Abstract
Recent arsenic pollution of drinking-water wells across Lebanon, northeastern USA has led to a growing concern about possible impact of agricultural activities on the hydrologic system. This study assessed the concentrations and distributions of arsenic and ten other elements (Al, Cd, Cr, Cu, Fe, K, Mn, P, Pb, and Zn) in stream sediments. The overall goal is to determine the extent of these elements within the fluvial systems, as well as overall sediment quality. A total of 65 stream sediments samples were collected, and analyzed for particle size distributions, organic matter contents, trace, and major elements concentrations. Results showed spatial variability in the concentrations of trace elements due to variation in sediments grain sizes, organic matter content, as well as land use activities within the study area. Calculation of sediment enrichment with respect to As, Cd, Cr, Cu, Mn, Pb, and Zn showed that about 48–52% of all the sampling locations are not enriched, nevertheless, approximately 2–11% of all the sampling locations are significantly enriched, an indication of anthropogenic input. However, results of ecological risk assessment showed no connection with sediment enrichment as most sampling locations have concentrations below the threshold probable effect concentration (PEC) value. Statistical analysis using principal component analysis (PCA) extracted three significant components explaining over 72% of total variance covering elements having origin in both natural and anthropogenic sources, thus suggesting that the concentrations and distribution of these elements within stream sediments are related to a combination of weathering processes on the bedrock geology, and anthropogenic activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The input of trace elements into drainage systems could be through natural sources relating to rock weathering and soil formation processes (Robinson and Ayotte 2006; Sharma and Rajamani 2000) or anthropogenic sources relating to human activities. For example, the use of organoarsenicals (roxarsone) as poultry feed additives has been known as a major source of arsenic within predominantly agricultural regions (Oyewumi et al. 2014; Rutherford et al. 2003). Roxarsone is often added to poultry feed at concentrations between 27 and 45 g per ton in order to boost growth, and prevent survival of cossidia intestinal parasites (O’Connor et al. 2005). However, roxarsone uptake in poultry tissue is low (Anderson and Chamblee 2001), as most of the roxarsone is excreted resulting in elevated concentration (15–77 mg/kg) of roxarsone in poultry litter (Anderson and Chamblee 2001; Garbarino et al. 2003). Studies have shown application of poultry litter containing roxarsone as fertilizer have led to the release of arsenic to agricultural farmlands (Codling et al. 2008), soil water (Brown et al. 2005; Kibet et al. 2013; Oyewumi and Schreiber 2012), drainages ditches (Church et al. 2010), and air from nearby poultry farms (O’Connor et al. 2005; Taupe et al. 2016). Other potential sources of trace and major elements include the use of biosolids, pesticides, and soil fumigations (Böhlke 2002; Gray et al. 1999), mining activities (Garcia-Sanchez and Alvarez-Ayuso 2003), gasoline traffic emission (Won et al. 2007), urbanization (Sutherland 2000; Wong et al. 2006), landfills (Looser et al. 1999), coal and oil combustion, manufacturing, and refuse incineration (Nriagu and Pacyna 1988).
The contamination of soils, sediments, water resources and biota by trace elements is of major concern because of their toxicity, and persistency in the environment (Ikem et al. 2003). Over the last decade, the geochemistry of stream sediments and soil within predominantly agricultural area, such as North Dakota, USA (Holmgren et al. 1993), northern Bangladesh (Bhuiyan et al. 2010), Gascogne region, France (N’Guessan et al. 2009), Algeria (Maas et al. 2010), Berlin, Germany (Säumel et al. 2012), China (Tang et al. 2010; Yu et al. 2012) have been studied for spatial and temporal assessment of the impact of human activities (Rubio et al. 2000; Zhao et al. 2014). Stream sediments are important environmental sinks and play critical roles in controlling the mobility of trace elements within fluvial systems (Ikem et al. 2003; Pekey 2006; Rubio et al. 2000; Segura et al. 2006; Semrod and Gourley 2014). However, the overall concentration of trace elements in stream sediments do not only depend on the pollution sources, but on the general character of solutes being transported as well as the physical composition of the sediments itself, such as grain size, surface area, surface charge (Presley et al. 1980; Viers et al. 2009). Incorporation of these solutes in sediments is further influenced by the pH, dissolved oxygen, geomorphologic, and hydrologic factors of the fluvial system (Emmerson et al. 1997; Pekey 2006; Semrod and Gourley 2014).
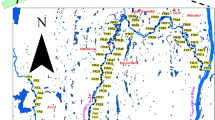
Lebanon, Connecticut is a predominantly agricultural community within the Northeastern USA (Fig. 1). It remains one of Connecticut’s largest agricultural community despite residential growth pressure (Lebanon 2010), and home to one of the largest egg and broiler poultry farm (Kofkoff), with over 1.2 million yearly chickens production. Manure from Kofkoff poultry farm is often used throughout Lebanon to fertilize corn and hay fields, nurseries and greenhouses, orchards, and Christmas tree farms. Routine testing of drinking water in 2013 at the Lebanon Elementary School revealed traces of arsenic (As) in drinking fountains. In addition, various drinking wells in Lebanon showed elevated concentration of As above EPA standard of 10 μg/L (Metcalf 2015). While various studies on trace elements chemistry in groundwater and topsoil have been conducted within Lebanon area (Kim et al. 2015), the source(s) of arsenic to Lebanon water wells is yet to be fully understood. In order to understand the impact of agricultural activities in Lebanon, CT, it is necessary to elucidate the distribution, concentration and overall fate and transport of trace and major elements in various environments. This study examined the geochemical signature and spatial distribution patterns of arsenic and ten other elements of interest (Al, Cd, Cr, Cu, Fe, K, Mn, P, Pb, and Zn) within stream sediments of a predominantly agricultural watershed in Lebanon, CT.
