Abstract
This study addresses the spatial variability in mesozooplankton biomass and composition in the Central and Western Bay of Bengal (India) during the summer monsoon season of 2001. Perennially warmer sea surface temperatures (>28°C), stratified top layer (sea surface salinity, 28–33 psu), high turbidity, and low nutrient concentrations due to weak/null upwelling and light limitation make the Bay of Bengal a region of low primary productivity. Despite this, mesozooplankton biomass values, i.e. 2.9–104 mg C m−3 in the Central Bay and 1.3–31 mg C m−3 in the Western Bay, observed in the mixed layer (2–51 m) during the summer monsoon were in the same range as reported from the more productive Arabian Sea. Mesozooplankton biomass was five times and density 18 times greater at stations with signatures of cold-core eddies, causing a higher spatial heterogeneity in zooplankton distribution. Among the 27 taxonomic groups recorded during the season, Copepoda was the most abundant group in all samples followed by Chaetognatha. The dominant order of Copepoda, Calanoida, was represented by 132 species in a total of 163 species recorded. Oncaea venusta was the key copepod species in the Bay. In the Central Bay, the predominant copepod species were carnivorous/omnivorous vis-a-vis mostly herbivores in the Western Bay. Pleuromamma indica increased to its maximum abundance at 18°N in the Central Bay, coinciding with the lowest dissolved oxygen concentrations. The Central Bay had higher mesozooplankton biomass, copepod species richness and diversity than in the Western Bay. Although zooplankton biomass and densities were greater at the eddy stations, correlation between zooplankton and chl a was not statistically significant. It appears that the grazer mesozooplankton rapidly utilize the enhanced phytoplankton production in cold-core eddies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature, salinity, and food supply are some of the important factors that are known to cause spatial changes in zooplankton populations (Lawrence et al. 2004). Warming of surface waters and fresh water input are both significant factors in the stratification in semi-enclosed water bodies (Rabalais et al. 2002). These environmental parameters have an impact on breeding and hence affect the density and composition of zooplankton (Greenwood et al. 2001).
The Bay of Bengal (BoB) receives voluminous riverine inflow (~1.62 × 1012 m3 yr−1; UNESCO 1988) and the precipitation exceeds evaporation (Han and Webster 2002). A strong and stable stratification results from the warmer sea surface temperature that mostly exceeds 28°C, lower surface-water salinity (28–32 psu) and weaker winds (<10 m s−1) (Shenoi et al. 2002). Consequently, nutrient-rich subsurface waters are prevented to come up and mix with the surface (Prasanna Kumar et al. 2002). Perennial cloud cover and the high-suspended terrigeneous matter brought in by rivers limit irradiance light, and reduce primary production in shallow euphotic waters in the Bay (Madhupratap et al. 2003). The continental shelf on the eastern margin of India being narrow, the sporadic mild upwelling (Shetye et al. 1991) is restricted to a narrow band in the Western Bay. However, mesoscale processes such as cold-core eddies are reported to increase production in this generally low productive region (Prasanna Kumar et al. 2004). Since primary production can significantly increase within eddies (Falkowski et al. 1991), such processes can greatly determine the spatial distribution of the grazer populations of mesozooplankton. However, effects of such cold-core eddies have not yet been studied in the Bay.
Data on distribution of zooplankton in the Atlantic Ocean (Deevey and Brooks 1977; Madin et al. 2001; Gaudy et al. 2003; Alcaraz et al. 2007) and the Pacific Ocean (Roman et al. 1995; White et al. 1995; Saltzman and Wishner 1997; Kang et al. 2004) are considerable. From the Indian Ocean, such studies have been carried out mostly in the Arabian Sea (Madhupratap and Haridas 1990; Smith et al. 1998; Hitchcock et al. 2002; Smith and Madhupratap 2005). Unlike in the Bay of Bengal, the high zooplankton biomass in the central and eastern Arabian Sea during summer monsoon is sustained by high primary productivity induced mainly by open ocean and coastal upwelling (Smith and Madhupratap 2005).
Studies on the zooplankton distributions in the Bay of Bengal during and after the International Indian Ocean Expedition (1960–1965) are scarce and those available are mostly from the coastal areas (Achuthankutty et al. 1980; Nair et al. 1981; Rakhesh et al. 2006). Of the total marine fish catch in Indian waters, ~31.5% comes from the Bay of Bengal (CMFRI 1970). Understanding zooplankton distribution is essential because they play a pivotal role in the trophic link between primary production and predators such as macrozooplankton, fishes, and marine mammals.
As a part of the Bay of Bengal Process Studies (BOBPS) programme, mesozooplankton were collected from the open ocean and shelf/slope waters of the Bay of Bengal during the summer monsoon. BOBPS was a national programme aimed at understanding the biogeochemical processes with a view to investigate the carbon flux potential in this oligotrophic and uniquely located basin (see for example in Madhupratap et al. 2003; Gauns et al. 2005). An important goal of this investigation was to understand the zooplankton dynamics in the open ocean and the near-coastal waters of this sparsely studied area. In addition to measuring the biomass and abundance in the upper 1,000 m, we also analysed the composition of the mesozooplankton community at group level, and copepods to generic/species level. The general hypotheses that were tested during this study are: (1) mesozooplankton abundance differs in coastal and offshore waters; (2) species diversity is high in warm, less productive region of the Bay; and (3) zooplankton distribution is closely related to hydrography of the region. In order to test the hypotheses, measurements of biological and hydrographical parameters were carried out in the Central and Western Bay. Upon taxonomic identification of the copepod species, various diversity indices were computed.
