Abstract
A cyclonic eddy (CE) in the southwestern Bay of Bengal (SWBoB; 10–15° N; 81–87° E) during winter monsoon 2005 and associated changes in the open ocean hydrography and productivity patterns were studied using satellite observations and in situ measurements. Analysis of the satellite-derived sea surface height anomaly (SSHA) indicated the existence of a large eddy (10–15° N; 81–87° E) from November to January, with its core centered at 13° N and 82° E. The large positive wind stress curl (~1.8 × 10−7 N m−2) and resultant Ekman pumping (~3 × 10−2 cm s−1) were identified as the prominent forcing mechanisms. In addition, the cyclonic storms and depressions experienced in the region during the study period seem to have served to maintain the strength of the CE through associated Ekman pumping. The cold (~26.6 °C), nutrient-enriched (NO3 > 2 μM, PO4 > 0.73 μM and SiO4 > 3 μM) upwelled waters in the upper layers of the CE enhanced the biological production (chl. a > 0.56 mg m−3). Dissolved oxygen in the surface waters was > 200 μM. The phytoplankton and zooplankton biomass recorded during the season was comparable or perhaps higher than the peak values reported from the northeastern Arabian Sea (chlorophyll a concentrations of 0.2 to 0.4 mg m−3 and zooplankton biovolume 0.6 ml m−3) during winter. Occurrence of a higher mesozooplankton biovolume (0.8 ml m−3) and relatively low abundance of microzooplankton indicates the prevalence of a short food chain. In conclusion, high biological production, both at primary as well as secondary level, suggests the prevalence of an efficient food web as a result of physical forcing and subsequent ecological interactions evident up to tertiary level in an oligotrophic basin like BoB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Bay of Bengal (BoB) is a unique tropical basin, situated in the eastern part of the northern Indian Ocean, occupying an area of 6 % of the world oceans. The oceanographic characteristics of this basin exhibit seasonality due to semi-annually reversing monsoon winds and currents (Schott and McCreary 2001; Shankar et al. 2002). The basin receives large quantity of fresh water through river runoff from Brahmaputra, Ganges, Mahanadi, Godavary, Krishna and Cauvery rivers and precipitation (200–240 cm year−1). Annual runoff is computed as 1.625 × 1012 m3 year−1 (Subramanian 1993), and excess precipitation over evaporation is ~2 m year−1 (Prasad 1997). Dispersal of fresh water over the basin leads to low-saline upper water column with strong stratification throughout the year. Along with this, extensive cloud cover, sediment load and narrow shelf (Qasim 1977; Sengupta et al. 1977; Radhakrishna et al. 1978; Gomes et al. 2000) makes the basin relatively less productive than its western counterpart Arabian Sea. In contrast to Arabian Sea, major physical processes like coastal upwelling, winter convection, etc. are not active; instead, episodic events like eddies and gyres make the region locally productive.
Studies on biological production of the basin were carried out as part of major programmes viz., International Indian Ocean Expedition (IIOE), Marine Research-Living Resources (MR-LR) and Bay of Bengal Process Studies (BoBPS). Recent studies suggest that eddies and gyres have a promising role in governing the biological production of the oligotrophic bay, by influencing the vertical transport of nutrients to the photic zone (Prasannakumar et al. 2004, 2007, 2010; Vinayachandran et al. 2005; Muraleedharan et al. 2007; Fernandes 2008; Ramaiah et al. 2010; Fernandes and Ramaiah 2013, 2014; Sabu et al. 2015). Most of these studies deal with the influence of episodic events on the biological production, particularly in primary level (Prasannakumar et al. 2004, 2007, 2010; Vinayachandran et al. 2005; Ramaiah et al. 2010).
In the secondary level, planktonic components such as micro- and mesozooplankton play a key role in the marine food web by occupying an intermediate position between the primary and tertiary levels. Their distribution, abundance and community composition depend on the physical and biological processes, either together or independently (Haury et al. 1978). Hence, the spatial and temporal scales of variations in atmospheric as well as oceanographic conditions determine heterogeneity in zooplankton population and in turn the food web structure (Parsons 1980). Recently, few studies have documented the zooplankton response to the various physicochemical processes in the BoB (Muraleedharan et al. 2007; Fernandes 2008; Fernandes and Ramaiah 2013, 2014). Muraleedharan et al. 2007 emphasized the importance of basin scale (warm gyre) as well as mesoscale process (eddy and upwelling) in the biological production (both primary and secondary) during the summer monsoon. Although these studies highlight the impact of eddies on zooplankton community, trophic response of such events is least studied until date. In view of this, the present study describes the impact of a large cyclonic eddy on the planktonic structure, in the SWBoB during winter 2005.
Materials and methods
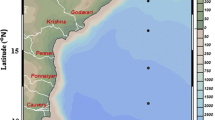
Data for the present study samples were collected on-board the FORV Sagar Sampada during the winter monsoon (December 2005) along the SWBoB (Fig. 1). Seabird CTD (SBE 911 Plus) was used to collect vertical profiles of temperature and salinity in the upper 1000-m water column. Salinity values from CTD were corrected against the values derived from the Autosal (Guild line 8400) on-board. The sea surface temperature (SST) was measured using a bucket thermometer. The mixed layer depth was determined as the depth where the density difference from the sea surface was 0.2 kg m−3 (Shetye et al. 1996). The thermocline layer was demarcated as the depth at which the temperature reached 15 °CFootnote 1.