Materials and method
Sample collection and analyses
A total of 65 stream sediment samples were collected from the Lebanon fluvial system between June 2014 and July 2015 (Fig. 1). Sampling locations were based on the distribution of land use activities as well as accessibility. Each sampling location was marked with the aid of Garmin GPS unit for creation of location map with GIS software. The sampling method employed at all sites were consistent: stream sediment samples were collected using a sterile stainless hand trowel and shovel, which were cleaned with alconox between locations. Samples were collected from the exposed and submerged edges of both sides and bottom of rivers/streams; with samplings depth ranges from the surface to about 10 cm below the surface. In locations where bridges cross river, we made every attempt to sample upstream from the bridge. The samples were later thoroughly mixed in a bowl to get a representative sample. During this process, twigs, leaves and other large debris were removed from the samples before packaging them into a labeled ziploc bags for further laboratory analysis.
In the laboratory, sediment samples were air dried, thoroughly homogenized, and sifted through a 2-mm sieve size. Fractions of the dried samples were analyzed for organic atter (OM) fraction using loss on ignition (Dean 1974; Konen et al. 2002). Particle size analysis (PSA) was also carried out using the ASTM 152H hydrometer method after dispersion of the soil with sodium hexametaphosphate (Calgon) for 24 hours (After Dane and Topp 2002). In addition, portion of those sample already sifted through 2 mm sieves were acid-digested using aqua regia based on EPA 3052 method (After Agazzi and Pirola 2000) in a CEM Mars 6 Express microwave. The acids used were not aggressive enough to dissolve the silicate lattice of the samples, only the adsorbed ions by clays, OM, and oxides were extracted into solution. The digested solution was analyzed for arsenic and ten other chemical elements (Al, As, Cd, Cr, Cu, Fe, K, Mn, P, Pb, and Zn) using inductively coupled plasma-atomic absorption spectrometry (ICP-AES) at Virginia Tech Soil Testing Laboratory, Blacksburg, Virginia. For each individual sample, triplicate measurements were recorded by the ICP-AES detector and any sample that showed poor precision were repeated. In addition, initial, and continuing verification of calibration curve was performed for every 10 samples analyzed. Certified standard reference soil (Ref 2587) obtained from National Institute of Standards and Technology (NIST, Gaithersburg MD 20899) was also extracted with the batch using the same method to determine the degree of recovery of the method.
Assessment of sediment chemistry
Sediment enrichment factors
A number of approaches have been used to assess anthropogenic contributions to stream sediments in many related studies (Caeiro et al. 2005; Chabukdhara and Nema 2012; Loska 2004; Oyewumi et al. 2014; Sutherland 2000). One such approach is the estimation of enrichment factor (EF) defined as the ratio of the concentration of a particular element in stream sediment samples to its natural background concentrations or concentrations from pristine unpolluted areas as shown in the equation below.
where C m is the measured concentration of elements in sediment, while B m is the background reference concentration of the same element. The world average shale concentration is often used as background values, while some studies have also used average concentrations from uncontaminated area (Bhuiyan et al. 2010; Lee 1994; Oyewumi et al. 2014). In this study we used the average concentrations of trace elements at the headwater of streams located within forested area (of the same watershed) as our background reference concentrations.
Another important aspect of EF calculation involves compensation for a possible influence of grain size distribution and provenance on the trace element concentrations in the sediment. This is often done by normalization with a reference conservative element. Previously used reference elements for normalization include Mn (Yongming et al. 2006), Al (Oyewumi et al. 2014; Pokrovsky et al. 2006; Sutherland 2000), Ca (Loska 2004), Fe (Bhuiyan et al. 2010; Schiff and Weisberg 1999), Sc (Lee 1994; N'Guessan et al. 2009) and Li (Loring 1990). For this study we estimated EF by normalizing all chemical elements’ concentration to Al concentration in stream sediments to compensate for the effect of grain size and mineralogy variation. Thus the EF takes the simple form (After Oyewumi et al. 2014; Sutherland 2000)
where C n_sample is the element concentration in the sediment sample, C Al_sample is the Al concentration in the sample, C n − background is the average concentration of element in the reference samples, and C Al_background is the average concentration of Al in the reference samples.
Various studies have recognized different descriptive classes of enrichment; however, Oyewumi et al. (2014) pointed out an issue with absolute scale of enrichment due to difficulty in determining the actual threshold of enrichment because of the natural variability of elements from one region to another. In this study, we used population percentile values from frequency distribution plots of enrichment factors to operationally define three enrichment categories (After Oyewumi et al. 2014). The EF values that fall between zero and 50th population percentiles (EF ≤ 50th percentile) are classified as not enriched (which indicates no/to low enrichment), while EF values within 50th percentile < EF <90th percentile are classified as moderately enriched, and EF values greater than EF ≥ 90th percentile are considered as significantly enriched.
Evaluation of consensus–based sediment quality
We determined the impact of trace element concentrations on benthic (bottom dwelling) organisms by comparing trace element concentrations to consensus sediment quality guidelines (SQG), based on consensus threshold effect concentrations (TEC), and threshold probable effect concentrations (PEC). The TEC is the level of concentration of elements in sediment below which no effect on the benthic organisms is expected, while the PEC is the sediment concentration above which negative effects are expected frequently (After MacDonald et al. 2000). Locations with concentrations below TEC values do not require any attention, while locations with concentrations above PEC values indicate significant contamination, and thus requires monitoring due to a possible impact on benthic ecosystem within such streams.
Statistical analyses
All statistical analyses were carried out using SAS 9.2 with significant level of α = 0.05(JMP®, Version 12), and statistical package for social sciences (SPSS, Version 22). Geochemical data were tested for normality using Shapiro-Wilk W test (p < 0.05). Non-normal data were log-transformed to obtain a more symmetrical distribution that meet assumption of normality and variance homogeneity of a parametric statistics. Descriptive data analyses such as mean, median, standard deviation, minimum and maximum concentrations were determined.
