Abstract
Stratification and loading of fecal indicator bacteria (FIB) were assessed in the main tidal channel of the Ballona Wetlands, an urban salt marsh receiving muted tidal flows, to (1) determine FIB concentration versus loading within the water column at differing tidal flows, (2) identify associations of FIB with other water quality parameters, and (3) compare wetland FIB concentrations to the adjacent estuary. Sampling was conducted four times during spring-tide events; samples were analyzed for FIB and turbidity (NTU) four times over a tidal cycle at pre-allocated depths, depending on the water level. Additional water quality parameters measured included temperature, salinity, oxygen, and pH. Loadings were calculated by integrating the stratified FIB concentrations with water column cross-sectional volumes corresponding to each depth. Enterococci and Escherichia coli were stratified both by concentration and loading, although these variables portrayed different patterns over a tidal cycle. Greatest concentrations occurred in surface to mid-strata levels, during flood tides when contaminated water flowed in from the estuary, and during ebb flows when sediments were suspended. Loading was greatest during flood flows and diminished during low tide periods. FIB concentrations within the estuary often were significantly greater than those within the wetland tide channel, supporting previous studies that the wetlands act as a sink for FIB. For public health water quality monitoring, these results indicate that more accurate estimates of FIB concentrations would be obtained by sampling a number of points within a water column rather than relying only on single surface samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetland systems provide a variety of beneficial ecosystem services (Millennium Ecosystem Assessment 2005), a key one being water purification through nutrient reduction by plants, settling and reduction of particulate matter, inactivation of pathogenic organisms through exposure to ultraviolet light, and predation by microorganisms (Mitsch and Gosselink 2008). Many constituent and pathogen indicators have been developed to monitor water quality, chiefly those assessing risk to human health and tracking impairments to beneficial uses of water bodies. Such impairments may lead to economic losses due to beach closures or illnesses. Fecal indicator bacteria (FIB), notably enterococci and Escherichia coli, often are used as a proxy for human pathogen indicators and are the basis for the US EPA’s new recreational water quality criteria (U.S. EPA 2012). In a study of 15 constructed wetlands, Rifai (2006) showed that these systems successfully reduce densities of FIB by 88.3 %, thereby, acting as a sink for these bacteria. Hence, wetlands are widely recognized as providing valuable water cleansing services and have been used to effectively treat municipal wastewaters (Kadlec and Knight 1996; Schueler and Holland 2000).
FIB enter waterways and coastal wetlands through a variety of sources, including point sources (e.g., discharges of wastewater effluents) and nonpoint sources (e.g., surface water runoff and feces from wildlife). Rapid turnover rates, numerous bacteria sources, and complex biological factors contribute to highly variable patterns in the surface concentrations of FIB that are often not discernible with a limited sampling design (Kim et al. 2004). Surface water concentrations of FIB have been shown to be affected by urban runoff (Ki et al. 2007; Surbeck et al. 2008), sediment suspension (Fries et al. 2005; Sanders et al. 2005; Fries et al. 2008; Brown et al. 2013), biological factors (Ricca and Cooney 1998; Alderisio and DeLuca 1999; Sanders et al. 2005), general water quality parameters (e.g., salinity, turbidity, or temperature; Fries et al. 2005), and other hydrodynamic processes (Ki et al. 2007).
FIB concentration patterns are further complicated in some urban estuarine systems which can contain a mixture of contaminated freshwater runoff inputs from developed watersheds mixing with marine water. Coastal salt marshes and embayments have been shown to act as both sinks (Grant et al. 2001; Steets and Holden 2003; Mill et al. 2006) and sources (Grant et al. 2001; Steets and Holden 2003; Jeong et al. 2005) of fecal indicator bacteria depending on tidal flows, freshwater inputs, sediment association and transport, solar radiation, rainfall patterns, and many additional factors (Jeong et al. 2005; Evanson and Ambrose 2006; Dorsey et al. 2013). Excessive contaminated runoff can overwhelm the effectiveness of salt marsh processes to reduce FIB loads thus allowing contamination of adjacent ocean beaches (Jeong et al. 2008; Sanders et al. 2005). Additionally, FIB-laden suspended sediment can be exported from the salt marsh to adjacent ocean beaches during strong ebb tidal flows (Sanders et al. 2005; Dorsey et al. 2010). It is therefore important to understand how FIB densities fluctuate within salt marsh systems whose outflowing water can impact adjacent recreational beaches, especially in urban areas where illness from swimming in contaminated water coupled with beach closures can result in significant economic losses (Given et al. 2006).
