Abstract
In this study, solidified floating organic drop microextraction (SFODME) by 1-undecanol was combined with slotted quartz tube flame atomic absorption spectrometry (SQT-FAAS) for the determination of cadmium at trace levels. Formation of a complex with 4,4′-dimethyl-2,2′-bipyridine facilitated the extraction of cadmium from aqueous solutions. Several chemical variables were optimized in order to obtain high extraction outputs. Parameters such as concentration of the ligand, pH, and amount of buffer solution were optimized to enhance the formation of cadmium complex. The SFODME method was assisted by dispersion of extractor solvent into aqueous solutions using 2-propanol. Under the optimum extraction and instrumental conditions, the limit of detection and limit of quantitation values obtained for cadmium using the combined methods (SFODME-SQT-FAAS) were found to be 0.4 and 1.3 μg L−1, respectively. Matrix effects on the method were also examined for tap water and wastewater, and spiked recovery results were found to be very satisfactory.

SFODME-SQT-FAAS system for sensitive determination of cadmium
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium is known to be a toxic element occurring in the environment naturally, and also from different types of human activities such as fossil fuel consumption, untreated mine waste, welding, smelting, and cigarette consumption (Organization 2010; Bakırdere et al. 2016; O’neill et al. 2015; Johnson and Kilburn 1983; Barnhart and Rosenstock 1984; Tellez-Plaza et al. 2013). The transition of cadmium into soil, plants, water sources, and other animals’ tissues finds its way into the human body through inhalation, dermal contact with suspended particles, drinking water, and nutritional supplies (Tavakkoli and Khanjani 2016; Koyu et al. 2006; Järup and Åkesson 2009). When compared with other heavy metals, cadmium exposure is one of the most common in industrial sites due to its low melting point. It accumulates in the kidney over time and if exposure is excessive, a progressive form of chronic renal disease characterized by tubular atrophy may occur (Agency for Toxic Substances and Disease Registry 25/08/2014; Hong et al. 2004). Cd and its compounds have been classified as carcinogenic to humans (Turan et al. 2017; Feist et al. 2008; International Agency for Research on Cancer Monographs, vol. 58, Cadmium, IARC Press, Lyon, 1993, pp. 119–238). Occupational cadmium exposure is associated with lung cancer and prostate cancer. Other target sites for cadmium carcinogenesis in humans include the urinary bladder, kidney, liver, and stomach (Godt et al. 2006).
Flame atomic absorption spectrometry is not as sensitive as modern-day metal detection devices that can detect ng/mL levels without any pre-concentration step. Some techniques that have been used for determinations of Cd at trace levels include graphite furnace atomic absorption spectrometry, anodic stripping voltammetry, atomic fluorescence spectrometry, and inductively coupled plasma mass spectrometry (ICP-MS) (Leao et al. 2016; Cao et al.; Zhang et al. 2016; Chen et al. 2016). With the aim of improving the sensitivity of flame atomic absorption spectrometry (FAAS) instruments, quartz tubes have been used to increase the residence time of atoms in the flame, and trapping (pre-concentration) atoms on the quartz inner walls (Bakirdere et al. 2011; Kılınç et al. 2013). Increased residence time in the flame means increased interaction between atoms and light beam, which results in higher absorbance values (Yaman 2005; Keskin et al. 2015; Özzeybek et al. 2007).
For further sensitivity improvements, trap studies have been conducted where metals with higher melting points than analytes of interest are used to coat the inner walls of the quartz tube (Kılınç et al. 2013). Trapping of atoms is achieved by using fuel-lean flames, after which a small-volume organic solvent is used to induce the revolatilization and atomization of analyte species, resulting in a transient signal (Demirtaş et al. 2015).
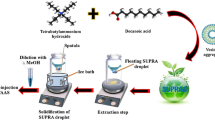
Recent microextraction techniques have been commonly used as sample preparation and analyte preconcentration because they offer rapid extractions, use low toxic solvent volumes, and give high enhancement factors (Biparva 2012). SFODME is one of the microextraction/preconcentration method which involves the use of a microdrop of organic solvent to extract an analyte-ligand complex from aqueous solution. The organic drop is solidified by keeping it in a freezer or an ice bath for a few minutes, then taken into a clean tube for instrumental determination (Khalili Zanjani et al. 2007). Matrix effect on analyte signal could be reduced and sensitivity is improved using this extraction/preconcentration method.