Water samples were collected using Niskin bottles from 11 standard depths (viz. 0, 10, 20, 30, 50, 75, 100, 150, 200, 250 and 300 m (Sverdrup et al. 1942)) for the estimation of dissolved oxygen (DO) and nutrients. DO was estimated by the Winkler method (Strickland and Parsons 1972) and nutrients (Grasshoff et al. 1983) by Auto-Analyzer (SKALAR) on-board FORV Sagar Sampada. For the estimation of chlorophyll-a, 1 l of water sample was collected from 7 standard depths (surface, 10, 20, 50, 75, 100 and 120 m) and filtered through a GF/F filter (pore size 0.7 μ). Chlorophylla was extracted with 10 ml of 90 % acetone and analysed spectrophotometrically (Perkin-Elmer UV/Vis at 640 nm) (Strickland and Parsons 1972). Total column (upper 120 m) chlorophyll a (mg m−2) concentrations were estimated by integrating the profile values.
For estimating microzooplankton, 5–8 l of water sample was collected from 7 standard depths (surface, 10, 20, 50, 75, 100 and 120 m). Water samples were initially prefiltered through a 200-μm bolting silk (Hydro-Bios) to eliminate the mesozooplankton, and the filtrate was carefully collected into black polythene bottles and fixed in 3–8 % of acid Lugol’s iodine. Although the screening of samples through a 200-μm sieve may disturb large and fragile mesozooplankton, this process is widely used in MZP sampling for discarding the mesozooplankton (Froneman and McQuaid 1997; Putland 2000; Stelfox–Widdicombe et al. 2004). These samples were then concentrated by gravity settling and siphoning methods. The organisms present in the samples were identified and categorized into heterotrophic dinoflagellates (HDS), ciliates (CTS), sarcodines (SDS), and crustacean nauplii (CNP) (Kofoid and Campbell 1939; Marshall 1969; Steidinger and Williams 1970; Subrahmanyan 1971; Maeda and Carey 1985).
Mesozooplankton were collected using Multiple Plankton Net (MPN, HYDRO - BIOS, 0.25 m2 mouth area, 200-μm mesh size). The net was towed vertically through five strata: the upper mixed layer, thermocline layer, bottom of thermocline to 300, 300–500 and 500–1000 m at a speed of 1 ms−1. The mesozooplankton biovolume was calculated by the displacement volume method (Postel et al. 2000) and was preserved in the 4 % formalin–seawater solution for detailed examination in the shore lab. The sample was primarily sorted into various taxonomic groups based on published references (ICES 1947; Newell and Newell 1973; Todd and Laverack 1991). Zooplankton abundance was estimated by counting all the individuals present in the sample or the aliquot depending on the volume. All abundance data were converted to density (ind. m−3) using the volume of water filtered by the net.
In addition to the in situ data, monthly averaged sea surface height anomaly (SSHA) from AVISO (http://www.aviso.oceanobs.com) and monthly mean chlorophyll data from MODIS (http://www.oceancolor.gsfc.nasa.gov) were also used.
Results and discussion
Thermohaline structure along 11° N, 13° N and 15° N showed a doming feature between 81° E and 83° E, especially in the upper 300 m (Fig. 2a, b and c). The intensity of the doming was at a maximum along 13° N with a vertical displacement of 25 °C isotherm from 60 m to surface (Fig. 2b). This doming feature in the thermohaline structure showed the presence of a large, intense cyclonic eddy (CE) in the study area. The weekly average SSHA along with geostrophic currents during the study period (December) confirmed the presence of a large CE centred at 13° N and 82° E, embedded in a large cyclonic circulation (90 cm s−1) between 80° and 85° E and 10° and 18° N belt (Fig. 3). The presence of an intense CE in the southwest BoB during winter, 2005, has been reported earlier (Chen et al. 2013). The monthly mean SSHA from November 2005 to February 2006 shows that this CE formed in November 2005 is a part of a large cyclonic gyre (CG, Fig. 4). The CG then intensified as an eddy and became centred more towards the southern side of the BoB during December. The presence of a CG in the SWBoB during the northeast monsoon has been reported earlier (Vinayachandran and Yamagata 1998; Schott and McCreary 2001).
The CE is forced primarily by upward Ekman pumping (EP) caused by positive wind stress curl and resultant upwelling within this gyre, cooling the sea surface (Vinayachandran and Mathew 2003). In order to illustrate the role of wind stress and EP, the 3 days mean wind from July to December 2005 in the CE region was analysed. It can be seen that wind stress curl showed noticeable variation between October and December (Fig. 5). The observed positive wind stress curl in the CE region suggests that local wind stress curl is the dominant driving force behind the formation of CE. The figure also illustrates the associated positive Ekman velocity (green-line) representing upward motion or condition for upwelling. The low-frequency variability of the wind stress has induced strong Ekman pumping producing a divergence in the upper water column, which may result in upward movement of deep water to the surface. This short-term variability of wind in the CE region was due to frequent cyclones and depressions occurring in the BoB (Fig. 6). During November–December, 2005 two cyclonic storms (‘BAZZ’—28 November to 2 December and ‘Fanoos’—6 to 10 December) and two depressions (20 to 22 November and 15 to 22 December) were experienced in the southern BoB. The wind stress curl (~12 × 10−7 Nm−3) and Ekman velocity (~32 × 10−3 cm s−1) increased significantly in the first week of December with the passage of these two tropical cyclones (Fig. 5).