Correlation and cluster analyses were used to determine strength of geochemical association or relationship among different chemical elements and various components of the sediments such as organic matter (OM), clay, and silt fractions. Understanding such relationship is very important to determining possible partitioning of these elements within the fluvial system. In addition, principal component analysis (PCA) was applied to geochemical data in order to explore possible similarities in behavior of the metals(After de Andrade Passos et al. 2010; Song et al. 2011), and to reduce dimensionality of the geochemical data, and possible sources (Borůvka et al. 2005; de Andrade Passos et al. 2010). Significant principal components (PC) were selected using the scree plot generated from the PCA as well as eigen values greater than 1 (>1) as suggested by the Kaiser criterion (Kaiser 1974). The components were further examined using varimax rotation, and loading weight of elements greater than 0.50 were further considered significant and relevant to each component selected.
Results and discussions
Stream sediment characteristics and concentrations
Table 1 presents summary of geochemical data in stream sediments for eleven chemical elements (Al, As, Cd, Cr, Cu, Fe, K, Mn, P, Pb, and Zn) in mean values, standard deviation, minimum and maximum concentrations. All sediment concentrations were reported on a dry weight basis. Results showed Fe has the highest mean concentrations of 7900 mg/kg followed by Al with an average concentration of 5500 mg/kg, respectively. Concentrations of P and K are widespread with over 95% of the sampling locations having P and K concentrations well above 200 mg/kg. The observed distribution of P and K concentrations are likely due to input from agricultural activities within the study area due to the use of inorganic (phosphate) and organic (poultry and animal manure) fertilizer. Studies have shown poultry litter contain about 1700 to 24,900 mg/kg P concentration (Jackson et al. 2003; Oyewumi and Schreiber 2012; Rutherford et al. 2003), and variable concentrations of K ranging from about 8792 to 59,940 mg/kg (Jackson et al. 2003).
Trace elements such as As, Cd, Cr, Cu, Mn, Pb, and Zn have generally low mean concentrations of 4.1, 0.3, 65, 7.3, 171, 10, 23 mg/kg respectively. Concentrations of trace elements have been attributed to a number of factors such as the presence of polluting sources (Förstner and Salomons 1980; Pekey 2006) like agricultural activities, and sewage disposal facilities. Other factors include the type and relative abundance of organic matter content in soil and sediments (Lair et al. 2007), type and abundance of clay minerals (Shaheen et al. 2013), and metal oxides (Hering et al. 2014; Stollenwerk 2003), which influence the quantity of available binding sites (Tyler and McBride 1982) for sorption. Also, basic physicochemical properties of the soil like the pH (Lukman et al. 2013), redox conditions (Pant and Reddy 2001), as well as overall concentrations and speciation of specific elements within the sediments could influence their competition for available binding sites (Alloway 2013).
Arsenic concentrations are generally low (average: 4 mg/kg), with many locations showing concentrations values below the instrument detection limit of 0.6 mg/kg and. We expected As concentration in stream sediment to be higher due to high concentration determined in top soil (Kim et al. 2015), as well as elevated concentrations (above EPA drinking standard of 10 μg/L) detected in many dug and drilled wells across Lebanon (Metcalf 2015). Also, it was expected that high Fe concentration in fluvial sediments (Average: 7900 mg/kg) would enhance high As adsorption due to well reported affinity between arsenic and iron oxyhydroxides in soil and sediment (Chen et al. 2007; Giménez et al. 2007; Stollenwerk 2003). The low As concentration could be attributed to a possible periodical dissolution of trace elements transported within agricultural runoff, or dilution from transported bed load derived from upper uncontaminated drainage areas, similar to the finding of Chen et al. (2004), or competitive adsorption between As and P, which has been shown to influence their overall concentrations in soils and sediments (Liu et al. 2001; Smith et al. 2002; Zhang and Selim 2008).
Chromium was detected from all sampling locations with a range of 3.6 to 1400 mg/kg. The highest Cr concentration value (1400 mg/kg) was determined at a downgradient side of a cemetery (SL 60). High Cr concentration in soil and sediments have been related to leachate discharge from cemeteries (Amuno 2013; Spongberg and Becks 2000), wastewater discharge (Singh et al. 2004), and abandoned landfill sites (Kemerich et al. 2012; Yusmartini and Setiabudidaya 2013).
The average concentrations of trace elements analyzed in this study are comparable to the background concentration values in shale (Turiekian and Wedepohl 1961) and other studies as shown in Table 2. The overall order of abundance of trace elements of interest in this study are Cr > Zn > Pb > Cu > As > Cd.
Grain size distribution in stream sediments varies across the study area. Information about grain size distribution is very important because it has been shown to influence sediment concentrations in many estuary systems (Miller and Miller 2007; Oyewumi and Schreiber 2012). As expected within stream sediments, 92% of the sampling locations are mostly sand, while clay contents are generally low with the exception of few locations: SL12 (16%), SL13 (17%), and SL23 (8%), respectively. Locations with reasonably high clay content also contained high concentration of various elements as follows: Cu (SL12: 25 mg/kg), Cd (SL13: 0.96 mg/kg), and Zn (SL13: 91 mg/kg). This is not surprising because clay fractions have been known to control abundance and distribution of trace element concentrations due to the presence of suitable binding sites (Shaheen et al. 2013). However, two notable locations (SL60 and SL62) with low clay contents (0.14 and 0.2%) contained high concentration of Cr (1400 mg/kg) and Cu (23 mg/kg), respectively. This unusual high concentration reflects possible anthropogenic point source of these elements at these locations.
