Abstract
Concentrations of 25 heavy metals (Ag, Al, As, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, Pb, Sb, Se, Sr, V, Zn and Hg) in surface sediments along the Doha Bay from 10 transects each with five stations were studied. Significant differences were observed in metal concentrations between the sampling locations and durations. Higher concentrations were observed in areas where there are a lot of anthropological activities. The distribution of selected metals was presented in contour maps showing the variation between the two periods. In order to further study particle size effect on metal uptake, two different grinding times were administered on four randomly selected samples and the results showed no significant difference on the analysis in the inductively coupled plasma-optical emission spectrometry (ICP-OES) instrument. The overall results of metal analyses were within the international standards criteria, and the results were comparable to the previous studies conducted around Qatar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution in marine environments has become a worldwide problem (Mashal et al. 2014; Guevara et al. 2005; Allen 1995). This is because of their toxicity, extensive causes and accumulative characteristics. With the fast industrial development in the east coastal region of Qatar, especially in the Doha Bay area, heavy metals are expected to be presented into the coastal environment and be combined within sediments together with organic matters, clay, oxides and sulphides (Wang and Chen 2000; Malins 1984). Marine sediments act as an excellent adsorbent for heavy metals and frequently offer an outstanding sink of man’s impact (Malins 1984). However, the metal fixation is not permanent, and some of the metals might be released into the water body under variable conditions through several processes of remobilization (Irabien and Velasco 1999; Mashal et al. 2014). Consequently, sediments would play a vital role in the transport and storage of potentially toxic metals in the marine environment (Langston 2000; Redfern 2006).
The concentration of heavy metal in the marine environment depends on the source of pollution, input and its speciation (Fergusson 1990) as well as to the sediment characteristics, grain size distribution, reactions at particle surfaces that effect the amount of metal adsorbed, adsorptive properties of clay minerals, specific surface area and reduction/oxidation reactions (Williamson and Wilcock 1994; Luoma 2000; Krumgalz et al. 1992; Mashal et al. 2014). The metal release from sediments can occur as the water salinity increases due to the high ionic strength of seawater or reduction/oxidation condition changes (Luoma 2000). This would be controlled by the oxygen concentration and its dynamics and movement from the water to the sediments and by the readsorption processes in various oxides such as Fe and Mn oxides. In addition, fine particulates would be trapped in coastal environments that have low hydrodynamic energy, while they are moved on in areas where hydrodynamic energy is high. Accordingly, three main zones can be eminent based on the following processes (Williamson and Wilcock 1994): (i) deposition, (ii) transportation and (iii) areas. However, the deposition area is the intermediate fate for most metals where the finest particulates generally accumulate and the others become immobilized due to the decomposition of organic material as insoluble sulfide precipitates (Luoma 2000). Various factors would affect the physical, chemical and biological properties of sediments. Sediment disturbance (bioturbation) is more important in muddy sediments than sandy sediments, and it has a major impact on the chemistry of muddy sediments. This could be occurred by various animals such as rays, crabs, worms and gastropods (Williamson and Wilcock 1994; Mashal et al. 2014).
Understanding the environmental fate of contaminants is of importance in order to predict potential impacts on human health and ecosystems and how contaminants are transformed by biological, geological and chemical processes over time (Williamson and Wilcock 1994; Mashal et al. 2014). Authors design and manage sampling and analysis methods in order to recognize the key factors that may be occurring and to identify the main sources of environmental risks. This procedure would be used in a risk management setting for industries and regulators and to decide whether remediation is suitable and determine the best remediation methodology. Moreover, sediment chemistry, metal concentration and toxicity, as sediment quality, are considered as important environmental forensics components. This would help us to understand and evaluate the risk posed by sediment-associated chemicals to aquatic organisms.