Materials and methods
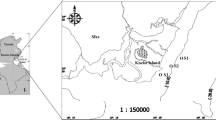
Sampling was carried out during the cruise 166 of ORV “Sagar Kanya” from the Central Bay of Bengal between 9°N and 20°N along 88°E and the Western Bay between 12°N and 19°N, 81°E and 85°E (Fig. 1) from 6 July to 2 August, 2001. The central transect in the Bay of Bengal represented an open ocean environment, with a lesser/no influence of boundary currents and upwelling events. In contrast, the Western Bay that comprises the shelf/slope waters is influenced by the northward-moving East Indian Coastal Current/Summer Monsoon Current during this season, and receives inflows from the rivers Mahanadi, Godavari, Krishna and Pennar and continental runoff from terrestrial precipitation.
One litre water sample was collected for chlorophyll a (chl a) measurements from each of the eight depths (surface, 10, 20, 40, 60, 80, 100 and 120 m) in the upper 120 m at eight stations (Fig. 1). Chl a was measured fluorometrically (Turner Designs 10 AU, USA) following standard JGOFS Protocols (UNESCO 1994).
Mesozooplankton samples were collected around noon and midnight from the same eight stations. Using a pressure-sensor-fitted multiple plankton net (MPN, Hydro-Bios, mouth area 0.25 m2, mesh size 200 μm), vertical hauls were made from five discrete depths in the upper 1,000 m. The thickness of sampling strata was based on temperature profiles obtained from Conductivity, Temperature, Depth profiler. The samples were collected from five strata: mixed layer, top of thermocline (TT) to base of thermocline (BT), BT to 300 m, 300–500 m, and 500–1,000 m. The net was hauled up at a speed of 0.8 m s−1 and the volume of water filtered was calculated by multiplying the sampling depth with the mouth area of the net.
Zooplankton biovolume (ml) was estimated by the standard displacement volume method (ICES 2000). Large gelatinous plankton such as salps, medusae and siphonophores were separated from the rest of the sample and the biovolume measured by the same method. This was done to calculate the carbon values separately for non-gelatinous (sensu, crustacean ICES 2000) and gelatinous (cnidarians) forms. The biomass was expressed per unit volume of water filtered by the net. Thereafter, the samples were fixed with 4% buffered formaldehyde-seawater solution and brought to the laboratory for further analyses. When the sample size was large, as usually in the first and second strata, it was split using a Folsom splitter, and in general, 25% aliquot was used for density measurement and identification of taxa. Often, when the densities in the deeper layers were low, we analysed the entire sample for counting and species identification.
Conversion factors for deriving zooplankton carbon biomass from the displacement volume of zooplankton used were: (1) 1 ml zooplankton = 0.075 g dry wt and (2) 1 g dry wt zooplankton = 0.342 g carbon of zooplankton (Madhupratap et al. 1981). All the samples were sorted using broad taxonomic groupings from Phylum to Family level (UNESCO 1968; ICES 2000). Only the copepods were identified to genus/species level (Tanaka 1956; Bradford and Jillett 1980) using a stereo zoom microscope (Zeiss, Germany) with 90× magnification.
Species diversity index (H′; Omori and Ikeda 1984), species number (S), species richness (d; Margalef 1951) and evenness (J′; Heip 1974) were calculated for examining spatial differences in the abundance of copepod species in the upper 1,000 m depth.
Biomass, abundance and taxonomical data were subjected to Wilcoxon matched pairs test to detect any variability arising due to day and night differences. ANOVA was performed to test spatio-temporal variability of various parameters. Spearman correlation between zooplankton biovolume and abundance versus environmental parameters was tested to understand which factor influences the zooplankton biovolume, abundance and distribution.
Results
Hydrography
Results on hydrography published in Prasanna Kumar et al. (2004) and Sardessai et al. (2007) are briefly described here.
Temperature
In the Central Bay (88°E), the sea surface temperature (SST) was 29°C between 9°N and 15°N and, was 28.5°C until 20°N. The mixed layer depth (MLD) was ~15 m at 9°N, and 51 m at 12°N but decreased markedly to <4 m at 20°N. The vertical thermal structure in the upper 300 m showed oscillations within the thermocline. Isotherms were tilted upwards around 9°N and 20°N, lowering the temperature at 60 m by at least 5°C, indicating signature of subsurface cold-core eddies.
In the Western Bay, SST averaged 30°C and was higher than in the Central Bay. MLD shoaled from 25 m at 12°N to ~2 m at 19°N. The doming of isotherms was around 17°N, where a cold-core eddy was evident. For details on the occurrences of cold-core eddies during this season in the Bay of Bengal, see Prasanna Kumar et al. (2004), and for satellite image of these eddies, see Sardessai et al. (2007).
Salinity
Sea surface salinity (SSS) in the Central Bay was 33.5 psu between 9°N and 15°N, and it decreased to 28 psu at 20°N. The salinity gradient in the upper 50 m at 9°N was about 0.02 psu m−1, while that at 20°N was about 0.14 psu m−1, indicating a strong signal of freshwater influx in the northern region. In the Western Bay, the SSS was 34 psu at 12°N, and it decreased northwards to reach 24 psu at 19°N. The salinity gradient of 0.2 psu m−1 in the upper 50 m was stronger at 19°N 85°E.
Dissolved oxygen
Dissolved oxygen (DO) depletion was observed at 150 m in the Central Bay. This oxygen minimum zone (5 μM) extended to greater depths north of 15°N (up to 700 m at 20°N). Below 700 m, the oxygen levels gradually increased. The subsurface DO concentrations decreased significantly towards the north in the CB. In the Western Bay, the oxygen minimum zone (OMZ) was observed at all stations at depths between 150 and 500 m.
