Abstract
In the present study, levels of metal contamination caused by former lead mining area were figured out. For this purpose, Pb, Cd, Cu, Ni, and Mn were determined not only in sediment samples taken from different places of the mining area but also in some plants taken around the mining place. In the digestion of plant samples, dry ashing procedure was applied. Flame atomic absorption spectrophotometer (FAAS) was used in the determination of analytes of interest. All the parameters in digestion and detection procedures were optimized to obtain efficient digestion and high sensitivities for analytes. Standard addition and direct calibration methods were applied to find whether there was any matrix interference to affect the determination of analytes. Mn concentration was found to be the highest for each sample analyzed. Lead concentration was found to be between 41 and 249 mg/kg in soil/sediment samples and between 2.2 and 1003 mg/kg in plant samples. The highest contamination levels for all of the analytes with the exception of Cd were found in current sediment sample.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination has become an increasing global environmental problem for the last decades. In recent years, determination of a variety of heavy metals in different environmental samples has been performed by many scientists all over the world due to their adverse health effects. It has been reported that excessive dietary accumulation of heavy metals may cause serious systemic health problems in the human body (Oliver 1997; Adabanija and Oladunjoye 2014; Yaman 2006). Lead and cadmium as analytes in this study are members of the most serious heavy metal family. These elements are common toxic metals in the environment. Human beings are exposed to these elements through many sources such as ambient air, drinking water, food, industrial materials, mining facilities, and consumer products (Zhai et al. 2015). Cd and Pb exposure cause a broad range of adverse health effects such as renal, hepatic, and reproductive disorders in humans and animals (Hong et al. 2004; Koyu et al. 2006; Rehm and Waalkes 1988). High amounts of Pb and Cd are still present in the environment and continue to cause health concerns (Ozen et al. 2003; Yaman et al. 2000, 2005, 2007a, b; Yaman 1999; Yaman and Gucer 1995; Er et al. 2013; Kaplan and Yaman 2009; Kaplan et al. 2011a, b; Kaya and Yaman 2008). Copper is known as an essential micronutrient at low concentrations, but it becomes toxic at higher concentrations (Upadhyay 2014). Common industrial sources of Cu are reported as municipal garbage, agricultural chemicals, construction sites, electrical equipment, and vehicles (Bertram et al. 2002; Kaplan et al. 2011a, b; Akkus et al. 2013). In literature, nickel is described as a potential essential trace element for plants and animals and identified as essential micronutrient for activation of urease in higher plants. Natural geological activities such as weathering, volcanoes, and some industrial processes such as mining, milling, melting, metallurgical processes, and the combustion of fossil fuels result in nickel emission into the environment. It was also reported that this element can be accumulated by plants and animals (Schaumlöffel 2012; Yaman and Bakirdere 2003). Manganese is known as one of the essential elements for humans and animals. This element is essential for many functions such as development of the skeletal system, energy metabolism, function of reproductive hormones, activation of certain enzymes, and antioxidant functions. In case of deficiency of this element, poor bone formation, birth defects, and increased susceptibility to seizures may occur (Eum et al. 2014; ATSDR 2008; Aschner and Aschner 2005; Papavasilious et al. 1979; Keen et al. 1999; Aschner 2000). In addition, neurotoxic effect of environmental exposure to manganese for children and infants was reported in literature (Molina-Villalba et al. 2015).
There are many studies in literature to figure out the effects of mining facilities on the contamination of the environment (Xu et al. 2015; Paoliello et al. 2002; Yang et al. 2006). Mining activities are undoubtedly one of the biggest sources for many toxic elements to the environment. Mining facilities may release various metals including lead and cadmium into the surrounding environment and cause contamination in air, ground/surface waters, plants, and soils. Different health problems appear among local residents, and these effects persist long after ceasing the mining activities (Varrica et al. 2014). Hence, studies related with the determination of contamination level caused by mining area are very important.
