Abstract
This study is a part of the European LIFE +2010 Project “ZeoLIFE—Water pollution reduction and water saving using a natural zeolitite cycle”. It characterizes the application of Italian zeolite-rich pyroclastic rocks (zeolitites) as soil conditioner. Laboratory experiments will be tested on an experimental field in the Codigoro area, Ferrara district (North-East Italy). The samples investigated are chabazite- and phillipsite-rich and are all collected in quarries from Central Italy: (1) Grosseto area (Sorano and Sovana); (2) Viterbo area (Farnese, Grotte Santo Stefano, Corchiano, Nepi), and (3) Rome area (Riano). All samples are characterized by more than 30 % of zeolite content, together with volcanic glass, feldspars, pyroxenes, and micas. The quantitative mineralogical characterization of soil samples from Codigoro shows variable proportions of quartz, illite, plagioclase, K-feldspar, calcite, dolomite, chlorite, serpentine, kaolinite, gypsum together with an amorphous residual. Collected data confirm that conditioning of soils with selected zeolitite can be extremely promising for a well evident improvement of the soil quality, and contribute to define a standard approach which can surely find a general application well above the boundaries of the selected area for the field test.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
This study reports the preliminary results of the European LIFE +2010 Project “ZeoLIFE—Water pollution reduction and water saving using a natural zeolitite cycle”. ZeoLIFE project is conceived to test an innovative and integrated use of zeolitic tuffs (zeolitite), aimed to: (1) reduce pollutants content in livestock effluents, (2) correct agricultural soils, and (3) economize both irrigation water and fertilizers. The final expected result is a contribution to sustainable development achieved improving the economic efficiency of the livestock and agronomic activities without negatively impacting on the environment.
In developed countries, the soil use for agriculture and farming requires significant consumption of water, thus introducing concerns over its long time sustainability. Similarly, the intensive and indiscriminate use of chemical, nitrogen based, fertilizers is a primary source of pollution in surface and groundwater, also leading to eutrophication of soils. Zeolitites are rocks containing more than 50 % zeolites and are, thus, capable to exchange their extra framework cations (mainly Na, K, Ca) with NH4 from solutions and to release it gradually under appropriate conditions. In particular, zeolitites can uptake NH4 from municipal and/or zootechnical wastewaters and, thanks to their high cation exchange capacity, releases it gradually when, for example, they are spread in soil. Release rate of NH4 from zeolitites is optimal when compared to synthetic fertilizers, since NH4 absorbed in zeolite is only marginally leached after intensive raining. A controlled NH4 release contributes to avoid soils eutrophication, and to mitigate the negative effects associated to traditional intensive agriculture (Bish and Ming 2001; Eberl et al. 1995; Lewis et al. 1984; Ming and Allen 2001; Passaglia 2008). ZeoLIFE project foresees that results from laboratory experiments will be large-scale tested in an experimental field located in the Codigoro area (Ferrara district, North-East Italy), over a time scale of 2 years. This target area was selected because of its vocation for intensive agriculture and farming, and for the presence of different environmental landscapes, being thus representative of a much larger field of application, encompassing numerous areas in the world.
1.1 Environmental issue of the target area
Codigoro area is located in North-Eastern Italy, close to the Po River delta. The area comprises various landscapes and natural fragile environments: from alluvial plains (both over and below sea level) to swampy areas, coastal lagoons, and wetlands. Soils are mainly constituted by alluvial/lacustrine sediments showing grain sizes from sand to clay (Bianchini et al. 2002; Bianchini et al. 2012). The area is exposed to significant nitrate contamination arising from in site and upstream (along the Po River) intensive farming. According to the EC directive 91/676/CEE, known as “Nitrates Directive”, adopted in 1991 to protect groundwater threatened by overexploitation of agricultural soils and accumulation of nitrates, this area is classified as “very highly vulnerable”. Demands from this standard could not, however, be matched in target area so far, thus requiring an application to EU for temporary derogation from the limits stated in the above-mentioned directives.
