Abstract
Loss of green cover, and increasing pollution is a prime global concern. The problem calls for screening of pollution-tolerant tree species that can be integrated into plantation drives. Recognizing this, the study analyzed bio-indicator responses and performance of commonly occurring plant species along a pollution gradient in western Himalaya. Based on distance from the road, three sites viz., highly polluted (HP), moderately polluted (MP), and least polluted (LP), were identified. From these sites, leaves of commonly occurring 26 tree species were collected and analyzed for dust accumulation, total chlorophyll, relative water content (RWC), ascorbic acid, and pH using standard protocols. Later, assessment of Air Pollution Tolerance Index (APTI) and Anticipated Performance Indices (API) was carried out. The results revealed variations in biochemical characteristics. The pH, RWC, and total chlorophyll increased with decreasing pollution while ascorbic acid increased with increasing pollution. Dust capturing potential of Ficus carica (1.191 mg/m2) and Toona ciliata (0.820 mg/m2) was relatively higher. Based on the results of APTI, Grevillea robusta was classified as tolerant. It scored significantly higher values (21.06, 21.19, and 19.61 in LP, MP, and HP sites, respectively). Quercus floribunda, G. robusta (68.75% each), Juglans regia (68.7%), and T. ciliata (62.50%) were good performers in HP sites. Acer caesium, Betula utilis, and Morus alba that had low API scores (43.75%) were predicted as poor performers. Thus, G. robusta, Q. floribunda, J. regia, T. ciliata, and F. carica were evaluated as best performers. They could be integrated into plantations drives for environmental management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Loss of forest cover and ever-increasing pollution, which are primarily a result of unplanned development, and unregulated human use, are contemporary global problems (Pandey et al. 2015; Wagh et al. 2006; Tang et al. 2007). Air pollution represents a major concern that has far-reaching implications on health and environment of a region (Ali and Athar 2008; Sharma and Uniyal 2016). Considering that no physical and chemical methods are available to ameliorate large-scale arrival of air pollutants, air pollution becomes a complex phenomenon (Dwivedi and Tripathi 2007; Mondal et al. 2011; Rai et al. 2013). This not only calls for reducing emissions, but also prioritized greening of the landscape. In developing countries such as India, urbanization and developmental activities that include expansion of road network and exponential growth in automobiles have been cited as prime reasons for increasing air pollution and decreasing greenery (Goyal et al. 2006; Prajapati and Tripathi 2008a, b; Kaler et al. 2016; Devi et al. 2015; Sharma and Uniyal 2016). Green cover along the roadsides not only helps in minimizing the effects of pollutants, but also acts as a barrier that limits dispersion of pollutants by decreasing airflow exchange (Jeanjean et al. 2017). Being static, plants are continuously exposed to pollutants and provide a large area for dust, particulate, and phytoaccumulation of air pollutants (Noor et al. 2015). Plants growing in a dusty environment generally have a higher particulate deposition on their leaves, and thus leaves are more sensitive to air pollution (Kaler et al. 2016). Different plant species respond differently to pollution, while some plants can tolerate fairly high levels of pollution, the others are sensitive (Nadgórska-Socha et al. 2016; Pathak et al. 2011; Radhapriya et al. 2012).
Susceptibility to air pollution varies among plants, which in turn is related to biochemical characteristics of plants such as leaf chlorophyll, ascorbic acid, leaf extract pH, and relative water content (Rai and Panda 2014; Lakshmi et al. 2009). Studying these parameters is therefore important for understanding pollution-induced changes in plants, and suggesting suitable plants for greening. In the early 1990s, Air Pollution Tolerance Index (APTI) approach that uses biochemical characteristics of plants was advocated to identify tolerance levels of different plant species (Singh et al. 1991). In the recent times, various workers have advocated the use of Anticipated Performance Index (API) for assessing performance of species growing in polluted environ(s) (Ogunkunle et al. 2015; Noor et al. 2015; Nagajyoti et al. 2010; Karbassi et al. 2016). For assessing API, information on biological and socioeconomic characteristics of species are also taken into consideration, and added to the results of APTI. Both these methods ultimately guide urban greening and plantation initiatives in polluted air environ(s).
