Abstract
Biomarker responses and histopathological lesions have been documented in laboratory mammals exposed to elevated concentrations of lead and cadmium. The exposure of white-footed mice (Peromyscus leucopus) to these metals and the potential associated toxic effects were examined at three contaminated sites in the Southeast Missouri Lead Mining District and at a reference site in MO, USA. Mice from the contaminated sites showed evidence of oxidative stress and reduced activity of red blood cell δ-aminolevulinic acid dehydratase (ALAD). Histological examinations of the liver and kidney, cytologic examination of blood smears, and biomarkers of lipid peroxidation and DNA damage failed to show indications of toxic effects from lead. The biomagnification factor of cadmium (hepatic concentration/soil concentration) at a site with a strongly acid soil was 44 times the average of the biomagnification factors at two sites with slightly alkaline soils. The elevated concentrations of cadmium in the mice did not cause observable toxicity, but were associated with about a 50% decrease in expected tissue lead concentrations and greater ALAD activity compared to the activity at the reference site. Lead was associated with a decrease in concentrations of hepatic glutathione and thiols, whereas cadmium was associated with an increase. In addition, to support risk assessment efforts, we developed linear regression models relating both tissue lead dosages (based on a previously published a laboratory study) and tissue lead concentrations in Peromyscus to soil lead concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead was mined in the Old Lead Belt of the Southeast Missouri Lead Mining District from 1721 until the last operating mine closed in 1972 (Seeger 2008). Deep mining began in 1953 and is ongoing in the New Lead Belt, also known as the Viburnum Trend. Studies have documented metal contamination in rivers and streams. Riparian mammals, birds, and amphibians from the area contain elevated concentrations of lead and cadmium (Niethammer 1985), and aquatic invertebrates contain elevated concentrations of lead, cadmium, and zinc (Besser 2007). Metals in water and sediments from mining activities in the area are associated with elevated concentrations in fish (Czarnezki 1985) and reduced populations of crayfish (Allert et al. 2008). Terrestrial sites have also been contaminated with lead and other metals. Ground-feeding songbirds from the area showed a reduction in the activity of the biomarker red blood cell δ-aminolevulinic acid dehydratase (ALAD) (Beyer et al. 2013). Kidneys of some of the American robins (Turdus migratorius) from the site had histological lesions associated with very high renal concentrations of lead. Lead is the metal of most concern in the Old Lead Belt, although Gale and Wixson (1979) documented that particulate emissions from two lead smelters in the Viburnum Trend were a significant source of environmental contamination from cadmium. The uptake of lead and cadmium by organisms from soil and the associated toxic effects have been well studied.
Lead is associated with the organic fraction of soil and is generally only marginally taken up by plants (Kabata-Pendias and Mukherjee 2007). Soil ingestion is generally the principal means of an animal’s exposure to lead, and concentrations of lead from soil generally decrease with trophic level in food chains (National Research Council 2005). In humans, lead is most persistent in bone, with a half-life of decades, compared to a half-life of about 30 days in blood (Agency for Toxic Substances and Disease Registry 2007). Cadmium in soil is generally associated with soil colloids, is taken up by plants mainly at low soil pH, and may precipitate in alkaline soils (Agency for Toxic Substances and Disease Registry 2012). Cadmium is poorly absorbed by mammals but is stored in renal tissue where it exhibits a long half-life. Although the results from studies are not all consistent, the Agency for Toxic Substances and Disease Registry (2004) concluded that the weight of evidence indicates that exposure to cadmium decreases tissue concentrations of lead in an animal. Reviews by Ma (2011, lead) and Cooke (2011, cadmium) relate tissue metal concentrations to toxic effects in mammals.
Toxic effects of lead on mammalian blood have been well studied. Nonregenerative anemia, often accompanied by distinctive changes in erythrocyte morphology, is a common manifestation of lead toxicity in many species (George and Duncan 1979). Lead binds to the sulfhydryl moieties of several enzymes important in the biosynthesis of heme, thereby impairing the production of hemoglobin within the developing erythrocyte. Lead also causes several other detrimental effects on hematopoiesis (Wardrop and Weiss 2010). The most notable effects of lead on the biosynthesis of heme are the inhibition of the cytosolic enzyme ALAD and the mitochondrial enzyme ferrochelatase (Wardrop and Weiss 2010). Inhibition of ferrochelatase by lead results in decreased hemoglobin production, as well as retention of iron within the mitochondria (Kaneko et al. 2008). These iron-laden mitochondria can be visualized as basophilic stippling when affected Wrights-Giemsa stained erythrocytes are viewed by light microscopy. When stained with Prussian blue, a ferric iron stain, affected erythrocytes are termed siderocytes (Wardrop and Weiss 2010). The presence of basophilic stippling with Wrights-Giemsa stain, resulting from inhibition of a nucleotidase leading to retention of ribosomes, is also a sign of lead toxicity under some conditions (George and Duncan 1979). Lead also increases the fragility and decreases the lifespan of erythrocytes (Kempe et al. 2005). Finally, increased membrane fragility leads to an increase in poikilocytosis, or abnormal erythrocyte shapes.
Decreased activity of ALAD is a sensitive biochemical measure of effects from toxic exposure to lead (Agency for Toxic Substances and Disease Registry 2007). The activity of ALAD is normally elevated in 21-day-old Peromyscus but remains relatively constant by the time the mice are 50 days old (McBride 2007). Inhibition of ALAD by lead, in addition to interfering with synthesis of heme, leads to an accumulation of δ-aminolevulinic acid (δ-ALA), which begins at the threshold of inhibition of ALAD (Sakai and Morita 1996). This compound interferes with the neurotransmitter gamma-aminobutyric acid and may be oxidized to generate free radicals (Agency for Toxic Substances and Disease Registry 2007; Ahamed and Siddiqui 2007).
Biochemical effects of lead and cadmium are well understood. Although there are differences in the way in which lead and cadmium affect the antioxidant defense system (Ercal et al. 2001), both interact with sulfhydryl groups of enzymes and proteins, generating reactive oxygen species, inhibiting the activity of enzymes involved in the antioxidant defense system of the liver, and depleting antioxidant reserves (Ercal et al. 2001). Our study includes five biomarkers of intracellular redox state—glutathione (GSH), glutathione disulfide (GSSG, which is the oxidized form of glutathione), the ratio of GSSG/GSH, thiols, and protein-bound thiols (total thiols less GSH). Oxidative stress, as shown by glutathione status and thiol concentrations, has been associated with lipid peroxidation and damage to DNA in controlled lead-dosing studies on rats (Flora et al. 2003; Alya et al. 2015; Liu et al. 2012). However, since animals may physiologically compensate for moderate exposure by increasing antioxidant defenses (Monaghan et al. 2009), values of these biomarkers are not necessarily directly related to exposure. For example, Salińska et al. (2012) failed to detect changes in either GSH or in thiobarbituric acid reactive substances (TBARS) in bank voles (Myodes glareolus) exposed to lead. Agrawal et al. (2014) did find an induction of lipid peroxidation in rats exposed to lead acetate, but no related changes in GSH levels. Changes in biomarkers may be difficult to interpret in field studies, in which animals are examined once and compared to reference animals that may not be as well matched as in controlled studies. Based on a meta-analysis of the impact of contaminants such as metals on wild animals, Isaksson (2010) concluded that GSH generally produces the strongest biomarker response of the oxidative markers examined and that although TBARS showed a tendency to increase in nine studies examined, the increase was not statistically significant.
