Abstract
The major constituents and trace metals in the surface sediments collected from the Western Harbor and El-Mex Bay along the Egyptian Mediterranean Coast were studied. The concentrations of major constituents decreased in the following order: Ca > Si > Mg > Na > K for the Western Harbor and El-Mex Bay. Additionally, the ranking order of trace metals was Fe > Al > Pb > Zn > Mn > Cu > Sn > V > As > Cd > Se for the Western Harbor. For El-Mex Bay, the decreasing order was Fe > Al > Mn > Sn > Pb > Zn > Cu > V > As > Cd > Se. Fe, Al, Zn, Pb, Cu, V, Cd and Sn in the Western Harbor occurred in higher concentrations than in El-Mex Bay. A higher concentration of Mn was observed in El-Mex Bay. Two pollution indicators, enrichment factor (EF) and metal pollution index (MPI), and several sediment quality guidelines (SQGs) were used to evaluate the status of metal pollution. Based on the mean EF values of the studied metals, surface sediments of the Western Harbor and El-Mex Bay revealed that they are enriched with metals from anthropogenic sources. An analysis of variance (ANOVA) test showed that the mean measurements for all metals across the Western Harbor and El-Mex Bay are significantly different at a 0.05 significance level. Principal components analysis (PCA) was applied in result interpretation. The spatial distribution of the different parameters was illustrated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals are primarily deposited in sediments, and in some cases, sediments hold over 99 % of the total amount of metals present in quantities several times higher than their natural background levels particularly in polluted sediments in regions near large industrial and urban areas (Ademoroti 1996). Consequently, sediments enriched with heavy metals constitute a threat to the health of aquatic organisms (Law and Singh 1991). Heavy metals are regarded as severe pollutants because of their toxicity, persistence and bioaccumulation problems. Because they are not chemically or biologically degraded, the pollutants remain in the environment for long periods and can enter the food chain (Tam and Wong 2000). Thorough knowledge of trace metal distribution in water and sediments plays a key role in detecting the pollution sources in aquatic systems (Shriadah 1991; El-Sikaily et al. 2004; Idodo-Umeh and Oronsaye 2006; Shreadah et al. 2006; Abdallah and Abdallah 2008; El-Zokm et al. 2012; El-Said and Youssef 2013; Abdel Ghani et al. 2013a; Khalil et al. 2013; Younis et al. 2014; El Zokm et al. 2015; Okbah et al. 2015).
Area of investigation
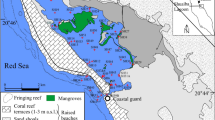
The Western Harbor is a complex harbour approximately 31 km2 west of Alexandria City along the Mediterranean Coast of Egypt (Fig. 1). It is connected to the open sea by a narrow strait. The average water depth is 7 m. The Harbour was naturally formed during Pre-Holocene subsidence of the coast and the subsequent transgression of the sea (Mostafaa et al. 2004). This harbour is the major trade port of the Northern Territory of Egypt and handles approximately 75 % of all the shipborne cargo of the country. It has received considerable amounts of treated and/or untreated industrial, agricultural and domestic wastes (Shriadah and Tayel 1991). Because the harbour is a semi-enclosed basin with limited water circulation, it may serve as an entrapment for wastes introduced from land-based sources and from the Harbour itself because of shipping activities. The future development and continued operation of the Alexandria Harbour are of great economic importance to this region; however, these activities may also impact its ecological functioning (Shreadah et al. 2006; Shriadah 1992; Shreadah and Emara 1992). El-Mex Bay is part of the Alexandria coast on the Mediterranean Sea. It is adjacent to the centre of Alexandria that is populated with about six million inhabitants and is considered to be one of the main fishing sources in Egypt. Contaminants such as heavy metals are apparently biologically available and are introduced by waterways and several land-based sources. El-Mex Bay has several industrial plants near the coast which directly discharge their effluents into it (Shriadah and Emara 1991; Emara and Shriadah 1991; Shreadah and Emara 1992; Emara et al. 1992; Said et al. 1994; Shreadah et al. 2006; Okbah et al. 2013). Not much is known about the levels of several metals and trace elements in particular Se, Sn, As, V and Al in the Western Harbor and El-Mex Bay. The most comprehensive records were obtained from surveys performed by El Sayed et al. (1988), Tayel et al. (1996), Mostafaa et al. (2004), Shreadah et al. (2006, 2014), Abdallah (2007, 2008) and Ahdy and Khaled (2009).
Aim of the work
This study aims to set up a monitoring programme for determining the current concentration of different types of major constituents and heavy metals in the surface sediments of the Egyptian western Mediterranean Coast. The evaluation of contamination levels was performed using different pollution indices. Factor analysis was used to discuss the distribution of major and trace metals. The impact on humans and the environment was also of a concern.
Material and methods
Sampling
Sediment samples were collected using a grab sampler in January 2010 from 21 locations distributed along the investigated area (Fig. 1, Table 1) from the Western Harbor (n = 11) and El-Mex Bay (n = 10). The sampling stations were chosen carefully to provide good area coverage. Samples were sliced from the grab centre, using a plastic spoon to avoid contamination by the metallic part of the grab and placed in self-sealing polyethylene bags for analysis. Sediment samples were freeze-dried using freeze-drier (Labconco, England), ground with agate mortar and stored at room temperature.
Chemicals and reagents
All reagents used were analytical grades. Milli-Q water was used throughout the study. All glassware were soaked in detergent, rinsed with water, soaked in 10 % HNO3 for 5 days, rinsed with Milli-Q water and stored in an oven at 110 °C until required. Precision was determined by three replicate analyses of one sample and expressed as a coefficient of variation (CV), and the results consistently within 10 % were considered to be precise. Validation of the method and sample quality control was checked using reference material (IAEA-356). The results of trace metal analysis of the reference material are shown in Table 2.