Organic matter (OM) content varies from a low value of 0.4% at location SL56 to a high value of 13% at location SL13. Most locations with high clay content also have relatively high OM content such as locations SL8 (OM 6%, clay 7%), SL12 (OM 11%, clay 16%), SL13 (OM 13%, clay 17%), SL23 (OM 8%, clay 8%). As observed, there is a somewhat linear relationship between OM and clay fractions in sediments (R 2 = 0.6). Overall, results of this study showed that the distribution of trace and major elements are related to the abundance of OM contents, and fine grain sediment sizes.
Trace elements enrichment and ecological assessments
Table 3 presents result of enrichment factor (EF) for trace elements of interest. Approximately 48–52% of all the sampling locations have no to low enrichment with respect to As, Cd, Cr, Cu, Mn, Pb, P, and Zn. In addition, 40–43% of the sampling locations are moderately enriched, while only 1.5 to 11% of sampling locations are significantly enriched. Sediments with low enrichment suggest a possible reflection of natural source (Yongming et al. 2006) or natural variability in mineralogical composition of the crustal material (N'Guessan et al. 2009; Sutherland 2000), while sediments with moderate and significant enrichment suggest possible influence of anthropogenic input (Oyewumi et al. 2014). From the data, it is apparent that Cu has the most significantly enriched (EF ≥ 90th percentile) sites with 11% of sampling locations compared to 9% of sampling locations for As, Cd, Mn, P, Pb, and Zn, and 1.5% of the sampling locations for Cr. Spatial distribution of enriched locations for As, Cr, P and Pb are presented in Fig. 2. As shown, there is a wide distribution of enriched locations within and outside agricultural area. This might be due to conversion of many agricultural farmland areas due to residential pressure. Sediment enrichment within agricultural area may reflect physical remobilization of trace elements through erosion and transport from agricultural farmland to fluvial systems, and possible adsorption to sediments; while those outside agricultural area might be due to contribution from bedrock geology, or transport of fine grain sediment and associated trace elements during river flow from agricultural areas. However, other potential sources of sediment enrichment cannot be ruled out, though they are much less likely at this study area.
Spatial distribution of sediment enrichment factor for As, Cr, P and Pb within Lebanon fluvial system. Sampling locations are shown in Fig. 1
Table 4 presents result of ecological assessment of sediment chemistry. This is based on numerical sediment quality guidelines which are often used to evaluate the degree to which sediment concentrations might adversely affect aquatic organisms. Stream sediments quality were determined by comparing the level of sediment concentrations with the consensus–based sediment quality (CBSQ) guidelines using TEC and PEC values for As, Cd, Cr, Cu, Pb, and Zn (After Caeiro et al. 2005; MacDonald et al. 2000). Despite the widespread number of sampling locations with moderately enriched sediments, results of ecological assessment showed that all sampling locations were below the threshold PEC levels for As, Cd, Cu, Pb, and Zn except one location for Cr. Also, only four locations have concentrations above the TEC values for As and Pb. Overall, about 94% of all sampling locations have tolerable concentrations (below TEC levels) of As and Pb, 100% for Cd and Zn, and about 98.5% for Cr, respectively.
Source apportionment
Table 5 showed correlation analysis on all geochemical data and soil constituents (OM, clay, and silt fractions). Geochemical data were initially log transformed before statistical analysis in order to ensure reliability of statistical results (After Oyewumi et al. 2014); however, soil constituents such as OM and sediment grain sizes were not log transformed. Positive significant (r > 0.5, p < 0.05) correlation between Al with seven elements were observed such as Cd(0.71), Cr(0.52), Cu(0.59), Fe(0.83), K(0.54), P(0.71), and Zn(0.80). This possibly confirms association of these elements with fine fraction in sediments, since Al is a well-known proxy element for clay minerals (Hanson et al. 1993; Pekey 2006). Similar to Al, there is a significant positive correlation between Fe and most elements such as Al(0.83), Cd (0.58), Cr (0.59), Cu (0.58), K( 0.64), Mn (0.57), P (0.64), and Zn (0.77). This also possibly confirms association with iron oxide minerals within the sediments (Cornel and Schwertmann 1996; Li and Thornton 2001; McBride 1994). In addition, there is a significant positive correlation among elements such as Pb and Cu (0.51), P and Cr (0.50), P and Cu (0.58), Zn and Cd (0.65), Zn and Cu (0.67), Zn and P(0.63).
High positive significant correlation also exist between soil components and most elements such as positive significant correlation between OM fraction and Al(0.57), As(0.51), Cd(0.62), Pb(0.54), and Zn(0.59). Also, between clay and Al(0.53), Cd(0.62), and Zn(0.49). Overall, high correlation among various elements might be due to their similar ionic radii (Chen et al. 1999), or possible affinity between these elements by virtue of similar source (Ghrefat et al. 2011), similar adsorptive characteristics between elements and minerals within the sediment (Eggleton and Thomas 2004), or similar biogeochemical processes that are active in influencing their mobility. Similar affinity of elements have been observed in many studies such as As, Cd, Cu, Mn and Zn affinity with the oxides and hydroxides of Fe and Mn (Prego and Cobelo-Garcıa 2003; Smedley and Kinniburgh 2002; Stollenwerk 2003); also, affinity between Cu, Zn and Pb to humic substances within sediments (Sekhar et al. 2004). The observed interelatioship between these elements is further ilustrated with the cluster analysis (Fig. 3) using Ward linkage and Euclidean distance coefficient. The dendrogram clearly showed association between these elements and soil constituents similar to the observed relationship with correlation analysis.