The aim of this study was to optimize a solidified floating organic drop microextraction method and couple it with slotted quartz tube flame atomic absorption to develop a sensitive and accurate analytical method for the determination of cadmium. To the best of our knowledge, this is the first study in literature where slotted quartz tube flame atomic absorption spectrometry (SQT-FAAS) has been combined with SFODME to further enhance the sensitivity for cadmium determination.
Experimental
Chemicals and reagents
A 1000-mg/L cadmium standard stock solution (High-Purity Standards, USA) was diluted into lower concentrations and used for the method development. All dilutions and cleaning were done with ultrapure water obtained from a Milli-Q® Reference Ultrapure Water Purification System. Buffer solutions in the pH range of 7–11 were prepared using the proper combinations of potassium dihydrogen phosphate, sodium hydroxide, sodium bicarbonate, di-sodium tetraborate decahydrate, and hydrochloric acid. The complexing ligand used (4,4′-dimethyl-2,2′-bipyridine purchased from Aldrich, USA) was prepared in methanol. Other chemicals including 1-undecanol, 1-dodecanol, 1-chlorooctadecane, 1-bromohexadecane, ethanol, methanol, 2-propanol, sodium chloride, potassium chloride, and potassium nitrate were all obtained from Merck, Germany.
Apparatus
A slotted quartz tube fitted Analytik Jena NovAA 300 flame atomic absorption spectrometer was used for the determination of Cd. Dimensions of lab-made quartz tube were 16 mm internal diameter, 18 mm outer diameter, 13 cm length, 5.5 cm entry slot, and 3.0 cm exit slot. An OHAUS Pioneer PA214C precision scale was used for weighing purposes. Hanna Instruments Edge® Multiparameter pH Meter-HI2020 was used for pH measurements. An HAPA M-100 model ultrasonicator and Kermanlar brand mechanical shaker were used in the mixing optimization step. An Andreas Hettich EBA20 centrifuge device was used to facilitate heterogeneous mixture separation and a freezer was used for the solidification of the organic drop. The custom-made SQT was aligned manually with a Cd hollow cathode lamp operating at 2.0 mA and a 0.50-nm spectral bandpass. The 228.8-nm cadmium analytical line was used for absorption measurements and background signal correction was achieved with a deuterium (D2) lamp.
Ligand complexing and SFODME procedures
The cadmium complex was formed by adding 0.50 mL buffer solution and 1.0 mL of ligand to 8.0 mL aqueous sample/standard solution in a centrifuge tube. The dispersive/extractant mixture (1.5 mL 2-propanol/200 μL 1-undecanol) was prepared in another tube, taken up with a syringe and injected into the aqueous solution. The injection was done rapidly to ensure a homogenous dispersion of extraction solvent. The tube was centrifuged at 3461g for 2.0 min in order to separate extraction solvent from the aqueous solution. The tube was then placed in a freezer for a few minutes for the solidification of extractor solvent after which the floating solid was collected into a clean tube. Due to the relatively high viscosity of 1-undecanol, 250 μL methanol was added for dilution before sending it to the SQT-FAAS system for cadmium determination. The purpose of this was to avoid any possible clogging of the nebulizer unit and to make easier the transport of sample to the flame.
Samples
Tap water samples were taken from Davutpasa, Istanbul, Turkey. Municipal wastewater was taken from an advanced biological wastewater treatment plant and stored at − 4.0 °C. Before use, wastewater samples were left under room temperature for approximately 1.0 h and then filtered with 125-mm filter paper.
Results and discussions
Stepwise optimizations were carried to obtain high outputs from all experimental parameters. A three-stage optimization was carried for complex formation, SFODME, and slotted quartz tube. All parameters under each stage were optimized with proper concentration of cadmium while other parameters were held constant. Optimum parameters were selected based on the highest average absorbance for triplicate measurements.