The Himalayan state of Himachal Pradesh is rich in biodiversity and attracts nature, adventure, and religious tourists. Its scenic beauty, lush green forests, snowbound mountains, and divine places have resulted in a boom of tourists from across the globe (Kuniyal 2002). One of the highly visited tourist places in the state is Rohtang pass. It is a gateway to the cold desert region Ladakh and motorable only for 6 months. For the rest 6 months, it is under snow. According to Himachal Pradesh Tourism Development Corporation, on an average, annually ~ 60 million people visit areas around Rohtang (HPTDC 2012; NGT 2013). It has resulted in heavy vehicular emissions, air pollution, and dust accumulation on the foliage of plants. Traffic congestion has further aggravated the problem. The National Green Tribunal (NGT), India has taken a serious note of the environmental hazards, and therefore imposed stringent regulations on movement of vehicles in the region (NGT 2013). Consequently, widening of National Highway (NH-21) on which this pass lies has already started (Sharma et al. 2011). Additionally, the Government is now focusing on the use of biodiesel, and compressed natural gas in vehicles that pass through this region (NGT 2013), and initiating suitable plantations. Consequently, the present study focused on (1) assessing dust accumulation on the leaves of tree species growing along NH 21, (2) analyzing biochemical characteristics of the leaves of these tree species, and (3) identifying bio-indicator responses and performance of naturally growing tree species along the NH-21 highway.
Materials and methods
Study area
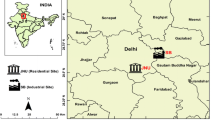
The study was carried out along the 243-km stretch of national highway (NH-21) in Himachal Pradesh (India) that encompasses a gradient from plains to high altitude passes. In general, mean annual temperature varies from extremely cold (−3 °C) at 3300 m asl to 38 °C in areas around 500 m asl. The Rohtang pass experiences heavy snowfall during winter months (December to February), and is closed for vehicles from mid-November to mid-May (Sharma et al. 2011). Twenty-nine locations between 31°24′40.85″-71°51′38.81″ to 32°21′8.34″-77°13′32.63″ with altitude ranging from 500 m to 3300 m amsl were selected for sampling vegetation (Fig. 1). At all the sampling sites, starting from the fringe of the road, three pollution gradients were identified. These have been named as Highly Polluted (HP), i.e., area within 0–15 m distance from the road boundary, Moderately Polluted (MP) represents area 15–30 m away from the road boundary, while Least Polluted (LP) represents area > 45 m away from the road. A schematic representation is provided in Fig. 2.
Sample collection
In each of the three sites (HP, MP, and LP), quadrats (plots) of 10 × 10 m were laid, and their four corners marked with red paint. Twenty-six commonly occurring plant species namely Abies pindrow Royle, Acacia catechu Willd., Acer caesium wallich ex D. Don, Betula utilis D. Don, Bombax ceiba L., Callistemon citrinus Skeel, Cedrus deodara G. Don, Dalbergia sissoo Roxb.ex DC., Ficus carica L., Grevillea robusta A.Cunn. ex R.Br, Grewia optiva J.R. Drumm ex Burrett, Juglans regia L., Mallotus philippensis Mull.Arg, Melia azedarach L., Morus alba L., Murraya koenigii Spreng., Picea smithiana Bioss., Pinus roxburghii Sargent, Populus deltoides Bartr. ex Marsh., Prunus cornuta Steud., Punica granatum L., Quercus floribunda Lindl. ex A.Camus, Robinia pseudoacacia L., Salix alba L., Toona ciliata Roem., and Zanthoxylum alatum Roxb. were selected on the basis of their abundance in quadrats. The height of trees occurring in the quadrats was measured using a digital clinometer (Nikon make). Fully mature 12 to 14 leaf samples were collected from commonly occurring plants in each plot. The leaves along with their petioles were collected from roadside facing branches of plants from an average height of 1.5 to 2.5 m (Chen et al. 2017), and placed in thermoplastic vials (Tarson make). Vials were labeled with tough tags, marked, and immediately placed in a liquid nitrogen container (P-21-Biological, Indian oil make). For estimation of dust accumulation, fully mature plant leaves in LP, MP, and HP sites were thoroughly cleaned with a camel hair brush. Identification marks were put on them, and they were left as such for 24 h. Later, with the help of a brush, dust accumulated on the leaves was collected in butter paper bags. Bags were sealed with airtight seal.
After field sampling, leaf and dust samples were brought to the laboratory for analytical investigations. Vials containing leaf samples were transferred to CRYO-CUBE F570 (Eppendorf, India make) ultra-low temperature freezer (− 80 °C) in ice boxes, and dust sample was kept in a desiccator (SECADORTM, USA make).
Laboratory analyses
Dust estimation
Dust samples were weighed on a digital balance ME104 (Mettler-Toledo), and dust accumulation was calculated following Kaler et al. (2016) using the equation:
Where W is dust content (g/m2), Wa initial weight of butter paper bag, Wb final weight of butter paper bag with dust, and A total area of the leaf (m2).