Zooplankton biomass and density
Mesozooplankton biomass and density did not vary significantly between day and night (P > 0.05) in both transects. However, both decreased significantly with increasing depth (P < 0.05). At some stations, biomass was high in the surface layers in both transects due to huge Pyrosoma swarms. The higher biomass in MLD in the Central Bay was found at 9°N and 20°N, with lesser values at stations in between (Fig. 2a). It ranged from 0.05 to 104 mg C m−3 in the upper 1,000 m. The density ranging from 0.008 to 963 ind. m−3 was the highest in MLD at 9°N and was moderate at 12°N and 20°N. In the Western Bay, the biomass and density ranged from 0.08 to 31 mg C m−3 and from 0.008 to 4,621 ind. m−3, respectively, with their highest values at 17°N 83°E (Fig. 2b).
Chlorophyll a and 0–1,000 m depth integrated zooplankton carbon biomass and abundance
In the Central Bay, chlorophyll a (chl a) varied from 0.07 ± 0.04 to 0.12 ± 0.11 mg m−3 in upper 120 m (Fig. 3a), with higher values at 9°N and 20°N. In the Western Bay, chl a concentrations were higher and varied from 0.09 ± 0.11 to 0.16 ± 0.11 mg m−3. However, the concentrations did not significantly differ between the stations along both transects (P > 0.05). The satellite pictures of cold-core eddies during this study period (Sardessai et al. 2007), clearly show that our sampling was mostly in the peripheries of these eddies.
In the Central Bay, zooplankton carbon biomass and densities in the upper 1,000 m ranged from 492 to 1,332 mg C m−2 and from 0.59 to 3.7 (×104 ind. m−2), respectively. Both were nearly two times higher at the eddy-influenced stations (9°N and 20°N) compared with the stations away from these eddies (15°N; Fig. 3b). Similarly, in the Western Bay, the column biomass (range, 185–1,110 mg C m−2) and density (range, 0.16–5.31 (×104) ind. m−2) were the greatest at 17°N 83°E by five and 18 times, respectively (Fig. 3b). The average biomass in the Central Bay was twice that in the Western Bay due to the frequent swarms and higher biovolumes of pyrosomes in the former. Because the individuals in pyrosome swarms could not be counted, they are not considered in the overall numerical abundance. Excluding the pyrosomes, the mesozooplankton density did not vary significantly between transects.
Mesozooplankton composition and spatial distribution
Among the 27 groups of mesozooplankton that we identified (Table 1), copepods were the most abundant taxon at all depths (Fig. 4). They contributed to the total zooplankton density 76–89% in the Central Bay and 80–99% in the Western Bay. The next important taxa in decreasing order of abundance were: chaetognaths, ostracods, polychaetes, appendicularia and euphausiids. While the density maxima of Copepoda were observed above (87.2–87.3%) and below (84.1–88.5%) the 200–300 m stratum (76.3%) in the Central Bay, they accounted for 99% of the total individuals in the thermocline stratum in the Western Bay. Chaetognaths, polychaetes and ostracods were found in all the sampled strata. Appendicularia were found mostly above 300 m and euphausiids below 200 m. Remaining groups, such as siphonophores, medusae and salps, were rare in the thermocline in both transects.
Composition and spatial distribution of copepods
Orders Calanoids were the predominant copepods in the Bay of Bengal (Fig. 5), with their maximum in the 200–300 m stratum (range, 18–68%) in the Central Bay and in the deepest stratum (range, 33–89%) in the Western Bay. Poecilostomatoids were significant above and below the thermocline in both transects. Mormonilloida were more abundant in the thermocline in the Central Bay (70%) and Cyclopoida (32%) in the Western Bay. Harpacticoida ranged from 1 to 18% among the Copepoda below 200 m in both transects.
Families The abundance of copepod families in the Bay of Bengal varied with depth. In the Central Bay, Oncaeidae were found dominant at all depths (Table 2) except in the thermocline where only Mormonillidae predominated (70%). In addition, herbivorous Clausocalanidae were found dominant in the MLD, members of Metridinidae and Lucicutiidae between 200 and 500 m and Miraciidae were important in the deepest stratum.
In the Western Bay, Paracalanidae dominated in the top 300 m, and Oncaeidae were found mostly in the MLD and between 300 and 500 m. Oithonidae were found dominant in the thermocline and Corycaeidae in the 200–300 m stratum. Metridinidae and Lucicutiidae were the only dominant families in the deepest stratum.
Vertical distribution of the dominant copepods
Vertical distribution patterns in the Central and Western Bay of the most abundant copepod species (species that contributed ≥2% of the total copepod densities in Central and Western Bay) are shown in Fig. 6. In the Central Bay, Clausocalanus arcuicornis was abundant in the MLD, with a small peak in the deep stratum. Mormonilla minor was encountered mostly in the thermocline and Eucalanus monachus in the upper 300 m. Oncaea venusta and Oithona similis were distributed throughout the water column, and Eucalanus elongatus, Lucicutia flavicornis, Pleuromamma indica, Macrosetella gracilis and Conaea gracilis increased in abundance below 200 m (Fig. 6a).