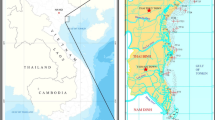
This paper aimed at providing knowledge on the environmental characteristics and properties in the location where many developments are constructed such as residences, marinas and other facilities within the coast of Doha Bay. This part which this study is concerned is to investigate metal concentration within the area. This bay is categorized as an impounded water body which is isolated partially or totally from the main water system due to human intervention (e.g. urbanization). This kind of water system may lay beneath many speculated environmental problems such as water stratification, high biological oxygen demand (BOD), siltation (release of mud and silt-sized sediment to the water) which increase the heavy metal load attracted by silts, change of pH as more organic contents increase the water becomes more acidic, increase in salinity due to higher evaporation and less water movement, increase in nutrient as more P (phosphorous) and N (Nitrogen) would be added to the water body with minimum recharging, accumulation of heavy metals, accumulation of organic pollutants, death of the native species, introduction of new exotic species, deterioration of water quality and value loss of estates (Denton et al. 2001). Fifty marine sediment samples for two time periods (May and December 2012) were collected from selected sites located at the coastal area north of Doha, north of the Pearl and south of Al Safeliya on the eastern coast of Qatar. These samples are subjected to simultaneous determination of minerals and heavy metals (Ag, Al, As, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, Pb, Sb, Se, Sr, V, Zn and Hg) using inductively coupled plasma-optical emission spectrometry (ICP-OES). The two sampling times are called phase I and phase II; respectively. Figure 1 shows the locations and the details of the 50 stations.
Experiment and materials
Site description
The selected site of the study stretches the whole bay of Qatar comprising the coastal area north of Doha, north of the Pearl and south of Al Safeliya on the eastern coast of country (Fig. 1). This area is under ongoing development by Lusail Real Estate Development Company where coastal residences inclusive of a number of facilities will be developed. Major construction work on channels for a marina and work on the seafloor will be materialized. The impact of these activities will result in environmental stresses. To ensure a factual knowledge on the area, this study is realized and designed as a baseline impact assessment analysis which will be used as reference for future studies within the area.
Sampling method
Fifty marine sediment samples for two time periods (May and December 2012) were collected from selected sites located at the coastal area north of Doha, north of the Pearl and south of Al Safeliya on the eastern coast of Qatar (Fig. 1). These samples are subjected to simultaneous determination of minerals and heavy metals (Ag, Al, As, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, Pb, Sb, Se, Sr, V, Zn and Hg) using ICP-OES. The two sampling times are called phase I and phase II, respectively.
All samples are collected by the Environmental Studies Centre (ESC)/Qatar sampling team. They were taken by a diver using Teflon beaker and then were kept in glass jars. All glass jars were labelled and pre-treated to suit this experiment. The obtained samples meet the requirements of the sampling program and handle so that they do not deteriorate or become contaminated before it is analysed; hence, samples were transformed to a freezer at temperature of −4 °C (set by ESC) for preservation required until analysis.
Sample preparation
The main principle of the sample preparation was to ensure that the samples were in the best condition required for the analysis. The sample preparation includes the following steps: (i) drying using freeze dryer (Stoppering Tray Dryer, LABCONCO), (ii) grinding (Retsch model PM400), (iii) digestion by a mixture of strong acids using Hot Block System (Environmental Express) and (iv) cooling and dilution.
Before the hot block digestion step, four randomly selected dried sediment samples were ground in agar mortar with Retsch PM400 model grinder at 100 rpm for two different grinding times of 15 and 30 min. Size distribution of ground sediment samples were analysed by using Mastersizer 2000, Hydro 2000S size distribution analyser. Sediment samples were subjected to a different grinding time to investigate if there is any significant difference between the metal concentration and the grain size.
Sample analysis
All samples were fully analysed using ICP-OES, Perkin-Elmer, Optima 5300 DV located at ESC/Qatar. The representative marine sediment samples are accurately weighed and treated with acids using hot block system; thus, the total recoverable element concentration will then be determined. After cooling, the sample was made up to the volume with deionized water and filtered if turbid. The sample solution is aspirated through nebulizer and the resulting aerosol is transported to the plasma torch where excitation occurs. Element specific emission spectra are produced by radio-frequency inductively coupled plasma. The spectra are dispersed by a grating spectrometer, and intensities of the line spectra are monitored at specific wavelengths by a charged coupled detector (Channeltron electron multiplier). A fitted background correction is used to correct the blank signal and matrix effect. The standard operational procedure number is SOP-ESC-ICP-01 which was developed using the USEPA method 3051A for marine sediment digestion and the USEPA method 6010b for sample analysis. In addition, the effect of grinding time was considered in the design of this experiment to study the consequence of grain size on the metal uptake.