Total chlorophyll
Chopped and crushed leaves (50 mg) were placed in vials containing 7 ml of dimethyle sulfoxide, and incubated at 65 °C for half an hour. After this step, centrifugation was performed, and extracted aliquot was transferred to another graduated tube, and final volume was made 10 ml in dimethyle sulfoxide. Samples were assayed using a spectrophotometer at 645 and 663 nm wavelength against a blank (Hiscox and Israelstam 1979).
Where V is volume of aliquot, a length of the light path in the cell (1 cm), w weight of the sample taken, and A645 and A663 are an absorbance at 645 and 663 nm, respectively.
Ascorbic acid
Chopped and crushed leaves (10 g) were homogenized in standard meta-phosphoric acid solution, and final volume was made to 100 ml. This solution was titrated against indophenol dye. The appearance of rose pink color was the endpoint. The procedure of AOAC (1980) was followed, and ascorbic acid calculated using the formula given below:
Where DF is volume of dye used, TR titration reading, v volume made, w weight of sample taken, and V volume used for titration.
Leaf extract pH
Chopped and crushed leaves (5 g) samples were mixed with 10 ml double distilled water. The supernatant obtained after centrifugation was subjected to estimation of pH by digital pH meter (0.001) (Singh et al. 1991).
Relative water content
For relative water content (RWC), after taking fresh leaf weight, the leaf was immersed in water and left overnight. Next day, it was blotted dry to get the turgid weight. Leaf was then dried overnight in an oven at 60 °C, and reweighed to obtain dry weight (Govindaraju et al. 2012).
Where FW is fresh weight, DW dry weight, and TW turgid weight of leaves.
For all analytical work, quality assurance and quality control (QA/QC) standard protocols were followed to validate the accuracy and repeatability of results. Double distilled water and reagents of analytical (AR) grade procured from Central Drug House (P) LTD, India, an ISO 9001: 2008 certified company, were used. All glassware was thoroughly cleansed with 1% HCl, and dried overnight in an oven at 60 °C. For maintaining calibration and precision of spectrophotometer, standards were periodically run after ten test sample analysis. Errors of instrumental readings were checked and corrected by running reagent blanks after every five test sample determinations.
The APTI was determined by incorporating values of the above-mentioned parameters in the formula used by Govindaraju et al. (2012) and Ogunkunle et al. (2015):
Where A is ascorbic acid (mg/g), T total chlorophyll (mg/g), P leaf extract pH, and R relative water content of leaves.
Bio-indicator response of plant species was recorded based on APTI ratings (< 11 is sensitive, 11–17 is intermediate, and > 17 is tolerant) obtained by the plant species.
By combining grades of APTI with biological and socioeconomic characters (plant habit, type of plant, canopy structure, size of the plant, leaf structure, and hardiness), API index was worked out. All studied plants were scored with respect to the obtained grades. Gradation scale obtained by both methods is presented in Table 1. Socioeconomic importance of plants was noted from available literature, and also recorded during our field surveys.
Grades (0 to 7) and scores (0 to 100) obtained by the species help in determining their performance in polluted environments (Padmavathi et al. 2013) (Table 2).
Statistical analyses
Descriptive statistics, two-way ANOVA (detailed results are given in supplementary material), and multiple regression (linear function model) were used for data analyses of biochemical parameters using XL-STAT-2017 (version-2017.1). Multiple regression predicts the estimates of dependent variables from original values of independent variables. A best-fit regression line is always used to accomplish this estimation. The functional form of this line is known as linear function model of multiple regression. Further validation of estimates provided by regression coefficients called coefficients of multiple determination (R2). Certain errors involved during this prediction, estimated by the standard error of estimates.
This model for the multiple regression is as follows (1):
Where Y is APTI of plant species, X1 is leaf extract pH, X2 is total chlorophyll (mg/g), X3 is relative water content (%), X4 is the ascorbic acid (mg/g), and e is a stochastic error term.
A linear functional form of the above model was used to assess absolute changes in APTI (dependent variable) governed by biochemical parameters (independent variables) of plant species, and altered along vehicular air pollution gradient as follows (2):
Where Y is a linear function of APTI, βo is slope factor, and β1X1, β2X2, β3X3, β4X4 are regression coefficients of biochemical characteristics explained in Eq. 1.
