Abstract
This study aims to evaluate the ecological risk and distribution of heavy metals in sediment, plants and fish in a seriously polluted water reservoir in Krompachy, Slovakia. Special attention was given to the different food web positions of individual fish species (predators, omnivores) and their size. The degree of heavy metal contamination in sediments decreased in the order Cu > Pb > Cr > Hg > Cd, and their mutual proportion was largely consistent with concentrations found in aquatic plants, i.e. water sedges (Carex acutiformis). Of the seven fish species investigated, piscivorous perch (Perca fluviatilis) accumulated higher quantities of metal than fish situated at lower trophic levels. Interestingly, co-equal levels of heavy metals to those found in perch (P. fluviatilis) also occurred in rudd (Scardinius erythrophthalmus). The Hg values in some fish muscles exceeded the maximum permissible limits suggesting a persistent problem of old environmental burden from former mining activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Contaminants, such as heavy metals, are of great environmental health concern. Many of them have genotoxic-mutagenic effect, which is sometimes induced already at relatively low concentrations (Šestinová and Findoráková 2017). Due to increasing anthropic activities, heavy metals are generated in environments and disrupt the continuity of essential natural processes. Heavy metals present at the bottom of water basins or the surrounding environment can reach significant concentrations due to their resistance to natural degradation over time (Pliešovská et al. 1997), and such sediments represent a large pool of these risky contaminants. Aquatic plants having permanent contact with the sediment through their roots absorb high amounts of heavy metals from the bed material, and they may therefore reflect the toxicity of the surrounding environment quite well (Jamnická et al. 2006). A polluted wildlife habitat providing food for fish and other aquatic animals directly facilitates the gradual accumulation of heavy metals in their bodies through the prey relationships in aquatic food webs (Demirak et al. 2006; Richardson and Kimura 2017). Fish represent the top consumers on a trophic pyramid in aquatic ecosystems, and as a consequence, they are highly endangered by diet-borne pollutants. Bioaccumulation of heavy metals in fish depends on various physiological and ecological factors (Pourang 1995). Among them, specific differences between individual fish species, like feeding behaviour, play an especially important role in the heavy-metal uptake (Dallinger et al. 1987). Other biological factors also affecting the contaminant magnification in fish include the growth rate, age, sex and habitat preferences of individual species (Pourang 1995). Ecological needs and the size of fish have also been found to affect their predisposition to metals accumulation (Yi and Zhang 2012).
Areas of extensive metallurgy, mining and mineral processing of iron and copper ores are the most heavily polluted places in eastern Slovakia. The highest levels of pollution in this region come from the former copper smelter near the town of Krompachy (Hančuľák et al. 2014; Musilová et al. 2016; Šalamún et al. 2017; Demková et al. 2017). Even though, the metal transfer among individual food web components has been attracting scientists for a long time (e.g. Demirak et al. 2006; Cui et al. 2011; Weber et al. 2013), it has never been studied in this model locality. Hence our broad goal was to study the associations of selected heavy metals between the sediment, water plants and fish in a small water reservoir located near a mineral processing plant known for its long-lasting copper pollution. The closer aim was to evaluate the amount of metal in fish belonging to different trophic groups. Interrelations between metal accumulation and fish size were also considered.
Material and Methods
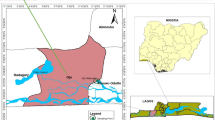
The research was conducted in the territory of the central Spiš, a region of north-eastern Slovakia historically known for intensive mining and ore processing activities. The studied reservoir (48°55′21.6" N, 20°52′07.6" E) is located in the town of Krompachy, near the former metallurgical plant Kovohuty Co., which processed raw materials for copper production with several breaks from 1843 (Musilová et al. 2016).
In the period between May 2014 and April 2016, 15 sediment samples of approximately 500 g were taken from 10 sampling sites in the reservoir using a dip sampler (TeleScoop, stainless steel V2A, capacity of 1000 ml), according to STN ISO 5667–12. In addition, 10 samples of rooted aquatic vascular plants (the swamp sedge Carex acutiformis) were collected from the same sampling sites. Each sample contained the whole plant, i.e. perennial organs located in the sediments (roots) and ephemeral parts submerged in water (leaves and the stem). The individual samples (sediment, plant tissues) were stored separately in plastic bags at 4 °C until analysed for the presence of heavy metals. During the same period, 45 fish of seven species and two families (Cyprinidae, Percidae) were caught in the reservoir (Table 1). Fishes were transported to the laboratory alive, then killed by severing the spinal cord and weighed and measured and examined for heavy metals. In total, 126 tissue samples of muscle, liver (hepatopancreas in cyprinids) and kidney were taken from the fish and stored individually in plastic bags at − 20 °C until further processing for trace elements. The scientific and common names of the fish and their major food items were stated according to the FishBase database (Froese and Pauly 2020) and Baruš and Oliva (1995).