While some FIB concentration patterns, such as an increase in FIB during storm and wet-weather events (Fries et al. 2005; Brown et al. 2013), are extensively reviewed in the literature and exhibit analogous trends, other patterns are more complicated and less studied. One such pattern is the fluctuation and stratification of FIB and other water quality parameters in the water column of coastal wetlands. Since the source of estuarine waters varies considerably based on freshwater runoff, tide cycle, and seasonal effects, in addition to biological factors such as predation, stratification may cause water quality conditions to vary considerably within the water column. For example, stratification of FIB is also likely to occur based on differential decay rates from exposure to differing frequencies and penetration of ultraviolet light into the water column. FIB are most often assessed by sampling the top of the water surface using grab samples and then determining their concentrations (most probable number or colony-forming units per 100 mL). Less is known about the mass loading of these bacteria based on an assessment of the entire water column, especially as related to tidal cycling and water column stratification within a wetland system. Such assessments are beneficial, as they provide further insight of bacterial mass in the column strata or overall water column. This information would greatly refine estimates of bacterial loading to and from a wetland with respect to an adjacent water body thus allowing the assessment of potential large-scale impacts and whether or not the wetland acts as a source or sink for FIB.
We assume that FIB would be unevenly distributed throughout the water column in a tidal salt marsh, especially if that system is impacted by contaminated freshwater runoff and that stratification patterns based on FIB concentrations would differ from those based on loading at different tidal heights. To test these hypotheses, we designed a study in an urbanized coastal salt marsh with the following objectives:
-
(1)
Determine potential FIB stratification within the water column throughout tidal cycles in terms of concentration and mass loading,
-
(2)
Identify associations FIB may have with other water quality parameters, and
-
(3)
Determine the implications of stratification with regard to water quality monitoring recommendations.
The general approach for this study was to sample FIB and several water quality parameters at different strata within the water column of the main tidal channel in the Ballona Wetlands Ecological Reserve (BWER or Reserve). Sampling was conducted during spring-tide conditions to measure FIB across the greatest range of water levels. Reference samples of surface water were collected in the adjacent estuary to compare with BWER water.
Methods
Study site
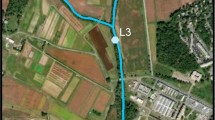
The BWER is one of the last major wetlands in Los Angeles County and is undergoing extensive restoration planning to reduce the degradation from urbanization impacts over the last several centuries (PWA 2006; Johnston et al. 2011, 2012). The BWER and adjacent Ballona Creek estuary (BCE) lie at the bottom of the Ballona Creek watershed that drains 340 km2 and is approximately 80 % urbanized (Bay et al. 1999; Fig. 1). The Ballona Creek watershed feeds directly into the cement-lined Ballona Creek, a highly channelized storm drain system, before flowing into the BCE upstream of the BWER and then entering the Santa Monica Bay (Bay et al. 1999). The channel bottom of Ballona Creek changes from concrete to sediment in the estuarine portion of the creek.
Both the BWER and BCE systems receive runoff contaminated with FIB both during dry weather (Dorsey 2006; Dorsey et al. 2010; Stein and Tiefenthaler 2005) and wet weather (Brown et al. 2013; Stein et al. 2007). Decades of adjacent urbanization and impacts to the BWER have left a small western salt marsh habitat portion of the Reserve and several tidal channels receiving muted tidal flows from the BCE through two self-regulating tide gates (Johnston et al. 2011, 2012; PWA 2006; Fig. 1). During flood tides, the tidal gate system allows a maximum tidal height of 1.1 m. The received BWER estuarine waters are a combination of fresh or brackish water runoff from the creek and tidal oceanic waters from the bay. The muted nature of the wetland allows for a unique set of surveys in a controlled system that is closed during high tides, allowing suspended particles to settle out of the water column.
Field procedures
Cross-sectional survey of the tidal channel
A survey was performed on 8 October 2010 to determine the cross-section elevations of the tidal channel where water samples and measurements were collected (Fig. 2). Using a level transit and a stadia rod, elevation measurements were taken every 50 cm and at every break in side slope at the sampling station. Elevation data were surveyed in the National Geodetic Vertical Datum of 1929 (NGVD29; adjusted 1985). Benchmark leveling (vertical control survey) was conducted using a Trimble GPS, tilting level, a tripod, and a no. 1 SK rod with 0.01-ft graduations. The resulting profile data were used to calculate channel volume and loading measurements at different tidal heights.