Results and discussion
Density, height, and dust capturing potential of plant species
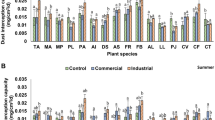
Tree density and height of the studied plant species in HP, MP, and LP sites ranged from 1 to 6 (individuals/10 m2) and 2 to 31 m, respectively. Dust accumulation loads varied among the species. In HP site, maximum dust load was noticed on leaves of F. carica (1.191 mg/m2) followed by T. ciliata (0.820 mg/m2), P. cornuta (0.308 mg/m2), J. regia (0.249 mg/m2), and B. ceiba (0.116 mg/m2) (Table 3). Negligible dust accumulation was noted on the leaves of C. citrinus, D. sissoo, and C. deodara (0.001 mg/m2 each). Similar trends were noticed in MP, where dust load ranged from 0.001 to 0.099 mg/m2. Leaves of plants in LP sites accumulated minimal dust when compared to leaves of plants in the MP and HP sites. Highly polluted sites were relatively more disturbed due to the continuous movement of vehicles, and thus a large amount of dust was captured by the plant species growing in this site (Joshi and Swami 2007). On the other hand, among species, broad and hairy leaves of F. carica facilitated higher dust accumulation. Thakar and Mishra (2010) also reported that leaves which are broad, rough, and hairy accumulate more dust. Height could also be an important factor governing patterns of dust accumulation on the foliage of plant species (Kaler et al. 2016).
Variations in biochemical characteristics of plant species
The biochemical characteristics of the studied plant species varied significantly (P < 0.05) among themselves, and the sites (see supplementary table). In all the three sites, B. ceiba reported highest leaf extract pH, i.e., 6.35 ± 0.006, 7.57 ± 0.017, and 7.72 ± 0.012 in HP, MP, and LP sites, respectively. On the other hand, minimum pH in all the three sites was recorded for C. deodara, i.e., 3.05 ± 0.002, 3.12 ± 0.005, and 3.16 ± 0.008 in HP, MP, and LP sites, respectively. Leaf extract pH was recorded to vary significantly between the sites (P < 0.05), and in the majority of the species, it ranged between 4 to 6 (Table 4). In general, for all the species, pH increased with decreasing pollution. In HP sites, Z. alatum (3.31 ± 0.006), P. smithiana (3.50 ± 0.008), P. granatum (3.64 ± 0.010), P. roxburghii (3.68 ± 0.008), and B. utilis (3.71 ± 0.007) had higher leaf extract pH values. In MP sites, pH of Z. alatum, P. smithiana, P. granatum, P. roxburghii, and B. utilis was 3.77 ± 0.006, 3.88 ± 0.003, 4.39 ± 0.012, 4.05 ± 0.010, and 4.17 ± 0.015, respectively, that was significantly higher (P < 0.05) than their respective values in HP sites. For other species also, similar trends were noted. In plant species, pH of leaf extract acts as an indicator of sensitivity to air pollution. Plants growing in polluted sites are reported to have acidic pH (Tripathi and Gautam 2007). We also observed higher acidic pH in plants growing in HP sites as compared to those growing in the LP sites (Table 4). Plants growing in highly polluted sites have high particulate matter deposition that puts them under stress, and thus higher acidic pH of leaf extract indicates higher air pollution (Tripathi and Gautam 2007).
With respect to RWC, in comparison to other species, F. carica reported the highest RWC values in all the three sites, i.e., 117.63 ± 0.141, 109.61 ± 0.202, and 96.57 ± 0.095% in LP, MP, and HP sites, respectively. The needles of A. pindrow retained less water when compared to all other species, and therefore reported significantly lower (P < 0.05) RWC values in LP (17.16 ± 0.227%), MP (12.82 ± 0.441%), and HP sites (8.64 ± 0.084 %) (Table 4). Alike pH, RWC also increased with decreasing pollution (Table 4). Relatively lower pollution in LP was a testimony to higher RWC in plants at this site when compared to RWC in plants growing in HP sites. Other species that recorded high RWC in LP were G. robusta (106.22 ± 0.168%), G. optiva (97.95 ± 0.542%), R. pseudoacacia (97.42 ± 0.173%), and S. alba (87.69 ± 0.089%). In HP, G. robusta (94.29 ± 0.063%), G. optiva (74.06 ± 0.190%), R. pseudoacacia (84.61 ± 0.116%), and S. alba (75.12 ± 0.049%) reported significantly lower values (P < 0.05). In HP sites, owing to dust on leaves, stomata generally remain closed during most of the time. This reduces transpiration thereby decreasing the RWC (Thawale et al. 2011). Similar results of low RWC values in dust-laden leaves have also been reported by Joshi and Swami (2007) from Haridwar, India.
