Abstract
This study presents the total concentrations and chemical fractionations of metals (Cu, Pb, Zn, Ni, Fe, and Mn) in 54 surface sediment samples collected from the Huaihe River (Anhui Province) in eastern China. Compared with the average shale values, Zn and Pb exhibited the most substantial anthropogenic enrichment, especially in Fengtai and Huainan areas, the main industrial districts along the Huaihe River (Anhui Province). Low levels of Cu and Ni were observed in the sediments. Based on risk assessment code (RAC), the metals associated with weak acid soluble (F1) in the Huaihe River sediments followed the order: Mn > Zn > Cu > Pb > Ni > Fe. Manganese presented the most potential for releasing into the aqueous environment and can easily enter the food chain. Copper, zinc, nickel, and iron were found dominant in the residual fraction, implying that these four metals were strongly bound to the sediments. Lead showed a different partitioning pattern from that of other metals studied, with a large percentage in Fe-Mn oxide fraction, indicating that slight redox potential changes may make significant influence on the removability of Pb. Moreover, Cu in oxidizable (F3) and residual (F4) fractions presented high positive correlation with organic matter, which can explain the high percentage of Cu in these two fractions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals are introduced into the aqueous system by two primary pathways: natural sources and human activities along rivers (Almeida et al. 2004; Jain 2004). However, with the rapid population growth, industrialization, and urbanization in recent decades, metals resulting from anthropogenic inputs now significantly exceed those from natural sources in many locations, leading to widespread environmental contamination (Ip et al. 2007). Generally, metals entering river systems can accumulate in sediments and suspended particles via physical, chemical, and biological processes, eventually precipitating and becoming incorporated in bottom sediments (Li et al. 2001; Suthar et al. 2009; Nasr et al. 2015a). Such accumulation of metals in sediments can pose serious problems to water quality and are potentially toxic to human health and the ecosystem in the long term (Ip et al. 2007). Therefore, it is important to study the geochemical distribution of heavy metals in sediments and undertake appropriate measures to control heavy metal contamination.
Generally, the total concentration is employed as an initial criterion to assess the potential risk of metal contamination (Tessier et al. 1979; Singh et al. 2005; Rodriguez et al. 2009). However, the mobility, bioavailability, and potential toxicity of metals depend not only on their total concentration but also on their specific chemical forms (Surija and Branica 1995; Bi et al. 2007; Rodriguez et al. 2009; Davutluoglu et al. 2010; Sundaray et al. 2011; Nasr et al. 2015b). Cuong and Obbard (2006) asserted that metal speciation offered a more realistic estimate of actual environmental impact. Dating back to 1954, the concept of speciation was first introduced to improve understanding of the biogeochemical cycling of trace elements in seawater (Jain 2004). To date, a great variety of extraction procedures have been developed to assess partitioning of sediment metals (Bi et al. 2007). Considering the diversity of procedures, global results obtained are often non-comparable (Fuentes et al. 2008). For this reason, the European Community (EC) Standards, Measurements and Testing Programme (formerly European Community of Bureau of Reference, BCR) proposed the BCR sequential extraction procedure and harmonized the methodology for the analysis of soils and sediments in 1992 (Fernandez et al. 2004; Fuentes et al. 2008; Yuan et al. 2004; Cuong and Obbard 2006). The BCR system also provides certified reference materials and has been successfully applied to a variety of matrices (Kubova et al. 2004). In this study, a modified BCR sequential extraction procedure is therefore used for extracting metal fractionations.
The Huaihe River, one of the seven largest rivers in China, is an important energy source and marketable grain base in China and is a key water resource for domestic, agricultural, and industrial purposes. The Huaihe River quality is vital for riparian economic development, community health, and the wellbeing of residents on both banks. The middle reach (Anhui Province) of the Huaihe River crosses farmland important to China. In addition, two major coalfields with abundant coal stocks are located in this area. Unfortunately, extensive mining activities and fast growing industrial development have severely reduced the water quality (Z. Yang et al. 2009). Large amounts of wastewater containing trace metals from chemical industries, power plants, and mining activities have significantly contributed to the increased contamination of the Huaihe River. By the end of 1995, 80 % of the Huaihe River was contaminated, and catastrophic pollution incidents occurred frequently (Tan et al. 2005).
A limited number of studies of the Huaihe River have been conducted, but little information concerning trace metal distributions and chemical forms is available. In this paper, a modified BCR sequential extraction method is utilized for trace metal speciation, with the following objectives: (i) to analyze the distribution and enrichment of trace metals in the Huaihe River (Anhui Province) and (ii) to evaluate the mobility, bioavailability, and toxicity of trace metal contamination.