Vertical distribution of the average percent abundance of major copepod species (≥2% of total copepods in a sample) in the Central (a) and Western (b) Bay of Bengal. Numerals on y-axis indicate the five sampled strata (1, surface-mixed layer depth; 2, top of thermocline–base of thermocline; 3, base of thermocline-300 m; 4, 300–500 m; 5, 500–1,000 m). Average integrated copepod density for the transect in the upper 1,000 m during day (D) and night (N) is indicated on top of the panel
In the Western Bay, Paracalanus aculeatus was mostly observed in the MLD. Paracalanus indicus and Eucalanus monachus were abundant in the upper 300 m; Oithona similis, though was present throughout the column, showed a prominent peak in the thermocline. Mormonilla minor and Lucicutia flavicornis were abundant in near-surface layers as well as the deepest stratum. Corycaeus danae, Euterpina acutifrons and Lucicutia maxima were most abundant in the 200–300 m stratum. Oncaea venusta, Macrosetella gracilis and Paracandacia truncata were more abundant in the 300–500 m stratum, and Pleuromamma indica was most abundant in the deepest stratum (Fig. 6b).
Diversity indices of copepods
Of the 163 copepod species observed, 132 were calanoids, 17 poecilostomatoids, six cyclopoids, six harpacticoids, and two were mormonilloids. Remarkably, of the 163 copepods species, 153 species were present in the Central Bay but only 57 were found in the Western Bay. In general, species diversity and richness were greater in the MLD and the deepest stratum sampled (500–1,000 m); also evenness was the highest in the deepest stratum.
Both the number of species (S) and species richness (d) were the highest at 9°N (S = 91). Species numbers decreased northwards (S, 34 at 20°N) in the Central Bay (Table 3). In contrast, evenness increased northwards and reached its maximum at 20°N. Species diversity (H′) was high at 9°N (3.86), decreasing northwards (at 20°N: 2.72). The number of species found in the Western Bay was relatively low (range, 25–33), with a low variability between the stations. The H′ was much lower in the Western Bay (2.65–2.95); however, J′ was relatively higher (range, 0.73 to 0.85).
Oncaea venusta was the dominant copepod species at all stations in the Central Bay except at 18°N where Mormonilla minor and Pleuromamma indica were dominant. At 15°N, M. minor, Macrosetella gracilis and O. venusta were dominant. In the Western Bay too O. venusta was dominant at all the three stations; in addition, Oithona similis and Paracalanus indicus dominated at 12°N 81°E and 19°N 85°E respectively.
Correlation analyses
Mesozooplankton biomass and densities correlated positively and significantly with temperature and dissolved oxygen, and negatively with salinity in the upper 200 m (Table 4). However, the mesozooplankton biomass and density from the MLD did not show any correlation with integrated chl a (P > 0.05) in the upper 120 m.
Discussion
Our results show that mesozooplankton biomass and densities are highly enhanced in the MLD at stations influenced by cold-core eddies, i.e. at two locations in the Central Bay (9°N and 20°N) and one in the Western Bay (17°N, 83°E). The zooplankton biomass and the physico-chemical features seem to be closely related in the Bay of Bengal. The biomass values in the open ocean areas during this study are higher than those reported by Muraleedharan et al. (2007) during July 2003. Also, in the location influenced by cold-core eddy in the Western Bay, the biomass is much higher than that reported earlier (Nair et al. 1981). The latter may be attributed to the absence earlier of the eddies. In the central and eastern Arabian Sea, high biomass of zooplankton are reportedly sustained by nutrient upwelling and higher primary production rates caused by physical processes such as open ocean and coastal upwelling (Smith and Madhupratap 2005). Though higher nutrient concentrations were observed at the eddy-influenced locations during this season in both transects in the Bay of Bengal (Sardessai et al. 2007), the chl a concentrations at various stations were similar, irrespective of the eddy influence. Thus in MLD, a lack of correlation of mesozooplankton biomass and density with chl a (P > 0.05) is mostly attributable to grazing by herbivorous zooplankton on phytoplankton (Gasparini and Castel 1999). As Banse (1994) also suggests, such a steady state of chl a concentrations, despite regional differences in subsurface nutrient input, is achieved due to zooplankton grazing. Thus, the phytoplankton increase in eddy-influenced regions seems to be rapidly decimated by its grazing by herbivorous/omnivorous zooplankton.
Also carnivorous zooplankton increase in such areas, as they feed on the herbivorous microzooplankton. In fact, during this study, the carnivorous/omnivorous Oncaeidae (Kattner et al. 2003) were the dominant copepods in the MLD apart from herbivorous Clausocalanidae and Paracalanidae. Madhupratap et al. (1996) reported that high zooplankton biomass in Arabian Sea was sustained by microzooplankton and bacteria, during the low chl a periods. Mean bacterial biomass is reportedly higher in the Bay of Bengal, compared with the Arabian Sea (Gauns et al. 2005). Also, alternative food sources such as detritus brought in by the rivers in the northern and Western Bay (Khodse et al. 2007) could be additional nutrition for the omnivorous/detritivorous zooplankton (see also Heinle et al. 1977). The warmer SSTs (>28°C) and low SSSs (23–33 psu) that keep the upper 50 m stratified could increase the residence time of the available particulate organic matter, and thus increasing its availability for the zooplankton in the upper water column.