In the determination of elements of interest, different instruments such as ICP-MS (Candeias et al. 2015), ICP-OES (Beeram et al. 2012), GFAAS (Bakirdere et al. 2013), and flame atomic absorption spectrophotometer (FAAS) (Waterlot et al. 2013) have been used in literature. All these instruments have some advantages over others. FAAS offering moderate sensitivity and high selectivity has attracted considerable interest as an alternative way to monitor and quantify many heavy metals in different food and environmental samples (Keskin et al. 2013; Bakirdere and Yaman 2008).
This study was conducted to assess metal contamination levels caused by a former lead mining area. In the determination of Pb, Cd, Cu, Ni, and Mn, FAAS was used under optimum conditions.
Experimental
Apparatus and reagents
In the determination of analytes, ATI UNICAM 929 Model FAAS equipped with hollow cathode lamps was used. Experimental conditions of FAAS instrument are summarized in Table 1.
All of the chemicals and reagents used in this study were in high purity grade. Double-distilled water was used in all standard preparations and dilutions. All of the containers were washed up with nitric acid and rinsed with double-distilled water before using. All glass beakers and containers were kept in 1.0 mol/L HNO3 to eliminate/reduce any possible contamination when not in use. In the preparation of working standard solutions, proper dilutions were performed by 1.0 mol/L nitric acid from the stock solutions of Pb, Cd, Cu, Ni, and Mn (1000 mg/L).
Sampling area
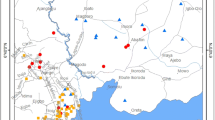
The sampling area known as “Derebacı Gallery” was one of the most important mining areas for lead and some other elements/minerals. The production of lead went on here for a long period of time. This gallery is located about 2 km south part of the Keban Town of Elazığ and extends eastward (Fig. 1, http://cografyaharita.com/turkiye_mulki_idare_haritalari2.html). Surface and underground water in this mining area flows to the Karakaya Dam Lake. Some of the minerals have been carried by water, accumulating at the entrance and inside part of the gallery and produced about 0.5-m sediments in depth.
Collection and digestion of samples
In order to perform the determination of Pb, Cd, Cu, Ni, and Mn, soil and sediment samples were collected from different distances to mining area. In addition, current sediment and contaminated soil samples from outside the drift were collected for analysis. Samples were taken from the surface and 25 cm deep to find out the rate of deposition with time. In the analysis, samples were dried at 105 °C. After the homogenization step, finely powdered and homogenized 0.40 g sample was taken and 2.0 mL of HNO3/H2O2 mixture (2/1) was added. Samples were slowly shaken and heated on a hot plate till dryness. After cooling the sample, 2.0 mL of 0.75 mol/L nitric acid was added to the beaker and sample was centrifuged to obtain a clear solution separated from the solid part. After the proper amount of dilution, solutions containing the analytes of interest were analyzed by FAAS system. Blank measurement was also performed using the same procedure in the digestion step.
Different plant samples were also collected around the mining gallery to find the contamination levels. For this purpose, fresh/dry bulrush and moss were selected due to the high abundance of these species in the mining area. In the digestion step, samples were washed with tap water and distilled water to remove any possible soil and dust remnants and then dried at 100 °C. Proper amounts of dried samples were taken and put into pyrex beakers, and 1.0 mL H2SO4 was added to each beaker to eliminate/minimize the volatilization losses during the ashing procedure. Samples were inserted in the ashing furnace and ashed at 500 °C for 5 h. Nitric acid (2.0 mL of 0.75 mol/L) was added to the white ash, and samples were filtered to obtain a clear solution. Blank sample was also prepared using the same procedure. Determination of Pb, Cd, Cu, Ni, and Mn in clear solution was performed by FAAS system.
Result and discussion
Mining activities have been going on in several parts of Turkey. Some of the mining activities were stopped, but heavy metal contamination continues. In this study, the effect of a closed lead mining gallery on the environment was tried to be figured out. In order to find the best digestion and measurement conditions, all of the system parameters including acid type(s) and ratio in the digestion procedure, flame type, and sample flow rate in the FAAS system were optimized. Under the optimum conditions, digested samples were sent to the FAAS system for quantitative measurement of Pb, Cd, Cu, Ni, and Mn in the soil, sediment, and plant samples. In order to figure out whether there was any matrix interference on the analyte signal, standard addition method was applied to one sediment and plant samples. It was observed that there was no change in the slopes of linear calibration plot and standard addition method; therefore, direct calibration plot was applied throughout this study.