Materials and methods
Study area
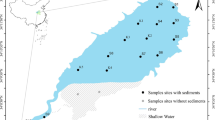
The Huaihe River is located between the Yellow River and the Yangtze River in eastern China, with a basin area of 270,000 km2. The river originates from Tongbai Mountain in Henan Province, flowing eastward for approximately 1000 km through five provinces, i.e., Henan, Hubei, Anhui, Jiangsu, and Shandong, before joining the Yangtze River in Sanjiangying (Jiangsu Province). Anhui Province is located in the middle reach of the Huaihe River, which covers 66,900 km2 and flows for 430 km (43 % of the total extent). In this paper, four areas (32° 34′ 9″∼32° 57′ 32″ N; 116° 39′ 47″∼117°15′ 40″ E) in Anhui Province, i.e., Shouxian, Fengtai, Huainan, and Huaiyuan, containing a total river length of 131.5 km, were studied for the metal distribution and fractionation.
Shouxian is located in the center of Anhui Province on the southern bank of the Huaihe River, with a population of 1.37 million. The total area is 2986 km2, of which 40.86 % is used for agriculture, primarily rice, wheat, and cotton. Tanning, dyeing and weaving, and printing are the dominant industries.
Fengtai (1030 km2) and Huainan (1566.40 km2) are important industrial areas in Anhui Province. Mineral resources, especially coal, are abundant in these two districts. Several collieries and coal-fired power stations are distributed along the river banks.
Huaiyuan, a domestic marketable grain base, lies in the north of Anhui Province, with a population of 1.30 million and a total area of 2400 km2. Huaiyuan is in the vicinity of two famous coalfields, namely Huainan and Huaibei. Certain coal storage and magnetite resources are also to be found in Huaiyuan.
It is reported that Huainan is the main electricity supplier to East China for the existence of abundant coal and coal-fired power plants since the 1940s (Tang et al. 2013). Great impact on the water quality of the Huaihe River (Huainan section) was observed by receiving a certain pollution load from the coal-fired power plants, mining activities, and domestic sewage. Moreover, a large number of ships and docks were also observed spreading along the river, which presented the potential of metal pollution by ship voyages and waste discharge.
The four districts of Anhui Province alongside the Huaihe River are situated in a climate transition zone in China: the area north of the river belongs to the warm, temperate, semi-humid region, while that south of the river is subject to a northern subtropical, humid climate. The annual average temperature and rainfall range from 11 to 16 °C and 600 to 1400 mm, respectively.
Sample collection and preparation
Fifty-four surface sediment samples (to a depth of 10 cm) in the Huaihe River (Anhui Province) from Shouxian (S1-S10), Fengtai (F11-F21), Huainan (H22-H41), and Huaiyuan (Y42–Y54) were collected with a grab sampler, from July 6 to 12, 2013. Sufficient sediments had been collected from a particular site (1 kg). To avoid metal interaction between sample sites, the grab sampler was washed with clean water after every sampling (Doherty et al. 2000). Immediately after collection, all samples were stored in labeled and sealed polyethylene bags and then transported to the laboratory. The sample sites (Fig. 1) were deliberately selected with consideration for the distribution of pollution sources, to investigate their effects on the spatial variation of trace metals and the gradients of contamination.
Prior to physical and chemical analysis in the laboratory, all samples were air dried at room temperature. After the coarse debris was removed, all samples were ground with an agate mill until all particles could pass through a 100-mesh nylon sieve. Then, all specimens were stored at 4 °C in polyethylene containers for further analysis.
Physicochemical analysis
The ratio of 1:5 of sediment to ultrapure water was used for the determination of pH values of all the surface samples. The organic matter content was obtained by calculating the difference between the dry weight of sediment samples before and after ashing in a muffle furnace at 550 °C for 5 h (Arain et al., 2008; Kazi et al., 2005). Detail description of the analysis of pH and organic matter (OM) can be found in Wang et al. (2015).
Analytical methods and quality control
Sediment samples (0.10 g) were weighted for the determination of total metal content, with the mixed acid digestion of 2 ml HNO3 (AR grade), 1 ml HClO4 (AR grade), and 5 ml HF (AR grade) according to Li et al. (2000). The samples were heated on an electrothermal plate at a temperature of 90–190 °C for 16 h. After cooling, the residual solutions were then filtered into polyethylene volumetric tubes and diluted to 25 ml with deionized water for further detection of Cu, Pb, Zn, Ni, Fe, Mn, and Al concentrations.
Metal fractionations were conducted by the modified BCR sequential extraction method proposed by Rauret et al. (1999). This sequential extraction procedure consists of four extractions: weak acid soluble (exchangeable and carbonate bound) (F1), reducible (Fe-Mn oxides) (F2), oxidizable (organic-sulfide bound) (F3), and residual (F4). The extractants and analytical conditions are given in Table 1. All the samples, with extractants added, were shaken at a speed of 180 ± 20 rpm. After shaking for 16 h at each step, the samples were centrifuged at 7000 rpm for 10 min at room temperature, and the supernatant was separated with a pipette. Then the residue was washed with 8 ml deionized water. After centrifuging for 10 min at 7000 rpm, the wash water was discarded. The supernatant obtained was stored in polyethylene containers for further analysis.