It was revealed that, in all the targeted plant species, total chlorophyll increased with decreasing pollution (Table 4). In LP site, maximum total chlorophyll (7.87 ± 0.011 mg/g) was recorded in P. smithiana, followed by P. cornuta (7.30 ± 0.005 mg/g), and Q. floribunda (7.26 ± 0.020 mg/g). Minimum total chlorophyll content in HP sites was recorded in P. cornuta (1.32 ± 0.004 mg/g) followed by R. pseudoacacia (1.72 ± 0.010 mg/g), G. optiva (1.85 ± 0.079 mg/g), and Z. alatum (1.94 ± 0.029 mg/g). The difference was found to be significant (P < 0.05). The degree of pollution plays an important role in guiding total chlorophyll content in plant species. In polluted sites, dust load on leaves and high concentration of noxious gasses have been cited to result in degradation of photosynthetic pigments (Rai and Panda 2014). Individuals of same species growing in polluted sites have been reported to have less chlorophyll as compared to individuals growing in relatively less polluted sites (Agbaire 2009). Thus, higher chlorophyll in plants strengthens tolerance to environmental pollutants whereas less chlorophyll makes them sensitive (Das and Prasad 2010; Govindaraju et al. 2012).
When compared to total chlorophyll, a reverse trend was noticed for ascorbic acid. Ascorbic acid increased with increasing air pollution. In each of the three sites, G. robusta and S. alba reported higher values of ascorbic acid. In HP, G. robusta and S. alba had 9.66 ± 0.008 and 6.86 ± 0.006 mg/g of ascorbic acid, respectively. Their respective values in MP were 9.18 ± 0.014 and 6.64 ± 0.008 mg/g, respectively, whereas in LP the same was 8.69 ± 0.015 and 5.83 ± 0.009 mg/g, respectively (Table 4). C. deodara, on the other hand, had minimum ascorbic acid (0.31 ± 0.001 mg/g) in LP site. The high ascorbic acid in G. robusta and S. alba in HP sites indicated their tolerance towards air pollutants. In a polluted environment, ascorbic acid inhibits oxidation of tree leaves, and also plays an important role in preventing early aging of tree leaves (Prajapati and Tripathi 2008a, b). Thus, for maximizing defense, species growing in HP sites were found to have higher levels of ascorbic acid as compared to their respective values in less polluted sites. Tolerant plants are reported to contain higher ascorbic acid in comparison to sensitive ones (Mondal et al. 2011).
Linear function model of multiple regression
With respect to statistics, the computed R2 value was 0.995 meaning that 99.5% of the variability in the study could be explained by linear function model. The coefficients of determination (β-coefficients) for leaf extract pH, total chlorophyll, RWC, and ascorbic acid were positive, and significantly related with APTI (P < 0.05) (Table 5). One unit change in pH, total chlorophyll, RWC, and ascorbic acid was increasing APTI values of plant species by 0.16, 0.10, 0.31, and 1.03 times, respectively. These results are in line with the findings of Rai and Panda (2014), and Mondal et al. (2011) who studied variations in biochemical characteristics in relation to APTI by employing regression linear function model. Linear functional model bi-plots of APTI with each biochemical parameter are shown in Figs. 3, 4, 5, and 6. Interestingly positive relationship existed between APTI with pH (R2 = 0.3265), total chlorophyll (R2 = 0.4180), relative water content (R2 = 0.5032), and ascorbic acid (R2 = 0.7997), respectively. This indicates the importance of biochemical characteristics that ultimately define the tolerance of the plant species.
Bio-indicator responses and APTI of plant species
Bio-indicator response of the studied species (Table 4), and its interpretation based on economic value, morphological traits along with APTI values (Tables 6 and 7), showed that G. robusta had significantly (P < 0.05) higher values, i.e., 21.06, 21.19, and 19.61 in LP, MP, and HP sites, respectively. The bioindicator response of G. robusta (> 17) indicated that it was tolerant to air pollution in each of the sites. It has been pointed out that species with high values of APTI (> 17) are ideal for plantations in urban areas (Babu et al. 2013; Ogunkunle et al. 2015), and can form an important component of green belt around polluted sites such as the present area. In the present study, minimum APTI value was recorded for C. deodara (2.23) in LP sites. This points to its sensitivity towards air pollution. With low APTI values (< 11) (Table 6), bio-indicator response of P. deltoides, P. roxburghii, J. regia, A. caesium, A. pindrow, P. smithiana, and B. utilis puts them under sensitive category in each of the sites. G. optiva, F. carica, and S. alba showed an intermediate response, with APTI values lying between 12 to 16. On the other hand, D. sissoo, and Q. floribunda, gave mixed responses, i.e., they were sensitive in HP sites, and intermediate in LP sites.
Anticipated performance index of plant species
API is an advancement over APTI (Table 7). In this method, a plant species can obtain a maximum of up to 16 grades (Mondal et al. 2011; Prajapati and Tripathi 2008a, b). Here socioeconomic and biological characters of the targeted plant species along with their APTI values revealed that G. robusta (68.75%), J. regia (68.75%), Q. floribunda (68.75%), and T. ciliata (62.50%) were good performers in HP sites along the national highway. Performance of P. smithiana, C. deodara, and P. deltoides was moderate in each of the sites. A. pindrow (50.00%), A. caesium (43.75%), B. utilis (43.75%), G. optiva (43.75%), M. philippensis (50.00%), M. alba (43.75%), and M. koenigii (43.75%) having low API scores were poor performers in their respective sites (Table 7).