Zooplankton are known to vertically migrate even in the presence of a weak oxygen minimum zone. In this study, the subsurface DO concentration in the Central Bay varied from suboxic (10 μM) levels in the south to hypoxic (5 μM) in the north between 150 and 700 m; however, a thick hypoxic zone of 150–300 m was lying between suboxic waters in the Western Bay. Since there was no significant diel difference in density of zooplankton in any stratum, it appears that the OMZ hindered the vertically migrating organisms in both transects. While zooplankton groups did not seem to be affected by the OMZ in the Central Bay, a prevalence of only copepods was seen at the upper boundary of the OMZ in the Western Bay. As Childress (1975) suggests, some crustaceans can adapt to survive in low oxygen environment. It is also thought that chaetognaths found in the OMZ may switch to anaerobic metabolism (Childress and Thuesen 1992). Similar to observations in the equatorial Pacific (Saltzman and Wishner 1997), the organisms unable to adapt to the low oxygen may be excluded from this zone as evidenced from the reduction of biomass and density below the MLD in our study. This could also be inferred from the very poor occurrence of siphonophores, medusae and salps from the thermocline and below up to the 300 m strata. These gelatinous forms are known to lack physiological adaptations for low oxygen tolerance (Childress and Thuesen 1992). Also the vertical distribution patterns of the dominant groups in this study indicate their differential tolerance to the DO levels.
Twenty-seven taxonomic groups of zooplankton were observed in this tropical Bay, copepods forming the bulk of abundance followed by chaetognaths, as also reported by many authors previously (Madhupratap and Haridas 1990; Padmavati et al. 1998; Koppelmann and Weikert 2000). The proportions of various taxa recorded in this study corroborate well with other regions of prominent oxygen minimum zone such as the northern Arabian Sea (Madhupratap et al. 2001) and Equatorial Pacific (Saltzman and Wishner 1997). Similar to earlier reports, Calanoida was the dominant order among the copepods followed by Poecilostomatoida (Deevey and Brooks 1977; Madhupratap and Haridas 1990). The copepod species richness reported in this study is much higher than in the temperate waters (Berasategui et al. 2005); Hwang et al. (2007) also observed higher copepod species richness in lower latitudes of the northern South China Sea. From these observations, it is believed that higher species richness in the tropics is strongly coupled to temperature (Rutherford et al. 1999). From the total of 163 copepod species that we recorded in the Bay of Bengal, 153 are from the Central Bay, and only 57 from the Western Bay. This clearly indicates that copepod species richness is much higher in the oceanic compared to the coastal waters. Lower species richness and diversity in the northern Central Bay and throughout the Western Bay appears to be a manifestation of the OMZ. However, the higher diversity and evenness in the deepest stratum could be because of the stable environment there, as suggested by Padmavati et al. (1998).
Generally, Oncaea venusta predominated at most stations and depths. Its food ranges from toxic dinoflagellates (Turner and Tester 1997; Wu et al. 2004) to marine snow (Alldredge 1972). Turner (1986) considers it to be an omnivore while Yamaguchi et al. (2002) considers it as a detritivore. According to Go et al. (1998), it also feeds on much larger zooplankton, such as chaetognaths and appendicularians. It is known to go into a pseudopelagic mode, i.e. reduce swimming speeds in an attempt to reduce respiratory losses (Nishibe and Ikeda 2008). The diverse feeding habits as well as respiratory adaptation of O. venusta seem particularly to play a role in the successful colonization of this species in food limited conditions as well as over a wide latitudinal range and hydrographical regime including the OMZ.
Mormonilla minor, the meso-bathypelagic particle feeder (Boxshall 1985) that occurred in large numbers in the thermocline at 15°N and 18°N (88°E), seems to feed on the partially decomposed particles and reside at the upper boundary of oxycline. It may shift vertically upwards to avoid the anoxic layers (Bottger-Schnack 1996; Saltzman and Wishner 1997). Pleuromamma indica, the indicator of OMZ (Saraswathy and Iyer 1986; Goswami et al. 1992), increased to its maximum abundance at 18°N 88°E where oxygen minimum water was brought to the shallower depths by cold-core eddies. A large number of individuals of herbivorous species such as Eucalanus monachus, Paracalanus indicus and Clausocalanus arcuicornis were found to be dominant at stations in the Western Bay probably due to the higher availability of phytoplankton biomass. Their predominance in the upper 300 m in the Bay corroborates many earlier findings (Kouwenberg 1994; Saltzman and Wishner 1997).
Oncaea venusta, Oithona similis, Lucicutia flavicornis and Macrosetella gracilis were generally distributed throughout the water column as also reported earlier (Bottger-Schnack 1994; Weikert 1982). Pleuromamma indica, Eucalanus elongatus, Lucicutia maxima and Conaea gracilis were abundant below 200 m as also observed by Madhupratap et al. (2001). The stations influenced by cold-core eddy differed from the stations without eddy in having higher mesozooplankton biomass and densities. Though the mesozooplankton density in the two transects did not greatly differ, the copepod species assemblages varied considerably.
In conclusion, the oceanic Central Bay supports higher mesozooplankton biomass, copepod species richness and diversity than the coastal Western Bay during the summer monsoon. Mesozooplankton biomass, abundance and copepod diversity seem to be strongly coupled to hydrography, dissolved oxygen in particular. In the stratified waters of the Bay of Bengal, cold-core eddy mediated phytoplankton biomass seems to elevate and sustain the zooplankton biomasses and abundances, which in turn are indicative of sustaining the fisheries resources.