Sediment, plant and fish samples were analysed for Cd, Cr, Cu, Hg and Pb (Tables 2 and 3) using the methods described by Šalamún et al. (2017) and Findoráková et al. (2017). The total concentration of heavy metals in the sediment was determined by X-ray fluorescence (SPECTRO XEPOS, Spectro). To assess the XRF analysis accuracy we used the certified river sediment reference material LGC6187 as the standard at the beginning and end of the measurements. The accumulation of metals in plant samples (separately in roots and stems with leaves) and fish tissues (muscle, liver, and kidney) was determined by ETA-AAS (Zeenit 700P, Analytik Jena) after microwave digestion of all tissues separately in an HNO3:H2O2 mixture in a closed system (Ethos One, Milestone). For assessment of the Hg concentration, samples of plants and an aliquot of approximately 300 mg of wet fish tissues were analysed using the hydride generation atomic absorption spectrometry (HG AAS) method. To assess the AAS analysis accuracy, we used the certified reference material NCS ZC 73,032 (Celery) for plant samples and CBW10018 (Chicken) for the individual fish samples as standards. Each determination was replicated three times. The overall recovery rates (mean ± SD) of Cd, Cr, Cu, Hg and Pb were 98 ± 2.4, 93 ± 2.6, 90 ± 3.4, 102 ± 4.3 and 95.4 ± 2.1 respectively.
The basic descriptive statistics are given for the heavy metal concentration in sediments, plant samples and fish tissues. Non-detected values of the heavy metal concentration in fish tissues (about 13%) and plant samples (below 7%) were replaced by zero. The detection limits set for individual heavy metals were as follows: Pb – 8.7 µg/L; Cr – 0.85 µg/L; Cd – 0.77 µg/L; Cu – 1.65 µg/L and Hg – 1.02 µg/L. Considering the number of samples and the fact that data did not meet the normal distribution, nonparametric statistical methods were applied using the Statistica software (StatSoft Inc. 2013). The Spearman’s rank coefficient was calculated to test relations between the heavy metal concentration in the sediment and plant roots and the metal concentration in individual fish organs and the fish size (weight and total length). The correlation between heavy metals in fish organs and the fish size was calculated for three fish species: Common carp (n = 5), European perch (15) and Roach (15). Another fish species did not meet the required number for statistical analysis. Only significant relations (positive or negative) are shown in Table 4. Differences between metal concentrations in sediments, roots and stems with leaves were analysed using a Wilcoxon paired test and Friedman ANOVA with consequent Dunn’s multiple post-hoc comparisons test. Differences among metal concentrations in fish tissues were tested for individual fish species using ANOVA χ2. Kendall’s coefficient of concordance was used to assess similarities/dissimilarities in the mode of metal accumulation by fish organs. Differences in metal concentrations established in tissues of the two dietary fish groups (piscivores and omnivores) were tested using the Kolmogorov–Smirnov test for cumulative distribution and Mann–Whitney test for median.
Results and Discussion
The heavy metal concentrations detected in sediments and two parts of sedges (root and stem with leaves) are summarized in Table 2. Concentrations of heavy metals significantly differed between sediments, roots and stems (Friedman ANOVA, P < 0.05, Table 2). Copper was found to be the predominant element in the reservoir sediments, followed by Pb, Cr, Hg and Cd, respectively. Concentrations of Cu (427.7 ± 117 µg·g−1 dry weight) and Hg (72.7 ± 62.4 µg·g−1 dry weight) were more than six and seven-times, respectively higher than the maximum permissible concentrations in sediments (MPC for Cu = 73 µg·g−1; Hg = 10 µg·g−1 dry weight) set under the Guidance document (MP SR 1998). The results showed repeat-ability within 5%. The RSDs did not exceed 2%. In many ways, the present data correspond to results reported previously by Šalamún et al. (2012); Musilová et al. (2016) and Findoráková et al. (2017). Those studies also indicated Cu as the predominant element, followed by Pb, Cr and Cd in water, sediments and surrounding soils.