Optimization of complex formation
Chemical variables that effect complex formation include the pH and amount of buffer solution, concentration of ligand, and complexing period. Metals are easily extracted when they exist in molecular coordination with ligands. pH of aqueous solution can however affect the state (ionic or molecular) that an analyte exists in. Buffer solutions between the pH ranges of 7.0–11 were added to the standard solution and extracted. The highest absorbance value was recorded for pH 10; on the other hand, relatively lower absorbance values were obtained at lower pH values. This might be attributed to the competition of hydrogen ion for the complex formation sites where Cd2+ ions can be bound to. At higher pH values, ligand’s hydroxyl groups are deprotonated and this ends up with more net negative charges (Amin and Selmy 2017; Wong et al. 2003). Hence, the amount of the pH 10 buffer solution was examined by adding 0.50, 1.0, 1.5, 2.0, and 2.5 mL volumes. The results obtained showed that buffer solution volumes above 0.50 mL had no significant effect on the complex formation. Therefore, 0.50 mL of pH 10 buffer was chosen for further optimizations.
Optimum concentration of 4,4′-dimethyl-2,2′-bipyridine was investigated for five different concentrations: 100, 250, 500, 1000, and 1800 mg/L. The absorbance values showed an increasing up to 1000 mg/L (optimum concentration), after which a gradual decrease occurred. Mixing of sample/standard solution after ligand addition is important because it facilitates a rapid and complete complex formation. To determine whether the mixing period would create any difference on Cd-ligand complex yield or not, 15-s, 5.0-min, 10-min, 20-min, and 45-min periods of mechanical shakings were applied, and 15 s gave the best result. The extraction process was therefore carried out after 15 s of mechanical mixing.
Type and amount of extraction solvent
Solidified floating organic drop microextraction requires an organic solvent that has a melting point around 10–30 °C and it must be immiscible in analyte matrix (Thongsaw et al. 2017). In light of this information, four different organic solvents namely 1-undecanol, 1-dodecanol, 1-chlorooctadecane, and 1-bromohexadecane that meet the criteria were tested for their extraction outputs. No experimental data was recorded for 1-bromohexadecane because white precipitates settled at the bottom of the tube after centrifugation. The highest absorbance signal was recorded for 1-undecanol and its optimum volume was tested between 100 and 300 μL, with 50 μL increments. An ideal extractor solvent amount collects sufficient amounts of analyte from aqueous solution. Relatively low absorbance values recorded for 100 and 150 μL suggest inadequate extractions compared to 200 μL which recorded the highest absorbance values. In addition, relatively lower absorbance recorded for 250 and 300 μL volumes indicate dilution of analytes after the 200 μL optimum volume.
Type and amount of dispersive solvent
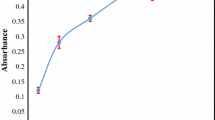
In order to increase the extracting yield of the SFODME method, 1-undecanol was dispersed into the analyte solution with the aid of solvents which are miscible with both solutions. The dispersion of extraction solvent helps to increase the surface area for an effective extraction and also results in a rapid and effective extraction. Methanol, ethanol, and 2-propanol were thus tested for their dispersive efficiencies. The highest absorbance value was recorded for 2-propanol, and its optimum volume of 1.5 mL was determined after testing 1.0, 1.5, 2.0, 2.5, and 3.0 mL. The significant decrease in absorption after 1.5 mL as shown in Fig. 1 can be attributed to overdispersion of extraction solvent which results in losses in the aqueous phase.
Type and amount of salt
Adding a right type and proper amount of salt to a liquid-liquid extraction process may result in high extraction output of analyte(s). The high solubility of salts in aqueous solutions tends to increase the free movement of analytes into the extracting organic solvent. For this purpose, sodium chloride, potassium chloride, and potassium nitrate were tried and compared to a no-salt added sample. According to the absorbance values, the added salts negatively impacted on the extraction output of cadmium complex. All three added salt results were over five times lower than the no-salt result. This could be attributed to the increase in solution density which limits the interaction between extraction solvent and cadmium complex. In addition, the increased density might have limited the complete separation of extraction solvent to the surface of aqueous solution. For these reasons, further optimizations were carried out without salt additions.