Chemical analyses and instrumentation
An exact weight (0.5 g) of freeze-dried homogenized sediments was completely digested in Teflon vessels using a mixture of HNO3, HF and HClO4 (3:2:1 v/v) at 80 °C (Ajay and Van Loon 1989). The final solution was diluted to 25 ml with distilled de-ionized water. All digested solutions were analysed in triplicate using the required instruments. A graphite furnace with atomic absorption (GFA-EX 7; Shimadzu AA-6800) was used to determine V, Se and Sn. Hydride/ASS (HVG-1; Shimadzu AA-6800) was used for As determination. Al was determined by applying burner head flame mode with N2O/C2H2 system (Shimadzu AA-6800). Other trace metals were determined using the AAS/flame mode (Shimadzu AA-6800). Na and K were determined using the flame photometer (Jenway PEP7). Ca and Mg were determined volumetrically using the EDTA standard solution Erio-Chrome T and Murexide indicator (APHA 1975). Carbonate content was determined according to Alexjev (1971). Si was determined according to Grasshoff (1979) using a double-beam UV/V spectrophotometer (SPEKOL 1300 Analytikjena).
Statistical analysis
Analysis of variance (ANOVA) was applied to test for significant differences in heavy metal concentrations at different sampling stations in the Western Harbor and El-Mex Bay. Principal components analysis (PCA) and factor analysis as varimax normalization rotation were applied with SPSS version 20.0. The number of factors was determined by the total variance explained, i.e. communality; usually, more than 85 % was necessary.
Results and discussion
Distribution of major and heavy metals
Concentrations of major constituents and trace metals in the surface sediments of the Western Harbor and El-Mex Bay were investigated. Results revealed that concentrations of major constituents (Table 3) decreased in the following order for mean values: Ca > Si > Mg > Na > K for both regions, Western Harbor and El-Mex Bay. Si concentrations in the Western Harbor ranged from 3.97 to 25.72 %; however, in El-Mex Bay, it ranged from 4.64 to 18.81 %. El-Said (2005) reported Si concentrations of 22.5 and 1.12 % for Abu Qir Bay and Marsa Matruh, respectively.
The concentrations of trace metals (Table 4) decreased in the following order: Fe > Al > Pb > Zn > Mn > Cu > Sn > V > As > Cd > Se for the Western Harbor. The decreasing order for El-Mex Bay was as follows: Fe > Al > Mn > Sn > Pb > Zn > Cu > V > As > Cd > Se. The horizontal distribution for all parameters is illustrated in Fig. 2a, b. High concentrations of Ca were measured in the outer Harbor where the composition of sediments is mainly carbonate. Large variation in Al and Na in the study area was observed (Table 3). Variations in Al concentrations can also be attributed to a change in mineralogy or the grain size of sediments. The high Al content indicates a terrigenous source by aluminosilicates such as clay minerals and feldspars (Massoud et al. 2012). High Fe and K concentrations were measured in the inner Harbor. Total organic matter (TOM) exhibited higher values in the inner Harbor, especially at stations 1 and 2, when compared to that at other stations because of the activities of the Alexandria Petroleum Company (Fig. 2a). Sediment samples collected from locations in the narrow inlets of the inner Harbour exhibited higher concentrations of heavy metals when compared to those from other locations because of residential wastewater runoff of into the area. The surface area of sediments is grain-size dependent and controls the adsorption of metals in the sediment (Mayer and Fink 1980). Fe, Al, Zn, Pb, Cu, V, Cd and Sn measured in the Western Harbor are higher than those observed in El-Mex Bay because the combined effect of relatively deep water and weak tidal currents indicated by moderately sorted sediments in the inner harbour allows the deposition of greater than 90 % of fine-grained sediments that were smaller than 63 μm in particle size (Mostafaa et al. 2004). Mean size (phi) = −2 log 2X; X: the grain size is in millimetre (Folk et al. 1957). In the Western Harbor, size ranged from −1.0 to 14.0 Ø (Abou-Mahmoud 2011). However, in El-Mex Bay, most of the sediments are rich in sand fraction. These sediments are exposed more to the sea, and current actions lead to good sorting and dominance of the coarser sandy fraction (Shreadah et al. 2014). The high Pb concentration in the Western Harbor may be related to the continuous runoff of residential wastewater (especially from painting factories and ship parking) Although Egypt has stopped using any more leaded gasoline, its effects still remain in sediments owing to its half-life time of 22.20 years, and sediments act as reservoirs of pollutants. Lead has no biodegradable nature. Williams (1995) suggested that anthropogenic Pb is mostly associated with the slowest settling fraction of particulate matter and so has potential to be transported far from its source and not settle in the sediment until reaching areas of high water stability. It is possible that anthropogenically derived lead is being transported to these near shore areas and deposited in the bottom sediments in regions with weak currents such as stations 2, 8 and 10 (Mostafaa et al. 2004).
a Horizontal distribution of total carbonate (TCO 3 ), total organic matter (TOM) and major constituents in the Western Harbor and El-Mex Bay. b Horizontal distribution of heavy metals in the Western Harbor and El-Mex Bay (Fe and Al in percent and Mn, Zn, Pb, Cu, V, Cd, Sn, As and Se in milligramme per kilogramme)
Mn was mainly concentrated in the boundary area between the Western Harbor and El-Mex Bay resulting from the El Nobariya drain, the Cement Company and the polyethylene factory. High concentrations of Cd were obtained in the outer Harbor and El-Mex Bay because of the presence of the Alexandria Petroleum Company and the polyethylene factory (Shreadah and Emara 1992; Said et al. 1994; Shreadah et al. 2006).