It is well documented that Hg and Cd represent the highest risk in biota because of their rapid and very effective accumulation in the food chain (Ravera 2001; Musilová et al. 2016; Ali et al. 2019). Our results confirm this fact, since the highest accumulation rate was observed for cadmium (Table 2). The positive correlation between concentrations of Cd in sediment and plant roots of the sedge Carex acutiformis (R = 0.8; p < 0.05) demonstrated the very effective penetration of Cd from sediments to plants (Das et al. 1997).
Heavy metal concentrations detected in the muscle, liver and kidney of individual fish are presented in Table 3 and show a predominance of Cu in the organs of almost all fish species. However, significant differences were shown in the remaining heavy metals accumulated in individual tissue samples (ANOVA χ2, p < 0.001, n = 45). Some metals showed a strong tendency towards specific tissues of fish (Table 3). Concentrations of Cd, Cr and Pb were mostly highest in the kidney; the highest overall concentrations of Cd and Cr were present in the kidney of perch (0.509 ± 0.94 and 27.4 ± 44 μg·g−1, respectively), and the largest amounts of Pb were established in the kidney of rudd (2.48 μg·g−1). The liver was found to be a key target organ for Cu accumulation, with the maximum concentrations in rudd (25.4 μg·g−1). Mercury was amassed in fish organs in a nearly similar proportion, with the highest values attained in the liver and kidney of rudd (0.53 and 0.52 μg·g−1, respectively). Kendall’s coefficient of concordance revealed that Cd (n = 45; r = 0.737) and Cu (n = 45; r = 0.857) were accumulated in all fish organs in a similar way. The different accumulation capacity of toxic elements in the investigated fish organs can be attributed to the specific features of the tissues. While the liver and kidney are considered metabolically active organs, playing an important role in heavy metal storage, redistribution, detoxification and elimination of many harmful substances from the body (Al-Yousuf et al. 2000), muscle represents one of the least metal-containing tissues with minimum metabolic activity (Allen-Gil and Matynov 1995; Farkas et al. 2002). The preferential accumulation of heavy metals in different organs of fish has been confirmed many times (see Pourang et al. 1995; Brázová et al. 2012; Jia et al. 2017).
Piscivorous perch situated at the top trophic level and, surprisingly, also the rudd occupying the level below, possessed the highest quantities of heavy metals in their organs. Terra et al. (2008), who studied heavy metal distribution in the muscle and gonads of carnivores, omnivores and detrivorous fish in the River Paraíba do Sul in Brazil, found that the highest concentrations of heavy metals occurred in carnivores, which is similar to our results.
Cyprinids, such as carp, goldfish and bream with rather similar ecology (benthic) and food preferences (plant material, benthic invertebrates, plankton; Froese and Pauly 2020) of rudd, accumulated much lower amounts of metals. Rudd may be considered as an omnivorous fish, with an evident preference for the plant food, particularly in older fish (Baruš and Oliva 1995). Our findings revealed that heavy metal concentrations in swamp sedge corresponded well with those assessed in the liver and kidney of rudd. This relationship was the most observable in the case of toxic Pb. Because we investigated only adult species of rudd expected to prioritize plant food (macrophytes), we assumed that rudd biomagnified heavy metals mainly via ingestion of plants. Data by Baruš and Oliva (1995), who analysed the intestinal content of rudd, could support this idea. Another explanation could be that the local contamination might play a more important role in metal magnification than the feeding habits of fish (Terra et al. 2008).