References
Achuthankutty CT, Madhupratap M, Nair VR, Nair SRS, Rao TSS (1980) Zooplankton biomass and composition in the western Bay of Bengal during late Southwest monsoon. Indian J Mar Sci 9:201–206
Alcaraz M, Calbet A, Estrada M, Marrase C, Saiz E, Trepat I (2007) Physical control of zooplankton communities in the Catalan Sea. Prog Oceanogr 74:294–312. doi:10.1016/j.pocean.2007.04.003
Alldredge AL (1972) Abandoned larvacean houses: a unique food source in the pelagic environment. Science 177:885–887
Banse K (1994) On the coupling of hydrography, phytoplankton, zooplankton and settling organic particles offshore in the Arabian Sea. In: Lal D (ed) Biogeochemistry of the Arabian Sea, pp 27–63. Reprinted from Proceedings of the Indian Academy of Sciences (Earth and Planetary Sciences) (1994) 103:27–63
Berasategui AD, Ramirez FC, Schiariti A (2005) Patterns in diversity and community structure of epipelagic copepods from the Brazil–Malvinas confluence area, southwestern Atlantic. J Mar Syst 56:309–316. doi:10.1016/j.jmarsys.2004.12.002
Bottger-Schnack R (1994) The microcopepod fauna in the Eastern Mediterranean and Arabian seas: a comparison with the Red Sea fauna. Hydrobiologia 292/293:271–282. doi:10.1007/BF00229951
Bottger-Schnack R (1996) Vertical structure of small metazoan plankton, especially non-calanoid copepods. 1. Deep Arabian Sea. J Plankton Res 18:1073–1101. doi:10.1093/plankt/18.7.1073
Boxshall GA (1985) The comparative anatomy of two copepods, a predatory calanoid and a particle-feeding mormonilloid. Philos Trans R Soc Lond B 311(1150):303–377. doi:10.1098/rstb.1985.0155
Bradford JM, Jillett JB (1980) The marine fauna of New Zealand: pelagic calanoid copepods: Family Aetideidae. NZ Oceanogr Inst Mem 86:1–102
Childress JJ (1975) The respiratory rates of midwater crustaceans as a function of depth of occurrence and relation to the oxygen minimum layer off southern California. Comp Biochem Physiol 50 A:787–799
Childress JJ, Thuesen EV (1992) Metabolic potential of deep-sea animals: regional and global scales. In: Rowe GT, Pariente V (eds) Deep-sea food chains and the global carbon cycle. NATO ASI Series, Kluwer Academic, Dordrecht, pp 217–236
CMFRI (1970) Annual report of central marine fisheries research institute, ICAR
Deevey GB, Brooks AL (1977) Copepods of the Sargasso Sea off Bermuda: species composition, and vertical and seasonal distribution between the surface and 2000 m. Bull Mar Sci 27:256–291
Falkowski PG, Ziemann D, Kolber D, Biefalg PK (1991) Role of eddy pumping in enhancing primary production in the ocean. Nature 352:55–58. doi:10.1038/352055a0
Gasparini S, Castel J (1999) Autotrophic and heterotrophic nanoplankton in the diet of the estuarine copepods Eurytemora affinis and Acartia bifilosa. J Plankton Res 19:877–890. doi:10.1093/plankt/19.7.877
Gaudy R, Youssara F, Diaz F, Raimbault P (2003) Biomass, metabolism and nutrition of zooplankton in the Gulf of Lions (NW Mediterranean). Oceanol Acta 26:357–372. doi:10.1016/S0399-1784(03)00016-1
Gauns M, Madhupratap M, Ramaiah N, Jyothibabu R, Fernandes V, Paul JT et al (2005) Comparative accounts of biological productivity characteristics and estimates of carbon fluxes in the Arabian Sea and the Bay of Bengal. Deep Sea Res Part II Top Stud Oceanogr 52:2003–2017. doi:10.1016/j.dsr2.2005.05.009
Go YB, Oh BC, Terazaki M (1998) Feeding behavior of the poecilostomatoid copepods Oncaea sp. on chaetognaths. J Mar Syst 15:475–482. doi:10.1016/S0924-7963(97)00038-9
Goswami SC, Gajbhiye SN, Padmavati G (1992) Distribution of Pleuromamma (Copepoda: Metridinidae) in the Western Indian Ocean. In: Desai BN (ed) Oceanography of the Indian Ocean. Oxford/IBH, New Delhi, pp 157–166
Greenwood A, O’Riordan RM, Barnes DKA (2001) Seasonality and vertical zonation of zooplankton in a semi-enclosed sea lough. J Mar Biol Assoc UK 81:213–220
Han W, Webster PJ (2002) Forcing mechanisms of sea level interannual variability in the Bay of Bengal. J Phys Oceanogr 32:216–239. doi:10.1175/1520-0485(2002)032<0216:FMOSLI>2.0.CO;2
Heinle DR, Harris RP, Ustach JF, Flemer DA (1977) Detritus as food for estuarine copepods. Mar Biol (Berl) 40:341–353. doi:10.1007/BF00395727
Heip C (1974) A new index measuring evenness. J Mar Biol Assoc UK 54:555–557
Hitchcock GL, Lane P, Smith S, Luo J, Ortner PB (2002) Zooplankton spatial distributions in coastal waters of the northern Arabian Sea, August, 1995. Deep Sea Res Part II Top Stud Oceanogr 49(12):2403–2423. doi:10.1016/S0967-0645(02)00042-5
Hwang JS, Dahms HU, Tseng LC, Chen QC (2007) Intrusions of the Kuroshio Current in the northern South China Sea affect copepod assemblages of the Luzon Strait. J Exp Mar Biol Ecol 352:12–27. doi:10.1016/j.jembe.2007.06.034