Geochemical data were further examined using multivariate method of principal component analysis (PCA) shown in Table 6. Three principal components (PC) were extracted based on eigenvalue greater than one (using Kaiser Criterion: Kaiser 1974). All the three selected components account for 72.49% of the total variance in the sediment data (Table 6). Elements with rotated component matrix (eigenvector weight) greater than 0.50 were further selected as influential to each component. The first component (PC 1) accounts for 51.44% of total variance in the data set. Eigenvector weight were positive (>0.50) for Al, Cr, Cu, Fe, K, P, Pb, and Zn; while only As, Cd, and Mn were below the threshold value of 0.50 in PC1. The second component (PC II) accounts for 11.45% of the total variance in the data set and eigenvector weight are positive (>0.50) for only Fe (0.63), and Mn(0.89). The third component (PC III) accounts for 9.60% of variance in data sets and eigenvector weight were positive for As(0.81), and Cd(0.76). Analysis of regional concentrations of most elements in PCI showed high concentrations of Fe and Al outside of agricultural area, while other elements have high concentrations within agricultural area of Lebanon. Previous petrographic and XRF analyses on Lebanon rock formations showed significant amount of Al and Fe (Kim et al. 2015) as the main component of various meta-igneous rock formations at the study area. However, significant loading of trace elements such as As, Cd, and Mn in components (PCII and III) other than PCI is an indication of alternative sources of these elements within the study area. Spatial representation of the three rotated components is shown in Fig. 4. The wide distribution of element positions further illustrate different alternative sources within the study area.
Component plot in rotated space (components 1, 2, and 3) of major and trace elements within stream sediment of Lebanon fluvial system. The variance percentage of each component is shown in Table 6
Conclusion
Trace and major elements chemistry from Lebanon fluvial systems are shown to be due to a combined influence of bedrock geology and anthropogenic activities across the area. This is illustrated by the distribution of elements as well as calculation of sediment enrichment factors (EF), which showed that about 48 to 52% of the sampling locations have low enrichment with respect to As, Cd, Cr, Cu, Mn, P, Pb and Zn; while about 40 to 43% of the sampling locations are moderately enriched, and only 1.5 to 11% are significantly enriched. The low enrichment in sediment is a possible reflection of natural variability in mineralogical composition of the crustal material. Despite elevated arsenic concentrations detected in many drinking-water wells, and topsoil across Lebanon, our results showed low sparsely distributed concentrations of arsenic in stream sediments across Lebanon. Analysis of potential ecological risk of sediments showed that only one site (1.5%) exceeded PEC value for Cr, while four locations (6.2%) exceeded TEC values for both As, and Pb. Our results did not identify connection between sediment enrichment and ecological risks possibly due to low number of sites with significant enrichment. The reason for this is not fully understood; there is a possibility of preferential adsorption of trace elements to stream particulate, and dissolution in water column as they are transported downstream. Overall, there is no general ecological risk for benthic organisms within the study area. It is imperative to note that geochemical assessment of agricultural landscape could be complicated by many factors such as changes in the land use pattern over time due to residential pressure; changes in weather pattern such as the effect of heavy rainfall and hurricane on stream sediment transport and enrichment. Thus, result from this study might not fully reflect overall impact of agricultural activities; it is therefore necessary for continuous monitoring of stream sediments concentrations so that safe threshold limits are not exceeded over time. Also, further study is warranted to examine geochemistry of trace elements associated with stream particulates, runoff from farmland, and agricultural soils in order to aid complete assessment of the impact of agricultural activities on Lebanon fluvial systems.
References
Agazzi, A., and Pirola, C. (2000). Fundamentals, methods and future trends of environmental microwave sample preparation. Microchemical Journal, 67(1–3), 337–341.
Alloway, B. J., 2013, Bioavailability of elements in soil, Essentials of medical geology, Springer, p. 351–373.
Amuno, S. (2013). Potential ecological risk of heavy metal distribution in cemetery soils. Water, Air, and Soil Pollution, 224(2), 1–12.
Anderson, B. K., and Chamblee, T. N. (2001). The effect of dietary 3-nitro-4-hydroxyphenylarsonic acid (roxarsone) on the total arsenic level in broiler excreta and broiler litter. Journal of Applied Poultry Research, 10(4), 323–328.
Bhuiyan, M. A. H., Parvez, L., Islam, M. A., Dampare, S. B., and Suzuki, S. (2010). Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. Journal of Hazardous Materials, 173(1–3), 384–392.
Böhlke, J.-K. (2002). Groundwater recharge and agricultural contamination. Hydrogeology Journal, v. 10(1), 153–179.
Borůvka, L., Vacek, O., and Jehlička, J. (2005). Principal component analysis as a tool to indicate the origin of potentially toxic elements in soils. Geoderma, 128(3), 289–300.
Brown, B. L., Slaughter, A. D., and Schreiber, M. E. (2005). Controls on roxarsone transport in agricultural watersheds. Applied Geochemistry, v. 20(1), 123–133.
Caeiro, S., Costa, M. H., Ramos, T. B., Fernandes, F., Silveira, N., Coimbra, A., Medeiros, G., and Painho, M. (2005). Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecological Indicators, 5(2), 151–169.
Chabukdhara, M., and Nema, A. K. (2012). Assessment of heavy metal contamination in Hindon River sediments: a chemometric and geochemical approach. Chemosphere, 87(8), 945–953.
Chen, M., Ma, L. Q., and Harris, W. G. (1999). Baseline concentrations of 15 trace elements in Florida surface soils. Journal of Environmental Quality, 28(4), 1173–1181.
Chen, W., Parette, R., Zou, J., Cannon, F. S., and Dempsey, B. A. (2007). Arsenic removal by iron-modified activated carbon. Water Research, 41(9), 1851–1858.
Chen, Z., Saito, Y., Kanai, Y., Wei, T., Li, L., Yao, H., and Wang, Z. (2004). Low concentration of heavy metals in the Yangtze estuarine sediments, China: a diluting setting. EstuarineCoastal and Shelf Science, 60(1), 91–100.