The pollutant loads in the muscle, liver and kidney of two fish groups are presented in Fig. 1. Mean heavy metal concentrations in the muscle tissue of piscivores and omnivores were rather similar. The most pronounced differences among different fish groups in heavy metal content were observed in the Cd content in fish liver (Figs. 1a, b). An evident progressive decline of this element was seen across the food chain from the highest rates in piscivores (0.348 ± 0.34 μg·g−1) towards significantly lower values in omnivores (0.079 ± 0.11 μg·g−1) (Mann–Whitney test, p < 0.05) (Fig. 1a). A further notable difference was detected between predators and omnivorous fish, in the kidney of which concentrations of Cr attained 24.9 ± 43.3 and 1.09 ± 1.53 μg·g−1, respectively (Kolmogorov–Smirnov test p˂0.05; Fig. 1a, 1b). Jia et al. (2017) investigated the storage capacity of muscle, liver and gills for heavy metals in three fish species of different trophic positions from the Xiang River near the city of Changsha in southern China. The piscivorous Pelteobagrus fulvidraco amassed much larger amounts of As, Cd and Pb than the omnivorous Carassius auratus and Squaliobarbus curriculus in all organs. They explained the disparity in various organ accumulation capacities by the specific morpho-anatomical and functional features of fish tissues. Other studies, e.g. Kenšová et al. (2010), reported the opposite data (higher Cd concentrations in non-predatory fish).
Boxplot of the pollutant load (μg.g−1 wet weight) in the muscle, liver and kidney of piscivorous (N = 17, grey box) and omnivorous (N = 28, white box) fish groups. Significant differences between medians are indicated by asterisk from Mann–Whitney test (p ˂ 0.05). Lines in box refer to the median; filled diamonds represent the arithmetic mean, and circles indicate outliers
Spearman’s rank correlation coefficient was used to describe the relationships between fish size (weight and total length) and degree of heavy metal accumulation in individual fish organs (Table 4). A significant increase of Hg concentrations in the muscle of perch (r = 0.726 L and 0.706 W; p < 0.005) and carp (r = 0.975 L/W; p < 0.005) with an increasing of their size were observed (Table 4). The increasing mercury levels with the fish (perch and carp) size could be explained to the high bioaccumulation capacity of this toxic element. Several studies (Burger and Gochfeld 2007; Cui et al. 2011; Zrnčić et al. 2013) clearly documented a positive relationship between mercury levels and animal sizes. Mercury is known as a serious human toxic agent, and the consumption of fish is significant source of Hg in animals and humans (Zhang et al. 2007). Cui et al. (2011) analysed trophic transfer of heavy metals for food webs in water, sediment, primary producers, invertebrates, fish and water birds in the newly-formed wetlands of the Yellow River Delta, China. They found that the trophic transfer is a predominant way for Hg accumulation in the higher trophic levels. In our research, particularly high concentrations of Hg in muscles were observed in all fish species, with the maximum values in roach flesh (0.352 ± 0.3 μg·g−1) (Table 3). The maximum levels for Hg in fish muscles (0.5 μg·g−1 wet weight) admitted in foodstuffs in European countries (Commission Regulation No 78/2005 for crustaceans, fish and fishery products) were slightly exceeded in seven individuals of benthic roach (0.51 – 0.79 μg·g−1) and four carnivorous perch (0.51 – 0.602 μg·g−1), respectively. In this respect, our data are of importance, because they indicate a potential harmful effect of Hg on human health of the local population.
The concentration of lead in the kidney also increased with increasing size of carp (r = 0.900 L/W; p < 0.05). Several authors (Wang and Rainbow 2008, Liu et al. 2015) also have stated that the main source of Pb is water uptake through fish gills rather than food, mainly due to the metal-binding sites located at the gill surface. Based on the findings of previous researches (e.g. Yi and Zhang 2012; Liu et al 2015) we assume, that a long lasting effect of higher Pb concentrations in surrounding water might result in the continued metal accumulation in fish and thus increase of Pb levels with fish age (and size). Opposite trend was shown in the muscle, liver and kidney of roach, where the concentrations of Pb were negatively correlated with fish weight but not with length. The negative relationships between fish size and heavy metal concentration (found in Cd, Cr, Cu and partly also in Hg) in perch and roach organs (see Table 4) were also observed in present study. Several earlier studies documented that the metabolic activity of younger fish is higher while accumulating more heavy metals than the older ones (e.g. Nussey et al. 2000; Canli and Atli, 2003). Canli and Atli (2003) who studied the relationships between heavy metal levels and the size of six Mediterranean fish species came to a conclusion that the negative relationships between fish size and metal levels in fish organs is a result of the difference between uptake and elimination of heavy metals and the fishes may control their tissue metal values with the growth.
Our results clearly demonstrate a persisting problem with old heavy metal loads in this locality. Above-limit concentrations of Cu and toxic Hg in the reservoir sediments as well as in some fish species indicate an effective transfer of heavy metals through the analysed water ecosystem compartments posing a potential harmful effect on human health.