Effect of mixing
Mixing the extraction solution could increase the interaction between extractor solvent and analyte, thus, the mass transfer of analyte into extraction solvent is enhanced. To investigate this possibility, mechanical shaking, ultrasonication, and hand shaking were performed for 30 s after dispersive injections and compared to no-mixing results. Absorbance values found were close to each other indicating minimal effects of mixing. Mechanical shaking after extraction was closest to no-mixing, and it was tested again at increasing mixing periods to ascertain whether a significant effect would be observed or not. The mixing periods tested were 15, 30, 60, 120, 240, 480, and 600 s but the absorbance recorded were still lower than no-mixing. The mixing step was therefore ignored in further experiments.
Effect of the viscosity and final dilution
Since the extraction solvent obtained after solidified floating organic drop microextraction is quite viscous, 200 μL methanol was added in order to facilitate its introduction to the flame through the nebulizer system. Even though this step is a necessity for the device, it is basically a dilution process that conflicts the underlying principle of microextraction methods, which is preconcentration. With that awareness, final dilution volume with methanol was also examined. The highest absorbance was recorded for 250 μL in comparison to 100, 150, 200, and 300 μL methanol dilutions as presented in Fig. 2. This suggests that the nebulization efficiency for 250 μL volume was high even though it was relatively more diluted than the lower volumes tested. Another observation was the decreasing trend in standard deviation of replicate results with increasing final volume.
Analytical figures of merit
Under the optimum experimental conditions of SFODME (Table 1), the analytical performance for FAAS and SQT-FAAS systems were determined and are presented in Table 2. Operating parameters of the SQT-FAAS system were obtained from Firat et al. (Fırat et al. 2017) and combined with the optimum extraction method. The enhancement in sensitivity of each system with regards to the FAAS was calculated using the limits of detection. It was found that the sensitivity of FAAS system increased by about four times when the SQT was attached. The combination of SFODME with FAAS (without SQT) resulted in about 42 times increase in sensitivity. This sensitivity increase is significant enough to use SFODME as a stand-alone preconcentration method for FAAS determinations in cases where SQT is neither available nor applicable. The high preconcentration factor obtained by the SFODME method was further boosted by the SQT to get a lower detection limit. The sensitivity increase obtained when SFODME was coupled with SQT-FAAS was 109 times, which correlates with the detection limit of 0.4 ng/mL. The precisions for the studied systems were calculated from six replicate measurements of the lowest concentrations in each calibration plot, and the results that were obtained ranged from 6.3–6.9%.
Recovery
Due to varying characteristics of different samples with different matrices, a developed analytical method may not be efficient in the determination of analyte of interest. In order to observe matrix effects on the quantitative determination of cadmium using developed SFODME-SQT-FAAS method, recovery studies were carried out for tap water and wastewater samples. Before the spiking of cadmium standard into the water samples, blank extractions were performed to see if they already contain Cd or not. No absorbance signal was recorded for both samples indicating the absence of cadmium, or below the detection limit of the method if present. Calibration standard was prepared in deionized water and used to determine the recoveries of tap water spiked and wastewater spiked to different concentrations within the linear calibration range. The percent recoveries recorded for all samples were very low (< 70%), indicating a significant impact of the sample matrices on cadmium recovery. In order to correct this, calibration standards were prepared in a similar water medium to match the sample matrix. The percent recoveries then obtained for tap water spiked to different concentrations using matrix-matching method are given in Table 3. It is clear in Table 3 that recovery results were higher than 90% for both matrices.
Conclusions
With the motivation to improve upon the sensitivity of conventional FAAS, an analytical method namely SFODME-SQT-FAAS was developed for the determination of cadmium at trace levels. The method needs only a slotted quartz tube additionally to a FAAS and very small amounts of organic solvents. This low requirements make the SFODME-SQT-FAAS technique very accessible, cheap, and easy to operate when compared with other advance and expensive devices such as ICP-MS. Good linearity over a wide range (1.0–60 μg/L) was obtained under the optimum conditions in addition to the low %RSD values (even at low concentrations) which indicate high precision for the both extraction process and instrumental measurements. The matrices of tap water and wastewater significantly lowered the recovery of cadmium but sample matrix matching was used over this to obtain appreciable results with high recoveries.
References
Agency for Toxic Substances and Disease Registry, A. (25/08/2014). Toxicological profile for cadmium. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15.