To better understand the hazards of lead and cadmium from mining to terrestrial wildlife and, as part of a Natural Resource Damage Assessment, we planned an ecotoxicological study on various small mammals in the mining district. Our traps, however, captured only a single species, the white-footed mouse (Peromyscus leucopus, Rafinesque), limiting our ability to understand the effects on the small mammal community. Subsequently, our aims were to (1) model exposure of mice to lead, by relating tissue concentrations and estimated dosages in the mice to lead concentrations in contaminated soils and to (2) examine the mice for the presence of toxic effects, by determining whether they showed signs of oxidative stress, pathological lesions, or a reduction of the activity of red blood cell ALAD. In addition, we considered whether measures of bioavailability of the metals to humans (in vitro bioaccessible lead), to earthworms (tissue concentrations) or to plants (differential extraction method) were useful in understanding observed differences in the accumulation of lead and cadmium in mouse tissues at the sites.
Methods
Sites and field sampling
Four forested sites were selected for study in Missouri (MO), USA. The “Reference” site was in the Prairie Fork Conservation Area (38.88199° N 91.73463° W), in eastern Callaway County, central MO. The three sites contaminated with metals associated with mining were in southeastern MO. The St. Francis State Park site (“River 1,” 37.95681° N 90.54126° W) and the Washington State Park site (“River 2,” 38.08731° N 90.67414° W) were located along the Big River in the Old Lead Belt of the Southeast Missouri Lead Mining District. Soils at these two sites were contaminated with lead mining waste deposited with sediments during flooding (Pavlowsky et al. 2010). These sediments had been contaminated by dam failures upriver that released dolomite tailings. A mineralogical analysis of such sediments in a previous study (Beyer et al. 2016) determined the lead to be mainly sorbed to minerals or present as lead carbonate. The “Smelter” site in Bixby, MO (37.65539° N 91.12199° W), in the Viburnum Trend, was located close to the vent of the Magmont Mine. The Smelter site was distinct in having more acidic soil and having been contaminated mainly with lead oxide emitted from the nearby Buick smelter, in Boss, MO (Gale and Wixson 1979). Analysis of soil collected near the same smelter determined that the lead was mainly organically bound, sorbed to minerals, or present as lead sulfate (Beyer et al. 2016). Both lead oxide and especially lead carbonate have high relative bioavailabilities in soil (Casteel et al. 2006).
Study sites were from 1.3 to 2.7 ha in area. Soil samples were collected by means of incremental soil sampling, in which each site was considered a unit and 30 subsamples to about a 4-cm depth were taken with a stainless steel scoop throughout the unit at randomly located points near traps. Soil sampling was repeated twice, providing estimates of mean soil metal concentrations with a standard error based on three determinations. Three replicate samples of earthworms were collected by digging at each site. Earthworms were rinsed with distilled water and then frozen before they purged themselves of ingested soil. Sixty-two mice were trapped at various times between May 22nd and Oct 17th 2014 in Sherman and Longworth live traps, set out in 10 m × 10 m grids. Traps were provided with Dacron® bedding and baited with peanut butter and rolled oats. They were set in the late afternoon or early evening and checked early the following morning. Mice were taken to Dr. Stan W. Casteel, DVM, for processing.
Procedures for collecting and processing of mice were approved by the USGS-Patuxent Wildlife Research Center Institutional Animal Care and Use Committee. Mice were euthanized under the combination of anesthesia and exsanguination as recommended by the American Veterinary Medical Association guidelines (Leary et al. 2013). Each mouse was put under deep anesthesia in a chamber under a slow flow of carbon dioxide, after which an axial cut with surgical scissors was made followed by collection of blood in heparinized capillary tubes. Hematocrit (1 μl in capillary tube) was determined and two blood smears were prepared (one drop on a glass slide). The remainder of the blood sample was transferred into cryovials for lead analysis (> 55 μl frozen at −80 °C) and determination of ALAD activity (> 110 μl frozen in liquid nitrogen). Liver, kidneys, pancreas and femurs were removed. A 70 mg portion of each liver was frozen at − 80 °C and saved for biomarker assays. Samples of liver, kidney, and pancreas for microscopic examination were fixed in formalin, cut to 4-μm thickness in a microtome, dried at 80 °C for 12 min, and stained with hematoxylin and eosin in a Leica XL autostainer. Renal tissue was also stained for detecting the presence of intranuclear inclusion bodies by the acid-fast Kinyoun method. Femurs and the remaining pieces of the hepatic and renal samples were stored at − 80 °C until they were prepared for metal analysis. Blood smears were examined at the School of Veterinary Medicine of the University of Wisconsin and slides of the other mouse tissues were examined at the Veterinary Medical Diagnostic Lab of the University of Missouri.
Species identification
Individual mice collected were identified genetically by species with the use of quantitative real-time polymerase chain reaction (qPCR) (Tessier et al. 2004). DNA was extracted from liver using the Gentra Puregene Tissue Kit (QIAGEN, Valencia, CA). DNA concentration and purity was assessed on a NanoDrop™ 8000 spectrophotometer (Thermo Scientific, Wilmington, DE). The qPCR was run in a 20-μl reaction containing 4 μl of “5× reaction buffer”, 0.2 μM of each deoxynucleotide (dNTP), 0.75 μM of each primer, 0.4 μl of Phire Hot Start II DNA Polymerase (Thermo Fisher Scientific, Pittsburgh, PA), 2 μl of 50 μM SYTO9 stain (Thermo Fisher), and 2 μl of template DNA (1 ng/ul). Each primer pair was amplified separately as follows: 98 °C 5.5 min followed by 35 cycles of 98 °C 10 s, 60 °C 10 s, and 72 °C 15 s. Melt curve analysis (ramp from 70 to 95 °C rising at 1 °C at each step) was used to determine which species-specific bands were present in a sample.
ALAD activity, oxidative status and DNA biomarkers
Activity of ALAD was measured in the white-footed mouse blood samples based on Pain’s (1987) modification of the original method by Burch and Siegel (1971) and scaled down in volume to 25 μl of whole blood. Effects of blood volume, incubation time, and buffer pH were investigated to ensure that the conditions were appropriate. Blood from both white-footed mice from the Reference site and from laboratory mice (Mus musculus) obtained from BioreclamationIVT, Westbury, NY, were used in this preliminary work. Blood samples were excluded from analysis if there was any evidence of clotting. Following a 60-min incubation with substrate (0.01 M δ-ALA in phosphate buffer at pH 6.45) at 38 °C, the assay was terminated by the addition of 10% trichloroacetic acid. Incubation tubes were then centrifuged at 1500×g, Ehrlich’s reagent was added to the supernatant and absorbance at 555 nm was measured at 90-s intervals up to 21 min using a BMG Labtech Fluorostar spectrophotometer. Peak performance occurred at about 15 min. Following the method of Berlin and Schaller (1974) that was used by Pain (1987), activity was expressed as μmol δ-ALA per min per liter of erythrocytes, which we use as a “unit.” Sample sizes were 22 (Reference site), 7 (River 1 site), 6 (River 2 site), and 7 (Smelter site). In preliminary work, the measured activity of ALAD in samples of white-footed mice collected from the Reference site was 22% of the activity of a pooled sample of blood from laboratory mice.
Liver tissue was analyzed for four measures of oxidative status: total thiols, GSH, total GSH (sum of GSH and GSSG), and TBARS, a biomarker of lipid peroxidation. Protein-bound thiols, GSSG ([total GSH − GSH]/2), and the ratio of GSSG to GSH (GSSG/GSH) were calculated using the measured endpoints.