The Egyptian Mediterranean Coast along Alexandria suffers from different types of pollutants from different land-based sources which makes it impossible to have a background reference station from the Alexandria coast of Egypt. Therefore, a comparative study between heavy metal concentrations in sediments of the Western Harbor and El-Mex Bay with those observed previously (Table 5) revealed that sediments of the Western Harbor and El-Mex Bay possessed lower concentrations of Fe, Al, Mn, Zn, Pb and Se than those reported by Mostafaa et al. (2004). By contrast, Cd and Sn exhibited higher concentrations as a result of industrial inputs inside the Western Harbor (Tayel and Shreadah 1996; Tayel et al. 1997; Emara and Shreadah 2009; Shreadah et al. 2011, 2012). It is worth mentioning that there is a floating dock with a (6000 t DW) capacity owned by the Egyptian company for ship repairing and building; there are also two dry docks with capacities of 10,000 and 85,000 t DW owned by the Alexandria shipyard, one floating dock with a 8500 t DW capacity owned by the Egyptian Navy and one dry dock owned by the Alexandria Port Authority with a capacity of 1200 t DW. Because Sn is used in antifouling material and Cd is used in painting, it is not surprising to find high concentrations of Sn and Cd in the Western Harbor. Mostafaa et al. (2004) stated that most of the sediments from the Western Harbor of Alexandria have elevated heavy metal concentrations that threaten benthic organisms. El-Mex Bay is one of Alexandria’s coasts but it differs in having several industrial plants that directly discharge their effluents into it (Emara and Shriadah 1991; Emara et al. 1992; Said et al. 1994; Fahmy et al. 1995; Shreadah and Emara 1996; Shobier et al. 2011; Fathy et al. 2012a, b). These effluents include petrochemical, cement, chemicals, tanneries, industrial dyes, ink and petroleum refining. Cd was used for a long time as a pigment and for corrosion-resistant plating on steel. Cd compounds were also used to stabilize plastic.
The concentrations of trace metals measured in the study area are comparable to those reported by Abdel Ghani et al. (2013b) in Abu Qir Bay and the Eastern Harbor but higher than those reported by El Zokm et al. (2013) in Marsa Matruh. Levels of V and As were comparable to concentrations reported by Mostafaa et al. (2004) in the Western Harbor. The levels of Sn in the Western Harbor (11.97–228.18 mg/kg) were near those of El-Mex Bay (24.35–226.70 mg/kg) and comparable to those reported by Abdel Ghani et al. (2013b).By contrast, concentrations of Sn measured in the study area were much higher than those found in the Western Harbor (2.10–15.30 mg/kg) as stated by Mostafaa et al. (2004). Se concentrations in the surface sediments of the Western Harbor (0.08–1.41 mg/kg) and El-Mex Bay (0.03–1.72 mg/kg) were comparable but lower than those reported by Mostafaa et al. (2004) for the Western Harbor (0.80–3.40 mg/kg). This can be attributed to industrial waste discharges into the study area. Comparing metal concentrations to those reported by Massoud et al. (2012) revealed comparable Fe values. By contrast, Al, Mn, Zn, Cd, Cu, Pb, Cu and Sn concentrations in the present study were higher when compared to those reported by Massoud et al. (2012) for the Alexandria coasts (El Muntaza-El Agami) of Egypt.
Al concentrations measured in the study area are higher than those in the western part of Alexandria but lower than those reported in Abu Qir Bay (El-Said et al. 2010). El-Said (2005) reported Al concentrations in Marsa Matruh near to those recorded in the study area (Table 5). Concentrations of Fe, Mn, Pb, Cd and Cu in the investigated area are higher than those observed by Abdallah (2008). Sediments from the western part of the Egyptian Mediterranean Sea exhibited lower concentrations of Fe, Mn, Zn, Pb, Cd and Cu compared to the investigated area (Ahdy and Khaled 2009). Sediments from the western part of the Egyptian Mediterranean Sea exhibited lower concentrations of Fe, Mn, Zn, Pb, Cd and Cu when compared to those from the investigated area (Abdallah 2008). The higher concentrations of heavy metals in the surface sediments of the Western Harbor and El-Mex Bay were mainly caused by the heavy industrial activities and urbanization in Alexandria. In addition to the continued discharge from agricultural, residential and industrial effluents into the area of investigation, shipping activities are major anthropogenic sources for heavy metals (Fahmy et al. 1995; Tayel and Shreadah 1996; Shreadah and Emara 1996; Tayel et al. 1997; Emara and Shreadah 2009; Shreadah et al. 2011, 2012; Shobier et al. 2011; Fathy et al. 2012a, b).
Enrichment factor
The enrichment factor (EF) was used to assess the contamination level and the possible anthropogenic impact in the sediments of the study area. Metal concentrations were normalized to Al or Fe to determine whether a sediment sample was enriched with metals when compared to the sample’s “natural” condition. Al has been used successfully to normalize heavy metal contaminants (Pekey et al. 2004). To differentiate between natural and anthropogenic components, the EF was calculated using the following formula (Ergin et al. 1991):
in which (M/Al) sample denotes the ratio of metal to Al concentration of the sample and (M/Al) crust is the ratio of metal and Al in the crust (Kremling and Streu 1993; Molinari et al. 1993; Liaghati et al. 2003). Because of the natural mineralogical differences of sediments and analytical uncertainty, only sediments with an EF greater than 2 were considered enriched (Liaghati et al. 2003). The values of metals for the surficial earth crust represent the average composition of the surficial rocks exposed to weathering that were cited in Martin and Meybek (1979) in Tables 6 and 7. The EF for each element was calculated to evaluate the anthropogenic influences on heavy metals in sediments of both study areas (Table 6). Results revealed very high mean EF values for Cd, Pb and Sn that were found in the sediments of the Western Harbor and El-Mex Bay, reflecting severe enrichment with these metals. Cd had the highest mean EF values among the ten metals studied. The mean EF values were 309.29 and 909.84 for Cd in the Western Harbor and El-Mex Bay, respectively (which were larger than 300, three hundred), indicating very severe enrichment in both areas, especially at stations (1, 3, 6, 8 and 11) in the Western Harbor and stations (12, 15, 16, 19, 20 and 21) in El-Mex Bay. Pb and Sn had the second highest EF values. However, Se, V, Mn and Fe exhibited the lowest EF values among the metals studied. Metal enrichment levels in the surface sediments from the Western Harbor decreased in the following order: Cd > Pb > Sn > Cu > Zn > As > Fe > Mn > V > Se. The levels in the surface sediments of El-Mex Bay decreased in the following order: Cd > Sn > Pb > Cu > As > Mn > Zn > V > Fe > Se. Based on mean EF values of the studied metals, it was shown that surface sediments of the Western Harbor and El-Mex Bay are enriched with metals from anthropogenic sources.