1.2 The integrated zeolitite cycle
The mineralogical composition, the different genetic environments, the worldwide occurrence, and the peculiar physical–chemical features of natural zeolitites are well known and thoroughly described in many scientific reports, recently reviewed by Bish and Ming (2001). Zeolitite addition to clayey and sandy soils was observed to improve their productiveness by increasing permeability, ventilation, and water retention capacity. Moreover, several studies demonstrated the high potential of zeolitites in selectively removing NH4 from wastewaters as effect of cation exchange (Kalló 2001; Passaglia 2008). Many investigations also documented small scale, laboratory experiments where both natural and NH4-exchanged zeolitites were used to reduce the amount of synthetic fertilizers and to correct substrates and agricultural soils (Passaglia 2008; Ming and Allen 2001). Furthermore, after complete release to the soils of NH4, treated zeolites can gain back their role sorbing NH4 in excess provided by fertilizers, thus retaining their positive NH4 control effect over long time and assuring a persistent reduction in chemical fertilization and irrigation, with an obvious economic and environmental return (Ming et al. 1995).
1.3 Aim of the work
This first paper provides a detailed chemical and mineralogical characterization of zeolitite from selected Italian quarries, together with quantitative mineralogical characterization of soil samples from the area where field test is planned. Mineralogical characterization of soils sample was carried out on the finest fraction (< 2 μm), where phases reactive to ions exchange are mostly concentrated. Over the next 2 years of project development, ions present in percolating solutions will be constantly monitored via an integrated system of piezometers and ion-selective electrodes, and related to soil mineralogical composition before and after zeolitite addition.
2 Analytical techniques and analyzed samples
An X’Pert PRO—PANAlytical diffractometer was employed to perform mineralogical quantitative phase analyses (QPA) on both zeolitites and soil samples. Quantitative mineralogical analyses were performed by the Rietveld-RIR (Reference Intensity Ratio) method using the General Structure Analysis System (GSAS) software package (Larson and Von Dreele 1994). The application of the Rietveld-RIR method represents a major step forward in QPA with respect to conventional methods, especially as far as accuracy and detection limits are concerned (e.g., Bish and Howard 1988; Bish and Post 1993; Von Dreele and Cline 1995; Hill 1991; Hill et al. 1993; Louër 1998; Riello et al. 1998; Snyder and Bish 1989; Young 1993). However, its use for soil QPA is not so common, as confirmed by the limited number of papers dealing with this subject (Alves and Omotoso 2009; Brinatti et al. 2010; Kaufhold et al. 2010).
Chemical analysis of zeolitite samples was carried out on pressed pellets of powdered rock via a wavelength-dispersive Philips PW 1480 X-ray fluorescence (XRF) spectrometer, using the methods of Franzini et al. (1975) and Leoni and Saitta (1976) for the determination of element concentration. Fe was assumed to be in its trivalent oxide form. Loss on ignition (LOI) was determined by heating sample under investigation in an oven at 1,100 °C.
Cation exchange capacity (CEC) of Ca2+, Mg2+, Na+, and K+ on zeolitite was determined via elution of suitably powdered samples in a Gooch filter with porosity 2 by a 1 N NH4 solution, obtained by dissolving analytical NH4Cl in Millipore water. The elution was carried out until the concentration of each single cation in the last 1 L flask was <0.5 mg/L. Measurements of Ca2+, Mg2+, Na+, and K+ concentrations were carried out on each 1 L flask of eluted solution by a Perkin-Elmer 303 atomic absorption spectrometer (AAS). The final CEC value was given by the sum of the total measured concentrations of Ca2+, Mg2+, Na+, and K+ in the overall eluted solution, each total cation concentration being divided by the corresponding equivalent cation weight. Estimated detection limit for this analytical procedure is 0.01 meq/g.
Apparent density (AD) of zeolitite samples was determined as ratio between the mass of ground sample which fills a measuring cup in loose conditions and the volume of the cup (i.e., 250 mL for this experiment). The ground sample was previously sieved to obtain two different grain sizes, i.e., <3 and 3–6 mm, and AD measurements were performed on both grain sizes.
Water retention (WR) of zeolitite samples was determined placing 250 g of ground sample in a 30-mm diameter column with the drain hose clamped, and afterward poured with 250 mL of deionized water. After 1 h, the drain hose was unclamped, and the drained water was collected in a measuring cup. WR was calculated by subtracting the volume of drained water from the original amount of water. As for AD, WR measurements were performed on both <3 and 3–6 mm grain sizes fractions.