Frozen liver samples were thawed and homogenized on ice in 2× solution of phosphate-buffered saline (PBS, pH 7.4; Fisher BioReagents, Waltham, MA, USA) at 200 μg/μl. After the homogenate was centrifuged at 10,000×g for 10 min at 4 °C, 60 μl aliquots of the supernatant were transferred to 0.2-ml tubes and frozen at − 80 °C until analysis. To quantify GSH, GSSG, and GSSG/GSH, we thawed the liver supernatant, diluted it to 12.5 μg/μl in 1× PBS and analyzed it with the DetectX® Glutathione Fluorescent Detection Kit (Arbor Assays, Ann Arbor, MI, USA), following the manufacturer’s protocol. To quantify TBARS, we thawed the supernatant on ice, diluted it to 25 μg/μl in 1× PBS, and analyzed the samples with the QuantiChrom™ TBARS Assay Kit (Bioassay Systems, Hayward, CA, USA), following the manufacturer’s instructions. TSH was determined in thawed supernatant that had been diluted to 12.5 μg/μl in 1× PBS using the Measure-iT™ Thiol Assay Kit (Life Technologies - Molecular Probes, Inc., Eugene, OR, USA), following the manufacturer’s instructions.
Levels of 8-hydroxy-deoxy-guanosine (8-OH-dG) were quantified in liver as a biomarker of DNA damage resulting from oxidative stress. DNA was purified from thawed liver samples using the QiaAMP DNA Mini Kit (QIAGEN, Gaithersburg, MD), following the manufacturer’s protocol. DNA concentration and purity was assessed on a NanoDrop™ 8000 spectrophotometer (Thermo Scientific, Wilmington, DE), and samples were normalized to a concentration of 0.15 μg/ul. Aliquots of the normalized samples (15 μg total DNA) were denatured by heating for 10 min at 100 °C and then cooled on ice for 5 min. Each tube received 50 μl of 40-mM sodium acetate and 0.4 mM ZnCl2, followed by 50 μl of 5 U/ml nuclease P1 (Sigma Aldrich, St. Louis, MO) and was incubated at 37 °C for 30 min. The pH was adjusted to 7.5–8.0 with 20 μl of 1 M Tris-HCl and 15 μl of 10 U/ml alkaline phosphatase was added to each tube. The samples were incubated at 37 °C for 30 min, heated for 10 min at 95 °C to inactivate the enzyme and then placed on ice. Concentrations of 8-OH-dG were determined by enzyme-linked immunosorbent assay (ELISA) using the Cayman Chemical 8-OH-dG EIA Kit (Cayman Chemical Company, Ann Arbor, MI), following the manufacturer’s protocol. Each plate was incubated for 120 min after the addition of Ellman’s reagent and absorbance was determined at 410 nm. The standards were fit using a 4-parameter logistic curve. The reporting limit was 10.3 pg/ml. Intra-assay variability of duplicates was 6.74 ± 4.9% (mean ± SD). Plates included blanks and two reference samples, composed of a mixture of DNA from the individual samples, used to monitor inter-assay variability (coefficients of variability = 1.86 and 2.04%, respectively; n = 3 assays).
Blood smears
Blood smears submitted to the Clinical Pathology Laboratory of the University of Wisconsin School of Veterinary Medicine were evaluated for hematologic manifestations of lead toxicity. An automated system (Westcore Aerospray® 7150, Logan, Utah) was used to stain blood smears with a modified Wrights-Giemsa stain (Thiazin and Eosin stain packs, Westcore®, Logan, Utah). Blood smears were examined for the presence of basophilic stippling, metarubricytes, poikilocytosis, and polychromasia. Briefly, for each blood smear, 500 erythrocytes were examined in microscopic fields chosen at random. Erythrocytes with basophilic stippling and metarubricytes were enumerated and the relative proportions expressed as percentages. Poikilocytosis and polychromasia were expressed semi-quantitatively using standards currently in use at the Clinical Pathology Laboratory. A classification of 1+ was given for specimens with 1–10 poikilocytes per field at 1000-fold magnification (×1000 field). Specimens with 10–20 poikilocytes per ×100 field were classified as 2+, and specimens with more than 20 poikilocytes per ×100 field were classified as 3+. Polychromatophilic erythrocytes were enumerated as the range of polychromatophils observed in 10 ×100 fields. For specimens in which basophilic stippling was detected, additional slides were stained with Prussian blue stain to determine whether the basophilic stippling was due to siderotic granules. Staining of blood with new methylene blue for ribosomal RNA could not be performed owing to inability to collect sufficient blood volume or to stain blood in the field. The clinical pathologist was blinded to the source of the specimens (contaminated versus reference site) throughout the duration of the study.
Chemical analytical methods
Concentrations of aluminum, cadmium, copper, iron, nickel, lead, and zinc in sieved soils (< 2 mm) and in earthworms from the sites were quantified at the USGS Columbia Environmental Research Center (Columbia, MO). These metals were extracted in a mixture of heated concentrated HNO3 and HCl and quantified by inductively coupled plasma mass spectrometry (ICP-MS), as described in Beyer et al. (2013). Recoveries from MESS-3 Marine Sediment Reference Material (from the National Research Council of Canada) were 33% of aluminum, 98% of cadmium, 100% of copper, 87% of iron, 85% of nickel, 96% of lead, and 98% of zinc. Recoveries of pre-digestion spiked samples of soil were 105% of cadmium, 99% of copper, 103% of nickel, 119% of lead, and 106% of zinc. All percent relative standard deviations from triplicate digestion and analyses of the same five elements were less than 4%.
A composite sample of soil from each site was sent to each of three laboratories. The Agricultural Analytical Services Laboratory at the Pennsylvania State University analyzed the soil for nutrients and determined the texture. The Soil, Water and Plant Testing Laboratory of Colorado State University determined the concentrations of metals extractable with ammonium bicarbonate-diethylenetriaminepentaacetic acid (AB-DTPA). This extraction solution, at pH 7.6, is appropriate for use in neutral and calcareous soils when estimating the potential for uptake by plants. The third composite sample was sent to Karen Bradham at the US Environmental Protection Agency’s (EPA) National Exposure Research Laboratory, where in vitro bioaccessible lead was estimated by the EPA method 3051A. This method was designed to estimate bioaccessibility to humans, but was modified to a pH of 2.5 and a particle size < 2 mm, making it more appropriate for wild mice. Bioavailability of lead to mice was estimated from a calibration regression relating bioavailability of lead to Japanese quail to bioaccessibility at pH 2.5 (Fig. 1 in Beyer et al. 2016 ).