The total content of Cu, Pb, Zn, Ni, Fe, Mn, and Al and concentrations of each extraction of the selected metals (Cu, Pb, Zn, Ni, Fe, and Mn) were measured using an inductively coupled plasma-optical emission spectrometer (ICP-OES, Perkin Elmer Optima, 2100DV), with the resonance lines at 324.8, 283.3, 213.8, 232.0, 396.2, 257.6, and 248.3 nm for Cu, Pb, Zn, Ni, Al, Mn, and Fe, respectively.
All the containers used in the experiments had been previously soaked in 10 % HNO3 for at least 24 h and then rinsed several times with deionized water (18 MΩ). In addition, the reagents used throughout the experiment were all of analytical grade. Standard working solutions of the studied trace metals were obtained by diluting the corresponding 1000 mg L−1 stock solutions.
The metal analysis quality control for the total concentrations and the sequential extraction procedure were assessed using reagent blanks, sample replicates, and standard reference material (GSD-9) (GBW07309). The Cu, Pb, Zn, Ni, Al, Mn, and Fe analytical results demonstrate good agreement with the standard reference material (recovery rates ranging between 85.12 and 108.79 %), and the standard deviations of the replicate samples were <8.89 %. In addition, no significant differences were observed between the cumulative concentrations of metals from the sequential extraction procedure and the independent total concentrations, with recovery rates ranging from 80.31 to 118.92 %.
Statistic analysis
The data manipulation and correlation analysis were performed using Microsoft Excel 2007 and the statistical software program SPSS 16.0 for Windows.
Results and discussion
Total metal concentrations
The statistic characteristics (pH, OM, and metal content) of the 54 surface sediments are plotted in Table 2.
Aluminum, iron, and manganese
The total concentrations of aluminum, iron, and manganese are in the range of 34,979.15–95,350.67 mg/kg, 18,927.61–46,279.05 mg/kg, and 354.91–1952.07 mg/kg, respectively. It is reported that Al, Fe, and Mn oxides in sediments present significant affection on the transportation and fate of trace metals (Dzombak and Morel 1987). Fe and Mn oxides in the sediments can participate in oxidation-reduction reactions that change the stability and bonding of adsorbed trace metals (Hem 1978; Kay et al. 2001). The high Al and Fe concentrations in the sediments of the Huaihe River indicated high clay and silt content, which had a higher adsorptive capability for metals due to their higher surface area per volume rates as compared to coarse materials (Davutluoglu et al. 2010).
Copper, lead, zinc, and nickel
Copper ranged from 11.16 to 52.77 mg/kg, with an average concentration of 31.30 mg/kg, which is lower than the Cu shale value. Generally, it is considered as a quick and practical method of evaluating metal enrichment to compare metal concentrations with the shale values (Ghrefat and Yusuf 2006; Jain 2004; Jain et al. 2008; Javan et al. 2015). Therefore, a comparison of Cu concentration with average shale value implied that on average, no Cu enrichment was observed in the Huaihe River (Anhui Province). However, Cu concentrations at H32 and H36 were found higher. These two sites are located in Huainan, which has been long known as active mining areas, and are the sites of power plants. The river in this part has received industrial effluent discharged by these power stations.
Lead exhibited relatively higher concentrations compared with the shale value, with a concentration range of 18.49–199.05 mg/kg. The average (53.43 mg/kg) Pb concentration was 2.67 times higher than the average shale value. In addition, the coefficient of variation of Pb (53.78 %) was comparatively high, indicating that its spatial distribution was heterogeneous. It suggests that Pb concentrations may have been, to some extent, influenced by anthropogenic activities. Lead concentrations greater than the average (53.43 mg/kg) were obtained from a high percentage (39 %) of the 54 sediment samples, which implies that great Pb enrichment has occurred at these sites. The maximum Pb concentration (199.05 mg/kg) was recorded at site Y48, under the Huaihe bridge. Atmospheric deposition of exhaust particulates from the automobile across the bridge may explain the significant high Pb level in site Y48 (Cuong and Obbard 2006).
Zinc exhibited considerable concentration variations (32.88–418.55 mg/kg), with the coefficient of variation (57.85 %) higher than 50 %. In particular, the average Zn concentration (183.57 mg/kg) was substantially higher than the shale value (95.00 mg/kg). Zn concentrations in 41 % of the 54 sediment samples were greater than the average, and this figure is similar to our Pb findings (39 %). Additionally, 55 % of the highly enriched sample sites were located in Fengtai and Huainan. The maximum Zn concentration (418.55 mg/kg) was collected at site H38, located downstream of the Tianjia’an power plant in Huainan.