Amin, A. E.-E. A. Z., & Selmy, S. A. H. (2017). Effect of pH on removal of Cu, Cd, Zn, and Ni by cement kiln dust in aqueous solution. Communications in Soil Science and Plant Analysis, 48(11), 1301–1308. https://doi.org/10.1080/00103624.2017.1341914.
Bakirdere, S., Aydin, F., Bakirdere, E. G., Titretir, S., Akdeniz, İ., Aydin, I., et al. (2011). From mg/kg to pg/kg levels: a story of trace element determination: a review. Applied Spectroscopy Reviews, 46(1), 38–66. https://doi.org/10.1080/05704928.2010.520179.
Bakırdere, S., Bölücek, C., & Yaman, M. (2016). Determination of contamination levels of Pb, Cd, Cu, Ni, and Mn caused by former lead mining gallery. [article]. Environmental Monitoring and Assessment, 188(3), 132. https://doi.org/10.1007/s10661-016-5134-5.
Barnhart, S., & Rosenstock, L. (1984). Cadmium chemical pneumonitis. Chest, 86(5), –789, 791. https://doi.org/10.1378/chest.86.5.789.
Cao, Y., Deng, B., Yan, L., & Huang, H. An environmentally-friendly, highly efficient, gas pressure-assisted sample introduction system for ICP-MS and its application to detection of cadmium and lead in human plasma. Talanta, 167, 520–525. https://doi.org/10.1016/j.talanta.2017.02.057.
Chen, G., Hao, X., Li, B. L., Luo, H. Q., & Li, N. B. (2016). Anodic stripping voltammetric measurement of trace cadmium at antimony film modified sodium montmorillonite doped carbon paste electrode. Sensors and Actuators B: Chemical, 237, 570–574. https://doi.org/10.1016/j.snb.2016.06.128.
Demirtaş, İ., Bakırdere, S., & Ataman, O. Y. (2015). Lead determination at ng/mL level by flame atomic absorption spectrometry using a tantalum coated slotted quartz tube atom trap. Talanta, 138, 218–224. https://doi.org/10.1016/j.talanta.2015.02.044.
Feist, B., Mikula, B., Pytlakowska, K., Puzio, B., & Buhl, F. (2008). Determination of heavy metals by ICP-OES and F-AAS after preconcentration with 2,2'-bipyridyl and erythrosine. Journal of Hazardous Materials, 152(3), 1122–1129. https://doi.org/10.1016/j.jhazmat.2007.07.095.
Fırat, M., Bakırdere, S., Fındıkoğlu, M. S., Kafa, E. B., Yazıcı, E., Yolcu, M., et al. (2017). Determination of trace amount of cadmium using dispersive liquid-liquid microextraction-slotted quartz tube-flame atomic absorption spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy, 129, 37–41.
Godt, J., Scheidig, F., Grosse-Siestrup, C., Esche, V., Brandenburg, P., Reich, A., et al. (2006). The toxicity of cadmium and resulting hazards for human health. Journal of Occupational Medicine and Toxicology (London, England), 1, 22–22. https://doi.org/10.1186/1745-6673-1-22.
Hong, F., Jin, T., & Zhang, A. (2004). Risk assessment on renal dysfunction caused by co-exposure to arsenic and cadmium using benchmark dose calculation in a Chinese population. Biometals, 17(5), 573–580.
Järup, L., & Åkesson, A. (2009). Current status of cadmium as an environmental health problem. [review]. Toxicology and Applied Pharmacology, 238(3), 201–208. https://doi.org/10.1016/j.taap.2009.04.020.
Johnson, J. S., & Kilburn, K. H. (1983). Cadmium induced metal fume fever: results of inhalation challenge. American Journal of Industrial Medicine, 4(4), 533–540.
Keskin, G., Bakirdere, S., & Yaman, M. (2015). Sensitive determination of lead, cadmium and nickel in soil, water, vegetable and fruit samples using STAT-FAAS after preconcentration with activated carbon. Toxicology and Industrial Health, 31(10), 881–889.
Khalili Zanjani, M. R., Yamini, Y., Shariati, S., & Jönsson, J. Å. (2007). A new liquid-phase microextraction method based on solidification of floating organic drop. Analytica Chimica Acta, 585(2), 286–293. https://doi.org/10.1016/j.aca.2006.12.049.