Water quality surveys
Sampling was conducted on 17 July and 12 August 2010 and 18 March and 15 April 2011 during spring-tide conditions (Table 1) within the main wetland channel of the BWER (Fig. 1). This site was positioned 50 m from the east tide gate in the deepest portion of the channel. A second reference site was located in the Ballona Creek estuary (BCE) 100 m upstream (east) of the tide gate. Only dry weather conditions were sampled, so measurements were not collected within 72 h of any rain event.
Sampling elevations in the water column at the wetland station were set at 0.05, 0.25, 0.50, and 0.75 m above the channel bottom (Fig. 2). Holes were drilled in a 3-in-diameter polyvinyl chloride (PVC) pipe at the selected elevations, and a half-inch rubber aquarium grade tubing was fed through and glued in place. The free end of the tubing was directed up the PVC pipe and across the channel where the end of each tube was labeled and secured on the bank to a peristaltic water pump sampler (American Sigma 900 Max Portable Sampler).
During each tidal cycle, four discrete samples were collected at approximately flood, slack-water high, ebb, and slack-water low tidal flows. At each sampling time, three replicate water samples were pumped from each elevation within the water column. Prior to sampling, water was allowed to free flow for 5 s to flush the tubes of residual and stagnant water before being directed into three sterile 125-mL polypropylene sampling bottles for each water depth. All depths were sampled within a 5-min time period. Three replicate surface water samples were also taken during each sampling time from both the wetland and the BCE sites. Surface samples were collected using a sterile sampling bottle attached to an extended pole. Immediately following each sampling time, water quality parameters including temperature (°C), salinity (ppt), pH, and dissolved oxygen (mg/L) were taken at each sampling elevation adjacent to the PVC pipe using an YSI 600QS sonde.
Laboratory methods
FIB concentrations (most probable number/100 mL) for E. coli and enterococci were determined using chromagenic substrate tests (APHA et al. 1998; Standard Methods Section 9223 B). IDEXX media Colilert®-18 was used for E. coli and Enterolert® media for enterococci (IDEXX Laboratories, Inc., Westbrook, ME). Tests were performed on samples diluted to 0.1 (10 mL of sample added to 90 mL of dilution water) and quantified using IDEXX Quanti-Tray® 2000 97-well trays. Additionally, one lab blank control to test for the sterility of dilution, water was analyzed for each batch of samples collected at each sampling time. Turbidity (NTU) was determined for each replicate using a HACH 2100N turbidimeter.
Channel volume measurements and mass loading
At the wetland station, water volume as a function of depth was calculated for the cross-sectional flow area. These results were later combined with measured FIB concentrations to determine mass loads at each tide level during each sampling event. The volume calculations involved several steps. The measured coordinates from the cross-sectional survey at the sampling station were combined with the trapezoid rule (Greenbaum and Chartier 2012) of numerical integration to estimate the cross-section’s volume per unit length of channel at several simulated depths. The predicted volumes were then correlated to the depths using regression analysis with a third-order polynomial equation to estimate volumes at any depth tested.
The concentration data collected at the five sampling depths (0.05, 0.25, 0.50, 0.75 m and water surface) were combined with the estimated volumes to determine the overall mass loading of bacteria (as MPN) at each water depth monitored. This step required the calculation of the mass loading contribution from each individual sampling depth by using the corresponding concentration and cross-sectional area of each layer (slice or stratum). The calculated volumes were combined with a numerical algorithm, which computed the weighed contribution from each sampling depth to the overall volume. The algorithm calculated each sampling port’s area of influence by determining the water depth boundaries that were within the volume of water within the strata closest to the port’s depth. As such, the algorithm determined the area (or volume per unit length of channel) of influence of each sampling port for any specific tide depth. The strata were defined based on an a priori null hypothesis that there was no stratification of FIB groups as the water column within the wetlands had never formerly been evaluated for stratification, especially across varying tide heights. The evaluation was conducted to assess, based on an even water column distribution, whether there was stratification, at what levels, and for which FIB groups. Additionally, the strata layers were defined based on area of influence of the sampling ports, which were set at pre-allocated depths.
The procedure above allowed the calculation of the volume per unit channel length of the water depth slices. Each slice volume was then multiplied by its corresponding measured concentration to determine the slice’s mass load contribution. The loadings from the slices were integrated to calculate an overall mass load for each sampling event (Eq. 1).
where M is the mass in MPN, C i is the average concentration in MPN/100 mL at each sampling port, ΔV i is the volume of each slice in cubic meters, and n is the number of slices.