Statistical analysis
About 10 % of total number of samples was subjected to quality control measurements. Moreover, the data which are obtained below the sensitivity of the method of analysis and instrument used were reported as below the limit of quantitation (LOQ). LOQ is defined as the lowest amount of analyte in a sample which can be quantitatively determined with suitable precision and accuracy of an individual analytical procedure. It is a parameter of quantitative assays for low levels of compounds in sample matrices and is used particularly for the determination of impurities and/or degradation products.
Data processing included the following: repeatability, reproducibility, recovery % and upper and lower acceptable limit. For the purpose of comparison, the study used a statistical analysis tool to establish and evaluate significant differences on the results obtained for each metal. Multivariate and repeated measures analysis of variance (ANOVA) are incorporated to delineate the differences among the 10 transects and between the study duration. Since all groups of data are expected to differ from each other to some degree, the extent to which groups differ can tell us whether they are intrinsically similar and can thus be assumed to be the same or whether they are effectively different. The primary tool used to make this determination is ANOVA. It distinguishes between groups of mean values by comparing variance. These variances are partitioned in ANOVA so that separate measures are provided for variation between the groups and within each group.
When interpreting a P value, it can be concluded that there is a significant difference between groups if the P value is small enough and less than 0.05 (5 %) is a commonly used cut-off value. In this case, 5 % is the significance level or the probability of a type I error. This is the chance of incorrectly rejecting the null hypothesis (i.e. incorrectly concluding that an observed difference did not occur just by chance) (Bland 2001) or more simply the chance of wrongly concluding that there is a difference between two groups when in reality there is no such difference. Post hoc test is performed to know which means of all the groups tested for ANOVA which rejected the null hypothesis. Tukey’s honestly significant difference is used as a post hoc test as this is the most sensitive robust analysis to identify the significant differences between and within the group being tested.
Results and discussion
It is important to look for ecological indicators to assess the condition of the environment. They are valuable in providing early warning signals, diagnose and formulate solutions on the causes of any environmental problem. There are several factors that influence the magnitude of the key indicators; it must be ideally selected to represent vital information about the structure, function and composition of the ecological system.
The data is represented by a box and whisker plot to show the spread of the values observed in each transect in a form of minimum, maximum, third quartile, first quartile, the median and the outlier. The central line indicated central tendency or the median; the large box indicated the variability around the central tendency. The standard error or the third quartile and first quartile and the whiskers around the box indicated the minimum and maximum range in the data. Moreover, the values obtained were compared with the international guidelines with its designated contaminant criteria.
Spatial variation of the metal concentration
Understanding the spatial distribution of data is essential in elucidating the dispersion of the contaminant within a geographic location. It provides visualization of the spatial pattern of the immensity of the pollution through the generation of a contour map using geographic information system (GIS) at ESC/Qatar. The emphasis of the spatial analysis is to measure properties and identify relationships and differences taking into account the spatial localization. The present study had incorporated spatial analysis on different transects analysed on different duration within the study area. It was found that significant differences were observed to most metals around the study area except Ba—Dec 2012 (F (9,40) = 1.054; p > 0.05); Cd—May 2012 (F (9,40) = 0.656; p > 0.05); K—Dec 2012 (F (9,40) = 1.951; p > 0.05); Li—Dec 2012 (F (9,40) = 1.160; p > 0.05); Mg—May 2012 (F (9,40) = 1.729; p > 0.05); Sb—May 2012 (F (9,40) = 1.558; p > 0.05); and Hg—Dec 2012 (F (9,40) = 1.462; p > 0.05). The transects E–J have significantly higher metal concentration as within these locations, it was evident that there are a lot of anthropological activities. Ongoing construction activities, presence of marinas, the four surface water discharge points along the coastline and the fact that the area is an enclosed location with an impounded water system are the contributory factors to the measured level.