Church, C. D., Kleinman, P. J. A., Bryant, R. B., Saporito, L. S., and Allen, A. L. (2010). Occurrence of arsenic and phosphorus in ditch flow from litter-amended soils and barn areas. Journal of Environmental Quality, 39(6), 2080–2088.
Codling, E. E., Chaney, R. L., and Mulchi, C. L. (2008). Effects of broiler litter management practices on phosphorus, copper, zinc, manganese, and arsenic concentrations in Maryland coastal plain soils. Communications in Soil Science and Plant Analysis, 39(7–8), 1193–1205.
Cornel, R., and Schwertmann, U. (1996). The iron oxides. Structure, properties, reactions and uses. Weinheim, Germany: VCH.
Dane, J. P., and Topp, G. C. (2002). Method of soil analysis. Part 4: Physical methods (pp. 1–1692). Madison, Wisconsin: Soil Science Society of America.
de Andrade Passos, E., Alves, J. C., dos Santos, I. S., Jose do Patrocínio, H. A., Garcia, C. A. B., and Costa, A. C. S. (2010). Assessment of trace metals contamination in estuarine sediments using a sequential extraction technique and principal component analysis. Microchemical Journal, 96(1), 50–57.
Dean Jr, W. E., 1974, Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods: Journal of Sedimentary Research, v. 44, no. 1.
Eggleton, J., and Thomas, K. V. (2004). A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environment International, 30(7), 973–980.
Emmerson, R., O'Reilly-Wiese, S., Macleod, C., and Lester, J. (1997). A multivariate assessment of metal distribution in inter-tidal sediments of the Blackwater Estuary,UK. Marine Pollution Bulletin, v. 34(11), 960–968.
Förstner, U., and Salomons, W. (1980). Trace metal analysis on polluted sediments: Part I: Assessment of sources and intensities. Environmental Technology, 1(11), 494–505.
Garbarino, J. R., Bednar, A. J., Rutherford, D. W., Beyer, R. S., and Wershaw, R. L. (2003). Environmental fate of roxarsone in poultry litter. I. Degradation of roxarsone during composting. Environmental Science and Technology, 37(8), 1509–1514.
Garcia-Sanchez, A., and Alvarez-Ayuso, E. (2003). Arsenic in soils and waters and its relation to geology and mining activities (Salamanca Province, Spain). Journal of Geochemical Exploration, 80(1), 69–79.
Ghrefat, H. A., Abu-rukah, Y., and Rosen, M. A. (2011). Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain Dam, Jordan. Environmental Monitoring and Assessment, 178(1–4), 95–109.
Giménez, J., Martínez, M., de Pablo, J., Rovira, M., and Duro, L. (2007). Arsenic sorption onto natural hematite, magnetite, and goethite. Journal of Hazardous Materials, 141(3), 575–580.
Gray, C. W., McLaren, R. G., Roberts, A. H. C., and Condron, L. M. (1999). The effect of long-term phosphatic fertiliser applications on the amounts and forms of cadmium in soils under pasture in New Zealand. Nutrient Cycling in Agroecosystems, 54(3), 267–277.
Hanson, P. J., Evans, D. W., Colby, D. R., and Zdanowicz, V. S. (1993). Assessment of elemental contamination in estuarine and coastal environments based on geochemical and statistical modeling of sediments. Marine Environmental Research, 36(4), 237–266.
Hering, J., Dixit, S., Campbell, K., and O'Day, P., 2014, Arsenic mobilization from contaminated sediments: a full-scale experiment in progress: Dams and Appurtenant Hydraulic Structures, p. 39.
Holmgren, G., Meyer, M., Chaney, R., and Daniels, R. (1993). Cadmium, lead, zinc, copper, and nickel in agricultural soils of the United States of America. Journal of environmental quality, 22(2), 335–348.
Ikem, A., Egiebor, N. O., and Nyavor, K. (2003). Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern Usa. Water, Air, and Soil Pollution, v. 149(1), 51–75.
Jackson, B. P., Bertsch, P. M., Cabrera, M. L., Camberato, J. J., Seaman, J. C., and Wood, C. W. (2003). Trace element speciation in poultry litter. Journal of Environmental Quality, 32(2), 535–540.
JMP®, Version 12,. SAS Institute Inc. , Cary, NC, 1989-2007. http://www.jmp.com/support/notes/35/282.html
Kaiser, H. F. (1974). An index of factorial simplicity. Psychometrika, v. 39(1), 31–36.
Kemerich, P. D. d. C., de Borba, W. F., Ferreira da Silva, R., Barros, G., Gerhardt, A. E., and Balestrin Flores, C. E. (2012). Anomalous values of heavy metals in soil of cemetery. Ambiente and Água-An Interdisciplinary Journal of Applied Science, v. 7(1), 140–156.
Kibet, L., Allen, A., Church, C., Kleinman, P., Feyereisen, G., Saporito, L., Hashem, F., May, E., and Way, T. (2013). Transport of dissolved trace elements in surface runoff and leachate from a coastal plain soil after poultry litter application. Journal of Soil and Water Conservation, v. 68(3), 212–220.
Kim, A., Weinsteiger, A., Costa, M., and OYEWUMI, O., 2015, Evaluation of Arsenic concentration in soil and rock units across Lebanon, CT: https://gsa.confex.com/gsa/2015AM/webprogram/Paper266915.html.
Konen, M. E., Jacobs, P. M., Burras, C. L., Talaga, B. J., and Mason, J. A. (2002). Equations for predicting soil organic carbon using loss-on-ignition for north central US soils. Soil Science Society of America Journal, v. 66(6), 1878–1881.
Lair, G., Gerzabek, M., and Haberhauer, G. (2007). Sorption of heavy metals on organic and inorganic soil constituents. Environmental Chemistry Letters, v. 5(1), 23–27.