Chemical analysis on zeolite crystalline aggregates was performed via an ARL-SEMQ electron microprobe in wavelength-dispersive mode, using the software package by Donovan (1995). Natural minerals were used as standards. Results are considered accurate within a 2–6 % confidence level. Calculated chemical formulae were based on 24 oxygen atoms. Zeolite water content was measured via thermogravimetric analyses using a TG–DTA Seiko SSC 5200 thermal analyzer on about 25 mg of ground sample.
2.1 Zeolite-bearing rocks
The selection of zeolite-bearing samples to be field tested included benchmarking among samples from seven localities, all located in Central Italy: (1) Grosseto area (Sorano and Sovana); (2) Viterbo area (Farnese, Grotte Santo Stefano, Corchiano, Nepi), and (3) Rome area (Riano). All these deposits are chabazite- and phillipsite-rich and are the closest to the Codigoro target area (from 380 to 450 km), thus, minimizing the transport cost and carbon footprint. Quarrying activity is commonly associated to the production of wastes (i.e., non-marketable materials, such as blocks with irregular shape or small dimensions), commonly disposed in dumps directly on site. The development of the project and its possible future applications require ground material, so the waste material disposed in dumps can then be recovered and exploited without any further impact on mining. Additional data on the geological setting and features of the selected quarries are given in Online Resource 1 (ESM_1).
2.2 Soil samples
The soil samples from the agricultural experimental field were taken at different depths from the surface down to about 4 m. Additional data on the sampling points are given in Online Resource 2 (ESM_2). Further characterizations on other chemical and physical properties (e.g., chemical composition, total organic content, particle size analysis, textures, water retention, etc.) of sampled soils will be carried out during the project implementation.
3 Results
3.1 Detailed characterization of zeolite-bearing samples from selected quarries
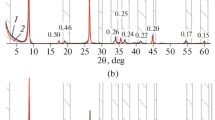
Quantitative phase analyses (with determination of the total zeolite content) and whole rock chemical analyses are reported in Tables 1 and 2, respectively; CEC, AD, and WR are reported in Table 3. Analcime, chabazite, phillipsite were found in the seven zeolitite samples in different amounts. Zeolites CEC values and species distribution in the rocks from the selected quarries are reported in Online Resource 3 (ESM_3).
QPA results (Table 1, ESM_3.1) indicate that the sample from Sorano shows the highest value of total zeolite content (>70 wt%), followed by the sample from Nepi, showing about 62 wt% zeolite content. Sample from Sorano also shows the highest chabazite content (68.5 wt%), representing 97 % of the whole zeolite fraction. In the sample from Nepi, chabazite represents the 85 % of the total zeolite fraction with subordinated phillipsite content (>8 wt%). Sample from Sorano shows also the highest Ca CEC value (1.46 meq/g, Table 3, ESM_3.2), while sample from Nepi shows the highest K CEC value (0.87 meq/g). K CEC values are comparable in samples from Sorano, Sovana, Grotte S. Stefano, Corchiano (varying between 0.62 meq/g for Farnese and 0.53 meq/g for Grotte Santo Stefano), with the exception of the sample from Riano, showing remarkably lower values (0.30 meq/g). Na CEC values range from 0.13 to 0.15 meq/g in samples from Farnese, Grotte Santo Stefano, and Corchiano. Lower values are observed in samples from other locations (0.12 meq/g for Sovana, 0.10 meq/g for Nepi and Riano, and 0.07 meq/g for Sorano). Among the studied zeolitites, sample from Sorano presents the most remarkable total CEC value, mainly due to its high zeolitic content.
Zeolitite from Sorano, thus, appears to be the best candidate for the target study, due to its high CEC and total zeolite content, thus suggesting to carry out a chemical analysis of the main zeolite species (i.e., chabazite, see Table 4). Obtained data confirm that exchangeable cations are almost entirely represented by Ca2+ and K+, being Na+, Mg2+, and Sr2+ quite negligible in chabazite composition. The theoretical CEC value for this chabazite is 3.59 meq/g, of which 2.04 due to Ca2+, 1.05 to K+, 0.35 to Mg2+, 0.14 to Na+ and 0.01 to Sr2+. Considering the total zeolite content in the zeolitite from Sorano (Table 1), it can be concluded that Ca2+ and K+ (and Na+ in minor amount) are almost entirely controlled by chabazite, thus, excluding almost completely the presence of these cations in the volcanic glass.