Ni concentrations ranged from 18.37 to 130.33 mg/kg, with an average of 32.79 mg/kg. Ni concentrations in 43 % of the 54 sediment samples were higher than the average (32.79 mg/kg). However, only one site (Y50, 130.33 mg/kg) was observed higher than the shale value. Therefore, the Huaihe River showed no Ni enrichment except in site Y50.
In general, the data presented in Table 2 suggested that metal concentrations in Huaihe River (Anhui Province) declined in this order: Zn > Pb > Cu, Ni. Greater attention should be paid to the increased Zn and Pb concentrations. The result was similar to the evaluated metal levels in coastal marine sediments from Singapore by Cuong and Obbard (2006).
Comparison with previous studies worldwide
The average trace metal (Cu, Pb, Zn, and Ni) concentrations in surface sediments from Yangtze River (Wuhan) (Z. Yang et al. 2009), Yellow River (Henan Province) (Luo et al. 2010), Coastal Bohai Bay, China (Gao and Chen 2012), East China Sea (Yuan et al. 2004), and world average level (Martin and Meybeck 1979) are presented in Table 3. Average Cu concentration in the Yangtze River (Wuhan) sediments was 1.74 times higher than that in the present study. Comparable Cu concentrations were observed in the Yellow River (Henan Province), world average, and the Huaihe River (Anhui Province). However, compared with that in the East China Sea (20.13 mg/kg), the average Cu concentration (31.30 mg/kg) in this present study was 1.55 times higher. Nickel concentration in the water systems listed above followed a similar trend to that of Cu.
The mean Pb concentration in the Huaihe River (Anhui Province) was the most seriously polluted among all water systems considered. Zinc concentration in the present study was exceeded only by that of the Yangtze River (Wuhan Tributaries).
Overall, comparisons of mean trace metal concentrations with those of the principal water systems in China and the world river average level also showed relatively high Pb and Zn contamination in the Huaihe River (Anhui Province).
Comparison with consensus-based SQGs
The consensus-based sediment quality guidelines (SQGs), including the threshold effect concentration (TEC) and probable effect concentration (PEC), are employed to assess sediment quality conditions by predicting the frequency of sediment toxicity (MacDonald et al. 2000). It is considered that adverse effects on sediment-dwelling organisms rarely occur when heavy metal concentrations are below the consensus-based TECs. On the contrary, adverse effects on sediment-dwelling organisms are expected to happen frequently when trace metal concentrations exceed the PECs. Therefore, these two indexes divide trace metal concentrations into three ranges (Fig. 2).
In our experiment, 55.56 % of the 54 sediment samples had Cu concentrations below the relevant TEC (31.60 mg/kg). Cu concentrations in the remainder lay between the TEC and PEC (149 mg/kg), which implies that adverse effects on sediment-dwelling organisms may occasionally occur. In comparison with Cu, more sediment samples were observed with individual trace metal concentrations varying between the relevant TECs and PECs for Pb, Zn, and Ni (40, 35, and 45 sediment samples, respectively). In addition, one site (Y48) and two sites (Y47, Y50) were found to have Pb and Ni concentrations above the corresponding PEC, respectively, meaning that adverse effects are expected to occur frequently in these three sites.
Generally, the sediment quality conditions were of great concern. In 98.15 % of the 54 sample sites, all except for Y44, at least one metal concentration higher than the corresponding TEC was recorded. Furthermore, 12 sample sites, seven of which are located in Fengtai or Huainan (F11, F12, F15, F17, H32, H36, H38), were found to have concentrations of all these four trace metals (Cu, Pb, Zn, Ni) exceeding the corresponding TECs. As a consequence, more attention should be paid to sediments in Fengtai and Huainan, where there is a high probability of adverse biological effects.
Metal speciation
Although the total concentration of trace metals is used as a criterion to evaluate the environmental impact of sediments polluted by metals, reliance on the total concentration alone is not sufficient (Morillo et al. 2004). The mobility, bioavailability, and toxicity of trace metals are determined by different chemical forms, which are controlled by chemical and geological conditions (Surija and Branica 1995; Z. Yang et al. 2009). In the present study, four chemical fractions (weak acid soluble, reducible, oxidizable, and residual) were obtained by conducting the modified BCR sequential extraction procedure (Rauret et al. 1999).
In the weak acid soluble fraction (exchangeable and carbonate bound, F1), trace metals are either weakly absorbed onto the sediment surface by weak electrostatic interaction or (co-)precipitated with the carbonates in the sediments (Rodriguez et al. 2009). Generally, it is considered that this fraction (F1), which can be directly uptaken by organisms, presents the most potential toxicity to the water quality with the highest mobility (Cai et al. 2007). The change of environmental conditions, such as pH, salinity, and the ionic composition of water, may present significant influence on the stability of weak acid soluble fraction.