Along the pollution gradient from LP to HP, the performance of F. carica and M. azedarach dropped from moderate (56.25%) to poor (50.00%) performer. On the other hand, J. regia and Q. floribunda were found to be good performers (68.75%) in HP site, and very good performers (75.00 %) in MP, and LP sites (Table 7). Pandey et al. (2015) have also shown that performance of a species may change with pollution loads. Pathak et al. (2011) and Rao et al. (2004) reported that plants exhibiting maximum API scores should be recommended for greening in air-polluted areas. In the present study, API appraisal of studied plant species showed that G. robusta, J. regia, Q. floribunda, and T. ciliata are tolerant, and thus perfect species for green belt development along the NH 21. Due to their dense/semi-dense canopy and high socioeconomic value, these plant species may be preferred for plantations, especially along the roadside.
Overall, the study thus shows that APTI is a good method to study sensitivity and tolerance of plant species based on their evaluated responses. The plant species which are more sensitive act as indicators of air pollution, whereas tolerant species can be used to mitigate air pollution. This method also helps to determine anthropogenic stresses faced by plants at biochemical levels in severely polluted environ(s). The method further screen large number of plants with respect to their susceptibility. While, API provides an improvement over APTI and incorporates the use of biological and socioeconomic uses along with biochemical parameters. Based on the performance, API evaluates the potential and capability of plant species to mitigate air pollution. With the help of these two indices, most suitable plant species for green belt development along roadsides can be identified and recommended.
Conclusions
The present study concludes that plant species vary in their responses towards air pollution, and APTI alone may not be ideal for recommending plantations around polluted areas. On the other hand, APTI values integrated with API could be of great importance in meeting the current requirements of suggesting suitable plants for greening. Significant values of β-coefficients showed that biochemical characteristics of plants had a pronounced positive effect on air pollution tolerance of plant species. We found that out of the 26 plant species growing naturally, G. robusta, J. regia, Q. floribunda, and T. ciliata were good performers and tolerant. Additionally, plant species such as F. carica and P. cornuta had higher dust accumulation potential. Thus, these plants species may be integrated into plantation programs in the present area.
References
Agbaire, P. O. (2009). Air pollution tolerance indices (APTI) of some plants around Erhoike-Kokori oil exploration site of Delta State, Nigeria. International Journal of Physical Sciences, 4(6), 366–368.
Ali, M., & Athar, M. (2008). Air pollution due to traffic, air quality monitoring along three sections of National Highway N-5, Pakistan. Environmental Monitoring and Assessment, 136(1–3), 219–226. https://doi.org/10.1007/s10661-007-9677-3.
AOAC. (1980). Official methods of analysis chemist, 13th ed. (W. Horwitz, ed.) Association of Analytical Chemists, 83: 617–623.
Babu, G. B., Parveen, S. N., Kumar, K. N., & Reddy, M. S. (2013). Evaluation of air pollution tolerance indices of plant species growing in the vicinity of cement industry and Yogi Vemana university campus. Indian Journal of Advances in Chemical Science, 2(1), 16–20.
Chen, L., Liu, C., Zhang, L., Zou, R., & Zhang, Z. (2017). Variation in tree species ability to capture and retain airborne fine particulate matter (PM 2.5). Scientific Reports, 7(1), 3206.
Das, S., & Prasad, P. (2010). Seasonal variation in air pollution tolerance indices and selection of plant species for industrial areas of Rourkela. Indian Journal of Environmental Protection, 30(12), 978–988.
Devi, U., Hoque, R. R., & Sarma, K. P. (2015). Accumulation of trace metals in soil and trees by the highway passing through an ecological heritage area. Journal of Environmental Research and Development, 9(3), 605–612.
Dwivedi, A. K., & Tripathi, B. D. (2007). Pollution tolerance and distribution pattern of plants in surrounding area of coal-fired industries. Journal of Environmental Biology, 28(2), 257–263.
Govindaraju, M., Ganeshkumar, R. S., Muthukumaran, V. R., & Visvanathan, P. (2012). Identification and evaluation of air-pollution-tolerant plants around lignite-based thermal power station for greenbelt development. Environmental Science and Pollution Research, 19(4), 1210–1223. https://doi.org/10.1007/s11356-011-0637-7.