References
Ali H, Khan E, Ilahi I (2019) Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J Chem 2019:14
Al-Yousuf MH, El-Shahawi MS, Al-Ghais SM (2000) Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci Total Environ 256:87–94
Allen-Gil SM, Martynov VG (1995) Heavy metal burdens in nine species of freshwater and anadromous fish from the Pechora River, northern Russia. Sci Total Environ 160–161:653–659
Baruš V, Oliva O (eds.) (1995) Mihulovci a ryby. Fauna R a SR. (Cyclostomata and fishes. Fauna of Czech Republic and Slovakia) 28 Academia Praha
Brázová T, Torres J, Eira C, Hanzelová V, Miklisová D, Šalamún P (2012) Perch and its parasites as heavy metal biomonitors in a freshwater environment: The case study of the Ružín water Reservoir. Slovakia Sensors 12(3):3068–3081
Burger J, Gochfeld M (2007) Risk to consumers from mercury in Pacific cod (Gadus macrocephalus) from the Aleutians: fish age and size effects. Environ Res 105:276–284
Canli M, Atli G (2003) The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut 121:129–136
Commission Regulation (EC) No. 78/2005 of January 19th 2005 Amending Regulation No. 466/2001 as Regards Heavy Metals
Cui B, Zhang Q, Zhang K, Liu X, Zhang H (2011) Analyzing trophic transfer of heavy metals for food webs in the newly-formed wetlands of the Yellow River Delta, China. Environ Pollut 159:1297–1306
Dallinger R, Prosi F, Segner H, Back H (1987) Contaminated food and uptake of heavy metals by fish: a review and a proposal for further research. Oecologia 73(1):91–98
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98(1):29–36
Demková L, Árvay J, Bobuľská L, Tomáš J, Stanovič R, Lošák T, Harangozo L, Vollmannová A, Bystrická J, Musilová J, Jobbágy J (2017) Accumulation and environmental risk assessment of heavy metals in soil and plants of four different ecosystems in a former polymetallic ores mining and smelting area (Slovakia). J Environ Sci Heal A 52(5):479–490
Demirak A, Yilmaz F, Tuna AL, Ozdemir N (2006) Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere 63:1451–1458
Farkas A, Salánki J, Specziár A (2002) Relation between growth and the heavy metal concentration in organs of bream Abramis brama L. populating Lake Balaton. Arch Environ Contam Toxicol 43:236–243
Findoráková L, Šestinová O, Kováčová M (2017) Assessment of potential sediment contamination using screening methods (XRF, TGA/MS) taking into account principles of greenchemistry. Eastern Slovakia Environ Earth Sci 76:119
Froese R, Pauly D (2020) FishBase. World Wide Web electronic publication, www.fishbase.org, version (07/2020). Accessed 1. June 2020
Hančuľák J, Kurbel T, Kupka D, Špaldon T, Šestinová O, Findoráková L, Fedorová E (2014) Influence of the copper smeltery in Krompachy (Slovakia) on atmospheric deposition. Inzynieria Miner 15(2):45–50
Jamnická G, Hrivnak R, Otahelova H, Skorsepa M and Valachovic M (2006) Heavy metals content in aquatic plant species from some aquatic biotopes in Slovakia. Proceedings of the 36th International Conference of IAD, September 4–8, 2006, Austrian Committee Danube Research/IAD, Vienna, Austria, pp: 366–370
Jia Y, Wang L, Qu Z, Wang Ch, Yang Z (2017) Effects on heavy metal accumulation in freshwater fishes: species, tissues, and sizes. Environ Sci Pollut Res 24:9379–9386
Kenšová R, Čelechovská O, Doubravová J, Svobodová Z (2010) Concentrations of Metals in Tissues of Fish from the Věstonice Reservoir. Acta Vet Brno 79:335–345
Liu J-L, Xu X-R, Ding Z-H, Peng J-X, Jin M-H, Wang Y-S, Hong Y-G, Yue W-Z (2015) Heavy metals in wild marine fish from South China Sea: levels, tissue- and species-specific accumulation and potential risk to humans. Ecotoxicology 24:1583–1592