In the reducible fraction (F2), trace metals are associated with Fe-Mn oxides, with a strong specific adsorption, which can result in the release of metals under reducing conditions. Trace metals in the oxidizable fraction (F3) may be associated with organic materials and sulfides by generating complexes (Rodriguez et al. 2009). Trace metals in this fraction will be released into the water column when the redox potential increases for the decomposition of organic matter. The residual fraction (F4) is the most chemically recalcitrant and least bioavailable form among the four different chemical species and occurs in the crystalline structure of some primary and secondary minerals in sediments (Dang et al. 2002; Kubova et al. 2004).
Generally, of the four chemical forms extracted in sediments, the first three fractions (F1, F2, and F3) are known as the biological effective forms, which are labile, and release trace metals when subject to environmental changes. Thorough investigation of the trace metal speciation present in sediments is therefore crucial, especially the biological effective fractions.
Copper, nickel, zinc, and iron
Metal speciation distributions in the Huaihe River (Anhui Province) are shown in Fig. 3. This diagram presents similar distribution patterns for Cu and Ni (F4 > F3 > F2 > F1) and Zn and Fe (F4 > F2 > F3 > F1). The dominant proportions of Cu, Zn, Fe, and Ni were all found in the residual fraction, with percentages of 55.29, 55.54, 69.18, and 71.51 %, respectively. These data suggest that a large proportion of these four metals were immobilized in mineral crystalline lattices. For Cu and Ni, the organic and sulfide fraction was the main scavenger among the non-lithogenic fractions, accounting for 28.40 and 15.39 % of the total, respectively. The high percentage of Cu in F3 can be explained by the high affinity of Cu to humic substances in the sediments (Morillo et al. 2004; Javan et al. 2015). The second greatest fraction among the non-residual phases for Cu and Ni was the Fe-Mn oxide fraction, containing 8.09 and 11.25 % of the total, respectively. For Zn and Fe, a converse result was observed. The reducible fraction was the main scavenger, followed by the oxidizable fraction for Zn and Fe. Similar chemical partitioning pattern of Fe was also reported by Morillo et al. (2002, 2004). The amount in the weak acid soluble fraction of Fe is negligible.
Lead
A different fractionation profile was observed for Pb (F2 > F3 > F4 > F1). The dominant proportion of Pb was found in the Fe-Mn oxide fraction (45.36 %) and followed by oxidizable (28.55 %) and residual (19.62 %) fractions. Thus, in the Huaihe River, iron and manganese play an important role in controlling the movement of Pb from sediments (Javan et al. 2015). The partitioning pattern of Pb was not unusual, and similar results were also reported by Morillo et al. (2004), Javan et al. (2015), and Dawson and Macklin (1998). The high total Pb concentration and high percentage in Fe-Mn oxides made this element of great interest for its high toxicity to aquatic organisms and fish.
Manganese
Manganese presents the highest percentage in the weak acid soluble fraction (exchangeable and carbonate, 46.52 %), which is considered as the most labile speciation. It is reported that manganese has special affinity towards carbonate for its similarity in ionic radii with that of calcium, which allows Mn to substitute Ca in carbonate, and co-precipitate with its minerals (Nasr et al. 2015b). Considerable Mn content was also observed in the Fe-Mn oxide fraction, accounting for 34.30 % of the total. Lower percentage of Mn (4.20 %) in oxidizable fraction was present in this study, and it was probably because of the competition between Fe-Mn organic complex and Fe-Mn oxide form.
Risk assessment code
The risk assessment code (RAC), first introduced by Perin et al. (1985), was employed in this study to determine the environmental risk of metals. The RAC was classified into five ranks (Table 4) based on the percentage of the weak acid soluble fraction (F1), which is a serious environmental concern, presenting the highest potential of environmental toxicity for easy equilibrium with aqueous phase, thus becoming more rapidly bioavailable (Sundaray et al. 2011). The metals associated with F1 in the Huaihe River sediments followed the order: Mn > Zn > Cu > Pb > Ni > Fe. Manganese (46.52 %) presented the highest percentage of the weak acid soluble fraction among the metals studied, indicating a high risk for the release into the water. Relatively high Mn concentration in F1 was also reported by Javan et al. (2015). Zinc (8.87 %), copper (8.33 %), lead (5.89 %), nickel (2.02 %), and iron (1.71 %) all indicated low risk with the percentages of F1 ranging between 1 and 10 %. These values indicated that Mn presented the highest mobility and bioavailability, posing a serious threat to benthic organisms in the Huaihe River, and can easily enter into the food chain. Kelderman and Osman (2007) reported that the concentration of the weak acid soluble fraction might be affected by recent pollution events (human activities). Further, Jain et al. (2008), Pempkowiak et al. (1999), and Y. Yang et al. (2012) also reported that the metals resulting from anthropogenic inputs primarily combined with the labile fraction, which is more easily uptaken by sediment-dwelling organisms. Consequently, it is of great importance to control the metal entry into the Huaihe River.