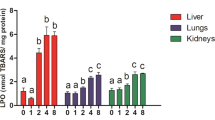
Mean red blood cell ALAD activity and 95% confidence intervals in white-footed mice from a reference site and from three sites contaminated with lead and cadmium located in the Southeast Missouri Lead Mining District. ALAD activity varied significantly among sites (Kruskal-Wallis, p < 0.05) and was significantly (p < 0.05) less at the River site 2 than at the Reference site (one-tailed Mann-Whitney p of 0.016 is less than Sidak alpha value of 0.017 corrected for multiple comparisons.) Although the mean activity at the Smelter site was greater than the activity at the Reference site, the confidence intervals overlapped
Tissue samples from the mice were extracted following the procedures in Casteel et al. (2006). Bones were dry ashed, ground to a powder, and dissolved in a 50% solution of nitric acid. Samples of blood were added to a mixture of 0.2% (v/v) ultrapure nitric acid, 0.5% (v/v) Triton X-100, and 0.2% (w/v) dibasic ammonium phosphate in deionized distilled water, as recommended by the Centers for Disease Control and Prevention. Liver and kidney samples were extracted at 90 °C with 70% nitric acid. Concentrations of lead in blood, liver, kidney, and bone and concentrations of cadmium in liver and kidney were quantified at the Missouri State Public Health Laboratory, MO, USA following the laboratory’s “Metals in Food by ICP-MS version 1.0” protocol. This procedure was based on EPA method 200.8 (Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma - Mass Spectrometry) and FDA document # T039 (Determination of Trace Elements in Aqueous Solutions by Inductively Coupled Plasma – Mass Spectrometry). It was first validated on test tissues and all blanks, spikes, duplicates, and standard reference materials (NIST SRM lot 955b – blood, NRCC TORT-2 - liver, NIST SRM 1400 - bone) run with samples were within acceptable limits for quality assurance. Reportable limits were 4 ng lead/ml in blood, 0.027 mg lead/kg and 0.022 mg cadmium/kg in liver, 0.085 mg lead/kg and 0.069 mg cadmium/kg in kidney, and 0.37 mg lead/kg in bone. Many of the concentrations detected in tissues of the 31 reference mice were below reportable limits (31 livers and 22 kidneys for lead and 6 livers for cadmium). These concentrations were approximated as half the reported concentrations for statistical purposes and means below reportable limits were reported as below reportable limits.
Statistics
Concentrations of lead and cadmium in tissues were summarized by site (mean plus or minus two standard errors). Lead and cadmium tissue concentrations were regressed on total, bioavailable (lead only), and AB-DTPA-extractable soil concentrations of the two metals. Because variances in lead and cadmium tissue concentrations were heterogeneous, increasing with the means, regressions were weighted by the reciprocal of the predicted value (weighted least squares regression). The method of weighted least squares was used also when regressing concentrations of lead in earthworms to concentrations of total lead in soil. We relied on a previously published 28-day dietary exposure study on deer mice (Peromyscus maniculatus) by McBride (2007; Table 2.4) to provide us with data to calculate regressions of hepatic and renal lead concentrations on dosages. McBride’s data on blood lead concentrations were not used because they were not linear, the slope decreasing with dosage. Means of biomarker values were examined for differences among the sites and for differences between particular sites and the Reference site. When the data were normally distributed with equal variances, then an ANOVA was run followed by the Holm-Sidak method. When the data were not normally distributed or the variances were not equal, then a Kruskal-Wallis test was run followed by Dunn’s method. Statistical calculations were performed with SigmaPlot® 13 software (Systat Software, San Jose, CA). Analytical and biochemical data on the individual mice may be accessed at https://doi.org/10.5066/F7JD4V85.
Results
Soils and earthworms
Soils from the three sites in the mining district contained elevated concentrations of total lead, cadmium, zinc, and copper (Table 1). Soil lead concentrations at the three contaminated sites (1500–2150 mg/kg) were well above the 17 mg/kg at the Reference site. On average, about 48% of the cadmium and 34% of the lead were extractable with AB-DTPA at the four sites. In vitro bioaccessibility of lead at the Reference site was 15.0%, well below the percentages at the River 1 site (68.4%), the River 2 site (51.2%), and the Smelter site (49.1%). Estimated concentrations of bioavailable lead and percent bioavailability were 2.81 mg/kg (17%) at the Reference site, 835 mg/kg (56%) at the River 1 site, 927 mg/kg (43%) at the River 2 site, and 803 mg/kg (42%) at the Smelter site.
Based on the Web Soil Survey of the USDA Natural Resources Soil Survey (http://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm), dominant soil types were Armster cobbly loam (Reference site), Haymond silt loam (River 1 site), Kaintuck fine sandy loam (River 2 site) and Viburnum silt loam (Smelter site). Haymond and Kaintuck soils were formed in alluvium, were frequently flooded, and had a slightly alkaline soil pH of 7.5 (Table 1). The Armster cobbly loam at the Reference site was slightly acid, with a pH of 6.5. The pH (5.3) of the strongly acid Viburnum silt loam soil is noteworthy because bioavailability of cadmium increases with soil acidity. This soil had a high organic matter content of 10.5% from a buildup of litter on the surface that overlaid a mineral soil that was platy and lacked organic matter. Soil cation exchange capacities of the sites were similar to each other (14–16 meq/100 g).
Earthworms from the Reference site were identified as Allolobophora rosea. Earthworms belonging to an unidentified species of Pheretima were most abundant at the River 1 and 2 sites, with some Eiseniella tetraedra (River 1) and Dendrobaena octaedra (River 2) present. No earthworms were found in the platy mineral horizon of the rocky Viburnum silt loam at the Smelter site, but some Lumbricus rubellus were collected in or just below the well-developed litter layer. Cadmium biomagnified in earthworms at all sites and zinc at the two sites with the most acidic soil (Table 2). Lead did not biomagnify in earthworms at any of the sites. The weighted (1/predicted) least squares regression relating lead concentrations in earthworms (Y) to soil (X) was Y = − 0.53 + (0.73 X) (r2 = 0.93, p < 0001, 11 degrees of freedom); the slope of 0.73 and the high correlation coefficient suggest that much of the lead measured in the earthworms could be attributed to the contaminated soil in the earthworms’ digestive tracts.
Lead and cadmium tissue concentrations in mice
The 62 mice trapped included subadults and adults weighing an average of 22 g (range of 14–37 g). All of these mice were genetically identified as P. leucopus. Mice were not separated by age or sex because of the small sample sizes at the sites. Mean concentrations of lead in blood, liver, and kidneys were much higher in mice from the contaminated sites than in mice from the Reference site (Table 3), and Eqs. 1–12 illustrate the relation of tissue lead concentrations to the three measures of soil lead concentrations. Although tissue lead concentrations in mice from the Smelter site tended to be less than those predicted by the regressions, all of the equations had good fits, significant at p < 0.05 and with r2 values of 0.61 to 0.80 (Table 4). Regressions on total and bioavailable lead explained greater fractions of the variances than did regressions based on the AB-DTPA extraction. In contrast to soil lead concentrations, total and AB-DTPA-extractable soil cadmium concentrations explained little or none of the considerable variance in cadmium concentrations in liver and kidney (Eqs. 13–16), as shown by the low r2 values (≤ 0.09). The ratios of the cadmium concentrations in liver to soil were strikingly different, varying more than a hundredfold (0.015 at the River 1 site, 0.066 at River 2 site, 0.72 at the Reference site and 1.8 at the Smelter site). The soil cadmium concentration at the River 1 site was 60 times the cadmium concentration at the Reference site, whereas the mean renal cadmium concentration was lower at the River 1 site than at the Reference site (Tables 1 and 3).
Regressions of McBride’s (2007) laboratory data of mean hepatic and mean renal lead concentrations on four dosages of lead (as lead acetate) fed to deer mice resulted in excellent fits, with r2 values of 1.0 and 0.97 (Eqs. 17 and 18). These two equations were used to convert lead concentrations detected in individual livers and kidneys to associated dietary dosages, which were then regressed on total and bioavailable soil lead concentrations, yielding Eqs. 19–22. The lead dosages estimated may be compared directly to a toxicity reference value to predict toxic effects associated with a given soil lead concentration. In theory, slopes of the regressions based on kidney and on liver metal concentrations should be similar and, in practice, they were within 10% of each other. Note that although the calculations were based on tissue residues, tissue residues do not appear as variables in Eqs. 19–22.
Histopathology
No lesions associated with lead or cadmium toxicity were observed in liver, spleen, or kidney of mice. Spleen and kidney were unremarkable with the exception of a kidney from a mouse collected from the Reference site that had a focal area of inflammation (macrophages and eosinophils) in the cortex. Liver of some the mice showed mild inflammation, variation in nuclei, or moderate cytoplasmic vacuolization.