Metal pollution index
Metal pollution index (MPI) was used to estimate the degree of pollution. It is calculated according to the following formula:
In which M is the metal concentration expressed in milligramme per kilogramme (Usero et al. 1996).
Table 4 presents MPI values for sediments of the studied areas. The lowest values were observed at stations 1, 4, 6 and 8 in the Western Harbor. By contrast, stations 12, 13, 16 and 18 in El-Mex Bay exhibited the highest MPI values. According to the classification of Gonçalves et al. (1992), the obtained MPI results indicated that sediments of all investigated sites were classed as seriously contaminated.
Sediment quality guidelines
The extent of sediment contamination and the environmental quality of the study areas were assessed using the “effects range low” (ERL) and the “effects range median” (ERM) as represented in Table 8. In the present study, the ERL and the ERM were calculated for some metals according to Long and Morgan (1990) and Long et al. (1995). Adverse biological effects occur rarely (<ERL, <10 % occurrence), occasionally (between ERL and ERM, 10–50 % occurrence) and frequently (>ERM, >50 % occurrence).
Concentrations of Zn at station 4 in the Western Harbor were lower than the ERL value (150 mg/kg), whereas all other sites possessed concentrations that exceeded the ERL. Conversely, Zn concentrations in the sediments of El-Mex Bay were below the ERL values for all studied sites, suggesting that El-Mex Bay could be considered an unpolluted area regarding Zn. Concentrations of Pb in sediments from stations 2, 8, 9, 10 and 11 in the Western Harbor were above the ERM value (218 mg/kg). This indicated that Pb would cause an adverse effect on benthic organisms at these sites. Moreover, several stations in El-Mex Bay exhibited Pb levels higher than the ERL value (46.70 mg/kg) but much lower than the ERM (218.00 mg/kg). Cu and Cd concentrations were above the ERL (34.00 and 1.20 mg/kg, respectively). By contrast, As concentrations were below the ERM (70.00 mg/kg) in all samples in the Western Harbor. Furthermore, all samples from El-Mex Bay had Cu and As concentration lower than the ERM, suggesting that sediment samples from this area have no adverse effects on benthic organisms. Moreover, Cd concentrations in sediments from station 3 (14.63 mg/kg) in the Western Harbor and stations 12 (10.35 mg/kg) and 16 (15.03 mg/kg) in El-Mex Bay were above the ERM, indicating that Cd could have significant adverse effects on benthic organisms.
The greatest certainty in predicting the absence or presence of sediment toxicity occurs at sediment contaminant concentrations that are lower than the threshold effect concentration (TEC) or greater than the probable effect concentration (PEC), respectively. The development of consensus-based sediment quality guidelines (CBSQGs) does not include determining the toxicity predictability related to specific contaminant concentrations in the gradient between TEC and PEC values. The TEC and PEC concentrations in the CBSQGs define three ranges of concentrations for each contaminant (< TEC > TEC < PEC > PEC). In assessing the degree of concordance that exists between the chemical concentrations in the three ranges and the incidence of toxicity, it has been demonstrated that for the most reliable consensus-based SQG contaminants, there is a consistent and incremental increase in the incidence of toxicity to sediment-dwelling organisms with increasing chemical concentrations (MacDonald et al. 2000a, b). It is recommended to interpret the potential impacts of contaminant concentrations between the TEC and PEC values of the CBSQGs or other guidelines, a midpoint effect concentration (MEC) be derived and qualitative descriptors be applied to the four possible ranges of concentration that will be created.
The qualitative descriptors would be termed “concern levels” and would be used as a relative gauge of the potential impacts to the benthic species at the level of contaminant and could be used to prioritize sites for additional studies. CBSQGs data for As, Cd, Cu, Pb and Zn revealed that the concentrations of As, Cu and Zn in (60, 60 and 80 %, respectively,) samples from El-Mex Bay were below the threshold effect concentration (TEL), and As concentration which is most (up to 81 %) of the Western Harbor samples was lower than the TEL. This suggests that most sediment samples from these areas may not have harmful effects on sediment-dwelling organisms. However, As and Zn concentrations could not exceed the probable effect concentration (PEC) in 100 % of El-Mex Bay and the Western Harbor samples. Moreover, up to 63 and 10 % of sediment samples from the Western Harbor and El-Mex Bay, respectively, exhibited high levels of pollution with Pb indicating that the concentration of Pb in the Western Harbor poses a threat to aquatic life.
