis, S., Charef, A., & Egoh, B. (2013). Current status and future prospects for the assessment of marine and coastal ecosystem services: a systematic review. PLoS One, 8(7), 67737.
Long ER, Morgan LG (1990) The potential for biological effects of sediment-sorbed contaminants tested in the national status and trends program. In: National Oceanic and Atmospheric Administration Technical Memorandum. NOS OMA. p. 52.
MacFarlane, G. R. (2002). Leaf biochemical parameters in Avicennia marina (Forsk.) Vierh as potential biomarkers of heavy metal stress in estuarine ecosystems. Marine Pollution Bulletin, 44, 244–256.
MacFarlane, G. R., & Burchett, M. D. (2000). Cellular distribution of Cu, Pb and Zn in the grey mangrove Avicennia marina (Forsk.) Vierh. Aquatic Botany, 68, 45–59.
Malaysian Food Regulations. (1985). In Hamid Ibrahim, Nasser and Yap ThiamHuat. Malaysian law on food and drugs. Kuala Lumpur: Malaysia Law Publisher.
Neves, R. C. F., Ferrari, J. E., Morales, P. M., & Padilha, P. (2011). Levels of copper in Nile tilapia from Brazil. Food Additives & Contaminants: Part B, 4(4), 238–243.
Ortega-Rubio A, Pinkus-Rendón MJ, Espitia-Moreno IC (2015) Natural protected areas and scientific research in Mexico. In: Centro de Investigaciones Biológicas del Noroeste S. C., La Paz B. C. S. University Autonomous of Yucatán and University of Michoacana de San Nicolás de Hidalgo. pp. 41–64.
Pollard, M. J., & Kingsford, M. J. (1999). Chemical marking of juvenile snapper, Pagrus auratus (Sparidae), by incorporation of strontium in dorsal spines. The Fishery Bulletin, 97, 118–131.
Prowe, F., Kirf, M., & Zauke, G. P. (2006). Heavy metals in crustaceans from the Iberian deep sea plain. Marine Sciences, 70, 271.
Ranjan, P., Ramanathan, A. L., Kumar, A., Singhal, R. K., Datta, D., & Venkatesh, M. (2018). Trace metal distribution, assessment and enrichment in the surface sediments of Sundarban mangrove ecosystem in India and Bangladesh. Marine Pollution Bulletin, 127, 541–547.
Romañach, S. S., DeAngelis, D., Koh, H. L., Li, Y., Teh, S. Y., Raja-Barizan, R. S., & Zhai, L. (2018). Conservation and restoration of mangroves: Global status, perspectives, and prognosis. Ocean and Coastal Management, 154, 72–82.
Shi, R., Lin, J., Ye, Y., Ma, Y., & Cai, M. (2015). The level and bioaccumulation of Cd, Cr, Cu and Zn in benthopelagic species from the Bering sea. Acta Ocanologica Sinica, 34(6), 21–25.
Spalding, M., Kainuma, M., & Collins, L. (2010). World atlas of mangroves. London: Earthscan ISBN 978-1-84407-657-4.
Theuerkauff, D., Rivera-Ingraham, G., Mercky, Y., Lejeune, M., Lignot, J. H., & Sucré, E. (2018). Effects of domestic effluent discharges on mangrove crab physiology: integrated energetic, osmoregulatory and redox balances of a key engineer species. Aquatic Toxicology, 196, 90–103.
Vrhovnik, P., Arrebola, J. P., Serafimovski, T., Dolenec, T., Šmuc, N. R., Dolenec, M., & Mutch, E. (2013). Potentially toxic contamination of sediments, water and two animal species in Lake Kalimanci, FYR Macedonia: Relevance to human health. Environmental pollution, 180, 92–100.
Waycott, M., Duarte, C. M., Carruthers, T. J., Orth, R. J., Dennison, W. C., Olyarnik, S., Calladine, A., Fourqurean, J. W., Heck, K. L., Hughes, A. R., & Kendrick, G. A. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences, 106(30), 12377–12381.
Weber, J. N. (1973). Incorporation of strontium into reef coral skeletal carbonate. Geochimica et Cosmochimica Acta, 37(9), 2173–2190.
Wu, J. P., & Chen, H. (2004). Effects of cadmium and zinc on oxygen consumption, ammonium excretion and osmoregulation of white shrimp (Litopenaeus vannamei). Chemosphere, 57, 1591–1598.
Wu, Q., Tam, N. F., Leung, J. Y., Zhou, X., Fu, J., Yao, B., ... & Xia, L. (2014). Ecological risk and pollution history of heavy metals in Nansha mangrove, South China. Ecotoxicology and environmental safety, 104, 143–151.
Acknowledgements
This research was supported by CONACYT (Consejo Nacional de Ciencia y Tecnología). We gratefully acknowledge CIMAV (Centro de Investigación en Materiales Avanzados) for providing laboratory equipment for the analysis of samples. We thank biologist Jaime Torres and our colleagues from the natural protected area “Estero El Salado” for their assistance during the collection of samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodríguez-Saldaña, V., González-Farías, F. & Miranda-Navarro, S.V. Bioaccumulation of metal(loid)s in Cardisoma crassum and pollution assessment in a mangrove protected area in Mexico. Environ Monit Assess 190, 732 (2018). https://doi.org/10.1007/s10661-018-7114-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-7114-4