ICES (2000) In: Harris RP, Wiebe PH, Lenz J, Skjoldal HR, Huntley M (eds) Zooplankton methodology manual. Academic Press, California, 684 pp
Kang JH, Kim WS, Chang KII, Noh JH (2004) Distribution of plankton related to the mesoscale physical structure within the surface mixed layer in the southwestern East Sea, Korea. J Plankton Res 26:1515–1528. doi:10.1093/plankt/fbh140
Kattner G, Albers C, Graeve M, Schnack-Schiel SB (2003) Fatty acid and alcohol composition of the small polar copepods, Oithona and Oncaea: indication on feeding modes. Polar Biol 26:666–671. doi:10.1007/s00300-003-0540-x
Khodse VB, Fernandes L, Gopalkrishna VV, Bhosle NB, Fernandes V, Prabhu Matondkar SG et al (2007) Distribution and seasonal variation of concentrations of particulate carbohydrates and uronic acids in the northern Indian Ocean. Mar Chem 103:327–346. doi:10.1016/j.marchem.2006.10.003
Koppelmann R, Weikert H (2000) Transfer of organic matter in the deep Arabian Sea zooplankton community: insights from d15N analysis. Deep-Sea Res II 47:2653–2672
Kouwenberg JHM (1994) Copepod distribution in relation to seasonal hydrographics and spatial structure in the northwestern Mediterranean. Estuar Coast Shelf Sci 38:69–90. Golfe du Lion. doi:10.1006/ecss.1994.1005
Lawrence D, Valiela I, Tomasky G (2004) Estuarine calanoid copepod abundance in relation to season, salinity, and land-derived nitrogen loading, Waquoit Bay, MA. Estuar Coast Shelf Sci 61:547–557. doi:10.1016/j.ecss.2004.06.018
Madhupratap M, Haridas P (1990) Zooplankton, especially calanoid copepods, in the upper 1000 m of the southeast Arabian Sea. J Plankton Res 12:305–321. doi:10.1093/plankt/12.2.305
Madhupratap M, Nair VR, Nair SRS, Achuthankutty CT (1981) Zooplankton abundance of the Andaman Sea. Indian J Mar Sci 10:258–261
Madhupratap M, Gopalakrishnan TC, Haridas P, Nair KKC, Aravindakshan PN, Padmavati G et al (1996) Lack of seasonal and geographical variation in mesozooplankton biomass in the Arabian Sea and its structure in the mixed layer. Curr Sci 71:863–868
Madhupratap M, Gopalakrishnan TC, Haridas P, Nair KKC (2001) Mesozooplankton biomass, composition and distribution in the Arabian Sea during the fall intermonsoon: implications of oxygen gradients. Deep Sea Res Part II Top Stud Oceanogr 48:1345–1368. doi:10.1016/S0967-0645(00)00142-9
Madhupratap M, Gauns M, Ramaiah N, Prasanna Kumar S, Muraleedharan PM, De Sousa SN et al (2003) Biogeochemistry of the Bay of Bengal: physical, chemical and primary productivity characteristics of the central and western Bay of Bengal during summer monsoon 2001. Deep Sea Res Part II Top Stud Oceanogr 50:881–896. doi:10.1016/S0967-0645(02)00611-2
Madin LP, Erich FH, Steinberg DK (2001) Zooplankton at the Bermuda Atlantic time-series Study (BATS) station: diel, seasonal and interannual variation in biomass, 1994–1998. Deep Sea Res Part II Top Stud Oceanogr 48:2063–2082. doi:10.1016/S0967-0645(00)00171-5
Margalef R (1951) Diversidad de especies en las communidades naturals. Publicaciones del Insitute de Biologia Aplicada 9:5–27
Muraleedharan KR, Jasmine P, Achuthankutty CT, Revichandran C, Dinesh Kumar PK, Anand P et al (2007) Influence of basin-scale and mesoscale physical processes on biological productivity in the Bay of Bengal during the summer monsoon. Prog Oceanogr 72(4):364–383. doi:10.1016/j.pocean.2006.09.012
Nair SR, Nair VR, Achuthankutty CT, Madhupratap M (1981) Zooplankton composition and diversity in western Bay of Bengal. J Plankton Res 3:493–508. doi:10.1093/plankt/3.4.493
Nishibe Y, Ikeda T (2008) Metabolism and elemental composition of four oncaeid copepods in the western Subarctic Pacific. Mar Biol (Berl) 153(3):397–404. doi:10.1007/s00227-007-0816-8
Omori M, Ikeda T (1984) Distribution and community structure. In: Methods in marine zooplankton ecology. Wiley-Interscience Publication, New York, pp 253–279
Padmavati G, Haridas P, Nair KKC, Gopalakrishnan TC, Shiney P, Madhupratap M (1998) Vertical distribution of mesozooplankton in the central and eastern Arabian Sea during the winter monsoon. J Plankton Res 20:343–354
Prasanna Kumar S, Muraleedharan PM, Prasad TG, Gauns M, Ramaiah N, DeSouza SN et al (2002) Why is the Bay of Bengal less productive during the summer monsoon compared to the Arabian Sea. Geophys Res Lett 29(24):2235. doi:10.1029/2002GL016013
Prasanna Kumar S, Nuncio M, Narvekar J, Kumar A, Sardessai S, DeSouza SN et al (2004) Are eddies nature’s trigger to enhance biological productivity in the Bay of Bengal? Geophys Res Lett 31:L07309. doi:10.1029/2003GL019274
Rabalais NN, Turner E, Wiseman WJ (2002) Gulf of Mexico Hypoxia, a.k.a. ‘The Dead Zone’. Annu Rev Ecol Syst. 33:235–263. doi:10.1146/annurev.ecolsys.33.010802.150513