Statistical analysis
A one-way ANOVA test was applied to Fe, Al, Mn, Zn, Pb, Cu, V, Cd, Sn, As and Se (Tables 9 and 10) showing that metal concentrations were significantly different between stations in both the Western Harbor and El-Mex Bay (p < 0.05). This could be because of mineralogical composition variation in sediments from one station to another (Mostafaa et al. 2004; Abou-Mahmoud 2011; Shreadah et al. 2014). El-Mex Bay receives a continuous direct discharge of industrial and agricultural drainage water from El-Mex pumping station; the effluent amount is approximately 2.4 × 109 m3 years−1 carrying agrochemicals, trace metals and domestic waste leading to variation in metal concentrations (Said et al. 1994; Okbah et al. 1998; Farag 2009). The Western Harbor is a semi-enclosed basin with limited water circulation, and it may serve as an entrapment and sediment deposition from land-based sources and from the Harbor itself (Shriadah 1991; Shriadah and Tayel 1991). Principal component analysis (PCA) was conducted to interpret the 18 parameter datasets that included carbonate, TOM, Si, Ca, Mg, K, Na, Fe and Al in addition to the potentially toxic metals: Mn, Pb, Cu, Cd, V, Cu, Sn, As and Se. Large absolute values >0.70 indicate a reliable correlation.
A—factor analysis for the Western Harbor region
Five factors explaining 83.28 % of total variance were adopted for these parameters in sediments, Table 11. PC1 explained that 29.73 % of the total variance had a high positive factor loading to Na (0.739), Al (0.855), Cu (0.735) and Sn (0.783) suggesting that most Cu and Sn detected in the samples were associated with aluminium-enriched clay minerals (Summers et al. 1996). PC2 (20.43 %) had high positive factor loading to TOM (0.861), Pb (0.836) and negative loading to carbonate (−0.77).
PC2 indicated that the geochemistry of the sediment in the Western Harbor was controlled by TOM concentration. Furthermore, the high loading of Pb concentration may be related to the continuous and sometimes massive runoff of residential wastewater into the Harbor, and the atmospheric emissions (leaded gasoline) from urban and industrial areas of Alexandria City which settled in the sediment for a long time (Mostafaa et al. 2004). PC3 (15.76 %) had positive factor loading for Mn (0.783), Zn (0.837) and Se (0.787). The higher loading to Mn, Zn and Se in the Harbor is mainly because of the presence of major sources of metal pollution and intensive human activities as stated by Mostafaa et al. (2004). PC4, which described 10.66 % of the total variance, had high positive factor loading for Mg (0.748) and Fe (0.731). The high loading of Fe in the study area could be attributed to the presence of many floating rusty stranded barges. These barges could be major sources for particulate Fe that settles in the bottom sediments. PC5 (7.11 %) yields high loading for Cd (0.940), V (0.704) and As (0.741). The loading of these metals in sediments of the Western Harbor appeared to be directly related to urban and industrial runoff discharged from the El Mahmoudia Canal.
B—factor analysis for El-Mex Bay region
PC1, PC2 and PC3 represented 82.02 % of the total variance adopted for TOM, Si, Ca, Mg, K, Na, Fe and Al in addition to the potentially toxic metals: Mn, Pb, Cu, Cd, V, Cu, Sn, As and Se, Table 12. PC1 (36.62 %) had positive factor loading with K (0.962), Na (0.955), Fe (0.958), Al (0.990), Mn (0.785), Zn (0.979) and V (0.943). PC1 indicated that the geochemistry of El-Mex Bay is highly affected by the geochemical cycle of K and Na. Furthermore, some metals yield significant linear correlation with Al, indicating that the major portion of heavy metals in the sediments was closely associated with fine-grained clay minerals and had common origins with Al (Summers et al. 1996). PC2 represented 28.5 % of the variation and had positive factor loading to carbonate (0.867), Ca (0.867), Pb (0.835), Cd (0.918) Cu (0.913) and Se (0.771). PC2 indicated that El-Mex Bay sediments mainly comprise Ca carbonate, and there are anthropogenic sources of Pb, Cd, Cu and Se which may be because of the industrial outfalls in the southern part of El-Mex Bay. Moreover, much increased concentrations of Pb, Cd, Cu and Se recorded in El-Mex Bay may be related to their release from the discharge of freshwater from El-Umum Drain carrying industrial, agricultural and sewage effluents. Sources of Cd in El-Mex Bay may include the cement industry (Portland Cement Factory), colourants or coal and oil combustion.
PC3 (14.92 %) had a positive factor loading to Sn (0.761) and As (0.768) revealing that As and Sn may come from the same anthropogenic source.
Conclusion
Major constituents and trace metals in sediments collected from El-Mex Bay and the Western Harbor of Alexandria were investigated. High concentrations of metals were measured in the study areas because of heavy industrial activities and urbanization in Alexandria. Enrichment factor (EF), metal pollution index (MPI) and consensus-based sediment quality guidelines (CBSQGs) revealed contamination. From the Western Harbor and El-Mex Bay, 63 and 10 % of sediment samples, respectively, exhibited high levels of pollution with Pb and pose a risk of adverse biological effects. EF for the Western surface sediments occurred in the order Cd > Pb > Sn > Cu > Zn > As > Fe > Mn > V > Se. However, the levels of surface sediments in El-Mex Bay decreased in the order Cd > Sn > Pb > Cu > As > Mn > Zn > V > Fe > Se revealing anthropogenic sources for these metals. The MPI of Mn, Zn, Pb, Cd, Cu, V, Sn, As and Se showed that the lowest values were observed at stations 1, 4, 6 and 8 in the Western Harbor. By contrast, the highest values were detected at stations 12, 13, 16 and 18 in El-Mex Bay. Results of CBSQGs indicated that up to 63 % of sediment samples from the Western Harbor exhibited high levels of Pb that pose a threat to aquatic life. The concentrations of As, Cu and Zn in most El-Mex Bay sediment samples were below the TEC suggesting that these metals may not have harmful effects on sediment-dwelling organisms.