Statistical analysis
The correlation analysis between fractionations of Cu, Pb, Zn, and Ni and OM, Fe, and Mn was listed in Table 5. Copper in F3 and F4 fractions presented high positive correlation (0.57 and 0.61, respectively) with organic matter. Moreover, Cu existed in F3 with a high percentage, which can be explained by the high correlation between Cu and OM. Morillo et al. (2004), Javan et al. (2015), and Nasr et al. (2015a) also reported that there was a high affinity of Cu to humic substances in the sediments. Zinc in F1 and F2 showed high correlations with organic matter and iron. Compared with OM and Fe, Mn presented insignificant correlation with fractionations of Cu, Pb, Zn, and Ni.
Conclusion
The total trace metal concentrations along the Huaihe River (Anhui Province) exhibited certain contamination by Zn and Pb, especially in Fengtai and Huainan areas. The low levels of Cu and Ni indicated that there were no/slight Cu or Ni pollution in this river. Comparison of trace metal concentrations among the principal water systems in China also revealed higher Pb and Zn levels in the Huaihe River.
The chemical partitioning of metals provided valuable information on the potential mobility of metals in the Huaihe River sediments. Among the metals studied, manganese is the most mobile for the highest percentage in the weak acid soluble fraction, presenting a high risk on the basis of RAC, and followed by the Fe-Mn oxide fraction. The residual fraction was found dominant for Cu, Zn, Ni, and Fe in the Huaihe River (Anhui Province), implying that these four metals are strongly bound to the sediments. Lead exhibited the highest percentage in the Fe-Mn oxide fraction. These five metals (Cu, Pb, Zn, Ni, and Fe) presented low risks (RAC, 1 % < F1 < 10 %) to the water. Copper in F3 and F4 presented high positive correlation with organic matter, which can explain the high percentage of Cu in the oxidizable and residual fractions.
References
Almeida, C. M. R., Mucha, A. P., & Vasconcelos, M. T. S. D. (2004). Influence of the sea rush Juncus maritimus on metal concentration and speciation in estuarine sediment colonized by the plant. Environmental Science & Technology, 38(11), 3112–3118. doi:10.1021/Es049932j.
Arain, M. B., Kazi, T. G., Jamali, M. K., Afridi, H. I., Jalbani, N., Sarfraz, R. A., et al. (2008). Time saving modified BCR sequential extraction procedure for the fraction of Cd, Cr, Cu, Ni, Pb and Zn in sediment samples of polluted lake. Journal of Hazardous Materials, 160, 235–239.
Bi, X., Feng, X., Yang, Y., Li, X., Sin, G. P. Y., Qiu, G., et al. (2007). Heavy metals in an impacted wetland system: a typical case from southwestern China. Science of the Total Environment, 387(1-3), 257–268. doi:10.1016/j.scitotenv.2007.07.059.
Cai, Q. Y., Mo, C. H., Wu, Q. T., Zeng, Q. Y., & Katsoyiannis, A. (2007). Concentration and speciation of heavy metals in six different sewage sludge-composts. Journal of Hazardous Materials, 147(3), 1063–1072. doi:10.1016/j.jhazmat.2007.01.142.
Cuong, D. T., & Obbard, J. P. (2006). Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Applied Geochemistry, 21(8), 1335–1346. doi:10.1016/j.apgeochem.2006.05.001.
Dang, Z., Liu, C. Q., & Haigh, M. J. (2002). Mobility of heavy metals associated with the natural weathering of coal mine spoils. Environmental Pollution, 118(3), 419–426. doi:10.1016/S0269-7491(01)00285-8.
Davutluoglu, O. I., Seckın, G., Kalat, D. G., Yılmaz, T., & Ersu, C. B. (2010). Speciation and implications of heavy metal content in surface sediments of Akyatan Lagoon–Turkey. Desalination, 260(1-3), 199–210. doi:10.1016/j.desal.2010.04.031.
Dawson, E. J., & Macklin, M. G. (1998). Speciation of heavy metals in floodplain and flood sediments: a reconnaissance survey of the Aire Valley, West Yorkshire, Great Britain. Environmental Geochemistry and Health, 20(2), 67–76. doi:10.1023/A:1006541724394.
Doherty, G. B., Brunskill, G. J., & Ridd, M. J. (2000). Natural and enhanced concentrations of trace metals in sediments of Cleveland Bay, Great Barrier Reef lagoon, Australia. Marine Pollution Bulletin, 41(7-12), 337–344. doi:10.1016/S0025-326x(00)00129-6.
Dzombak, D. A., & Morel, F. M. M. (1987). Adsorption of inorganic pollutants in aquatic systems. Journal of Hydraulic Engineering-Asce, 113(4), 430–475.
Fernandez, E., Jimenez, R., Lallena, A. M., & Aguilar, J. (2004). Evaluation of the BCR sequential extraction procedure applied for two unpolluted Spanish soils. Environmental Pollution, 131(3), 355–364. doi:10.1016/j.envpol.2004.03.013.