3.2 QPA of soil samples from Codigoro
Codigoro soil samples come from five drills made at the four corners and in the center of the experimental field, respectively (Online Resource 2). The drills were cut lengthwise in segments, and the mineralogical composition of each part was obtained via the Rietveld-RIR method.
Particular attention was devoted to the fine fraction of these sediments which, due to the high surface area and the particular nature of the related minerals (mainly clay minerals), may trap and concentrate ions present in aqueous solutions. Hence, for a better characterization of the constituent clay minerals, X-ray powder diffraction (XRPD) measurements were carried out on the <2 μm fraction of each selected sample where the clay minerals are concentrated. XRPD was carried out on randomly oriented samples and, for some selected samples, on ethylene glycol exposed (001) oriented mounts.
The results indicate that the <2 μm fraction is characterized by various proportions of quartz, illite, plagioclase, K-feldspar, calcite, dolomite, chlorite, serpentine, kaolinite, gypsum and by an amorphous residual. Irregular interstratified phyllosilicates are also present, as evidenced by the broad low angle peak [d (hkl) ≅ 14 Å, not fitted by chlorite reflection (001) alone]. Since, treatments with ethylene glycol do not enhance any appreciable changes in the low angle peaks of the pattern, it is conceivable that no expandable interstratified phyllosilicates are present (Brigatti et al. 2011).
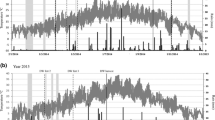
Histograms representing mineralogical composition and distribution of soil samples from each drill are shown in Figs. 1, 2, 3, 4 and 5 and reported as online resource as well (Online Resource 4—ESM_4). Obtained data suggest that the mineralogical composition in different samples is homogeneous from qualitative point of view, but highly variable in the quantity of identified phases.
4 Discussion and conclusion
4.1 Selection of the most suitable zeolitite quarry
Identification and quantification of zeolite different species and exchangeable cations in zeolitites are directly connected to the project aims since, for example, K is recognized as one of the primary plant macronutrient, whereas excessive Na contents can lead to nutritional imbalance and poor plant growth; thus, the knowledge of the base mechanisms controlling cation mobility is of paramount importance.
The performed experiments identify the zeolitite from Sorano as the most suitable raw material for ZeoLIFE Project implementation, as also supported by the stability in mineralogical composition of the Sorano samples over time. Table 5 reports QPA literature data for zeolitites from Sorano, Sovana, and Riano. Some deviations from data reported in this study can be observed in samples from Sovana and Riano, whereas a good agreement is observed in samples from Sorano. Mineralogical deviations over time can be explained as an effect of the progress of quarry front, which during exploitation meets areas where the varying ambient conditions (i.e., temperature, pressure and interacting solution) produced variations in the relative amount of phases crystallized during diagenesis.
Several other more practical aspects account for the choice of zeolitite from Sorano, such as its proximity to the area of field test, thus, limiting transportation cost and CO2 emission, the on-site availability of a grinding plant, and the presence of a large amount of raw materials in the dump that avoids the expansion of the existing quarries to support the project (Meggiolaro 2003).
4.2 Mineralogical characterization of soils
Mineralogical characterization of samples from the area hosting the experimental field identified soils that are mainly characterized by clay minerals. However, phases with appreciable CEC capacity (e.g., smectites) are almost completely lacking, thus, providing a scientific evidence for the need of intensive irrigation and fertilization which will likely have a negative impact on the quality of water resources. The sampling performed in the different points of the experimental field indicates that the composition of the soil is not constant, as far as distribution of the different mineral species is concerned (Figs. 1, 2, 3, 4, 5). Conditioning the top layer of the soil with zeolitite means inserting an element able to control the migration of cations both close to the surface and in depth, since preventing the percolation of leachable cations. In fact, current fertilization plans define a need for ammonium nitrogen varying between 120 and 170 kg/ha/year: the addition to soil of a relatively small amount of zeolitite is expected to drastically modify the permeability and release rate kinetics of the soil with respect to percolating solutions, thus, finally marking a net reduction in nitrates migration into groundwater.