Principle component analysis (PCA) showed that sediments of the Western Harbor have five factors explaining 83.28 % of total variance for TCO3, TOM, Si, Ca, Mg, K and Na in addition to Fe, Al, Mn, Pb, Cu, Cd, V, Cu, Sn, As and Se. All of the factors PC1, PC2, PC3, PC4 and PC5 had high positive loadings to these 18 parameters which are associated with different sources of metal pollution, intensive human activities and industrial runoff discharge. Three factors were obtained for sediments in El-Mex Bay representing 82.02 % of the total variance. Positive factor loading of PC1 with K, Na, Fe, Al, Mn, Zn and V indicated that the geochemistry of El-Mex Bay is highly affected by the geochemical cycle of K and Na. PC2 had positive factor loading to carbonate, Ca, Pb, Cd, Cu and Se suggesting that El-Mex Bay sediments mainly comprise CaCO3, and the anthropogenic source of Pb, Cd, Cu and Se is from industrial, agricultural and sewage effluents. PC3 had a positive factor loading to Sn and As assuming that they may be released from the same anthropogenic source.
References
Abdallah, M. A. M. (2007). Speciation of trace metals in coastal sediments of El-Mex bay south Mediterranean Sea-West of Alexandria (Egypt). Environmental Monitoring and Assessment, 132, 111–123.
Abdallah, M. A. M. (2008). Trace metal behavior in Mediterranean-climate coastal bay: El-Mex Bay, Egypt and its coastal environment. Global Journal of Environmental Research, 2, 23–29.
Abdallah, M. A. M., & Abdallah, A. (2008). Biomonitoring study of heavy metals in biota and sediments in the South Eastern coast of Mediterranean sea Egypt. Environmental Monitoring and Assessment, 146, 139–145.
Abdel Ghani, S. A., El Zokm, G., Shobier, A., Othman, T., & Shreadah, M. (2013a). Metal pollution in surface sediments of Abu-Qir Bay and Eastern Harbour of Alexandria, Egypt. Egyptian Journal of Aquatic Research, 39, 1–12.
Abdel Ghani, S., Shobier, A., & Shreadah, M. (2013b). Assessment of arsenic and vanadium pollution in surface sediments of the Egyptian Mediterranean coast. Journal of Environmental Technology and Management, 16(1/2), 82–101.
Abou-Mahmoud, M. M. (2011). A comparative study between seabed characteristic of the Western and Eastern Harbour of Alexandria, Egypt. Master in Geophysics Science, Faculty of Science, Alexandria University, Egypt.
Ademoroti, C. M. A. (1996). Standard methods for water and effluent analysis. Ibadan: Foludex Press Ltd. 182pp.
Ahdy, H., & Khaled, A. (2009). Heavy metals contamination in sediments of the western part of Egyptian Mediterranean Sea. Australian Journal of Basic and Applied Sciences, 3, 3330–3336.
Ajay, S. O., & Van Loon, G. W. (1989). Studies on redistribution during the analytical fractionation of metals in sediments. Science of the Total Environment, 87(88), 171–187.
Alexjev, V. (1971). Quantitative analysis.Mir. Publ. Moscow. American Public Health Association. (APHA) 1975 Standard methods of the examination of water and wastewater. Inc, New York.
APHA. (1975). Standard methods for the examination of water and waste water by American Public Health Association. Broadway: APHA.
El Sayed, M., El Wakeel, S. K., & Rifaat, A. (1988). Factor analysis of sediments in the Alexandria Western Harbor, Egypt. Oceanologica Acta, 11, 1–11.
El Zokm, G. M., Abdel Ghani, S. A., Shobier, A. H., Othman, T. S., & Shreadah, M. A. (2013). IR spectroscopic investigation, X-ray structural characterization thermal analysis decomposition and metal content of sediment samples along Egyptian Mediterranean Coasts. World Applied Sciences Journal, 23(6), 823–836.
El Zokm, G. M., Okbah, M. A., & Younis, A. M. (2015). Assessment of heavy metals pollution using AVS-SEM and fractionation techniques in Edku Lagoon sediments, Mediterranean Sea, Egypt. Journal of Environmental Science and Health, Part Atoxic/Hazardous Substance & Environmental Engineering. doi:10.1080/10934529.2015.994945.
El-Said, G. F. (2005). Distribution of fluoride content in some localities of Egyptian coastal water, Ph.D. Thesis, Chemistry Department, Faculty of Science, Alexandria University, Egypt, 355p.
El-Said, G., & Youssef, D. H. (2013). Ecotoxicological impact assessment of some heavy metals and their distribution in some fractions of mangrove sediment from Red Sea, Egypt. Environmental Monitoring and Assessment, 185(1), 393–404.
El-Said, G. F., El Sadawy, M., & Moneer, A. A. (2010). Incorporation of flouride and Boron into surface sediment along the Mediterranean coast. Egyptian Journal of Aquatic Research 36(4), 569–583.
El-Sikaily, A., Khaled, A., & El Nemr, A. (2004). Heavy metals monitoring using bivalves from Mediterranean Sea and Red Sea. Environmental Monitoring and Assessment, 98, 41–58.
El-Zokm, G. M., El-Gohary, S. E., & Abd-El-Khalek, D. E. (2012). Studies of some heavy metals in water and sediment in El-Mex Fish Farm, Egypt. World Applied Sciences Journal, 18(2), 171–180.
Emara, H., & Shreadah, M. (2009). Distribution and composition of aliphatic aromatic petroleum hydrocarbons at some hot spots of Alexandria coastal water, Egypt. International Workshop on Integrated Coastal Zone Management (Izmir-Turkey 20–22 Oct., 2009), 201–217.
Emara, H. I. & Shriadah, M. A. (1991). Manganese, iron, cobalt, nickel, and zinc in the Eastern Harbor and El-Mex Bey waters (Alexandria) International Proceeding Symposium of Marine Chemistry in the Arab Region, Suez, April, pp. 97–112.