Figure 2 shows the maps of the distribution of Zn metal surrounding the study area during the two sampling durations. Zn is commonly used for roof constructions, gutters, drainpipes, roof flashings and coverings. Cu and Pb have similar applications. According to Wood 1974 and Aprile and Bouvy 2008, Zn, Cu and Pb are three of the most toxic and relatively accessible metal which when exposed to the atmosphere and/or washed to the open waters will ultimately contribute to the pollution loading within the area. In the present study, these metals differ significantly in their dispersion within the study area (Tukey HSD, p < 0.05). Zn and Cu metals have an average concentration of 6.49 ± 2.72 and 2.70 ± 1.66 ppm respectively while Pb obtained concentrations below the LOQ. V and Ni are known to be the primary constituents in crude oil (Alloway 1995). The concentrations obtained for V and Ni in both sampling duration were found to be significantly different (Tukey HSD, p < 0.05). Higher concentrations were observed in southern part Doha Bay where the marina is located. The average concentration for V is 6.21 ± 3.38 ppm while for Ni is 3.97 ± 2.26 ppm. Some metal concentrations were observed to be below the LOQ of the instrument’s sensitivity. As, Be, Ca, Co, Mo, Pb, Se and Sr were recorded < LOQ to most stations during the May 2012 sampling while Be, Ca, Co, Pb, Se and Sr, for December 2012.
Seasonal variation of the metal concentration
Metals are classified into three criteria according to environmental impact. These classifications include their reactivity and availability to ecological system. Trace metals normally occur at very low levels in the environment. They exert a variable range of toxicity and effect, while some metals such as Mn, Fe, Cu and Zn are essential metal micronutrients; others such as Hg, Cd and Pb are not required even in small amount by any organism (Fergusson 1990; Hosono et al. 2011; Luoma 2000). These metals are stable and exist as persistent environmental contaminants which impose havoc to the organisms and the environment (Aprile and Bouvy 2008). They are known as heavy metals which are very toxic and tend to accumulate in the soils and sediments (Williamson et al. 2003). Overtime, these metals accumulate within the sediments, and the latter become an important reservoir of metal concentrations which provide input record of the pollution loading within a system.
The relationship was assessed using repeated measures ANOVA and further delineated using post hoc test. Al, Ba, Cd, Cr, Cu, K, Ni, Sb, Zn and Hg metals showed significant differences over the two period of the study (ANOVA, p < 0.05). Higher concentrations were observed during December 2012. Temperature variation within a season is one of the factors that affect metal concentration. According to Fergusson 1990 and Luoma 2000, the metal concentration in sediments is temperature dependent where the marine sediments are suspected to have less metal concentration during the summer season and higher concentration on water column due to vertical mixing. Qatar has an arid climate with May as the beginning of summer and December as the winter season. In addition to this abiotic factor that may influence the metal concentration measured during the study, there are an increased constructions and modifications within the area which contribute to the results, in fact, ongoing activities such as hotel and building constructions, i.e. Pearl Qatar, Lusail Development. Figure 3 shows the comparison of the two sampling duration and degree of distribution of the metal concentrations around the study area. Fe, Li, Mg, Mn and V metals showed no significant difference on the concentration over the two periods of the study (ANOVA, p > 0.05). However, among the transects, significant differences were observed having higher concentration within the location with most activities.
Size analysis for sediment samples
The results were comparable to both grinding times which were evident in the trace metal concentrations. Paired t test was used to evaluate the relationship. The sediment samples obtained from station A3 which was subjected under 15 min—grinding time did not differ significantly with A3 under 30 min (t (16) = −0.604, p > 0.05). Similarly, D3-15 min, E1-15 min and I1-15 min did not differ significantly with D3-30 min, E1-30 min and I1-30 min, respectively (t (16) = −1.834, −0.906, −1.256; p > 0.05). This might be affected from the digestion, indicating that samples were fully digested by the selected mixture of acids. In fact, smaller particle size is preferred as this has greater surface area which will enable complete digestion of the samples which is a preparation prior to metal analysis.