Of 58 blood smears examined, 9 showed evidence of basophilic stippling with Wright-Giemsa stain. Erythrocytes with basophilic stippling were present in low numbers (0.2–0.8%) and stained negatively with Prussian blue stain, indicating that the basophilic stippling was not due to siderotic granules. Poikilocytosis was present with a classification of 1+ in 18 specimens and 2+ in only 1 specimen. No metarubricytes were identified. Polychromasia was present in all specimens and was often pronounced, with 14 specimens containing 7–10 or more polychromatophilic erythrocytes per ×100 field. Based on the frequent polychromatophilic erythrocytes and absence of iron staining, the basophilic stippled cells were likely reticulocytes and likely represented the normal level of circulating immature erythrocytes or evidence of a regenerative response. Neither of these findings was indicative of erythrocyte morphologic manifestations of lead toxicity.
ALAD activity, oxidative status, and DNA biomarkers
Activity of ALAD varied significantly among sites (Kruskal-Wallis, p < 0.05, Fig. 1). The activity of 6.19 units at the Reference site was greater than the activity of 4.69 units (76% of value at Reference site) at the River 1 site and the activity of 1.97 units (32% of value at Reference site) at the River 2 site. The mean activity at the River 2 site was significantly less than the activity at the Reference site by the Mann-Whitney test with a multiple comparison Sidak alpha correction (p < 0.017). Although the mean ALAD activity at the Smelter site (14.4 units) was above the reference activity, the 95% confidence limits overlapped.
The mean TBARS value was greatest at the Reference site, indicating that neither lead nor cadmium caused lipid peroxidation in mouse liver at the three contaminated sites (Table 5). Mean values of 8-OH-dG did not differ significantly among sites, indicating that there was no perceptible damage to hepatic DNA in mice from contaminated sites. All hepatic biomarkers of exposure showed significant differences among groups. Concentrations of GSH and GSSG were significantly less at the River 2 site than at the Reference site, consistent with a negative response to lead exposure. Mice from the Smelter site, also contaminated with soil lead, had higher mean concentrations of GSH, thiols, and protein-bound thiols than detected at the Reference site, although the differences were not statistically significant. These biomarkers show the opposite trend (lower concentrations) at the River 1 and 2 sites.
Discussion
Relationship of tissue lead and cadmium concentrations to soil lead and cadmium concentrations
The combination of the soil lead concentrations, the tissue lead concentrations, and the laboratory data relating lead dosages to tissue lead concentrations provides us with a means of predicting exposures of Peromyscus to lead at mining sites directly from soil lead concentrations. Risk assessors evaluating hazards to terrestrial wildlife from lead typically use a general approach to predict exposure, relying on estimates of the lead concentration in dietary items, the soil ingestion rate of the animal and the bioavailability of lead in soil. The predicted exposure may then be related to a toxicity reference value estimated from published dosing studies on mammals exposed to lead. Equations 19–22 (Table 4), in contrast, rely on tissue lead concentrations to estimate exposure, without the need to estimate values of the three variables individually. Although these equations reduce the uncertainty associated with estimating exposure, their specificity means that they apply to white-footed mice or to species that may be assumed to have similar feeding habits and a similar toxicological response to lead. Note also that the lead in Eqs. 19–22 refers to the chemical form of lead acetate, which facilitates comparisons to toxicity reference values based on a soluble form of lead. Eqs. 19 and 20 may be modified to express dietary concentration (mg/kg diet), rather than dosage (mg/kg body weight day), by multiplying the right side of the equation by 2.26 (McBride 2007, from data on p. 13).
Measures of total (heated HNO3 and HCl) and bioavailable metals in soil were most closely correlated with tissue lead concentrations (Table 4). The pH of 7.6 of the AB-DTPA extraction solution, while appropriate for the River 1 and 2 sites, was too high for the Reference and Smelter sites and we suggest that a neutral salt extraction would have been more appropriate for comparing the four sites. Lead biomagnification factors for earthworms (concentration in earthworms/concentration in soil, Table 2) at the four sites were similar to each other (from 0.42 to 0.95). Cadmium was most readily taken up by earthworms in acidic soils, but the effect was not as pronounced as in mice. Cadmium and lead biomagnification factors of earthworms were not useful in explaining differences in tissue concentrations among mice at the four sites, possibly because the uptake by mice is mediated by uptake of the metals into plants, whereas earthworms ingest these metals directly from soil.
Mean tissue concentrations of lead in Peromyscus reported here are generally consistent with tissue concentrations reported at other sites. Lead concentrations in mice from our reference site are comparable to those reported by Levengood and Heske (2008). Lead concentrations in liver and kidney in our mice are also comparable to those in deer mice (Peromyscus maniculatus) from the Anaconda Smelter Superfund Site in Montana, once soil lead concentrations at the different sites are taken into account (Hooper et al. 2002). The tissue lead concentrations that we detected were below those reported by Stansley and Roscoe (1996) (4.98 mg/kg in liver, 34.9 mg/kg in kidney, 245 mg/kg in femur, all dry weight) in white-footed mice at a trap and skeet range where the soil was more severely contaminated (75,000 mg lead/kg).
Compared to most other species of wildlife, white-footed mice seemingly have low exposure to lead. Stansley and Roscoe (1996) suggested that soil ingestion was the main source of lead to white-footed mice at the trap and skeet range they studied. Diets of white-footed mice consist mainly of mast and other seeds, lepidopterous larvae, and other arthropods (Whitaker 1966) and less than 2% soil (Beyer et al. 1994). Small mammals such as moles and shrews, which prey on earthworms and other organisms closely associated with soil, have a much greater exposure to lead. Studying a polluted site in the Netherlands, Ma et al. (1991) concluded that mean renal and hepatic concentrations of lead in the insectivorous Sorex araneus were on the order of ten times those in the herbivorous vole Microtus agrestis and that the ratio for cadmium was about a hundred to one. White-footed mice in our study had lower tissue concentrations than did songbirds collected from the Southeast Missouri Lead Mining District (Beyer et al. 2013). American robins from the area, which have a spring diet of soil invertebrates, had a much higher mean hepatic lead concentration (33.6 mg/kg, dry weight) than did the mice.
Pathologic effects
The absence of pathologic lesions in the mouse tissues is consistent with the tissue lead concentrations detected. Most lesions from lead that have been reported in mammals are associated with lead concentrations greater than the mean lead concentrations (wet weight) of 207 ng/ml in blood, 1.8 mg/kg in liver, 3.51 mg/kg in kidney, and 171 mg/kg in bone that we detected at our most contaminated site (River 2). Wardrop and Weiss (2010) consider 300 ng/ml (30 μg/dl) in blood diagnostic of lead poisoning. The absence of renal intranuclear inclusion bodies from our mice is consistent with the findings of Goyer et al. (1970), who detected inclusion bodies when renal lead concentrations reached about 10 mg/kg, wet weight, in rats dosed with lead. A study on bank voles showed that 16 mg/kg dry weight in kidney was associated with renal injury (Ma 1989), which is also higher than the mean renal lead concentration at the River 2 site. In reviewing the literature on lead in mammals, Ma (2011) concluded that renal concentrations greater than 80 mg/kg, dry weight, were associated with weight loss and death.