Emara, H. I., Shriadah, M. A., Maoustafa, T. H., & El-Deek, M. S. (1992). Effects of sewage and industrial wastes on the chemical characteristics of the Western Harbor and El-Mex Bay Waters of Alexandria, Egypt. Science of the Total Environment 773–784.
Ergin, M., Saydam, C., Basturk, O., Erdem, E., & Yoruk, R. (1991). Heavy metal concentrations in surface sediments from the two coastal inlets (Golden Horn Estuary and Izmit Bay) of the Northeastern Sea of Marmara. Chemical Geology, 91, 269–285.
Fahmy, M., Tayel, F., & Shreadah, M. (1995). Effect of pollution on the water quality of Mex Bay and Dekhaila harbor of Alexandria. 1st Int. Conf. on present and future technology of navigation and marine science of Mediterranean and the Red Sea, 29–31 October 1995, El-Mahrousa, Alex., Egypt. Alex., Egypt.
Farag, M. M. (2009). Study on the spatial and temporal distributions of physical parameters at El Mex and Western Harbour areas, Alexandria, Egypt. Egyptian Journal of Aquatic Biology and Fisheries, 13(2), 17–34.
Fathy, S., Abdel Hamid, F., Shreadah, M., Mohamed, L., & El-Gazar, M. (2012a). Application of principal component analysis for developing water quality index for selected coastal areas of Alexandria Egypt. Recourses and Environment Journal, 2(6), 297–305.
Fathy, S., Abdel Hamid, F., Shreadah, M., Mohamed, L., & El-Gazar, M. (2012b). Effect of some environmental pollutants on enzymatic and total antioxidant activities in Tilapia niloticus. Blue Biotechnology Journal, 1(3), 433–443.
Folk, R. L., & Ward, W. C. (1957). Barazos river bar: A study in the significance of grain size parameters. J sed. petrol 27, 3–26.
Gonçalves, E. P. R., Boaventura, R. A. R., & Mouvet, C. (1992). Sediments and aquatic mosses as pollution indicators for heavy metals in the Ave River basin (Portugal). Science of the Total Environment, 142, 142–156.
Grasshoff, K. (1979). Methods of sea water analysis with contributions by T. Almgreen; R. Dowson; M. Ehrhaedt; S. H. Fonselius; B. Josefsson; F. Koroleff; K. kremling; Aufl weinheim New York Verlag Chemie. 317 pp.
Idodo-Umeh, G., & Oronsaye, J. A. O. (2006). Heavy metal pollution of the sediments from Eriora River in Olomoro town, Niger Delta, Nigeria. Journal of Sustainable Tropical Agricultural Research, 21, 74–81.
Khalil, M. K., El Zokm, G. M., Fahmy, M. A., Said, T. O., & Shreadah, M. A. (2013). Geochemistry of some major and trace elements in sediments of Edku and Mariut Lakes, North Egypt. World Applied Sciences Journal, 24(3), 282–294.
Kremling, K., & Streu, P. (1993). Saharan dust influence trace element fluxes in deep North Atlantic subtropical waters. Deep Sea Research, 40, 1155–1168.
Law, A. T., & Singh, A. (1991). Relationships between heavy metal contents and body weight of fish from the Kelang Estuary Malaysia. Marine Pollution Bulletin, 22, 86–89.
Liaghati, T., Preda, M., & Cox, M. (2003). Heavy metal distribution and controlling factors within coastal plain sediments, Bells Creek catchments, southeast Queensland, Australia. Environment International, 29, 935–948.
Long, E. R., & Morgan, L. G. (1990). The potential for biological effects of sediment-sorbed contaminants tested in the National Status and Trends Program. National Oceanic and Atmospheric Administration Technical Memorandum NOS OMA 52, National Ocean Service, Rockville, Maryland.cology, Vol. 39, 2000a, pp. 20–31.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidences of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000a). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31.
MacDonald, D. D., Moore, D. R., Pawlisz, A., Smorong, D. E., Breton, R. L., MacDonald, D. B., Thompson, R., Lindskoog, R. A., & Hanacek, M. A. (2000b). Calcasieu estuary remedial investigation/feasibility study (RI/FS): baseline ecological risk assessment (BERA). Volume I. Baseline problem formulation. Prepared for: CDM Federal Programs Corporation.
Martin, D. T., & Meybek, J. M. (1979). Elemental mass balance of material carried by major world rivers. Marine Chemistry, 7, 173–206.
Masoud M. S., Elsaraf, W. M., Abdel Halim, A. M., Aly A. E., & Hasan, M. H. I. (2012). Heavy metal accumulation in sediment of Alexandria coastal area. Bulletin of the Faculty of Science, 47(12), 12–28.
Mayer, L. M., & Fink, L. K. (1980). Granulometric dependence of chromium accumulation in estuarine sediments in Maine. Estuarine, Coastal and Shelf Science, 11, 491–503.
Molinari, E., Guerzon, S., & Rampazzo, G. (1993). Contribution of Saharan dust to the central Mediterranean Basin. Geological Society of America, Special Paper, 284, 303–312.
Mostafaa, A., Barakat, A., Qian, Y., & Wade, T. (2004). An overview of metal pollution in the Western Harbor of Alexandria, Egypt. Soil & Sediment Contamination, 13, 299–311.
Okbah, M. A., Mahmoud, T. H., & El-Deek, M. S. (1998). Assessment of trace metals in the sediments from the coastal zone of Alexandria, Egypt. Chemistry and Ecology, 14, 151–161.
Okbah, M. A., Ibrahim, A. M. A., & Gamal, M. N. M. (2013). Environmental monitoring of linear alkylbenzene sulfonates and physicochemical characteristics of seawater in El-Mex Bay (Alexandria, Egypt). Environmental Monitoring and Assessment, 185, 3103–3115.