The statistical analysis showed that the metal concentrations of these four sediment samples (A3, D3, E1, I1) did not differ significantly in terms of grinding time, but by visual inspection, each metal concentration in each station increases significantly (Fig. 4). Stations A3 and D3 had lower concentrations than E1 and I1 which was consistent with the conclusion observed on the spatial and temporal variation analysis that metal concentrations in the stations within transects E to I has significantly higher metal levels than transects A to D. Sediment grain size is one of the main controlling factors for the distribution of heavy metals in an aquatic ecosystem; however, based on the results obtained, there was no significant difference between the different grinding times of the sediment. All eight sediment samples had approximately same size distribution (Fig. 5). Most of all ground sediment particle sizes were found smaller than 100 μm. This was important for confirming reproducible results in the hot block digestion process and evaluating metal concentration variation dependence on grain size. According to the study of Aprile and Bouvy 2008, they found that the concentration of metals in sediments cannot be interpreted simply by a change in grain size but other factors must be taken into consideration such as anthropogenic influences. The grain size analysis made on this study did not give a whole representation of its effects on the concentration of the metals as there were only a few stations analysed, and it is important to note that a detailed size distribution analysis on all stations within each transect must be performed to comprehend its effect. Moreover, the chemistry of the sediment and the metal itself must also be evaluated and investigated as they are also contributory factors to the contaminant levels. These include adsorption from water column or vertical mixing, pH, oxidation of surface sediments and biological uptake by organic matter or organisms. Physicochemical adsorption direct from the water column happens in many different ways. It usually occurs when particulate matter directly adsorb heavy metals straight from the water and high organic matter is proportionate to high metal levels while chemical and biological adsorption are more complicated as they are controlled by many factors such as pH and oxidation. Schlinder (1991) suggested that the pH value oversees the adsorption of metal ions at surfaces. Higher pH values promote more adsorption than lower pH which the latter actually prevents the retention of metals by the sediment (Belize et al. 2004; Hosono et al. 2011). The increase in pH is affected by the action of sulphate-reducing bacteria under anaerobic conditions which are present in areas where there is a lack of tidal flushing and water circulation which can be postulated from the present study as there are areas which have an enclosed compounded water system. Under these conditions, it tend to favour the formation and retention of metals as sulphides and the deposition of organic matter in these enclosed sites is expected to increase, thus increasing the organic carbon due to limited mixing especially in lagoon areas. On the other hand, shallow areas which are exposed to oxygen (O2) during changing of tides (low tide) can dry up and can influence the oxidation-reduction potential of the sediment. Clark et al. (1998) showed that the redox potential of the sediment can affect the metal trapping directly through change in the oxidation state of the metal itself or indirectly through a change in the oxidation state of the ions that can form complexes with the metal. It further oxidizes sulphides present to sulphuric acid, thereby increasing the pH of the sediment pore water and allows mobilization of metals. In the present study, there are areas in Doha Bay which are consistent and comply with the description of the stated previous studies such as having an enclosed water system, shallow water and sources of anthropogenic activities which influence the level of metal concentration. It is therefore essential that a thorough investigation considering all these contributory factors must be made within the area to further understand the impact of the contaminants and establish comparison in future studies.
Comparison to international guidelines and previous studies
A number of international guidelines were consulted to identify if relevant published threshold criteria exist for each metal contaminant investigated during the present study. The consulted international sediment quality guidelines (SQG) with its designated contaminant criteria include the following: (i) Florida Department of Environmental Protection, 1994 (USA); threshold effects level (TEL); probable effects level (PEL), (ii) Canadian Environmental Quality Guidelines (Canada); Interim Sediment Quality Guideline (ISQG); PEL and (iii) National Oceanic and Atmospheric Administration (USA); effects range-low (ERL); effects range-median (ERM).
The contaminant analytes that can be compared with the guidelines are As, Cd, Cr, Cu, Ni, Pb, Zn and Hg. The results obtained were below the limit set by the three consulted guideline. Table 1 shows the summary of the data considered for each metal analyte during the two sampling duration while Fig. 6 shows the graphical representation for visual understanding. In addition, several previous studies had been published for metal analysis on sediment samples in different locations in Qatar, UAE, Saudi Arabia, Oman and the Middle East. Comparing the results of the present study to these previous published studies will provide information regarding the magnitude of the contamination surrounding the present study area. Table 1 shows the different concentrations obtained for metal analysis from different locations in the national, the other GCC countries and international results.