To explore further the forcing mechanisms behind the intensification of the CE, the monthly mean SSHA along with geostrophic currents during 2004, 2005 and 2006 for the month of December (Fig. 7) were analysed. It was observed that the intensity of the East India Coastal Current (EICC) was anomalously stronger in 2005 (>75 cm−1) than 2004 (<25 cm−1) and 2006 (<20 cm−1). Also, the year 2005 was found to be a negative Indian Ocean dipole (IOD) year (Grunseich et al. 2011); hence, the intensification of the EICC is due to the combined effect of intense eastward Wyrtki jet, strong summer monsoon currents and warm SST anomalies in the eastern equatorial Indian Ocean (EEIO) associated with negative IOD (Thompson et al. 2006). Hence, the increased number of cyclones/depressions in the study area and appreciable changes in the climatological circulation patterns in the EEIO appear to be linked to the IOD, in that a negative IOD made the CG more intense and active in winter 2005.
The distribution of dissolved oxygen (DO) and micronutrients in the upper waters showed prominent variability as a result of cyclonic eddy. The surface waters were well oxygenated (>200 μM) with an enhanced level of nitrate concentration (~2 μM; Fig. 8) in the CE region. The dissolved oxygen (DO) and nitrate concentration varied from 200 to 233 μM and 1.1 to 3.8 μM, respectively, with a maximum at the centre of the CE. The average concentrations of DO and nitrate in surface waters recorded were 217 and 2.12 μM, respectively. Vertical profiles showed upsloping of isolines from subsurface (50 m) to surface along 13° N and 15° N, while along 11° N, the upsloping feature was relatively less prominent and restricted to the upper 50 m (Fig. 9). The strong Ekman pumping due to frequent cyclonic events in the BoB resulted in the upwelling of nutrient-enriched subsurface waters to the surface. The unusual nutrient enrichment in the photic zone may trigger the proliferation of phytoplankton, which liberates sufficient amount of dissolved oxygen to the surrounding waters as a result of photosynthetic activity. Supply of nutrients to the photic zone from the deep through Ekman pumping due to atmospheric and physical forcing is a common phenomenon of nutrient transport in the basin, like BoB (Prasannakumar et al. 2004; Muraleedharan et al. 2007; Maneesha et al. 2011). The amount of nutrient entrainment depends on the strength as well as spatial and temporal variability of such mesoscale processes. In the present study, persistent and strong Ekman transport due to CE, along with successive physical events such as cyclones, depression and deep depression (Chen et al. 2013), provided sufficient amount of nutrients to the photic zone to trigger phytoplankton blooms.
Weekly composite of Chl. a imagery showed patches with high concentrations (>0.5 mg m−3) over the entire study area (Fig. 10). The in situ observation also showed more or less similar concentration in the upper 20 m (>0.22 mg m−3) irrespective of transects. In general, phytoplankton community in an enriched condition is usually dominated by diatoms. Madhu et al. 2006 reported the dominance of diatoms (91 to 95.4 %) from the SWBoB, mainly pennate diatoms like Thalassionema nitzschioids, Skeletonema costatum, Coscinodiscus spp., Thalassiothrix longissima, Nitzschia seriata, Chaetoceros spp. and Rhizosolenia spp. In our study, a slightly lower concentration along the 13° N transect could be attributed to the grazing by higher level organisms which was abundant along this transect. The peripheral regions (11° N and 15° N) sustained comparatively higher concentration than the core of the eddy (13° N). This high concentration at the periphery could be attributed to the frontal characteristics of the edges that support high phytoplankton standing stock as suggested by Franks (1992). A study by Vinayachandran and Mathew (2003) has reported the blooming of phytoplankton in the southern BoB during winter as a result of cyclonic gyre. Although winter blooming is an annual feature in the southern BoB, the extent, duration and Chl. a concentration may vary from year to year. In a similar event of super cyclone during October 1999 sustained high primary production (Madhu et al. 2002). The frequent cyclonic storms, depression and deep depressions in the study region ensure the persistent Ekman transport which ultimately results in the phytoplankton bloom. Such enhancement in the Chl.a concentration as noticed in the present study was due to the combined effect of cyclonic storms and deep depressions along with the CE in the SWBoB.
The response of secondary standing stock (both micro and meso) to CE was also evident in the present study. Microzooplankton (20–200 μm) abundance in the upper 120 m varied between 9 × 104 and 16 × 104 ind. m−2 at 11° N and 13° N, respectively (Fig. 11). The microzooplankton community was dominated by 73 % of ciliates (CTS) along 11° N, whereas heterotrophic dinoflagellates (HDF) were dominant (37 and 53 %) along 13° and 15° N, respectively. Ciliates contributed only 4 % along both these transects. The higher number of ciliates at 11° N might be due to the patch of preferable fraction of phytoplankton cells. Studies in marine systems indicate that the abundance of CTS is related with the availability of smaller phytoplankton (Godhantaraman and Krishnamurthy 1997; Heinbokel and Beers 1979). The cell size of phytoplankton is assumed to be linked with the physical state of the system, and in the present observation, it might be linked to the age of the eddy.