These data also confirm that conditioning of soils with zeolitite can be extremely promising for a well evident improvement of the quality of soils in terms of farming productivity. The suggested investigation can thus contribute in defining a standard approach, which can surely find a general application well above the boundaries of the selected area for the field test. Furthermore, by integrating results from scientific investigations with similar scope (see, for example, Bianchini et al. 2002; Bianchini et al. 2012; EU.WATER Project), a mapping of soil features, including chemical–mineralogical data, can be derived, thus enabling the definition of optimal soil treatment and the identification of possible geochemical anomalies induced by human activity.
References
Alves ME, Omotoso O (2009) Improving Rietveld-based clay mineralogic quantification of Oxisols using Siroquant. Soil Sci Soc Am J 73(6):2191–2197
Bianchini G, Laviano R, Lovo S, Vaccaro C (2002) Chemical–mineralogical characterisation of clay sediments around Ferrara (Italy): a tool for an environmental analysis. Appl Clay Sci 21:165–176
Bianchini G, Natali C, Di Giuseppe D, Beccaluva L (2012) Heavy metals in soils and sedimentary deposits of the Padanian Plain (Ferrara, Northern Italy): characterisation and biomonitoring. J Soil Sediment 12:1145–1153
Bish DL, Howard SA (1988) Quantitative phase analysis using the Rietveld method. J Appl Crystallogr 21(2):86–91
Bish DL, Ming DW (2001) Natural zeolites: occurrence, properties, applications. reviews in mineralogy and geochemistry, vol 45. The Mineralogical Society of America, New York
Bish DL, Post JB (1993) Quantitative mineralogical analysis using the Rietveld full-pattern fitting method. Am Mineral 78(9–10):932–940
Brigatti MF, Malferrari D, Laurora A, Elmi C (2011) Structure and mineralogy of layer silicates: recent perspectives and new trends. In: Brigatti MF and Mottana A (Eds) Layered mineral structures and their application in advanced technologies. EMU Notes in Mineralogy 11: 1–71
Brinatti MA, Mascarenhas YP, Pereira VP, De Moya Partiti CS, Macedo A (2010) Mineralogical characterization of a highly-weathered soil by the Rietveld Method. Scientia Agricola (Piracicaba, Braz.) 67(4):454–464
Carnevali R, Gualtieri A, Passaglia E (1994) Quantitative determination of zeolites component in Italian pyroclastites by the Rietveld analysis of X-ray powder patterns. Mater Eng (Modena, Italy) 5(2):211–221
de’ Gennaro R, Cappelletti P, Cerri G, de’ Gennaro M, Dondi M, Langella A (2004) Zeolitic tuffs as raw materials for lightweight aggregate. Appl Clay Sci 25(2004):71–81
Donovan JJ (1995) PROBRE: PC-based data acquisition and processing for electron microprobes. Advanced MicroBeam, Inc., Vienna
Eberl DD, Barbarick KA, Lai TM (1995) Influence of NH4-exchanged clinoptilolite on nutrient concentrations in sorghum-sudangrass. In: Ming DW, Mumpton FA (eds) Natural zeolite ‘93. Occurrence, properties, use. International Committee on Natural Zeolites, Brockport, pp 491–504
Franzini M, Leoni L, Saitta M (1975) Revisione di una metodologia analitica per fluorescenza—X, basata sulla correzione completa degli effetti di matrice. Rendiconti della Società Italiana di Mineralogia e Petrografia 31(2):365–378
Gualtieri AF, Brignoli G (2004) Rapid and accurate quantitative phase analysis using a fast detector. J Appl Crystallogr 37(1):8–13
Gualtieri AF, Marchi E, Passaglia E (1999) Zeolite content and cation exchange capacity of zeolite-rich rocks. Stud Surf Sci Catal 125:707–713 (Porous Materials in Environmentally Friendly Processes)
Gupta AK, Fyfe WS (1975) Leucite survival: the alteration to analcime. Can Mineral 13:361–363
Hill RJ (1991) Expanded use of the Rietveld method in studies of phase abundance in multiphase mixtures. Powder Diffr 6(2):74–77