Conclusion
The study analysed the concentrations of 25 metals around Doha Bay to evaluate the pollution loading and the magnitude of the impact that the contaminant had on the sediment samples within the area on two sampling durations. Significant differences were observed between the temporal variation which December 2012 sampling (phase II) recorded higher concentration than May 2012 (phase I). Moreover, significant differences of metal sediment accumulation were observed between the 10 transects. Higher metal concentrations were recorded in transects E to I compared with A to D. The areas with higher levels are characterized by many anthropological activities. However, the concentrations were still within limit to some metals which FDEP, CEQG and NOAA (international standards) had set permissible levels. Sediment grain size was also determined in the study which obtained no significant differences between the metal concentration and difference in grinding time (15 and 30 min). However, the data were not conclusive as there were only four sediment samples analysed although the results were consistent and representative of the transects with increased anthropological activities thus with higher metal concentrations: E1 and I1 and lower metal concentrations: A3 and D3. Based on this result, it was evident that sediment grain size not only affects the contaminant levels in the area but there may be other contributory factors that influence the measured concentrations. It is therefore recommended that a detailed comprehensive environmental impact assessment be undertaken within the study area and should be done annually to evaluate the temporal changes in the environmental status as it is predicted that increased activities will happen within the area due to commercialization and development. A number of actions are recommended to improve our understanding of heavy metal processing and its impacts in addition to enhance and improve the monitoring and reporting of results of this study. The following include
-
Deployment of current meters to measure the current direction, value and change during different weather conditions.
-
Conduct detailed monitoring scheme water quality survey and biota analysis in order to obtain representative values within the ecosystem of the area.
-
Execute hydrodynamic modelling to understand water hydrodynamic of the area.
-
Study siltation plume associated with dredging and the fate of the released sediments.
References
Abayachi, K. J, & Douabul, A. A. Z. (1986). Trace element geochemical association in Arabian Gulf. Marine Pollution Bulletin, 17, 353–356.
Ali, S. B., & Al-Lihaibi, S. S. (1993). Trace elements in sediments of the Western Gulf. Marine Pollution Bulletin, 27, 103–107.
Al-Hashimi A. H., & Salman H. H. (1985). Trace metals in the sediments of the northwestern coast of the Arabian Gulf. Marine Pollution Bulletin, 16, 118–120.
Allen, H. E. (1995). Metal contaminated aquatic sediments. Michigan: Ann Arbor Press.
Alloway, B.J. (1995). Heavy metals in soils. 2nd edn. Blackie Academic & Professional. 368p.
Anderlini, V., Samhan, O., & Zarba, M. (1987). Multivariate geochemical investigation of trace metal pollution in Kuwait marine sediments. Marine Environmental Research, 21, 31–48.
Aprile, F. M., & Bouvy, M. (2008). Distribution and enrichment of heavy metals in sediments at the Tapacura River Basin, Northeastern Brazil. Brazilian Journal of Aquatic Sciences and Technology, 12, 1–8.
Belize, N., Chen, Y., Gunn, J., & Dixit, S. (2004). Sediment trace metal profiles in lakes of Killarney Park, Canada: from regional to continental influence. Environmental Pollution, 130, 239–248.
Bland, J. M. (2001). Calculating confidence intervals. British Medical Journal, 312, 772.
Clark, M. W., McConkie, D., Lewis, D. W., & Saenger, P. (1998). Redox stratification and heavy metal partitioning in Avicennia-dominated mangrove sediments: a geological model. Chemical Geology, 149, 147–171.
Cosma, B., Frache, R., Baffi, F., & Dadone, A. (1982) Trace metals in sediments from the Ligurian Coast, Italy. Marine Pollution Bulletin 13, 127–132.
Denton, G. R. W., Bearden, B. G., Concepcion, L. P., Siegrist, H. G., Vann, D. T., & Wood, H. R. (2001). Contaminant Assessment of Surface Sediments from Tanapag Lagoon, Saipan, Water and Environmental Research Institute of the Western Pacific, Technical Report No. 93, University of Guam, Mangilao, Guam.
Eisler, R. & Hennekey, R. J. (1977). Acute toxicities of Cd2+, Cr6+, Hg2+, Ni2+, and Zn2+ to estuarine macrofauna. Archives of Environment Contamination and Toxicology, 6, 315–323.
Fergusson, J. E. (1990). The heavy elements: chemistry, environmental impact and health effects. Oxford: Pergamon Press.
Guevara, R., Rizzo, A., & Sanchez, R. (2005). Heavy metal inputs in northern Patagonia lakes from short sediment core analysis. Journal of Radioanalytical and Nuclear Chemistry, 265, 481–493.