Fuentes, A., Llorens, M., Saez, J., Isabel Aguilar, M. A., Ortuno, J. F., & Meseguer, V. F. (2008). Comparative study of six different sludges by sequential speciation of heavy metals. Bioresource Technology, 99(3), 517–525. doi:10.1016/j.biortech.2007.01.025.
Gao, X., & Chen, C. T. A. (2012). Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Research, 46(6), 1901–1911.
Ghrefat, H., & Yusuf, N. (2006). Assessing Mn, Fe, Cu, Zn, and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere, 65(11), 2114–2121. doi:10.1016/j.chemosphere.2006.06.043.
Hem, J. D. (1978). Redox processes at surfaces of manganese oxide and their effects on aqueous metal-ions. Chemical Geology, 21(3-4), 199–218. doi:10.1016/0009-2541(78)90045-1.
Ip, C. C. M., Li, X. D., Zhang, G., Wai, O. W. H., & Li, Y. S. (2007). Trace metal distribution in sediments of the Pearl River Estuary and the surrounding coastal area, South China. Environmental Pollution, 147(2), 311–323. doi:10.1016/j.envpol.2006.06.028.
Jain, C. K. (2004). Metal fractionation study on bed sediments of River Yamuna, India. Water Research, 38(3), 569–578. doi:10.1016/j.watres.2003.10.042.
Jain, C. K., Gupta, H., & Chakrapani, G. J. (2008). Enrichment and fractionation of heavy metals in bed sediments of River Narmada, India. Environmental Monitoring and Assessment, 141(1-3), 35–47. doi:10.1007/s10661-007-9876-y.
Javan, S., Hassani, A. H., Ahangar, A. G., & Soltani, J. (2015). Fractionation of heavy metals in bottom sediments in Chahnimeh 1, Zabol, Iran. Environmental Monitoring and Assessment, 187(6), doi:Artn 340. doi 10.1007/S10661-015-4510-X.
Kay, J. T., Conklin, M. H., Fuller, C. C., & O’Day, P. A. (2001). Processes of nickel and cobalt uptake by a manganese oxide forming sediment in Pinal Creek, globe mining district, Arizona. Environmental Science & Technology, 35(24), 4719–4725. doi:10.1021/Es010514d.
Kazi, T. G., Jamali, M. K., Kazi, G. H., Arain, M. B., Afridi, H. I., & Siddiqui, A. (2005). Evaluating the mobility of toxic metals in untreated industrial wastewater sludge using a BCR sequential extraction procedure and a leaching test. Analytical and Bioanalytical Chemistry, 383, 297–304.
Kelderman, P., & Osman, A. A. (2007). Effect of redox potential on heavy metal binding forms in polluted canal sediments in Delft (The Netherlands). Water Research, 41(18), 4251–4261. doi:10.1016/j.watres.2007.05.058.
Kubova, J., Stresko, V., Bujdos, M., Matus, P., & Medved, J. (2004). Fractionation of various elements in CRMs and in polluted soils. Analytical and Bioanalytical Chemistry, 379(1), 108–114. doi:10.1007/s00216-004-2505-5.
Li, X. D., Shen, Z. G., Wai, O. W. H., & Li, Y. S. (2001). Chemical forms of Pb, Zn and Cu in the sediment profiles of the Pearl River Estuary. Marine Pollution Bulletin, 42(3), 215–223. doi:10.1016/S0025-326x(00)00145-4.
Li, X. D., Wai, O. W. H., Li, Y. S., Coles, B. J., Ramsey, M. H., & Thornton, I. (2000). Heavy metal distribution in sediment profiles of the Pearl River estuary, South China. Applied Geochemistry, 15(5), 567–581. doi:10.1016/S0883-2927(99)00072-4.
Luo, B., Liu, L., Zhang, J. L., Tan, F. Z., Meng, W., Zheng, B. H., Zhao, X. G., & Zhang, Y. S. (2010). Levels and distribution characteristics of heavy metals in sediments in main stream of Huaihe River. Journal of Environmental Health, 27, 1122–1127 (in Chinese).
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39(1), 20–31.
Martin, J. M., & Meybeck, M. (1979). Elemental mass-balance of material carried by major world rivers. Marine Chemistry, 7(3), 173–206. doi:10.1016/0304-4203(79).
Morillo, J., Usero, J., & Gracia, I. (2002). Partitioning of metals in sediments from the Odiel River (Spain). Environment International, 28(4), 263–271. doi:10.1016/S0160-4120(02)00033-8.
Morillo, J., Usero, J., & Gracia, I. (2004). Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere, 55(3), 431–442. doi:10.1016/j.chemosphere.2003.10.047.