Lead
Lead is one of the most dangerous heavy metals causing many health problems even at trace levels. Possible contamination of environmental samples with this element could affect living organisms. The risk of lead poisoning through the food chain is getting higher as the soil lead level rises. According to a study conducted by Farag et al., significantly higher blood lead concentrations in a subsample of participants with hematuria in mining sites near Potosi, Bolivia were found (Farag et al. 2015). Hence, studies regarding the determination of contamination levels of this element are very important in scientific area. Results found in this study are seen in Tables 2 and 3. As seen in these tables, the highest concentration of lead was found in current sediment and from soil outside the drift. There was no big difference observed in the results of samples taken from surface and the depth of 25 cm. Among the plant samples analyzed, fresh moss taken 15.0 m away from gallery entrance having some ore residue had the highest lead level, 1003 ± 96 mg/kg, while a relatively low concentration of lead was found in the same plant taken from the gallery entrance. This shows that deposition of lead 15 m away from the gallery is higher than other sampling points. Due to the ore residue in this sampling point, this contamination level could be considered as normal. In addition, lead levels in dry bulrush at the gallery entrance and 8.0 m away from the gallery were found to be higher than fresh bulrush taken from the same points.
Cadmium
Cadmium has also been emitted to the environment from different mining facilities. There are many studies in literature to assess the contamination levels caused by mining activities. In one of the studies, heavy metal contamination including cadmium was evaluated by Pascauld et al., and they observed that cadmium contamination is very high even at very long distances from mining waste (Pascaud et al. 2015). In our study, results given in Table 2 reveal the differences in the level of cadmium in the sediments taken from the surface and a depth of 25 cm. It is clear that Cd levels in the inner gallery are higher than that in the surface sediments. In fresh moss taken from 15.0 m away from the gallery entrance (contaminated with ore residue), cadmium concentration was found to be the highest when compared with plants taken from other sampling points.
Copper
High amounts of copper have been released into the environment by mining activities. Many scientists have indicated the effects of mining activities on copper contamination in different environmental samples. O’neill et al. studied contamination with different heavy metals in surface water and sediments near the Tynagh silver mine site, and they found out that Cu, Cd, Fe, Mn, Pb, and Zn concentrations in both water and sediment were above guideline values downstream of the site (O’Neill et al. 2015). The results given in Table 2 showed that the copper concentrations in sediment and soil samples ranged from 0.6 to 11.5 mg/kg. In three sampling points, copper concentrations in 25 cm deep were higher than surface samples. This might be an indication of reduction in copper emission by the gallery. The plant samples in the studied area recorded Cu concentrations that varied from 2.2 to 34.5 mg/kg.
Nickel
There are some literature findings about the effect of mining activities on environmental Ni contamination. Ngole and Ekosse studied on copper, nickel, and zinc contaminations in soils within the mining area and landfilling environments. They observed that contamination level of elements of interest in soils around the mining area was considerably higher than those around the landfill. In that study, Ni concentration in the mining area was found to be 153.0 mg/kg while the level was 0.91 mg/kg in the landfill (Ngole and Ekosse 2012). This study is a good indication to show the effects of mining on environmental contamination of nickel. In our study, nickel concentrations were found to be significantly higher in current surface and deep sediments than soil and sediments taken from other sampling points. Among the plants collected, the highest Ni concentration (33.2 mg/kg) was observed in the fresh moss taken 15.0 m from the gallery entrance while the dry bulrush taken 8.0 m from the gallery entrance had the lowest Ni level, 0.70 mg/kg.