However, the total abundance of microzooplankton during the present study was markedly lower than earlier reports from the same area during the winter monsoon (Jyothibabu et al. 2008). This lower abundance can be attributed to two conditions; firstly, the relative colder surface waters during the winter monsoon compared to other seasons might have caused low abundance (Jyothibabu et al. 2008); and secondly, the nutrient enriched condition of the CE might have favoured dominance of large-sized phytoplankton cells (Cushing 1989). Hence, the low abundance of total microzooplankton in the study region suggests the prevalence of short food chain rather than microzooplankton-mediated food web in such gyre ecosystems.
Variations in mesozooplankton (200 μm –20 mm) biovolume, abundance and community structure were prominent in the CE. In the mixed layer, the mesozooplankton biovolume ranged between 0.35 and 1.4 ml m−3 (Fig. 12) with an average of 0.85 ml m−3. High biovolume (1.4 ml m3) in the mixed layer was observed at two locations (15° N, 81° E and 13° N, 82° E) of which 13° N, 82° E corresponds to the core region of the CE. In the thermocline layerFootnote 2, the biovolume ranged from 0.08 to 0.69 ml m−3 with the maximum at 13° N, 81° E. In the deeper strata viz., below the thermocline to 300, 300–500 and 500–1000 m, the values recorded were <0.13 ml m−3 (Fig. 13), except in a few locations attributed to swarming events of Pyrosoma. The increase in the mesozooplankton biovolume in response to the CE during the observation time was comparable to those reported by Sabu et al. 2015 from the cyclonic eddy region, whereas it was somewhat higher than that of Jyothibabu et al. 2008 (777 mg Cm−2; back calculated as ~3.5 ml m−2) from the western BoB during winter monsoon. Since the studies pertaining to the zooplankton during the winter monsoon were few, comparisons were made with the summer monsoon reports in order to understand its differential response to CE (Nair et al. 1981; Muraleedharan et al. 2007; Fernandes and Ramaiah 2009). It is found that the biovolume recorded in the SWBoB during winter monsoon 2005 was higher or sometimes comparable to the above reports (Table 1). Fernandes and Ramaiah 2014 has observed high biomass from the SWBoB during the same period and suggested upwelling like processes in the area. However, the higher biomass observed in the present study from the same region may be associated with the existence of a large cyclonic eddy which is evident from the close resolution sampling (1 °× 1°). Events such as CE enhance the biological production of the bay which is at times comparable to its western counterpart, Arabian Sea (Madhupratap et al. 1996), during winter monsoon.
Consistent to the biovolume, numerical abundance of mesozooplankton ranged between 70 and 4288 ind. m−3 and was considerably higher in the mixed layer. At the core region of CE, the mesozooplankton abundance recorded was >800 ind. m−3 (13° N; 82° E) which was comparably higher than the other stations along 13° N (Table 2). In general, mesozooplankton abundance was noticeably higher in the study region, and it can be attributed to the availability of rich phytoplankton standing stock for effective grazing. Patch of higher abundance (4288 ind. m−3) observed at 11° N, 83° E, which was many times higher than other stations, was contributed by 93 % (Fig. 14) of copepods (4000 ind. m−3). This higher abundant patch of copepods without corresponding increase in biovolume is due to the contribution by small-sized individuals. This can be attributed to the availability of high phytoplankton for grazing and also the occurrence of ciliates in the microzooplankton community at the same region, which is considered to be the preferred food of copepods (Calbet and Saiz 2005). However, in the thermocline layer, the mesozooplankton abundance was relatively low and varied from 4 to 498 ind. m−3. Along 13° N, thermocline layer supported relatively lower abundance of mesozooplankton than 11 and 15° N. In the deeper depth strata, the abundance was <100 ind. m−3. The total abundance of mesozooplankton showed significant variation (p < 0.05) between different depth strata which may be due to the low stock of phytoplankton towards deeper depths.
The mesozooplankton abundance in the core region (13° N; 82° E) of the CE sustained relatively higher mesozooplankton abundance (>800 ind. m−3) in the mixed layer with 93 % of copepods followed by 1.46 % chaetognaths (Fig. 14). However, in the thermocline layer, the abundance observed was very low, 19 ind. m−3, of which 66 % of the total was contributed by copepods followed by euphausiids (9 %) and chaetognaths (7 %). Higher abundance in the mixed layer and lower abundance in the thermocline layer in the core region can be attributed primarily to availability of nutrients and the resultant proliferation of phytoplankton in the mixed layer, which might have attracted zooplankton community chiefly for feeding purpose. In the water column above the thermocline layer, the biological activity goes on rapid rate (Banse 1964) and physically homogenous water column provide more congenial environment for zooplankton (Nair et al. 1977). In the present study, the upper column (mixed layer) of the eddy core region was highly active in order to support the zooplankton community compared to the thermocline layer.
Also at the locations other than the core of the CE, the community was dominated by copepods, followed by chaetognaths and appendicularians both in the mixed layer and the thermocline layer. Relative contribution of copepods ranged from 71 to 96 % in the mixed layer and 78 to 95 % in the thermocline layer. The contribution of chaetognaths and appendicularians in the mixed layer ranged from 1.4 to 10 % and <1 to 19 %, respectively. Along 15° N transect, locations with <80 % copepods were found to harbour a greater number of appendicularians (−11 %) followed by chaetognaths (−7 %). Similarly along 13 and 11° N transects, chaetognaths and appendicularians occupied the second and third ranks in the mesozooplankton community. The presence of carnivorous groups, like chaetognaths, suggests a high predation pressure on copepods, and the omnivorous group, like appendicularians (chiefly detritivory), was supported by the availability of dissolved organic matter either through the disposal of fine detrital/faecal matters or other decomposed organic material. Dominance of active grazers like copepods, carnivorous groups, like chaetognaths, and detritivorous, like appendicularians, in the study region denotes the prevalence of short classical food chain and supports the enhanced vertical transport of organic carbon from the surface to deep waters.