We suggest that the mean renal cadmium concentration of 21 mg/kg (wet weight) at the Smelter site was probably too low to cause histopathological lesions in the kidney, which is a critical target organ for cadmium. Cooke (2011) concluded that sensitive nephrotoxic effects in small mammals might occur at 30–60 mg/kg wet weight, but that 100 mg/kg wet weight would be a better threshold for serious adverse effects. Tubular damage occurs under chronic exposure at about 200 mg/kg, wet weight, in the renal cortex of kidneys of humans and laboratory mammals (Agency for Toxic Substances and Disease Registry 2012). Other researchers, however, have suggested lower thresholds of injury. Damek-Poprawa and Sawicka-Kapusta (2003) suggested that about 24 mg renal cadmium/kg, dry weight, is associated with renal lesions detected in yellow-necked mice (Apodemus flavicollis) collected near two zinc smelters in Poland.
Biomarkers of oxidative stress, DNA damage, and ALAD activity
The mice from the River 1 and 2 sites were under oxidative stress, presumably from exposure to lead. Consistent with effects of lead described in the literature (Ercal et al. 2001; Kasperczyk et al. 2013; Alya et al. 2015), concentrations of three biomarkers of oxidative stress (GSH, thiols, and protein-bound thiols) were less at the River 1 and 2 sites than at the Reference site. The oxidative stress from lead was not severe enough, however, to affect TBARS or 8-OH-dG. Activity of ALAD was significantly reduced at the River 2 site, similar to a reduction previously reported in white-footed mice exposed to lead from smelter emissions (Beyer et al. 1985) and to lead associated with a trap and skeet range (Stansley and Roscoe 1996). The severity of lead contamination at the River 2 site was greater than measured at the Anaconda Smelter Superfund Site A, where Reynolds et al. (2006) reported a reduction in ALAD activity in pocket gophers (Thomomys talpoides) but not in deer mice or meadow voles (Microtus pennsylvanicus). Previous studies on white-footed mice from reference sites have reported 14.2 and 10.8 units of ALAD activity (McFarland 2005), compared to our mean of 6.19 units (μmol δ-ALA per min per liter erythrocytes). Percent reductions in ALAD activity at the River 1 and 2 sites are consistent with previously published results. In a controlled laboratory dosing study on deer mice, McBride (2007) (Table 2.4) found that ALAD activity was reduced by 61% at a blood lead concentration of 0.19 μg/ml. The Agency for Toxic Substances and Disease Registry (2007) concluded that greater than 50% inhibition of ALAD activity was associated with a blood lead concentration in humans exceeding 0.20 μg/ml (20 μg/dl). Both conclusions agree with our finding of a 68% reduction in activity at a mean blood lead concentration of 0.207 μg/ml.
Cadmium’s effect on lead concentrations, ALAD activity, and GSH and thiol concentrations in mice
Cadmium concentrations in mice from the Smelter site were much greater than those in mice from the three other sites (Table 3). If hepatic cadmium concentrations were proportional to soil cadmium concentrations at the four sites, then the mean hepatic cadmium concentration in mice from the Smelter site would be about half of from the River 1 and 2 sites; instead, the mean concentration was about 22 times as great and the biomagnification factor (concentration in tissue/concentration in soil) was about 44 times the mean of the biomagnification factors for the River 1 and 2 sites. This disproportion may be attributed to the lower soil pH at the Smelter site compared to the River 1 and 2 sites (5.3 versus 7.5), since cadmium is almost insoluble in soil at pH 7.5 (Kabata-Pendias and Mukherjee 2007). In addition, cadmium at the Smelter site, emitted by the nearby smelter (Gale and Wixson 1979), may have been more bioavailable than cadmium washed downriver from piles of mining waste. Despite the considerable contamination of the soils, mice from the River 1 and 2 sites accumulated concentrations of cadmium in their livers that lie within the range of background concentrations for mice, rats, and voles (hepatic cadmium 0.2–1.5 mg/kg, dry weight) (Cooke 2011). The sharp differences in cadmium accumulation among the sites were not foreseen but are important because controlled studies have demonstrated cadmium influences both tissue lead concentrations and the biomarkers selected for study.
The uptake of lead by mice might be expected to be greatest at the Smelter site, based on the high AB-DTPA-extractable lead concentrations, the low soil pH, and the low calcium and phosphorus concentrations in the soil (Table 1). Instead, the ratio of the mean hepatic tissue lead concentration to the soil lead concentration at the Smelter site (0.00039) was little more than half (55%) of the average of the corresponding ratios for the River 1 (0.00060) and River 2 (0.00081) sites. This result is consistent, however, with the results of controlled studies, in which concurrent exposures to lead and cadmium have been found to reduce renal lead concentrations by about 50% in rats and 75% in goats compared to renal lead concentrations in those animals fed lead without cadmium (Mahaffey et al. 1981; Haneef et al. 1998). In laboratory mice, concurrent exposure to lead and cadmium decreased blood lead concentrations by 68% compared to exposure to lead alone (Smith et al. 2012).
The accumulation of cadmium in the mice from the Smelter site also seems to have affected the biomarkers. Concentrations of GSH, thiols and protein-bound thiols were lower at the River 1 and 2 sites than at the Reference site, consistent with a greater exposure to lead at these two sites (Table 5). In contrast, mean concentrations of these three biomarkers in mice from the Smelter site were higher not only than the mean concentrations at the River 1 and 2 sites, but also higher than the mean concentrations at the Reference site. As cells are exposed to cadmium, causing oxidative stress, a compensatory increase in synthesis of glutathione may occur, accompanied by an increase in tissue concentrations of glutathione. This has been shown, for example, in a study of glutathione concentrations in kidneys and livers of rats exposed to cadmium for a year (Kamiyama et al. 1995). Tissue concentrations of glutathione depend on both the magnitude and duration of the oxidative stress. When depletion of glutathione exceeds synthesis, tissue concentrations of glutathione decrease (Ercal et al. 2001). Induction of thiols may, in turn, explain the lower than expected tissue lead concentrations we observed. The thiol medications dimercaprol and D-penicillamine, for example, are used in chelation therapy for lead (Gurer and Ercal 2000). Finally, exposure of the mice to elevated cadmium concentrations seems to explain the greater activity of the thiol ALAD at the Smelter site (Fig. 1), consistent with similar findings in an in vitro study (Davis and Avram 1978) and in controlled dosing studies on rats and mice (Goering and Fowler 1987; Hogan and Razniak 1992).
In summary, we found that white-footed mice from three sites in the Southeast Missouri Lead Mining District had only moderately elevated tissue lead concentrations, despite high concentrations of lead in the soils, up to 1830 mg/kg. The moderately elevated concentrations are probably at least partly the result of a low rate of soil ingestion, which is an important route of exposure to lead in many wildlife species. Although lead caused significant decreases in concentrations of three biomarkers of oxidative stress and a decrease in the activity of ALAD, the exposure was not great enough to cause pathologic lesions or changes in biomarkers of lipid peroxidation and DNA damage. Two other results from the study stand out, however. Mice from the most acidic site had a biomagnification factor of cadmium that was over 40 times the average biomagnification factor at two sites with slightly alkaline soil. Thus, soil pH is a critical variable in terrestrial risk assessments involving cadmium. The effects of cadmium on the tissue concentrations of lead and the conflicting effects of cadmium from those of lead on the biomarkers should also be noted. Although cadmium’s effect on the biomarkers has been well documented in laboratory studies, such an effect may be difficult to recognize in the field. We were able to isolate the effects of cadmium because soils at the sites had very different pH values. Having knowledge of cadmium’s effects on lead and on biomarkers from controlled studies enabled us to offer a likely explanation of the results. We think that it would be difficult, however, to incorporate compensatory regulation of thiols from concurrent exposure to lead and cadmium into an ecological risk assessment.