Manganese
Manganese is another well-known metal released into the environment by mining facilities, and it should be studied in mining regions due to its potential accumulation both in the environment and living organisms. Wei et al. performed a novel study regarding molecular diversity of arbuscular mycorrhizal fungi associated with a Mn hyperaccumulator Phytolacca americana in Mn mining area (Wei et al. 2014). They found out high contamination mining environment with manganese. Molina-Villalba et al. reported the biomonitoring of arsenic, cadmium, lead, manganese, and mercury in urine and hair of children living near mining and industrial areas (Molina-Villalba et al. 2015). In our study, the mining area was found to be heavily contaminated with manganese (Tables 2 and 3). In every sampling point, Mn concentration was much higher than other elements determined. In current sediment taken from 25 cm deep, 15,000 mg/kg Mn was found while the level was 4900 mg/kg in current surface sediment. Similar findings were observed for plant samples. In each plant sample, Mn concentration was found to be much higher than others. In fresh moss, 1170 mg/kg Mn was detected. These results demonstrate high contamination level of mining region with Mn by mining gallery.
Conclusion
In this study, assessment of the metal contamination in sediment, soil, and plant samples taken from around the former lead mining gallery was performed. Results found show that the environment of the mining area is likely affected by leaching of metals. High concentrations of some metals were observed not only in sediment but also in plant samples collected around the mining gallery. Metals were released and deposited in the sediments and plant samples. Values of contamination level indicated that the metal pollution in the current surface sediment was on the order of Mn > Pb > Cd > Ni > Cu. High levels of lead, manganese, and some other metals in sediment and soil samples in mining area might lead to the potential contamination of different environmental samples including plants and water. It can be concluded that the population living near mining areas might have a variety of health concerns. Contamination within the precincts of the mining environment may be dramatically decreased by rehabilitation of the gallery region.
References
Adabanija, M. A., & Oladunjoye, M. A. (2014). Geoenvironmental assessment of abandoned mines and quarries in South-western Nigeria. Journal of Geochemical Exploration, 145, 148–168.
Akkus, S., Bal, T., Karaaslan, N. M., Yaman, E., Kilinc, E., & Yaman, M. (2013). Fractionation of Ni Cr and Cu in soil by sequential extraction procedure and determination by inductively coupled plasma optical emission spectrometry. Clean: Soil, Air, Water, 41, 1229–1234.
Aschner, M. (2000). Manganese: brain transport and emerging research needs. Environmental Health Perspectives, 108, 429–432.
Aschner, J. L., & Aschner, M. (2005). Nutritional aspects of manganese homeostasis. Molecular Aspects of Medicine, 26, 353–362.
ATSDR. (2008). Toxicological profile for manganese. Atlanta: Agency for Toxic Substances and Disease Registry.
Bakirdere, S., & Yaman, M. (2008). Determination of lead, cadmium and copper in roadside soil and plants in Elazig, Turkey. Environmental Monitoring and Assessment, 136, 401–410.
Bakirdere, S., Yaroğlu, T., Tırık, N., Demiröz, M., Fidan, A.K., Maruldalı, O., & Karaca, A. (2013). Determination of As, Cd and Pb in tap water and bottled water samples by using optimized GFAAS system with Pd-Mg and Ni as matrix modifiers, J. Spec., Article Number: UNSP 824817, http://dx.doi.org/10.1155/2013/824817.
Beeram, S., Morris, A., Hardaway, C. J., Richert, J. C., & Sneddon, J. (2012). Studies of whole crawfish shells for the removal of chromium, lead, and selenium ions in solution. Instrumentation Science and Technology, 40, 618–639.
Bertram, M., Graedel, T. E., Rechberger, H., & Spatari, S. (2002). The contemporary European copper cycle: waste management subsystem. Ecological Economics, 42, 3–57.
Candeias, C., Avila, P.F., da Silva, E.F., & Teixeira, J.P. (2015). Integrated approach to assess the environmental impact of mining activities: estimation of the spatial distribution of soil contamination (Panasqueira mining area, Central Portugal), Environ. Monit. Assess., 187, Article Number: 135.
Er, C., Senkal, B. F., & Yaman, M. (2013). Determination of lead in milk and yoghurt samples by solid phase extraction using a novel aminothioazole-polymeric resin. Food Chemistry, 137, 55–61.
Eum, J. H., Cheong, H. K., Ha, E. H., Ha, M. N., Kim, Y., Hong, Y. C., Park, H., & Chang, N. (2014). Maternal blood manganese level and birth weight: a MOCEH birth cohort study. Environmental Health, 13, 31. doi:10.1186/1476-069X-13-31.