Lebanon, 2010, Lebanon Planning and Zoning Commission: http://www.lebanontownhall.org/resources/pocd_2010.pdf Date Accessed, Sept 15, 2015.
Lee, D. S. (1994). Atmospheric concentrations of trace elements in urban areas of the United Kingdom. Atmospheric environment (1994), v. 28(16), 2691.
Li, X., and Thornton, I. (2001). Chemical partitioning of trace and major elements in soils contaminated by mining and smelting activities. Applied Geochemistry, v. 16(15), 1693–1706.
Liu, F., De Cristofaro, A., and Violante, A. (2001). Effect of pH, phosphate and oxalate on the adsorption/desorption of arsenate on/from goethite. Soil Science, v. 166(3), 197–208.
Looser, M. O., Parriaux, A., and Bensimon, M. (1999). Landfill underground pollution detection and characterization using inorganic traces. Water Research, v. 33(17), 3609–3616.
Loring, D. H. (1990). Lithium--a new approach for the granulometric normalization of trace metal data. Marine Chemistry, v. 29, 155.
Loska, K. (2004). Metal contamination of farming soils affected by industry. Environment International, v. 30(2), 159.
Lukman, S., Essa, M., Mu'azu, N. D., Bukhari, A., and Basheer, C. (2013). Adsorption and desorption of heavy metals onto natural clay material: influence of initial pH. Journal of Environmental Science and Technology, v. 6(1), 1.
Maas, S., Scheifler, R., Benslama, M., Crini, N., Lucot, E., Brahmia, Z., Benyacoub, S., and Giraudoux, P. (2010). Spatial distribution of heavy metal concentrations in urban, suburban and agricultural soils in a Mediterranean city of Algeria. Environmental Pollution, v. 158(6), 2294–2301.
MacDonald, D. D., Ingersoll, C. G., and Berger, T. A. (2000). Development and evaluation of concensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, v. 39(1), 20–31.
McBride, M. B. (1994). Environmental chemistry of soils. New York: Oxford University Press.
Metcalf, M., Examining Potential Sources of Arsenic in Bedrock Wells of Lebanon, Connecticut, in Proceedings 2015 NGWA Conference on Groundwater in Fractured Rock2015, Ngwa.
Miller, J. R., and Miller, S. M. O. (2007). Contaminated rivers: a geomorphological-geochemical approach to site assessment and remediation, Springer Science and Business Media.
Moore, J. N., Brook, E. J., and Johns, C. (1989). Grain size partitioning of metals in contaminated, coarse-grained river floodplain sediment: Clark Fork River, Montana, U.S.A. Environmental Geology, 14(2), 107-115.
N'Guessan, Y. M., Probst, J. L., Bur, T., and Probst, A. (2009). Trace elements in stream bed sediments from agricultural catchments (Gascogne region, S-W France). Where do they come from?: Science of The Total Environment, v. 407(8), 2939–2952.
Nriagu, J. O., and Pacyna, J. M. (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature, v. 333(6169), 134–139.
O'Connor, R., O'Connor, M., Irgolic, K., Sabrsula, J., Gurleyuk, H., Brunette, R., Howard, C., Garcia, J., Brien, J., Brien, J., and Brien, J. (2005). Transformations, air transport, and human impact of arsenic from poultry litter. Environmental Forensics, v. 6(1), 83–89.
Oyewumi, O., and Schreiber, M. E. (2012). Release of arsenic and other trace elements from poultry litter: Insights from a field experiment on the Delmarva Peninsula. Delaware: Applied Geochemistry.
Oyewumi, O., Schreiber, M., and Ciparis, S. (2014). The impact of poultry litter application on sediment chemistry of the Broadkill River estuary system, Delaware. Environmental Monitoring and Assessment, v. 186(1), 97–115.
Pant, H., and Reddy, K. (2001). Phosphorus sorption characteristics of estuarine sediments under different redox conditions. Journal of Environmental Quality, v. 30(4), 1474–1480.
Pekey, H. (2006). The distribution and sources of heavy metals in Izmit Bay surface sediments affected by a polluted stream. Marine Pollution Bulletin, v. 52(10), 1197–1208.
Pokrovsky, O. S., Schott, J., and Dupré, B. (2006). Trace element fractionation and transport in boreal rivers and soil porewaters of permafrost-dominated basaltic terrain in Central Siberia. Geochimica et Cosmochimica Acta, v. 70(13), 3239–3260.
Prego, R., and Cobelo-Garcıa, A. (2003). Twentieth century overview of heavy metals in the Galician rias (NW Iberian peninsula). Environmental Pollution, v. 121(3), 425–452.
Presley, B., Trefry, J., and Shokes, R. (1980). Heavy metal inputs to Mississippi Delta sediments. Water, Air, and Soil Pollution, v. 13(4), 481–494.
Robinson Jr., G. R., and Ayotte, J. D. (2006). The influence of geology and land use on arsenic in stream sediments and ground waters in New England, USA. Applied Geochemistry, v. 21(9), 1482–1497.
Rubio, B., Nombela, M. A., and Vilas, F. (2000). Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. Marine Pollution Bulletin, 40(11), 968–980.
Rutherford, D. W., Bednar, A. J., Garbarino, J. R., Needham, R., Staver, K. W., and Wershaw, R. L. (2003). Environmental fate of roxarsone in poultry litter. Part II. Mobility of arsenic in soils amended with poultry litter. Environmental Science and Technology, v. 37(8), 1515–1520.
Säumel, I., Kotsyuk, I., Hölscher, M., Lenkereit, C., Weber, F., and Kowarik, I. (2012). How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environmental Pollution, v. 165, 124–132.
Schiff, K. C., and Weisberg, S. B. (1999). Iron as a reference element for determining trace metal enrichment in Southern California coastal shelf sediments. Marine Environmental Research, v. 48(2), 161.