Hosono, T., Su, C.-C., Delinom, R., Umezawa, Y., Toyota, T., Kaneko, S., & Taniguchi, M. (2011). Decline in heavy metal contamination in marine sediments in Jakarta Bay, Indonesia due to increasing environmental regulations. Estuarine, Coastal and Shelf Science, 92(2), 297–306.
Irabien, M. J., & Velasco, F. (1999). Heavy metals in Oka River sediments (Urdaibai National Biosphere Reserve, Northern Spain): lithogenic and anthropogenic effects. Environmental Geology, 37, 54–63.
Krumgalz, B. S., Fainshtein, G., & Cohen, A. (1992). Grain size effect on anthropogenic trace metals and organic matter distribution in marine sediments. The Science of the Total Environment, 116, 15–30. Elsevier Science Publishers.
Langston, W. J. (2000). Toxic effects of metals and the incidence of metal pollution in marine ecosystems. In R. W. Furness & P. S. Rainbow (Eds.), Heavy metals in the marine environment (pp. 101–122). USA: Florida.
Luoma, S. N. (2000). Processes affecting metal concentrations in estuarine and coastal marine sediments. In R. W. Furness & P. S. Rainbow (Eds.), Heavy metals in the marine environment (pp. 51–66). Florida: CRC Press, Inc.
Malins, D. C. (1984). Chemical pollutants in sediments and diseases of bottom-dwelling fish in Puget Sound, Washington. Environmental Science & Technology, 18, 705–713.
Manheim, F. T. (1961). A geochemical profile in the Baltic Sea Geochimica et Cosmochimica Acta. 24, 52–70.
Mashal, K., Salahat, M., Al-Qinna, M. & Al-Degs, Y. (2014). Spatial distribution of cadmium concentrations in street dust in an arid environment. Arabian Journal of Geosciences. doi:10.1007/s12517-014-1367-1.
Muhammad, Sadiq. (2002). Metal contamination in sediments from a desalination plant effluent outfall area. Science of The Total Environment, 287, 37–44.
Redfern, F.M. (2006). Heavy metal contamination from landfills in coastal marine sediments: Kiribati and New Zealand, Thesis, The University of Waikato.
Roth, L., & Hornung H. (1977). Heavy metal concentration in water, sediments and fish from Mediterranean coastal area, Israel. Environmental Science Technology 11, 265–269.
Schlinder, P. W. (1991). The regulation of heavy metal concentrations in natural aquatic systems. In J.-P. Vernet (Ed.), Heavy metals in the environment (pp. 95–124). Amsterdam: Elsevier.
Secrieru, D. & Secrieru, A., (2002). Heavy Metal Enrichment of Man-made Origin of Superficial Sediment on the Continental Shelf of the North-western Black Sea. Estuarine, Coastal and Shelf Science, 54(3): 513–526.
Shiber, J. G. (1980). Deep Sea Research Part B. Oceanographic Literature Review. Soil Pollution 27, 860.
Shriadah, M. M. A. (1999). Heavy metals in mangrove sediments of the United Arab Emirates Shoreline (Arabian Gulf). Water, Air and Soil Pollution 116, 523–534.
Wang, F., & Chen, J. S. (2000). Relation of sediment characteristics to trace metal concentration: a statistical study. Water Research, 34, 694–698.
Williamson, R.B. and Wilcock, R.J. (1994). The Distribution and fate of contaminants in estuarine sediments: recommendations for environmental monitoring and assessment, Technical Publication, 47, New Zealand.
Williamson, B., Lewis, G., Mills, G., & Vant, B. (2003). Contaminants on the coast. In J. R. Goff, S. L. Nichol, & H. L. Rouse (Eds.), The New Zealand Coast: Te Tai o Aotearoa (pp. 237–259). Palmerston North: Dunmore Press.
Wood, J. M. (1974). Biological cycles for toxic elements in the environment. Science, 183, 10.
Compliance with ethical requirements
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. In addition, this study did not involve human participants and/or animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Naimi, H.A., Al-Ghouti, M.A., Al-Shaikh, I. et al. Metal distribution in marine sediment along the Doha Bay, Qatar. Environ Monit Assess 187, 130 (2015). https://doi.org/10.1007/s10661-015-4352-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4352-6