Farag, S., Das, R., Strosnider, W. H. J., & Wilson, R. T. (2015). Possible health effects of living in proximity to mining sites near Potosi, Bolivia. Journal of Occupational and Environmental Medicine, 57, 543–551.
Hong, F., Jin, T., & Zhang, A. (2004). Risk assessment on renal dysfunction caused by co-exposure to arsenic and cadmium using benchmark dose calculation in a Chinese population. Biometals, 17, 573–580.
Kaplan, O., & Yaman, M. (2009). Selective and sequential extraction of lead in soil samples and plant parts taken from a serpentine and copper mining area. Atomic Spectroscopy, 30, 1–9.
Kaplan, O., Ince, M., & Yaman, M. (2011a). Sequential extraction of cadmium in different soil phases and plant parts from a former industrialized area. Environmental Chemistry Letters, 9, 397–404.
Kaplan, O., Kaya, G., & Yaman, M. (2011b). Sequential and selective extraction of copper in different soil phases and plant parts from former industrialized area. Communications in Soil Science and Plant Analysis, 42, 2391–2401.
Kaya, G., & Yaman, M. (2008). Online preconcentration for the determination of lead, cadmium and copper by slotted tube atom trap (STAT)-flame atomic absorption spectrometry. Talanta, 75, 1127–1133.
Keen, C. L., Ensunsa, J. L., Watson, M. H., Baly, D. L., Donovan, S. M., Monaco, M. H., & Clegg, M. S. (1999). Nutritional aspects of manganese from experimental studies. Neurotoxicology, 20, 213–223.
Keskin, G., Bakırdere, S., & Yaman, M. (2013). Sensitive determination of lead, cadmium and nickel in soil, water, vegetable and fruit samples by using STAT-FAAS after preconcentration with activated carbon. Toxicology and Industrial Health. doi:10.1177/0748233713484650.
Koyu, A., Gokcimen, A., Ozguner, F., Bayram, D. S., & Kocak, A. (2006). Evaluation of the effects of cadmium on rat liver. Molecular and Cellular Biochemistry, 284, 81–85.
Molina-Villalba, I., Lacasana, M., Rodriguez-Barranco, M., Hernandez, A. F., Gonzalez-Alzaga, B., Aguilar-Garduno, C., & Gil, F. (2015). Biomonitoring of arsenic, cadmium, lead, manganese and mercury in urine and hair of children living near mining and industrial areas. Chemosphere, 124, 83–91.
Ngole, V. M., & Ekosse, G. I. E. (2012). Copper, nickel and zinc contamination in soils within the precincts of mining and landfilling environments. International Journal of Environmental Science and Technology, 9, 485–494.
Oliver, M. A. (1997). Soil and human health: a review. European Journal of Soil Science, 48, 573–592.
O’Neill, A., Phillips, D. H., Bowen, J., & Sen Gupta, B. (2015). Contaminants in surface water and sediments near the Tynagh silver mine site, County Galway, Ireland. Science of the Total Environment, 512–513, 261–272.
Ozen, O. A., Songur, A., Sarsilmaz, M., Yaman, M., & Kus, I. (2003). Zinc, copper and iron concentrations in cerebral cortex of male rats exposed to formaldehyde inhalation. Journal of Trace Elements in Medicine and Biology, 17, 207–209.
Paoliello, M. M. B., De Capitani, E. M., da Cunha, F. G., Matsuo, T., Carvalho Mde, F., Sakuma, A., & Figueiredo, B. R. (2002). Exposure of children to lead and cadmium from a mining area of Brazil. Environmental Research, 88, 120–128.
Papavasilious, P. S., Kutt, H., Miller, S. T., Rosal, V., Wang, Y. Y., & Aronson, R. B. (1979). Seizure disorders and trace metals: manganese tissue levels in treated epileptics. Neurology, 29, 1466–1473.
Pascaud, G., Boussen, S., Soubrand, M., Joussein, E., Fondaneche, P., Abdeljaouad, S., & Bril, H. (2015). Particulate transport and risk assessment of Cd, Pb and Zn in a Wadi contaminated by runoff from mining wastes in a carbonated semi-arid context. Journal of Geochemical Exploration, 152, 27–36.