Segura, R., Arancibia, V., Zúñiga, M. C., and Pastén, P. (2006). Distribution of copper, zinc, lead and cadmium concentrations in stream sediments from the Mapocho River in Santiago, Chile. Journal of Geochemical Exploration, v. 91(1–3), 71–80.
Sekhar, K. C., Chary, N., Kamala, C., Raj, D. S., and Rao, A. S. (2004). Fractionation studies and bioaccumulation of sediment-bound heavy metals in. Kolleru lake by edible fish: Environment International, v. 29(7), 1001–1008.
Semrod, K. A., and Gourley, J. R. (2014). Mapping the distribution of the bioaccessible fraction of trace metals in the sediments of an urban stream, Park River watershed, Connecticut. Water, Air, and Soil Pollution, v. 225(8), 1–15.
Shaheen, S. M., Tsadilas, C. D., and Rinklebe, J. (2013). A review of the distribution coefficients of trace elements in soils: Influence of sorption system, element characteristics, and soil colloidal properties. Advances in Colloid and Interface Science, v. 201, 43–56.
Sharma, A., and Rajamani, V. (2000). Weathering of gneissic rocks in the upper reaches of Cauvery river, south India: implications to neotectonics of the region. Chemical Geology, v. 166(3–4), 203–223.
Singh, K. P., Mohan, D., Sinha, S., and Dalwani, R. (2004). Impact assessment of treated/untreated wastewater toxicants discharged by sewage treatment plants on health, agricultural, and environmental quality in the wastewater disposal area. Chemosphere, v. 55(2), 227–255.
Smedley, P. L., and Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, v. 17(5), 517–568.
Smith, E., Naidu, R., and Alston, A. (2002). Chemistry of inorganic arsenic in soils. Journal of Environmental Quality, v. 31(2), 557–563.
Song, Y., Ji, J., Yang, Z., Yuan, X., Mao, C., Frost, R. L., and Ayoko, G. A. (2011). Geochemical behavior assessment and apportionment of heavy metal contaminants in the bottom sediments of lower reach of Changjiang River. Catena, v. 85(1), 73–81.
Spongberg, A. L., and Becks, P. M. (2000). Inorganic soil contamination from cemetery leachate. Water, Air, and Soil Pollution, v. 117(1–4), 313–327.
SPSS, Version 22, IBM Corp. Released. (2013). IBM SPSS statistics for windows, version 22.0. Armonk, NY: IBM Corp. https://www-01.ibm.com/support/docview.wss?uid=swg21476197
Stollenwerk, K. G., 2003, Geochemical processes controlling transport of arsenic in groundwater: a review of adsorption, Arsenic in ground water, Springer, p. 67–100.
Sutherland, R. A. (2000). Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environmental Geology, v. 39(6), 611–627.
Tang, W., Shan, B., Zhang, H., and Mao, Z. (2010). Heavy metal sources and associated risk in response to agricultural intensification in the estuarine sediments of Chaohu Lake Valley, East China. Journal of Hazardous Materials, v. 176(1), 945–951.
Taupe, N., Lynch, D., Wnetrzak, R., Kwapinska, M., Kwapinski, W., and Leahy, J. (2016). Updraft gasification of poultry litter at farm-scale–A case study. Waste Management, v. 50, 324–333.
Turiekian, K., and Wedepohl, K. (1961). Distribution of the elements in some major units of the earth’s crusts. Bulletin of the Geological Society of America, v. 72, 175–192.
Tyler, L. D., and McBride, M. B. (1982). Mobility and extractability of cadmium, copper, nickel, and zinc in organic and mineral soil columns. Soil Science, v. 134(3), 198–205.
Viers, J., Dupré, B., and Gaillardet, J. (2009). Chemical composition of suspended sediments in World Rivers: new insights from a new database. Science of The Total Environment, v. 407(2), 853–868.
Won, J. H., Park, J. Y., and Lee, T. G. (2007). Mercury emissions from automobiles using gasoline, diesel, and LPG. Atmospheric Environment, v. 41(35), 7547–7552.
Wong, C. S. C., Li, X., and Thornton, I. (2006). Urban environmental geochemistry of trace metals. Environmental Pollution, v. 142(1), 1–16.
Yongming, H., Peixuan, D., Junji, C., and Posmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Science of The Total Environment, v. 355(1–3), 176–186.
Yu, S., Zhu, Y.-g., and Li, X.-d. (2012). Trace metal contamination in urban soils of China. Science of the Total Environment, v. 421, 17–30.
Yusmartini, E. S., and Setiabudidaya, D., Characteristics of leachate at Sukawinatan Landfill, Palembang, Indonesia, in Proceedings Journal of Physics: Conference Series 2013, Volume 423, IOP Publishing, p. 012048.
Zhang, H., and Selim, H. M. (2008). Competitive sorption-desorption kinetics of arsenate and phosphate in soils. Soil Science, v. 173(1), 3–12.
Zhao, L., Xu, Y., Hou, H., Shangguan, Y., and Li, F. (2014). Source identification and health risk assessment of metals in urban soils around the Tanggu chemical industrial district, Tianjin, China. Science of the Total Environment, v. 468, 654–662.
Acknowledgements
This study was supported by faculty research grant from the Central Connecticut State University (CCSU). We are grateful to Dr. Madeline Schreiber, Department of Geoscience, Virginia Tech for access to CEM Microwave. We appreciate field assistance from Jonathan Bauer, and Amanda Cross. We also thank Dr. Mark Evans for his support; and anonymous reviewers for their useful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oyewumi, O., Feldman, J. & Gourley, J.R. Evaluating stream sediment chemistry within an agricultural catchment of Lebanon, Northeastern USA. Environ Monit Assess 189, 141 (2017). https://doi.org/10.1007/s10661-017-5856-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-5856-z