References
Agency for Toxic Substances and Disease Registry. (2004). Interaction profile for: arsenic, cadmium, chromium, and lead. Atlanta: U.S. Department of Health & Human Services.
Agency for Toxic Substances and Disease Registry. (2007). Toxicological profile for lead. Atlanta: U.S. Department of Health and Human Services.
Agency for Toxic Substances and Disease Registry. (2012). Toxicological profile for cadmium. Atlanta: U.S. Department of Health & Human Services.
Agrawal, S., Flora, G., Bhatnagar, P., & Flora, S. (2014). Comparative oxidative stress, metallothionein induction and organ toxicity following chronic exposure to arsenic, lead and mercury in rats. Cellular and Molecular Biology (Noisy-Le-Grand), 60(2), 13–21.
Ahamed, M., & Siddiqui, M. K. J. (2007). Low level lead exposure and oxidative stress: current opinions. Clinica Chimica Acta, 383(1–2), 57–64. https://doi.org/10.1016/j.cca.2007.04.024.
Allert, A. L., Fairchild, J. F., Di Stefano, R. J., Schmitt, C. J., Besser, J. M., Brumbaugh, W. G., & Poulton, B. C. (2008). Effects of lead-zinc mining on crayfish (Orconectes hylas) in Black River watershed, Missouri, USA. Freshwater Crayfish, 16, 97–111.
Alya, A., Bini Ines, D., Montassar, L., Najoua, G., & Saloua, E. F. (2015). Oxidative stress, biochemical alterations, and hyperlipidemia in female rats induced by lead chronic toxicity during puberty and post puberty periods. Iranian Journal of Basic Medical Sciences, 18(10), 1034–1043.
Berlin, A., & Schaller, K. (1974). European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Zeitschrift für Klinische Chemie und Klinische Biochemie, 12(8), 389–390.
Besser, J., Brumbaugh, W., May, T., & Schmitt, C. (2007). Biomonitoring of lead, zinc, and cadmium in streams draining lead-mining and non-mining areas, southeast Missouri, USA. Environmental Monitoring and Assessment, 129(1), 227–241.
Beyer, W., Pattee, O., Sileo, L., Hoffman, D., & Mulhern, B. (1985). Metal contamination in wildlife living near two zinc smelters. Environmental Pollution Series A, Ecological and Biological, 38(1), 63–86. https://doi.org/10.1016/0143-1471(85)90094-7.
Beyer, W., Connnor, E., & Gerould, S. (1994). Estimates of soil ingestion by wildlife. The Journal of Wildlife Management, 58(2), 375–382. https://doi.org/10.2307/3809405.
Beyer, W., Franson, J., French, J., May, T., Rattner, B., Shearn-Bochsler, V., et al. (2013). Toxic exposure of songbirds to lead in the Southeast Missouri lead mining district. Archives of Environmental Contamination and Toxicology, 65(3), 598–610. https://doi.org/10.1007/s00244-013-9923-3.
Beyer, W., Basta, N. T., Chaney, R. L., Henry, P. F., Mosby, D. E., Rattner, B. A., et al. (2016). Bioaccessibility tests accurately estimate bioavailability of lead to quail. Environmental Toxicology and Chemistry, 35(9), 2311–2319. https://doi.org/10.1002/etc.3399.
Burch, H., & Siegel, A. (1971). Improved method for measurement of delta-aminolevulinic acid dehydratase activity in human erythrocytes. Clinical Chemistry, 17(10), 1038–1041.
Casteel, S., Weis, C. P., Henningsen, G. M., & Brattin, W. J. (2006). Estimation of relative bioavailability of lead in soil and soil-like materials using young swine. Environmental Health Perspectives, 114(8), 1162–1171. https://doi.org/10.1289/ehp.8852.
Cooke, J. (2011). Cadmium in small mammals. In W. Beyer & J. Meador (Eds.), Environmental contaminants in biota: interpreting tissue concentrations (2nd ed., pp. 627–642). Boca Raton: Taylor and Francis. https://doi.org/10.1201/b10598-21.
Czarnezki, J. M. (1985). Accumulation of lead in fish from missouri streams impacted by lead mining. Bulletin of Environmental Contamination and Toxicology, 34(1), 736–745
Damek-Poprawa, M., & Sawicka-Kapusta, K. (2003). Damage to the liver, kidney, and testis with reference to burden of heavy metals in yellow-necked mice from areas around steelworks and zinc smelters in Poland. Toxicology, 186(1), 1–10. https://doi.org/10.1016/S0300-483X(02)00595-4.
Davis, J. R., & Avram, M. J. (1978). A comparison of the stimulatory effects of cadmium and zinc on normal and lead-inhibited human erythrocytic δ-aminolevulinic acid dehydratase activity in vitro. Toxicology and Applied Pharmacology, 44(1), 181–190. https://doi.org/10.1016/0041-008X(78)90297-1.
Ercal, N., Gurer-Orhan, H., & Aykin-Burns, N. (2001). Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Current Topics in Medicinal Chemistry, 1(6), 529–539. https://doi.org/10.2174/1568026013394831.
Flora, S. J., Pande, M., & Mehta, A. (2003). Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic lead intoxication. Chemico-Biological Interactions, 145(3), 267–280. https://doi.org/10.1016/S0009-2797(03)00025-5.
Gale, N., & Wixson, B. (1979). Cadmium in forest ecosystems around lead smelters in Missouri. Environmental Health Perspectives, 28, 23–37. https://doi.org/10.1289/ehp.792823.
George, J. W., & Duncan, J. R. (1979). The hematology of lead poisoning in man and animals. Veterinary Clinical Pathology, 8(1), 23–30. https://doi.org/10.1111/j.1939-165X.1979.tb00878.x.
Goering, P. L., & Fowler, B. A. (1987). Metal constitution of metallothionein influences inhibition of δ-aminolaevulinic acid dehydratase (porphobilinogen synthase) by lead. Biochemical Journal, 245(2), 339–345. https://doi.org/10.1042/bj2450339.
Goyer, R. A., Leonard, D. L., Moore, J. F., Rhyne, B., & Krigman, M. R. (1970). Lead dosage and the role of the intranuclear inclusion body. Archives of Environmental Health: An International Journal, 20(6), 705–711. https://doi.org/10.1080/00039896.1970.10665647.
Gurer, H., & Ercal, N. (2000). Can antioxidants be beneficial in the treatment of lead poisoning? Free Radical Biology & Medicine, 29(10), 927–945. https://doi.org/10.1016/S0891-5849(00)00413-5.
Haneef, S., Swarup, D., Dwivedi, S., & Dash, P. (1998). Effects of concurrent exposure to lead and cadmium on renal function in goats. Small Ruminant Research, 28(3), 257–261. https://doi.org/10.1016/S0921-4488(97)00083-7.
Hogan, G., & Razniak, S. (1992). Split dose studies on the erythropoietic effects of cadmium. Bulletin of Environmental Contamination and Toxicology, 48(6), 857–864.
Hooper, M. J., Cobb, G. P., & McMurry, S. T. (2002). Wildlife biomonitoring at the anaconda smelter site. Montana: Deer Lodge County http://www.fws.gov/montanafieldoffice/environmental_contaminants/abr/abr_final_report.pdf. Accessed 25 November 2017.
Isaksson, C. (2010). Pollution and its impact on wild animals: a meta-analysis on oxidative stress. EcoHealth, 7(3), 342–350. https://doi.org/10.1007/s10393-010-0345-7.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. New York: Springer. https://doi.org/10.1007/978-3-540-32714-1.