Nasr, S. M., Okbah, M. A., El Haddad, H. S., & Soliman, N. F. (2015a). Fractionation profile and mobility pattern of metals in sediments from the Mediterranean Coast, Libya. Environmental Monitoring and Assessment, 187(7), Artn 430. doi:10.1007/S10661-015-4668-2.
Nasr, S. M., Soliman, N. F., Khairy, M. A., & Okbah, M. A. (2015b). Metals bioavailability in surface sediments off Nile delta, Egypt: application of acid leachable metals and sequential extraction techniques. Environmental Monitoring and Assessment, 187(6), Artn 312. doi:10.1007/S10661-015-4548-9.
Pempkowiak, J., Sikora, A., & Biernacka, E. (1999). Speciation of heavy metals in marine sediments vs their bioaccumulation by mussels. Chemosphere, 39(2), 313–321. doi:10.1016/S0045-6535(99)00112-5.
Perin, G., Craboledda, L., Lucchese, M., Cirillo, R., Dotta, L., Zanette, M., Orio, A. (1985). Heavy metal speciation in the sediments of Northern Adriatic sea— a new approach for environmental toxicity determination. In Lekkas, T.D. (Ed), Heavy metals in the environment (Vol. 2, pp. 454–456). Edinburgh: CEP Consultants.
Rauret, G., Lopez-Sanchez, J. F., Sahuquillo, A., Rubio, R., Davidson, C., Ure, A., et al. (1999). Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. Journal of Environmental Monitoring, 1(1), 57–61. doi:10.1039/A807854h.
Rodriguez, L., Ruiz, E., Alonso-Azcarate, J., & Rincon, J. (2009). Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. Journal of Environmental Management, 90(2), 1106–1116. doi:10.1016/j.jenvman.2008.04.007.
Singh, K. P., Mohan, D., Singh, V. K., & Malik, A. (2005). Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges, India. Journal of Hydrology, 312(1-4), 14–27. doi:10.1016/j.jhydrol.2005.01.021.
Sundaray, S. K., Nayak, B. B., Lin, S., & Bhatta, D. (2011). Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: Mahanadi basin, India. Journal of Hazardous Materials, 186(2-3), 1837–1846. doi:10.1016/j.jhazmat.2010.12.081.
Surija, B., & Branica, M. (1995). Distribution of Cd, Pb, Cu and Zn in carbonate sediments from the Krka River estuary obtained by sequential extraction. Science of the Total Environment, 170(1-2), 101–118.
Suthar, S., Nema, A. K., Chabukdhara, M., & Gupta, S. K. (2009). Assessment of metals in water and sediments of Hindon River, India: impact of industrial and urban discharges. Journal of Hazardous Materials, 171(1-3), 1088–1095. doi:10.1016/j.jhazmat.2009.06.109.
Tan, B. Q., Wu, P. R., Song, G. J. (2005). Water pollution and control of the Huaihe River Basin. Journal of Water Resuorce and Protection 21(6), 4–10 (in Chinese).
Tang, Q., Liu, G., Zhou, C., & Sun, R. (2013). Distribution of trace elements in feed coal and combustion residues from two coal-fired power plants at Huainan, Anhui, China. Fuel, 107, 315–322. doi:10.1016/j.fuel.2013.01.009.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace-metals. Analytical Chemistry, 51(7), 844–851. doi:10.1021/Ac50043a017.
Wang, J., Liu, G., Lu, L., Zhang, J., & Liu, H. (2015). Geochemical normalization and assessment of heavy metals (Cu, Pb, Zn, and Ni) in sediments from the Huaihe River, Anhui, China. Catena, 129, 30–38. doi:10.1016/j.catena.2015.02.008.
Yang, Y., Chen, F., Zhang, L., Liu, J., Wu, S., & Kang, M. (2012). Comprehensive assessment of heavy metal contamination in sediment of the Pearl River estuary and adjacent shelf. Marine Pollution Bulletin, 64(9), 1947–1955.
Yang, Z., Wang, Y., Shen, Z., Niu, J., & Tang, Z. (2009). Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. Journal of Hazardous Materials, 166(2-3), 1186–1194. doi:10.1016/j.jhazmat.2008.12.034.
Yuan, C. G., Shi, J. B., He, B., Liu, J. F., Liang, L. N., & Jiang, G. B. (2004). Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environment International, 30(6), 769–783. doi:10.1016/j.envint.2004.01.001.
Acknowledgments
This work was financially supported by the Program for National Key Technology Research and Development Program, Ministry of Science and Technology, China (Grant no. 2010BAC10B02), Key Program for Science and Technology Development of Anhui Province (No. 12010402111), and the National Natural Science Foundation of China (No. 41373110). We acknowledge the editors and reviewers for polishing the language of the paper and for the in-depth discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Liu, G., Lu, l. et al. Metal distribution and bioavailability in surface sediments from the Huaihe River, Anhui, China. Environ Monit Assess 188, 3 (2016). https://doi.org/10.1007/s10661-015-5005-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-5005-5