Variations in the zooplankton distribution pattern in the mesoscale eddies depends on many factors like source water, age, size, intensity, forcing mechanisms, interaction with wind events etc. (Owen 1981; Benitez-Nelson and McGillicuddy 2008; Morales et al. 2010). In our study, the variations of chl. a and zooplankton biovolume, abundance and composition between the core and non-core regions of eddy can be influenced by the age, size, intensity, interaction of eddy with wind events as well as the propagation of the eddy. The eddy with strong cyclonic circulation was evolved in the area during November 2005 and subsequent wind events such as cyclonic storms, deep depression and depression intensified the CE. Thus, the large area was influenced by this 3- to 4-week-old CE and associated circulation which resulted in an enhancement in the phytoplankton standing stock of the area. The eastward propagation of core of the CE was also evident in the satellite imagery. Hence, the higher biovolume and abundance of mesozooplankton in the core region suggest the retention of zooplankton community in the core. Also, the high grazing activity of the retained mesozooplankton in the core resulted in relatively low in situ Chl. a in the upper column. Studies based on the cold core rings observed in the Gulf Stream also recorded higher primary production in the core region through the entrainment of surrounding water masses in the ring (Kennelly et al. 1985; Hitchcock et al. 1993; Arístegui et al. 1997; Leterme and Pingree 2008).
The alterations in the primary and secondary levels (due to changes in the environment, resulting from the episodic events such as eddies and gyres in oligotrophic region like BoB) are influential in determining the production at tertiary level. We considered the trends in oceanic tuna resources to relate the implications at tertiary level as several studies have documented that this resource is closely related to events like El Nino, La Nina (Satheesh Kumar et al. 2014) and Indian Ocean dipole events (Lan et al. 2012). In the Indian waters, oceanic tuna are represented by three species dominated by yellowfin tuna (94.6 %), dogtooth tuna (1.5 %) and big-eye tuna (3.9 %) (Abdussamad et al. 2012). Published results from National Fishery Data Collected by Central Marine Fisheries Research Institute (CMFRI) suggested that yellowfin tuna resources were remarkably higher along the southeast coast than northeast, northwest and southwest coasts. An increase in the tuna fishery along the southeast coast was observed during the years 2006 and 2007 (Abdussamad et al. 2012), which was subsequent to our observation period. To some extent, the increase in fishery resources during the subsequent years (2006 and 2007) of our observation (2005) can be attributed to such atmospheric and oceanic events such as cyclones and eddies as observed in the present study. Further in situ oceanographic observations integrated with fish landing information would provide better understanding on the influence of episodic events such as eddies and cyclones in the BoB on tertiary level production.
Conclusion
Physical processes such as cyclonic eddies during winter monsoon show strong inter-annual variations in the intensity. During winter 2005, cyclonic eddy circulation was prominent in the SWBoB, which significantly influenced the biological production. In addition, atmospheric disturbances such as depressions, deep depressions, cyclones etc. experienced during the period, maintained the strength of the cyclonic eddy. High turbulence due to the combined effect of frequent cyclonic storms and strong and large-scale cyclonic circulation delivered appreciable quantity of nutrients to the photic zone, leading to the development of the food web dominated with larger cells that transfer a greater proportion of primary productivity to higher trophic level (Legendre 1981; Michaels and Silver 1988; Legendre and Gosselin 1989; Kiørboe 1993). The zooplankton community structure during the present study also showed the prevalence of short traditional food chain in the region. The observed values of Chl. a and mesozooplankton biovolume was comparable to the productive region of the northern Indian Ocean, i.e. northeastern Arabian Sea during the winter monsoon. In the present study, high biological production, both at primary as well as secondary level suggests the prevalence of an efficient food web as a result of physical forcing and subsequent ecological interactions evident up to tertiary level in an oligotrophic basin, like BoB.
Notes
Thermocline layer is a zone of rapid increase in temperature, ending in a colder and relatively constant-temperature deeper layer (Levinton 1995). In our study area, the thermocline layer is demarcated from the bottom of the mixed layer to the depth at which 15 °C isotherm is observed. Below this, the temperature remains relatively constant.
Thermocline layer varied from 129 to 188 m in the study region.
References
Abdussamad, E. M., Rao, G. S., Koya, K. P. S., Rohit, P., Joshi, K. K., Sivadas, M., Kuriakose, S., Ghosh, S., Jasmine, S., Chellappan, A., & Koya, M. (2012). Indian tuna fishery—production trend during yesteryears and scope for the future. Indian Journal of Fisheries, 59(3), 1–13.
Arístegui, J., Tett, P., Hernández-Guerra, A., Basterretxea, G., Montero, M. F., Wild, K., et al. (1997). The influence of island generated eddies on chlorophyll distribution: a study of mesoscale variation around Gran Canaria. Deep-Sea Research, 44, 71–96.
Banse. (1964). On the vertical distribution of zooplankton in the sea. Progress in Oceanography, 2, 55–125.