Goyal, S. K., Ghatge, S. V., Nema, P. S. M. T., & Tamhane, S. M. (2006). Understanding urban vehicular pollution problem vis-a-vis ambient air quality—case study of a megacity (Delhi, India). Environmental Monitoring and Assessment, 119(1-3), 557–569. https://doi.org/10.1007/s10661-005-9043-2.
Hiscox, J. D., & Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany, 57(12), 1332–1334.
HPTDC. (2012). Tourism survey for the state of Himachal Pradesh (April 2011–March 2012), Final. Report.http://tourism.gov.in/sites/default/files/Other/Himachal%20Pradesh%20Final%20Report_%2. Accessed on 20 Dec 2017.
Jeanjean, R. P. A., Buccolieri, R., Eddy, J., Monks, S. P., & Leigh, J. R. (2017). Air quality affected by trees in real street canyons: the case of Marylebon neighbourhood in central London. Urban Forestry & Urban Greening, 22, 14–53. https://doi.org/10.1016/j.ufug.2017.01.009.
Joshi, P. C., & Swami, A. (2007). Physiological responses of some tree species under roadside automobile pollution stress around city of Haridwar, India. Environmentalist, 27(3), 365–374. https://doi.org/10.1007/s10669-007-9049-0.
Kaler, N. S., Bhardwaj, S. K., Pant, K. S., & Rai, T. S. (2016). Determination of leaf dust accumulation on certain plant species grown alongside National Highway-22, India. Current World Environment, 11(1), 77.
Karbassi, S., Malek, M., Shahriari, T., & Zahed, M. A. (2016). Uptake of metals by plants in urban areas. International Journal of Environmental Science and Technology, 13(12), 2847–2854. https://doi.org/10.1007/s13762-016-1110-4.
Kuniyal, J. C. (2002). Mountain expeditions: minimising the impact. Environmental Impact Assessment Review, 22(6), 561–581. https://doi.org/10.1016/S0195-9255(02)00031-8.
Lakshmi, P. S., Sravanti, K. L., & Srinivas, N. (2009). Air pollution tolerance index of various plant species growing in industrial areas. The Ecoscan, 2, 203–206.
Mondal, D., Gupta, S., & Kumar, J. (2011). Anticipated performance index of some tree species considered for green belt development in an urban area. Plant Science, 2(4), 99–106.
Nadgórska-Socha, A., Kandziora-Ciupa, M., Ciepał, R., & Barczyk, G. (2016). Robinia pseudoacacia and Melandrium album in trace elements biomonitoring and air pollution tolerance index study. International Journal of Environmental Science and Technology, 13(7), 1741–1752. https://doi.org/10.1007/s13762-016-1010-7.
Nagajyoti, P. C., Lee, K. D., & Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: a review. Environmental Chemistry Letters, 8(3), 199–216. https://doi.org/10.1007/s10311-010-0297-8.
NGT. (2013). Judgement of the National Green Tribunal regarding tourism impacts in the north-western Himalayas. http://www.greentribunal.gov.in/judgement239/finalorder.pdf. Accessed 22 June 2017.
Noor, M. J., Sultana, S., Fatima, S., Ahmad, M., Zafar, M., Sarfraz, M., Balkhyour, M. A., Safi, S. Z., & Ashraf, M. A. (2015). Estimation of anticipated performance index and air pollution tolerance index and of vegetation around the marble industrial areas of Potwar region: bioindicators of plant pollution response. Environmental Geochemistry and Health, 37(3), 441–455. https://doi.org/10.1007/s10653-014-9657-9.
Ogunkunle, C. O., Suleiman, L. B., Oyedeji, S., Awotoye, O. O., & Fatoba, P. O. (2015). Assessing the air pollution tolerance index and anticipated performance index of some tree species for biomonitoring environmental health. Agroforestry Systems, 89(3), 447–454. https://doi.org/10.1007/s10457-014-9781-7.
Padmavathi, P., Reddy, C. J., & Aniji, M. (2013). Impact of air pollution on crops in the vicinity of a power plant: a case study. International Journal of Engineering Research & Technology, 2(12), 3641–3651.
Pandey, A. K., Pandey, M., Mishra, A., Tiwary, S. M., & Tripathi, B. D. (2015). Air pollution tolerance index and anticipated performance index of some plant species for development of urban forest. Urban Forestry & Urban Greening, 14(4), 866–871. https://doi.org/10.1016/j.ufug.2015.08.001.
Pathak, V., Tripathi, B. D., & Mishra, V. K. (2011). Evaluation of Anticipated Performance Index of some tree species for green belt development to mitigate traffic generated noise. Urban Forestry and Urban Greening, 10(1), 61–66. https://doi.org/10.1016/j.ufug.2010.06.008.