Rakhesh M, Raman AV, Sudarsan D (2006) Discriminating zooplankton assemblages in neritic and oceanic waters: a case for the Northeast coast of India, Bay of Bengal. Mar Environ Res 61:93–109. doi:10.1016/j.marenvres.2005.06.002
Roman MR, Dam HG, Gauzens AL, Urban-Rich J, Foley DG, Dickey TD (1995) Zooplankton variability on the equator at 140ºW during the JGOFS EqPac study. Deep Sea Res Part II Top Stud Oceanogr 42(2–3):673–693. doi:10.1016/0967-0645(95)00025-L
Rutherford S, D’Hondt S, Prell W (1999) Environmental controls on the geographic distribution of zooplankton diversity. Nature 400:749–753. doi:10.1038/23449
Saltzman J, Wishner KF (1997) Zooplankton ecology in the eastern tropical Pacific oxygen minimum zone above a seamount: 2 .Vertical distribution of copepods. Deep Sea Res Part I Oceanogr Res Pap 44:931–954. doi:10.1016/S0967-0637(97)00006-X
Saraswathy M, Iyer KH (1986) Ecology of Pleuromamma indica Wolfenden (Copepoda-Calanoida) in the Indian Ocean. Indian J Mar Sci 15:219–222
Sardessai S, Ramaiah N, Prasannakumar S, DeSouza SN (2007) Influence of environmental forcings on the seasonality of dissolved oxygen and nutrients in the Bay of Bengal. J Mar Res 65(2):301–316
Shenoi SSC, Shankar D, Shetye SR (2002) Differences in heat budget of the near surface Arabian Sea and Bay of Bengal: Implications for the summer monsoon. J Geophys Res 107(C6):3052. doi:10.1029/2000JC000679
Shetye SR, Shenoi SSC, Gouveia AD, Michael GS, Sundar D, Nampoothiri G (1991) Wind-driven coastal upwelling along the western boundary of the Bay of Bengal during the Southwest monsoon. Cont Shelf Res 11:1397–1408. doi:10.1016/0278-4343(91)90042-5
Smith SL, Madhupratap M (2005) Mesozooplankton of the Arabian Sea: Patterns influenced by seasons, upwelling, and oxygen concentrations. Prog Oceanogr 65:214–239. doi:10.1016/j.pocean.2005.03.007
Smith S, Roman M, Prusova I, Wishner K, Gowing M, Codispoti LA, Barber R, Marra J, Flagg C (1998) Seasonal response of zooplankton to monsoonal reversals in the Arabian Sea. Deep Sea Res Part II Top Stud Oceanogr 45:2369–2403. doi:10.1016/S0967-0645(98)00075-7
Tanaka O (1956) The pelagic Copepods of the Izu Region, middle Japan systematic Account I. Families Calanidae and Eucalanidae. Publ Seto Mar Biol Lab V(2)
Turner JT (1986) Zooplankton feeding ecology: contents of fecal pellets of the cyclopoid copepods Oncaea venusta, Corycaeus amazonicus, Oithona plumifera and O. simplex from the northern Gulf of Mexico. P.S.Z.N.I Mar Ecol (Berl) 7:289–302. doi:10.1111/j.1439-0485.1986.tb00165.x
Turner JT, Tester PA (1997) Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol Oceanogr 42:1203–1214
UNESCO (1968) Zooplankton sampling. Monographs on oceanographic methodology. UNESCO Publication, No 2, 174 pp
UNESCO (1988) River inputs into ocean systems: status and recommendations for research. UNESCO Technical papers in marine science, No 55, Final report of SCOR Working Group 46, Paris, 25 pp
UNESCO (1994) Protocols for the Joint Global Ocean Flux Study (JGOFS). Man Guides 29:170
Weikert H (1982) The vertical distribution of zooplankton in relation to habitat zones in the area of the Atlantis II Deep, Central Red Sea. Mar Ecol Prog Ser 8:129–143. doi:10.3354/meps008129
White JR, Zhang X, Welling LA, Roman MR, Dam HG (1995) Latitudinal gradients in zooplankton biomass in the tropical Pacific at 140ºW during the JGOFS EqPac study: effects of El Nino. Deep Sea Res Part II Top Stud Oceanogr 42(2–3):715–733. doi:10.1016/0967-0645(95)00033-M
Wu CH, Hwang JS, Yang JS (2004) Diets of three copepods (Poecilostomatoida) in the southern Taiwan Strait. Zool Stud 43(2):388–392
Yamaguchi A, Watanabe Y, Ishida H, Harimoto T, Furusawa K, Suzuki S et al (2002) Community and trophic structures of pelagic copepods down to greater depths in the western Subarctic Pacific (WEST-COSMIC). Deep Sea Res Part I Oceanogr Res Pap 49:1007–1025. doi:10.1016/S0967-0637(02)00008-0
Acknowledgements
We thank Dr. S. R. Shetye, Director, NIO, for facilities and encouragement. This work was supported by the grants from Department of Ocean Development, New Delhi, under the BOBPS Programme. VF thanks CSIR for the SRF fellowship for carrying out this work and Dr. K. Altaff, New College, Chennai, and Dr. R. Stephen, Regional Centre of NIO, Kochi for their help in identification and confirmation of copepod species. Drs. S. Prasanna Kumar and S. Sardessai are acknowledged for the published data on physical and chemical parameters. This is NIO contribution number 4427.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandes, V., Ramaiah, N. Mesozooplankton community in the Bay of Bengal (India): spatial variability during the summer monsoon. Aquat Ecol 43, 951–963 (2009). https://doi.org/10.1007/s10452-008-9209-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-008-9209-4