Benitez-Nelson, C. R., & McGillicuddy, D. J., Jr. (2008). Mesoscale physical-biological biogeochemical linkages in the open ocean: an introduction to the results of the E-flux and EDDIES programs. Deep-Sea Research Part II, 55, 1133–1138.
Calbet, A., & Saiz, E. (2005). The ciliate–copepod link in marine ecosystems. Aquatic Microbial Ecology, 38, 157–167.
Chen, X., Delu, P., Yan, B., Xianqiang, H., Chen-Tung Arthur, C., & Zengzhou, H. (2013). Episodic phytoplankton bloom events in the Bay of Bengal triggered by multiple forgings. Deep-Sea Research Part I, 73, 17–30.
Cushing, D. H. (1989). A difference in structure between ecosystems in strongly stratified waters and in those that are only weakly stratified. Journal of Plankton Research, 11, 1–13.
Fernandes, V. (2008). The effect of semi-permanent eddies on the distribution of mesozooplankton in the central Bay of Bengal. Journal of Marine Research, 66, 465–488.
Fernandes, V., & Ramaiah, N. (2009). Mesozooplankton community in the Bay of Bengal (India): spatial variability during the summer monsoon. Aquatic Ecology, 43(4), 951–963.
Fernandes, V., & Ramaiah, N. (2013). Mesozooplankton community structure in the upper 1,000 m along the western Bay of Bengal during the 2002 fall intermonsoon. Zoological Studies, 52(4), 31. p.11.
Fernandes, V., & Ramaiah, N. (2014). Distributional characteristics of surface-layer mesozooplankton in the Bay of Bengal during the 2005 winter monsoon. Indian Journal of Geo-Marine Sciences, 43(2), 176–188.
Franks, P. J. S. (1992). Sink or swim: accumulation of biomass at fronts. Marine Ecology Progress Series, 82, 1–12.
Froneman, P. W., & McQuaid, C. D. (1997). Preliminary investigation of the ecological role of microzooplankton in the Kariega estuary, South Africa. Estuarine, Coastal and Shelf Science, 45, 689–695.
Godhantaraman, N., & Krishnamurthy, K. (1997). Experimental studies on food habits of tropical microzooplankton (prey–predator interrelationship). India Journal of Marine Science, 26, 345–349.
Gomes, H. R., Goes, J. I., & Saino, T. (2000). Influence of physical processes and freshwater discharge on the seasonality of phytoplankton regime in the Bay of Bengal. Continental Shelf Research, 20, 313–330.
Grasshoff, K., Ehrhardt, M. and Krembling, K. (1983). Methods of seawater analysis. p. 125–183.
Grunseich, G., Subrahmanian, B., Murty, V. S. N., & Giese, B. S. (2011). Sea surface salinity variability during the Indian Ocean dipole and ENSO events in the tropical Indian ocean. Journal of Geophysical Research, 116, C11013. doi:10.1029/2011JC007456.
Haury, L. R., McGowan, J. A., & Wiebe, P. H. (1978). Patterns and processes in the time- space scales of plankton distributions. In J. H. Steele (Ed.), Spatial pattern in plankton communities (pp. 277–327). New York, US: Plenum Press.
Heinbokel, J. F., & Beers, J. F. (1979). Studies on the functional role of tintinnids in the Southern California Bight. II. Grazing rates of field populations. Marine Biology, 47, 191–197.
Hitchcock, G. L., Mariano, A. J., & Rossby, T. (1993). Mesoscale pigments fields in the Gulf Stream: observations in a meander crest and trough. Journal of Geophysical Research, 98, 8425–8445.
ICES. (1947). Fiches d’Identification du Zooplankton. International Council for Exploration of Seas (ICES). Copenhagen.
Jyothibabu, R., Madhu, N. V., Maheswaran, P. A., Jayalakshmy, K. V., Nair, K. K. C., & Achuthankutty, C. T. (2008). Seasonal variation of microzooplankton (20–200 μm) and its possible implications on the vertical carbon flux in the western Bay of Bengal. Continental Shelf Research, 28(6), 737–755.
Kennelly, M. A., Evans, R. H., & Joyce, T. M. (1985). Small scale cyclones on the periphery of a Gulf Stream warmcore ring. Journal of Geophysical Research, 95(C5), 8845–8857.
Kiørboe, T. (1993). Turbulence, phytoplankton cell size, and the structure of pelagic food webs. Advances in Marine Biology, 29, 1–67.
Kofoid, C. A., & Campbell, A. S. (1939). Reports on the scientific results of the expedition to the eastern tropical pacific, in charge of Alexander Agassiz, US fish commission steamer “Albatross”, from October 1904 to March 1905. University California Publications in Zoology, 34, 1–403.
Lan, K.-W., Evans, K., & Lee, M.-A. (2012). Effects of climate variability on the distribution and fishing conditions of yellowfin tuna (Thunnus albacares) in the western Indian Ocean. Climatic Change, 2012, 1–15.
Legendre, L. (1981). Hydrodynamic control of marine phytoplankton production: the paradox of stability. In J. C. J. Nihoul (Ed.), Ecohydrodynamics. Proceedings of 12th international. Liege colloquium on ocean hydrodynamics. Amsterdam: Elsevier.
Legendre, L., & Gosselin, M. (1989). New production and export of organic matter to the deep ocean: consequences of some recent discoveries. Limnology and Oceanography, 34, 1374–138.