In addition to loading estimates, a weighted average concentration (MPN/100 mL) was calculated to provide an overall estimate of how FIB concentrations changed across the tide cycle during each sampling event. Each estimate was calculated by adding the FIB mass in all layers and dividing the resulting total mass by the corresponding total volume in the water column. This procedure resulted in an average concentration for each sampling event, which was weighed by the FIB concentration and volume in each layer.
Analysis methods
All data were log base 10 transformed prior to statistical analyses to meet normality requirements. One-way ANOVAs were calculated for both concentration and loading data using SYSTAT 13 software, and two-way ANOVAs and regression analyses were calculated using GraphPad Prism. Principal component analysis (PCA) was conducted using continuous input variables, including depth, turbidity, temperature, pH, dissolved oxygen, salinity, E. coli, and enterococci. Significance was based on an alpha value (α) <0.05.
Results
Water quality parameters
Chemical and physical water quality parameters displayed varying degrees of stratification within the water column during some part of a tidal cycle. Salinity displayed the most stratification (Table 2 and Fig. 3), followed by temperature during slack-water high conditions as the surface layer heated (Table 2). Patterns for these two parameters were evident by greater standard errors (SE) and ranges during periods of stratification (Table 2). As water flowed through the gate into the wetlands during flood tides, it generally was well mixed as reflected by narrow ranges and low SE values. By slack-water flood, stratification was evident and carried through the ebb flows. During the ebb flows, stratification began to break down so that by slack-water low, depths were usually less than 0.5 m with no or very little stratification of water quality parameters. Dissolved oxygen displayed little stratification, as indicated by the low ranges and SE values throughout all sampling periods, and did not appear to be associated with a particular stratum or tidal flow. pH exhibited the least variability of all parameters, with a total overall range of 7.86 to 8.70 and no apparent stratification. Turbidity was variable, often lower during the flood and slack-water high periods and highest during ebb flows, indicating suspended sediment within the water column.
FIB stratification and loading patterns in the wetland
Concentrations of FIB within the wetlands often differ up to three orders of magnitude (Table 3) and, based on one-way ANOVAs (Table 4), were significantly stratified within the water column over a tidal cycle 50 % of the time for E. coli and 25 % of the time for enterococci. Higher average concentrations of FIB were found during ebb flows and slack-water low periods relative to other times in the tide cycle (Table 3). This situation was most evident during the July and August sampling events. Greater concentrations of FIB also tended to occur during flood tides when water flowed into the wetlands from the adjacent estuary, best observed during the August and April surveys for both FIB groups. During these conditions, greater concentrations of FIB tended to be observed in the mid- to surface strata. For the other events, lower FIB concentrations often occurred during the slack-water flood conditions in the middle strata when the tide gates were closed but varied based on FIB group and the specific timing.
Similar patterns were evident when looking at the weighted average concentration of FIB over the four tidal periods during the sampling events (Fig. 4). To calculate the average concentration for each tidal period, the FIB concentration for a stratum was multiplied by the corresponding volume of water in that stratum to estimate its FIB mass (MPN). The masses of all strata were added to calculate the total mass in the water column, which was then divided by the volume of the water column to obtain an average concentration in MPN/100 mL for the tidal period. FIB concentrations tended to be elevated during the morning flood tide periods and then diminished over the day from slack-water high through the ebb flows. The only exception to this pattern was during the March survey when enterococci were elevated later in the day during the high tide. FIB spikes during low tide periods (slack-water low) were most pronounced for the July survey, less so for the August and not evident during March and April.
FIB mass loading was significantly stratified within the water column 94 % of the time for both E. coli and enterococci (Table 4). Only one ebb tide period for each FIB group was mixed enough to not exhibit significant stratification. Primary loading tended to occur within the middle strata for both FIB groups, although individual patterns varied throughout each sampling event (Fig. 5). For example, during the July event, loads were greatest for both FIB indicators in the upper strata of water flooding into the wetlands and at the surface during slack-water low. Alternatively, during the March and April surveys, FIB loads tended to diminish during the low ebb flows for both FIB groups. The greatest loadings occurred during periods of flood to slack-water high; therefore, greater loading was associated with deeper, cooler, more saline incoming water. Loading of both FIB groups diminished during low tides when we measured warmer, less saline, and more turbid water.