Rehm, S., & Waalkes, M. P. (1988). Cadmium-induced ovarian toxicity in hamsters, mice, and rats. Fundamental and Applied Toxicology, 10, 635–647.
Schaumlöffel, D. (2012). Nickel species: analysis and toxic effects. Journal of Trace Elements in Medicine and Biology, 26, 1–6.
Upadhyay, R. K. (2014). Metal stress in plants: its detoxification in natural environment. Brazilian Journal of Botany, 37, 377–382.
Varrica, D., Tamburo, E., Milia, N., Vallascas, E., Cortimiglia, V., De Giudici, G., Dongarrà, G., Sanna, E., Monna, F., & Losno, R. (2014). Metals and metalloids in hair samples of children living near the abandoned mine sites of Sulcis-Inglesiente (Sardinia, Italy). Environmental Research, 134, 366–374.
Waterlot, C., Bidar, G., Pelfrene, A., Roussel, H., Fourrier, H., & Douay, F. (2013). Contamination fractionation and availability of metals in urban soils in the vicinity of former lead and zinc smelters, France. Pedosphere, 23, 143–159.
Wei, Y., Hou, H., Li, J. N., ShangGuan, Y. X., Xu, Y. F., Zhang, J., Zhao, L., & Wang, W. Y. (2014). Molecular diversity of arbuscular mycorrhizal fungi associated with an Mn hyperaccumulator-Phytolacca americana, in Mn mining area. Applied Soil Ecology, 82, 11–17.
Xu, W., Ni, S., Gao, Y., & Shi, Z. (2015). Reconstruction of the cadmium contamination history of a river floodplain from Maoniuping mining area (China) by gamma Ray spectrometry and inductively coupled plasma mass spectrometry. Spectroscopy Letters, 48, 542–552.
Yaman, M. (1999). Determination of cadmium and lead in human urine by STAT-FAAS after enrichment on activated carbon. Journal of Analytical Atomic Spectrometry, 14, 275–278.
Yaman, M. (2006). Comprehensive comparison of trace metal concentrations in cancerous and non-cancerous human tissues. Current Medicinal Chemistry, 13, 2513–2525.
Yaman, M., & Bakirdere, S. (2003). Identification of chemical forms of lead cadmium and nickel in sewage sludge of waste water treatment facilities. Microchimica Acta, 141, 47–54.
Yaman, M., & Gucer, S. (1995). Determination of cadmium and lead in vegetables after activated-carbon enrichment by atomic-absorption spectrometry. Analyst, 120, 101–105.
Yaman, M., Dilgin, Y., & Gucer, S. (2000). Speciation of lead in soils and relation with its concentration in fruits. Analytica Chimica Acta, 410, 119–125.
Yaman, M., Atici, D., Bakirdere, S., & Akdeniz, I. (2005). Comparison of trace metal concentrations in malign and benign human prostate. Journal of Medicinal Chemistry, 48, 630–634.
Yaman, M., Kaya, G., & Simsek, M. (2007a). Comparison of trace element concentrations in cancerous and non-cancerous endometrium and over tissues. International Journal of Gynecological Cancer, 17, 220–228.
Yaman, M., Kaya, G., & Yekeler, H. (2007b). Distribution of trace metal concentrations in paired cancerous and non-cancerous human stomach tissues. World Journal of Gastroenterology, 13, 612–618.
Yang, Q. W., Lan, C. Y., Wang, H. B., Zhuang, P., & Shu, W. S. (2006). Cadmium in soil–rice system and health risk associated with the use of untreated mining wastewater for irrigation in Lechang China. Agricultural Water Management, 84, 147–152.
Zhai, Q., Narbad, A., & Chen, W. (2015). Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients, 7, 552–571.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Bakırdere, S., Bölücek, C. & Yaman, M. Determination of contamination levels of Pb, Cd, Cu, Ni, and Mn caused by former lead mining gallery. Environ Monit Assess 188, 132 (2016). https://doi.org/10.1007/s10661-016-5134-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5134-5