Leterme, S. C., & Pingree, R. D. (2008). The Gulf Stream, rings and north Atlantic eddy structures from remote sensing (altimeter and SeaWiFS). Journal of Marine Systems, 69, 177–190.
Levinton, J. S. (1995). The oceanic environment: marine biology function, biodiversity, ecology (p. 420). Oxford: Oxford University Press.
Madhu, N. V., Maheswaran, P. A., Jyothibabu, R., Revichandran, C., Balasubramanian, T., Gopalakrishnan, T. C., & Nair, K. K. C. (2002). Enhanced biological production off Chennai triggered by October 1999 super cyclone (Orissa). Current Science, 82, 1472–1479.
Madhu, N. V., Jyothibabu, R., Maheswaran, P. A., Gerson, V. J., Gopalakrishnan, T. C., & Nair, K. K. C. (2006). Lack of seasonality in phytoplankton standing stock (Chl.a) and production in the western Bay of Bengal. Continental Shelf Research, 26, 1868–1883.
Madhupratap, M., Prasannakumar, S., Bhattathiri, P. M. A., Dileep Kumar, M., Raghukumar, S., Nair, K. K. C., & Ramaiah, N. (1996). Mechanism of the biological response to winter cooling in the northeastern Arabian Sea. Nature, 384, 549–552.
Maeda, M., & Carey, P. G. (1985). An illustrated guide to the species of the family Strombidiidae (oligotrichida, ciliopora), free-swimming protozoa common in aquatic environments, vol. 19 (p. 68). Bulletin Ocean Research Institute: University of Tokyo.
Maneesha, K., Sarma, V. V. S. S., Reddy, N. P., Sadhuram, C. Y., Ramana Murty, T. V., Sarma, V. V., & Kumar, M. D. (2011). Meso-scale atmospheric events promote phytoplankton blooms in the coastal Bay of Bengal. Journal of Earth System Science, 120, 1–10.
Marshall, S. (1969). Protozoa, order Tintinnida. Zooplankton sheet 127, conseil international pour l’ exploration de la mer. Mer. Fiches Ident. Zooplankton Fishe, 117–127.
Michaels, A. F., & Silver, M. W. (1988). Primary production, sinking fluxes and the microbial food web. Deep-Sea Research, 35, 473–490.
Morales, C. E., Torreblanca, M. L., Hormazabal, S., Correa-Ramírez, M., Nuñez, S., & Hidalgo, P. (2010). Mesoscale structure of copepod assemblages in the coastal transition zone and oceanic waters off central-southern Chile. Progress in Oceanography, 84, 158–173.
Muraleedharan, K. R., Jasmine, P., Achuthankutty, C. T., Revichandran, C., Dinesh Kumar, P. K., Anand, P., & Rejomon, G. (2007). Influence of basin scale and mesoscale physical processes on biological productivity in the Bay of Bengal during summer monsoon. Progress in Oceanography, 72(4), 364–380.
Nair, V. R., Peter, G., & Paulinose, V. T. (1977). Zooplankton studies in the Indian Ocean I. From Bay of Bengal from the south-west monsoon period. Mahasagar Bulletin of National Institute of Oceanography, 10(1&2), 45–54.
Nair, S. R., Nair, V. R., Achuthankutty, C. T., & Madhupratap, M. (1981). Zooplankton composition and diversity in western Bay of Bengal. Journal of Plankton Research, 3(4), 493–508.
Newell, G. E., and Newell, R. C. (1973). Marine plankton. A practical guide. Revised edition, Hutchinson (Educational) Biological Monographs (pp 244). London.
Owen, R. W. (1981). Fronts and eddies in the sea: mechanisms, interactions and biological effects. In A. R. Longhurst (Ed.), Analysis of marine ecosystems (pp. 197–231). New York: Academic.
Parsons, T. R. (1980). Fundamentals of aquatic ecosystems. In M. Barns (Ed.), Blackwell scientific (p. 229). Oxford: Publications Oxford.
Postel, L., Fock, H., & Hagen, W. (2000). Biomass and abundance. In R. P. Harris, P. H. Wiebe, J. Lenz, H. R. Skjoldal, & M. Huntley (Eds.), ICES zooplankton methodology manual (pp. 87–89). San Diego, California, USA: Academic.
Prasad, T. G. (1997). Annual and seasonal mean buoyancy fluxes for the tropical Indian Ocean. Current Science, 73, 667–674.
Prasannakumar, S., Nuncio, M., Narvekar, J., Kumar, A., Sardesai, S., de Souza, S. N., Gauns, M., Ramaiah, N., & Madhupratap, M. (2004). Are eddies nature’s trigger to enhance biological productivity in the Bay of Bengal. Geophysical Research Letters, 31, L07309. doi:10.1029/2003GL019274.
Prasannakumar, S., Nuncio, M., Ramaiah, N., Sardessai, S., Narvekar, J., Fernandes, V., & Paul, J. T. (2007). Eddy-mediated biological productivity in the Bay of Bengal during fall and spring intermonsoons. Deep-Sea Research Part I, 54, 1619–1640.
Prasannakumar, S., Nuncio, M., Narvekar, J., Ramaiah, N., Sardessai, S., Gauns, M., Fernandes, V., Paul, J. T., Jyothibabu, R., & Jayaraj, K. A. (2010). Seasonal cycle of physical forcing and biological response in the Bay of Bengal. Indian Journal of Marine Sciences, 39, 388–405.