Kamiyama, T., Miyakawa, H., Li, J. P., Akiba, T., Liu, J. H., Liu, J.-H., et al. (1995). Effects of one-year cadmium exposure on livers and kidneys and their relation to glutathione levels. Research Communications in Molecular Pathology and Pharmacology, 88(2), 177–186.
Kaneko, J. J., Harvey, J. W., & Bruss, M. L. (2008). Clinical biochemistry of domestic animals. Burlington: Academic Press.
Kasperczyk, S., Blaszczyk, I., Dobrakowski, M., Romuk, E., Kapka-Skrzypczak, L., Adamek, M., et al. (2013). Exposure to lead affects male biothiols metabolism. Annals of Agricultural and Environmental Medicine, 20(4), 721–725.
Kempe, D. S., Lang, P. A., Eisele, K., Klarl, B. A., Wieder, T., Huber, S. M., et al. (2005). Stimulation of erythrocyte phosphatidylserine exposure by lead ions. American Journal of Physiology-Cell Physiology, 288(2), C396–C402.
Leary, S., Underwood, W., Anthony, R., Cartner, S., Corey, D., Grandin, T., et al. (2013). AVMA guidelines for the euthanasia of animals: 2013 edition. http://works.bepress.com/cheryl_greenacre/14/. Accessed 25 Nov 2017.
Levengood, J. M., & Heske, E. J. (2008). Heavy metal exposure, reproductive activity, and demographic patterns in white-footed mice (Peromyscus leucopus) inhabiting a contaminated floodplain wetland. Science of the Total Environment, 389(2), 320–328. https://doi.org/10.1016/j.scitotenv.2007.08.050.
Liu, C.-M., Ma, J.-Q., & Sun, Y.-Z. (2012). Puerarin protects the rat liver against oxidative stress-mediated DNA damage and apoptosis induced by lead. Experimental and Toxicologic Pathology, 64(6), 575–582. https://doi.org/10.1016/j.etp.2010.11.016.
Ma, W.-C. (1989). Effect of soil pollution with metallic lead pellets on lead bioaccumulation and organ/body weight alterations in small mammals. Archives of Environmental Contamination and Toxicology, 18(4), 617–622. https://doi.org/10.1007/BF01055030.
Ma, W.-C. (2011). Lead in mammals. In W. Beyer & J. P. Meador (Eds.), Environmental contaminants in biota: interpreting tissue concentrations (2nd ed., pp. 595–607). Boca Raton: Taylor and Francis.
Ma, W.-C., Denneman, W., & Faber, J. (1991). Hazardous exposure of ground-living small mammals to cadmium and lead in contaminated terrestrial ecosystems. Archives of Environmental Contamination and Toxicology, 20(2), 266–270. https://doi.org/10.1007/BF01055914.
Mahaffey, K. R., Capar, S. G., Gladen, B. C., & Fowler, B. A. (1981). Concurrent exposure to lead, cadmium, and arsenic. Effects on toxicity and tissue metal concentrations in the rat. Journal of Laboratory and Clinical Medicine, 98(4), 463–481.
McBride, T. (2007). Influence of metal mixtures on co-occurring toxic metal bioavailability and effects in adult and developing deer mice. PhD, Texas Tech University, Lubbock.
McFarland, C. (2005). Delta-aminolevulinic acid dehydratase (ALAD) characterization and use in metal exposure assessments. PhD, Texas Tech University, Lubbock.
Monaghan, P., Metcalfe, N. B., & Torres, R. (2009). Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecology Letters, 12(1), 75–92. https://doi.org/10.1111/j.1461-0248.2008.01258.x.
National Research Council. (2005). Mineral tolerance of animals (2nd ed.). Washington, DC: National Academy Press.
Niethammer, K., Atkinson, R., Baskett, T., & Samson, F. (1985). Metals in riparian wildlife of the lead mining district of southeastern Missouri. Archives of Environmental Contamination and Toxicology, 14(2), 213–223.
Pain, D. (1987). Hematological parameters as predictors of blood lead and indicators of lead poisoning in the black duck. Environmental Pollution, 60, 67–81.
Pavlowsky, R. T., Owen, M., & Martin, D. (2010). Distribution, geochemistry, and storage of mining sediment in channel and floodplain deposits of the big river system in St. Francois, Washington, and Jefferson Counties, Missouri. Ozarks Environmental and Water Resources Institute (OEWRI), Environmental Data Report, 10–002.
Reynolds, K., Schwarz, M., McFarland, C., McBride, T., Adair, B., Strauss, R., et al. (2006). Northern pocket gophers (Thomomys talpoides) as biomonitors of environmental metal contamination. Environmental Toxicology and Chemistry, 25(2), 458–469. https://doi.org/10.1897/05-130R1.1.
Sakai, T., & Morita, Y. (1996). δ-Aminolevulinic acid in plasma or whole blood as a sensitive indicator of lead effects, and its relation to the other heme-related parameters. International Archives of Occupational and Environmental Health, 68(2), 126–132.
Salińska, A., Włostowski, T., & Zambrzycka, E. (2012). Effect of dietary cadmium and/or lead on histopathological changes in the kidneys and liver of bank voles Myodes glareolus kept in different group densities. Ecotoxicology, 21(8), 2235–2243. https://doi.org/10.1007/s10646-012-0979-z.
Seeger, C. (2008). History of mining in the Southeast Missouri lead district and description of mine processes, regulatory controls, environmental effects, and mine facilities in the viburnum trend subdistrict. In M. Kleeschulte (Ed.), Hydrologic investigations concerning lead mining issues in southeastern Missouri: U.S. Geological Survey Scientific Investigations Report 2008–5140.
Smith, E., Gancarz, D., Rofe, A., Kempson, I., Weber, J., & Juhasz, A. (2012). Antagonistic effects of cadmium on lead accumulation in pregnant and non-pregnant mice. Journal of Hazardous Materials, 199, 453–456.
Stansley, W., & Roscoe, D. (1996). The uptake and effects of lead in small mammals and frogs at a trap and skeet range. Archives of Environmental Contamination and Toxicology, 30(2), 220–226. https://doi.org/10.1007/BF00215801.
Tessier, N., Noël, S., & Lapointe, F.-J. (2004). A new method to discriminate the deer mouse (Peromyscus maniculatus) from the white-footed mouse (Peromyscus leucopus) using species-specific primers in multiplex PCR. Canadian Journal of Zoology, 82(11), 1832–1835. https://doi.org/10.1139/z04-173.
Wardrop, J., & Weiss, D. (2010). Schalm’s veterinary hematology (6th ed.). Ames: Blackwell Publishing Ltd..
Whitaker, J. O. (1966). Food of Peromyscus maniculatus bairdi and Peromyscus leucopus in Vigo County, Indiana. Journal of Mammalogy, 47(3), 473–486. https://doi.org/10.2307/1377688.
Acknowledgements
We thank Catherine Maddox for assistance with the hepatic biomarker analyses. David Hoffman of the Patuxent Wildlife Research Center and 2 anonymous reviewers provided helpful suggestions on the manuscript. This work was partly funded by the Missouri Department of Natural Resources through the U.S. Department of the Interior’s Natural Resource Damage and Restoration Program. Use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. government. Supplemental data are available at the US Geological Survey ScienceBase-Catalog website at https://doi.org/10.5066/F7JD4V85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Beyer, W., Casteel, S.W., Friedrichs, K.R. et al. Biomarker responses of Peromyscus leucopus exposed to lead and cadmium in the Southeast Missouri Lead Mining District. Environ Monit Assess 190, 104 (2018). https://doi.org/10.1007/s10661-017-6442-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6442-0