Hill RJ, Tsambourakis G, Madsen IC (1993) Improved petrological modal analyses from X-ray powder diffraction data by use of the Rietveld method I. Selected igneous, volcanic, and metamorphic rocks. J Petrol 34(5):867–900
Kalló D (2001) Applications of natural zeolites in water and wastewater treatment. In: Bish DL, Ming DW (eds) Natural zeolites: occurrence, properties, applications. Rev Mineral Geochem 45: 519–550
Kaufhold S, Ufer K, Kaufhold AW, Stucki JS, Anastàcio A, Reinhold J, Dohrmann R (2010) Quantification of allophane from Ecuador. Clay Clay Miner 58(5):707–716
Larson AC, Von Dreele R (1994) General Structure Analysis System (GSAS). Los Alamos National Laboratory Report LAUR, 86–748
Leoni L, Saitta M (1976) X-ray fluorescence analysis of 29 trace elements in rock and mineral standards. Rendiconti della Società Italiana di Mineralogia e Petrografia 32(2):497–519
Lewis MD, Moore FD 3rd, Goldsberry KL (1984) Ammonium-exchanged clinoptilolite and granulated clinoptilolite with urea as nitrogen fertilizers. In: Pond WG, Mumpton FA (eds) Zeo-Agriculture. Use of Natural Zeolites in Agriculture and Aquaculture. Westview Press, Boulder, pp 105–111
Louër D (1998) Advances in powder diffraction analysis. Acta Crystallogr A A54(6, Pt. 1):922–933
Meggiolaro V (2003) The zeolite deposits of Piandirena Sorano—Central Italy. ZEOGYP-BOARD project (Project n. GRD1-2000-25244): Retrofitting existing plants for low cost production of high performance building boards. Project funded by the European Community under the ‘Competitive and Sustainable Growth’ Programme (1998–2002)
Ming DW, Allen ER (2001) Use of natural zeolites in agronomy, horticulture, and environmental soil remediation. In: Bish DL, Ming DW(eds) Natural zeolites: occurrence, properties, applications. Rev Mineral Geochem 45: 619–654
Ming DW, Barta DJ, Golden DC, Galindo C Jr, Henninger DL (1995) Zeoponic plant-growth substrates for space applications. In: Ming DW, Mumpton FA (eds) Natural zeolites ’93: occurrence, properties, use. International Committee on Natural Zeolites, Brockport, pp 505–513
Passaglia E (2008) Zeoliti naturali, Zeolititi e loro applicazioni. ARVAN srl, Mira
Riello P, Canton P, Fagherazzi G (1998) Quantitative phase analysis in semicrystalline materials using the Rietveld method. J Appl Crystallogr 31(1):78–82
Snyder RL, Bish DL (1989) Quantitative analysis. In: Bish DL, Post JE (eds) Modern powder diffraction. Mineralogical Society of America, Rev Mineral 20:101–144
Von Dreele RB, Cline JP (1995) The impact of background function on high accuracy Quantitative Rietveld Analysis (QRA): application to NIST SRMs 676 and 656. Adv X-Ray Anal 38:59–68
EU.WATER, Transnational integrated management of water resources in agriculture for the EU WATER emergency control. http://www.eu-water.eu/
Young RA (1993) The Rietveld method. IUCr Monographs on Crystallography, vol 5. Oxford University Press, Oxford
Acknowledgments
We acknowledge Verdi S.r.l. for giving us permission to consult the geologic study “The zeolite deposits of Piandirena Sorano Central Italy” (2003) by Dr. Vito Meggiolaro, and are grateful to Dr. Vito Meggiolaro for searching for the original files in his archive and making them available to us. This publication is made in the context of the European LIFE +2010 project “ZeoLIFE—Water pollution reduction and water saving using a natural zeolitite cycle” (project code: LIFE +10 ENV/IT/000321); we are therefore grateful to the EC for funding received.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malferrari, D., Laurora, A., Brigatti, M.F. et al. Open-field experimentation of an innovative and integrated zeolitite cycle: project definition and material characterization. Rend. Fis. Acc. Lincei 24, 141–150 (2013). https://doi.org/10.1007/s12210-013-0235-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-013-0235-3