MP SR (1998) Metodický pokyn MŽP SR z 27. Augusta 1998 č. 549/98–2 na hodnotenie rizík zo znečistených sedimentov tokov a vodných nádrží, Vestník MŽP SR, 6 (5) 1998. (Methodological Instruction of the Ministry of Environment of the Slovak Republic of 27 August 1998 no. 549 / 98–2 for the risk assessment from contaminated sediments of streams and water reservoirs. Bulletin of the Ministry of the Environment of the Slovak Republic, 6 (5) 1998)
Musilová J, Árvay J, Vollmannová A, Tóth T, Tomáš J (2016) Environmental Contamination by Heavy Metals in Region with Previous Mining Activity. Bull Environ Contam Toxicol 97:569–575
Norm STN ISO 5667–12: (2019) (75 7051) Water Quality. Part 12: Guidance on Sampling of Bottom Sediments from rivers, lakes and estuaries
Nussey G, Van Vuren JHJ, du Preez HH (2000) Bioaccumulation of chromium, manganese, nickel and lead in the tissues of the moggel, Labeo umbratus (Cyprinidae), from Witbank dam, Mpumalanga. Water Sa 26:269–284
Pliešovská N, Flórián K, Orlitová E (1997) Migration forms of heavy metals and their impact on water quality in the Hornád River basin. Acta Montan Slovaca 2:158–162
Pourang N (1995) Heavy-metal bioaccumulation in different tissues of 2 fish species with regards to their feeding-habits and trophic levels. Environ Monit Assess 35:207–219
Ravera O (2001) Monitoring of the aquatic environment by species accumulator of pollutants: a review. J Limnol 60:63–78
Richardson SD, Kimura SY (2017) Emerging environmental contaminants: Challenges facing our next generation and potential engineering solutions. Environ Technol Inno 8(2017):40–56
StatSoft, Inc (2013) STATISTICA (data analysis software system), version 12.0. www.statsoft.com
Šalamún P, Renčo M, Kucanová E, Brázová T, Papajová I, Miklisová D, Hanzelová V (2012) Nematodes as bioindicators of soil degradation due to heavy metals. Ecotoxicology 21:2319–2330
Šalamún P, Hanzelová V, Miklisová D, Šestinová O, Findoráková L, Kováčik P (2017) The effects of vegetation cover on soil nematode communities in various biotopes disturbed by industrial emissions. Sci Total Environ 592:106–114
Šestinová O, Findoráková L (2017) Assessment of Eastern Slovakia sediments genotoxicity and phytotoxicity using screening tests: chromotests and phytotoxkit fresen. Environ Bull 26:2454–2462
Terra BF, Araújo FG, Calza CF, Lopes RT, Teixeira TP (2008) Heavy metal in tissues of three fish species from different trophic levels in a tropical Brazilian River. Water Air Soil Pollut 187:275–284
Wang W-X, Rainbow PS (2008) Comparative approaches to understand metal bioaccumulation in aquatic animals. Comp Biochem Physiol C Toxicol Pharmacol 148(4):315–323
Weber P, Behr ER, De Lellis KC, Secretti Vendruscolo D, Flores MM (2013) Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchem J 106:61–66
Yi YJ, Zhang SH (2012) Heavy metal (Cd, Cr, Cu, Hg, Pb, Zn) concentrations in seven fish species in relation to fish size and location along the Yangtze River. Environ Sci Pollut Res 19:3989–3996
Zhang Z, He L, Li J, Wu Z (2007) Analysis of heavy metals of muscle and intestine tissue in fish — in Banan section of Chongqing from Three Gorges Reservoir, China. Polish J Environ Stud 16:949–958
Zrnčić S, Oraić D, Ćaleta M, Mihaljević Ž, Zanella D, Bilandžić N (2013) Biomonitoring of heavy metals in fish from the Danube River. Environ Monit Assess 185:1189–1198
Acknowledgements
We specially thank David McLean for help with English language corrections. We gratefully acknowledge the funding of this study by the Slovak Research and Development Agency, projects No APVV-18–0467 and Grant Agency of the Ministry of Education of the Slovak Republic and Slovak Academy of Sciences (VEGA), project No 2/0126/20.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brázová, T., Šalamún, P., Miklisová, D. et al. Transfer of Heavy Metals Through Three Components: Sediments, Plants and Fish in the Area with Previous Mining Activity. Bull Environ Contam Toxicol 106, 485–492 (2021). https://doi.org/10.1007/s00128-021-03114-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03114-w