Okbah, M. A., El Zokm, G. M., & Younes A. M. (2015). Heavy metals fractionation and acid volatile sulfide (AVS) in the Bardawil lagoon sediments, northern Sinai, Egypt Development in Analytical Chemistry (accepted for publications).
Pekey, H., Karakas, D., Ayberk, S., Tolum, L., & Bakoglu, M. (2004). Ecological risk assessment using trace elements from surface sediments of Izmir Bay (Northeastern Marmara Sea) Turkey. Marine Pollution Bulletin, 48, 946–953.
Said, M., El-Deek, M., Mahmoud, T., & Shreadah, M. (1994). Effect of pollution on the hydrochemical characteristics of different water types in El-Mex Bay area west of Alexandria, Egypt. Acta Adriatica, 34(1/2), 9–19.
Shobier, A., Abdel Ghani, S., & Shreadah, M. (2011). Distribution of total mercury in sediments of four semi-enclosed basins along the Mediterranean coast of Alexandria. Egyptian Journal of Aquatic Research, 37(1), 1–11.
Shreadah, M. A., & Emara, H. I. (1992). Major cations and alkalinity in the Eastern harbor and El-Mex Bey. Bulletin of the Faculty of Science, Alexandria University, 32(A), 156–174.
Shreadah, M., & Emara, H. I. (1996). Heavy metals (iron, manganese, nickel, cadmium, and lead) in the sediments from the Eastern harbor and El-Mex Bay of Alexandria, Egypt. Proc. 6th Int. Symp. Environ. Prot. is a must, 916927, Alexandria, 21–23 May, Egypt.
Shreadah, M. A., Said, T., Younis, A., & Farag, R. (2006). Speciation of organotin compounds in sediments of semi-closed areas along the Mediterranean Coast of Alexandria. Chemistry and Ecology, 22(5), 395–404.
Shreadah, M. A., Said, T. O., Abdel Moniem, M. I., Fathallah, E., & Mahmoud, M. (2011). PAHs in sediments along the semi-closed areas of Alexandria, Egypt. Journal of Environmental Protection, 2(6), 700–709.
Shreadah, M., Said, T., Othman, I., Fathallah, E., & Mahmoud, M. (2012). Polychlorinated biphenyls and chlorinated pesticides in sediments along the semi-closed areas of Alexandria, Egypt. Journal of Environmental Protection, 3(2), 141–149.
Shreadah, M. A., Masoud, M. S., Khattab, A. M., & El Zokm, G. M. (2014). Impacts of different drains on the seawater quality of El-Mex Bay (Alexandria, Egypt). Journal of Ecology and the Natural Environment, 8(8), 287–303.
Shriadah, M. A. (1991). Recent observations on some hydrographic and chemical aspects of Lake Edku waters, Egypt. Bulletin of the High Institute of Public Health, XXII(1), 185–201.
Shriadah, M. (1992). Major cations and alkalinity in the Western Harbor of Alexandria. Bulletin of the High Institute of Public Health, XXII(3), 481–497.
Shriadah, M., & Emara, I. (1991). The distribution of chromium, cadmium, and lead in areas of multi-polluting factors of Alexandria. Inter. Proc. Symp. Mar. Chem. In the Arab Region, Suez, April, 1991, p. 30–50.
Shriadah, M. A., & Tayel, F. R. (1991). Environmental condition of the Western Harbor of Alexandria: physico-chemical characteristics. Bulletin of the High Institute of Public Health, XXII(1), 213–228.
Summers, J. K., Wade, T. L., Engle, V. D., & Malaeb, Z. A. (1996). Normalization of metal concentrations in estuarine sediments from the Gulf of Mexico. Estuarine, 19, 581–594.
Tam, N. F. Y., & Wong, Y. S. (2000). Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Journal of Environmental Pollution, 110(2), 195–205.
Tayel, F., & Shreadah, M. (1996). Fe, Cu, Mn, Pb and Cd in some fish species from the Western harbor of Alexandria, Egypt. Bull. Nat. Inst. Oceanogr. & Fish., A. R. E., Vol. 22, p. 85–96.
Tayel, F., Fahmy, M., & Shreadah, M., (1996). Studies on the physicochemical characteristics of Mex Bay and New Dekhaila Harbor waters of Alexandria, Egypt. Bull. Nat. Inst. Oceanogr. & Fish., A.R.E., Vol. 22, p. 1–18.The University of Plymouth, Plymouth, U.K, 1995.
Tayel, F. Shreadah, M., & El-Shenawy, M. (1997). The occurrence of zinc, copper, cadmium, and lead in the seawater of Alexandria harbor, Egypt. Proc. of the 7th Int. Conf. on Environ. Prot. is a must, p. 106–116, Alex., 20–22 May, 1997.
Usero, J., Regalado, E. G., & Garcia, I. (1996). Trace metals in bivalve molluscs Chamelea gallina from the Atlantic Coast of Southern Spain. Marine Pollution Bulletin, 32, 305–310.
Williams, M. R. (1995). Particulate trace metals in British coastal waters. Ph.D Thesis.
Younis, A. M., El-Zokm, G. M., & Okbah, M. A. (2014). Spatial variation of acid-volatile sulfide and simultaneously extracted metals in Egyptian Mediterranean Sea lagoon sediments. Environmental Monitoring and Assessment, 186, 3567–3579.
Acknowledgments
The authors are grateful to Prof. Dr. Maged M. A. Hussein, Mr. Mohamed I. Abd El-Moneim National Institute of Oceanography and Fisheries for their kind help.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shreadah, M.A., Shobier, A.H., Ghani, S.A.A. et al. Major ions anomalies and contamination status by trace metals in sediments from two hot spots along the Mediterranean Coast of Egypt. Environ Monit Assess 187, 280 (2015). https://doi.org/10.1007/s10661-015-4420-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4420-y