Kılınç, E., Bakırdere, S., Aydın, F., & Ataman, O. Y. (2013). In situ atom trapping of Bi on W-coated slotted quartz tube flame atomic absorption spectrometry and interference studies. Spectrochimica Acta Part B: Atomic Spectroscopy, 89, 14–19. https://doi.org/10.1016/j.sab.2013.08.008.
Koyu, A., Gokcimen, A., Ozguner, F., Bayram, D. S., & Kocak, A. (2006). Evaluation of the effects of cadmium on rat liver. Molecular and Cellular Biochemistry, 284(1), 81–85.
Leao, D. J., Junior, M. M. S., Brandao, G. C., & Ferreira, S. L. C. (2016). Simultaneous determination of cadmium, iron and tin in canned foods using high-resolution continuum source graphite furnace atomic absorption spectrometry. Talanta, 153, 45–50. https://doi.org/10.1016/j.talanta.2016.02.023.
O'neill, A., Phillips, D., Bowen, J., & Gupta, B. S. (2015). Contaminants in surface water and sediments near the Tynagh silver mine site, County Galway, Ireland. Science of the Total Environment, 512, 261–272.
Organization, W. H. (2010). Exposure to cadmium: a major public health concern. Geneva: World Health Organization www.who.int/ipcs/features/cadmium.pdf (accessed 13 January 2015).
Özzeybek, G., Erarpat, S., Chormey, D. S., Fırat, M., Büyükpınar, Ç., Turak, F., et al. (2017). Sensitive determination of copper in water samples using dispersive liquid-liquid microextraction-slotted quartz tube-flame atomic absorption spectrometry. Microchemical Journal, 132, 406–410.
P. Biparva, AAM (2012). Microextraction techniques as a sample preparation step for metal analysis. In M. A. Farrukh (Ed.), Atomic Absorption Spectroscopy: InTech.
Tavakkoli, L., & Khanjani, N. (2016). Environmental and occupational exposure to cadmium in Iran: a systematic review. [review]. Reviews on Environmental Health, 31(4), 457–463. https://doi.org/10.1515/reveh-2016-0042.
Tellez-Plaza, M., Guallar, E., Howard, B. V., Umans, J. G., Francesconi, K. A., Goessler, W., et al. (2013). Cadmium exposure and incident cardiovascular disease. Epidemiology (Cambridge, Mass.), 24(3), 421–429. https://doi.org/10.1097/EDE.0b013e31828b0631.
Thongsaw, A., Chaiyasith, W. C., Sananmuang, R., Ross, G. M., & Ampiah-Bonney, R. J. (2017). Determination of cadmium in herbs by SFODME with ETAAS detection. Food Chemistry, 219, 453–458. https://doi.org/10.1016/j.foodchem.2016.09.177.
Turan, N. B., Chormey, D. S., Büyükpınar, Ç., Engin, G. O., & Bakirdere, S. (2017). Quorum sensing: little talks for an effective bacterial coordination. TrAC Trends in Analytical Chemistry, 91, 1–11. https://doi.org/10.1016/j.trac.2017.03.007.
Wong, K., Lee, C., Low, K., & Haron, M. (2003). Removal of Cu and Pb by tartaric acid modified rice husk from aqueous solutions. Chemosphere, 50(1), 23–28.
Yaman, M. (2005). The improvement of sensitivity in lead and cadmium determinations using flame atomic absorption spectrometry. [article]. Analytical Biochemistry, 339(1), 1–8. https://doi.org/10.1016/j.ab.2005.01.009.
Zhang, J., Fang, J., & Duan, X. (2016). Determination of cadmium in water samples by fast pyrolysis–chemical vapor generation atomic fluorescence spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy, 122, 52–55. https://doi.org/10.1016/j.sab.2016.05.009.
Acknowledgements
The authors thank Dr. Çiğdem Şahin for the ligand she supplied for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akkaya, E., Chormey, D.S. & Bakırdere, S. Sensitive determination of cadmium using solidified floating organic drop microextraction-slotted quartz tube-flame atomic absorption spectroscopy. Environ Monit Assess 189, 513 (2017). https://doi.org/10.1007/s10661-017-6232-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6232-8