Prajapati, S. K., & Tripathi, B. D. (2008a). Seasonal variation of leaf dust accumulation and pigment content in plant species exposed to urban particulates pollution. Journal of Environment Quality, 37(3), 865–870. https://doi.org/10.2134/jeq2006.0511.
Prajapati, S. K., & Tripathi, B. D. (2008b). Anticipated performance index of some tree species considered for green belt development in and around an urban area: a case study of Varanasi City, India. Journal of Environmental Management, 88(4), 1343–1349. https://doi.org/10.1016/j.jenvman.2007.07.002.
Radhapriya, P., Navaneetha, G. A., Malini, P., & Ramachandran, A. (2012). Assessment of air pollution tolerance levels of selected plants around cement industry, Coimbatore, India. Journal of Environmental Biology, 33(3), 635–641.
Rai, P. K., & Panda, L. L. S. (2014). Dust capturing potential and air pollution tolerance index (APTI) of some road side tree vegetation in Aizawl, Mizoram, India: an Indo-Burma hot spot region. Air Quality, Atmosphere and Health, 7(1), 93–101. https://doi.org/10.1007/s11869-013-0217-8.
Rai, P. K., Panda, L. L. S., Chutia, B. M., & Singh, M. M. (2013). Comparative assessment of air pollution tolerance index (APTI) in the industrial (Rourkela) and non industrial area (Aizawl) of India: an eco- management approach. African Journal of Environmental Science and Technology, 7, 944–948. https://doi.org/10.5897/AJEST2013.1532.
Rao, P. S., Gavane, A. G., Ankam, S. S., Ansari, M. F., Pandit, V. I., & Nema, P. (2004). Performance evaluation of a green belt in a petroleum refinery: a case study. Ecological Engineering, 23(2), 77–84. https://doi.org/10.1016/j.ecoleng.2004.06.013.
Sharma, A., & Uniyal, S. K. (2016). Heavy metal accumulation in Pyrrosia flocculosa (D. Don) Ching growing in sites located along a vehicular disturbance gradient. Environmental Monitoring and Assessment, 188(10), 547. https://doi.org/10.1007/s10661-016-5561-3.
Sharma, N. L., Kuniyal, J. C., Singh, M., Sharma, P., Chand, K., Negi, A. K., Sharma, M., & Thakur, H. K. (2011). Atmospheric ultrafine aerosol number concentration and its correlation with vehicular flow at two sites in the western Himalayan region: Kullu-Manali, India. Journal of Earth System Science, 120(2), 281–290.
Singh, S. K., Rao, D. N., Agrawal, M., Pandey, J., & Naryan, D. (1991). Air pollution tolerance index of plants. Journal of Environmental Management, 32(1), 45–55. https://doi.org/10.1016/S0301-4797(05)80080-5.
Tang, B., Wong, S., & Lee, A. K. (2007). Green belt in a compact city: a zone for conservation or transition? Landscape and Urban Planning, 79(3), 358–373. https://doi.org/10.1016/j.landurbplan.2006.04.006.
Thakar, B. K., & Mishra, P. C. (2010). Dust collection potential and air pollution tolerance index of tree vegetation around Vedanta Aluminium Limited, Jharsuguda. The Bioscan, 3, 603–612.
Thawale, P. R., Babu, S. S., Wakode, R. R., Singh, S. K., Kumar, S., & Juwarkar, A. A. (2011). Biochemical changes in plant leaves as a biomarker of pollution due to anthropogenic activity. Environmental monitoring and assessment, 177(1-4), 527–535.
Tripathi, A. K., & Gautam, M. (2007). Biochemical parameters of plants as indicators of air pollution. Journal of Environmental Biology, 28(1), 127–132.
Wagh, N. D., Shukla, P. V., Tambe, S. B., & Ingle, S. T. (2006). Biological monitoring of roadside plants exposed to vehicular pollution in Jalgaon City. Journal of Environmental Biology, 37(2), 419–421.
Acknowledgements
The authors are thankful to the Director CSIR-IHBT for facilities and encouragement. Head and staff members of High Altitude Biology Division are acknowledged for their support and valuable comments. We would like to thank the Editor and Reviewer(s) for their positive comments and suggestions that helped in improving the earlier version of this manuscript.
Funding
We thank the Ministry of Environment, Forests, and Climate Change for financial support via National Mission on Himalayan Studies through project number GAP-0199. This is CSIR-IHBT communication number 4148.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Kashyap, R., Sharma, R. & Uniyal, S.K. Bioindicator responses and performance of plant species along a vehicular pollution gradient in western Himalaya. Environ Monit Assess 190, 302 (2018). https://doi.org/10.1007/s10661-018-6682-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6682-7