Putland, J. N. (2000). Microzooplankton herbivory and bacterivory in Newfoundland coastal waters during spring, summer and winter. Journal of Plankton Research, 22, 253–277.
Qasim, S. Z. (1977). Biological productivity of the Indian Ocean. Indian Journal of Marine Science, 6, 122–137.
Radhakrishna, K. P., Bhattathiri, M. A., & Devassy, V. P. (1978). Primary productivity of Bay of Bengal during August–September 1976. Indian Journal of Marine Science, 7, 94–98.
Ramaiah, N., Fernandes, V., Paul, J. T., Jyothibabu, R., Mangesh, G., & Jayaraj, K. A. (2010). Spatio-temporal variability in biological productivity and carbon biomass in the surface layers of the Bay of Bengal. Indian Journal of Marine Science, 39, 369–379.
Sabu, P., Asha Devi, C. R., Lathika, C. T., Sanjeevan, V. N., & Gupta, G. V. M. (2015). Characteristics of a cyclonic eddy and its influence on mesozooplankton community in the northern Bay of Bengal during early winter monsoon. Environmental Monitoring and Assessment, 187(6), 330. doi:10.1007/s10661-015-4571.
Satheesh Kumar, P., Pillai, N. G., & Manjusha, U. (2014). El Nino southern oscillation (ENSO) impact on tuna fisheries in Indian Ocean. SpringerPlus, 3, 591.
Schott, F., & McCreary, J. P. (2001). The monsoon circulation of the Indian Ocean. Progress in Oceanography, 51, 1–123.
Sengupta, R., De Sousa, S. N., & Joseph, T. (1977). On nitrogen and phosphorous in the western Bay of Bengal. Indian Journal of Marine Sciences, 6, 107–110.
Shankar, D., Vinayachandran, P. N., & Unnikrishnan, A. S. (2002). The monsoon currents in the north Indian Ocean. Progress in Oceanography, 52, 63–120.
Shetye, S. R., Gouveia, A. D., Shankar, D., Shenoi, S. S. C., Vinayachandran, P., Sundar, N., Micheal, G. S., & Nampoothiri, G. S. (1996). Hydrography and circulation in the western Bay of Bengal during northeast monsoon. Journal of Geophysical Research, 101, 14011–14025.
Steidinger, K. A., Williams, J. (1970). A memoir of the hourglass cruises—dino-flagellates. Marine Research Laboratory (p. 249). Florida.
Stelfox–Widdicombe, C. E., Archer, S. D., Burkill, P. H., & Stefels, J. (2004). Microzooplankton grazing in phaeocystis and diatom dominated waters in the southern North Sea in spring. Journal of Sea Research, 51, 37–51.
Strickland, J. D. H., & Parsons, T. R. (1972). A practical handbook of seawater analysis, II ed. Bulletin-Fisheries Research Board of Canada, 167, 310.
Subrahmanyan, R. (1971). The dinophycea of the Indian seas, memoir II. Part 2, family Peridineacea. Marine Biological Association of India, Cochin, India.
Subramanian, V. (1993). Sediment load of Indian rivers. Current Science, 64, 928–930.
Sverdrup, H. U., Johnson, M. W., & Fleming, H. R. (1942). The oceans their physics, chemistry and general biology. New York: Prentice-Hall.
Thompson, B., Gnanaseelan, C., & Salvekar, P. S. (2006). Variability in the Indian Ocean circulation and salinity and its impact on SST anomalies during dipole events. Journal of Marine Research, 64, 853–880.
Todd, C. D., & Laverack, M. S. (1991). Coastal marine zooplankton: a practical manual for students (p. 106). Cambridge: Cambridge University Press.
Vidya, P. J., Prasanna Kumar, S., Gauns, M., Verenkar, A., Unger, D., & Ramaswamy, V. (2013). Influence of physical and biological processes on the seasonal cycle of biogenic flux in the equatorial Indian Ocean. Biogeosciences, 10, 7493–7507.
Vinayachandran, P. N., & Mathew, S. (2003). Phytoplankton bloom in the Bay of Bengal during the northeast monsoon and its intensification by cyclones. Geophysical Research Letters, 30(11), 1572. doi:10.1029/2002GL016717.
Vinayachandran, P. N., & Yamagata, T. (1998). Monsoon response of the sea around Sri Lanka: generation of thermal domes and anticyclonic vortices. Journal of Physical Oceanography, 28, 1946–1960.
Vinayachandran, P. N., McCreary, J. P., Jr., Hood, R. R., & Kohler, K. (2005). A numerical investigation of phytoplankton bloom in Bay of Bengal during northeast monsoon. Journal of Geophysical Research, 110, C12001. doi:10.1029/2005JC002966.
Acknowledgments
We are thankful to all the participants of Cruise No 240 FORV Sagar Sampada for the help rendered in sampling. We also greatly acknowledged Dr. Bill Peterson for his valuable help. This investigation was carried out under the Marine Research on Living Resource (MR-LR) programme. The altimeter products are produced by SSALTO/DUACS and distributed by AVISO.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayalakshmi, K.J., Sabu, P., Devi, C.R.A. et al. Response of micro- and mesozooplankton in the southwestern Bay of Bengal to a cyclonic eddy during the winter monsoon, 2005. Environ Monit Assess 187, 473 (2015). https://doi.org/10.1007/s10661-015-4609-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4609-0