Differences in loading between the flood and ebb flows were determined to assess if FIB mass diminished over the slack-water high period due to wetland processes. Loads compared between flood and ebb sampling alone could not be used because of the changes in channel volumes. Therefore, for each sampling event, an adjusted mean ebb load was calculated using the ebb flow load and channel volume from the previous flood flow. This calculation provided an estimate of what the load may have been if the ebb flow had been sampled at the same tidal height as during the flood flow. Finally, flood and adjusted ebb flows were compared by calculating the percent difference (Fig. 6). Both FIB groups resulted in an overall reduction from flood to ebb tidal conditions with the exception of the March sampling event where loading increased.
A comparison of mean FIB loads (MPN/m3) between the flood and ebb tidal heights at the time of sampling and the adjusted mean load at the same height during the ebb flow (denoted by the red arrows). Mean loads for flood and ebb flows were determined by averaging loads among the strata. Each adjusted ebb load was calculated proportionally by using the ebb flow load and volume of water in the tide channel during the earlier flood flow to estimate loads at the same tidal heights (red dashed line) as the tide fell
Multivariate patterns
The two-factor principal component analysis (PCA) indicated that turbidity and dissolved oxygen were the dominant components driving factor 1 (39.5 %), while water depth and temperature were the dominant components of factor 2 (19.3 %) (Fig. 7). Turbidity and DO had a strong relationship to each other, and both were inversely related to salinity. This relationship reflected flood tide flows where water was more saline and of low turbidity in contrast to ebb flows where water departing the wetlands was more turbid with suspended sediments and slightly less saline. Greater concentrations of dissolved oxygen during the afternoon ebb flows probably reflected the results of photosynthetic activity occurring throughout the day.
Loading of the FIB groups exhibited different patterns according to the multivariate results (Fig. 7). E. coli were loosely associated with depth and factor 2, indicative of the stratification patterns seen in the significance assessments, while enterococci were more associated with salinity and negatively with temperature and pH. The complicated nature of the variability within the stratification data does not lend itself to a clear definition of the factors. Elevated FIB loading was associated with both flood flows characterized by cooler temperatures and more saline water, while warmer, fresher water having increased turbidity was associated with some low tide spikes in FIB loading.
FIB trends—estuary vs. wetland surface data
Concentrations of FIB between the estuary and the wetland were compared using surface measurements since stratified sampling was not done in the estuary. Elevated concentrations of FIB previously have been correlated with increased turbidity during strong ebb tide flows in the wetlands (Dorsey et al. 2010) and a nearby lagoon (Dorsey et al. 2013), so these parameters were regressed here to see if a similar situation existed within the estuary and in the wetlands (Fig. 8).
Surface concentrations in the estuary often differed up to four orders of magnitude for both E. coli and enterococci over a tidal cycle (Fig. 8, Table 3). In contrast, wetland FIB concentrations generally displayed less variability during tidal cycles, differing by up to three orders of magnitude. Turbidity and both FIB groups displayed positive significant regressions only during the July sampling event and only in the estuary (Fig. 8). Mean E. coli concentrations were greater in the estuary than at the wetland site, based on two-way ANOVAs (Table 5), and significantly differed throughout the tidal period during all sampling events. In contrast, enterococci concentrations tended to be similar between the estuary and the wetland sites with the exception of the April survey where mean concentrations in the estuary exceeded those at the wetland site. Like E. coli, mean concentrations of enterococci differed significantly throughout the tidal periods for all surveys (Table 5).
Discussion
Stratification
The unique muted nature of the tide gate closure pattern allowed for an evaluation of the hydrologic processes and their relationship to the fecal indicator bacteria as a proxy for bacteria and other pathogens. Results of this study and previous work (Dorsey et al. 2010) indicated that the following events typically occur over a tidal period. The most mixing occurs during flood flows as water enters the wetlands from the estuary through the tidal gate. When the tidal level reaches a maximum of 1.1 m water depth, the gate closes, thus entering a period of high water slack. During this period, any suspended sediment settles, wetland processes reduce levels of FIB, and the water column becomes stratified. When the tide turns to ebb flow, the gate opens, and the water column remixes as water flows from the wetland channels back into the estuary. During the ebb flows, turbidity spikes and associated FIB concentrations can occur.
Prior studies have emphasized variations in fecal indicator bacteria concentrations due to tidal conditions, but this is the first study to the authors’ knowledge that evaluated both stratification within the water column and FIB loading within the system. While complex patterns of FIB began to emerge throughout the evaluation across all strata and sampling events, overarching results suggested that both FIB groups were found to be significantly stratified at least part of the time within the water column of the BWER. This pattern held true for both concentration and loading data, albeit in varying degrees; during periods of mixing and ebb flows, this stratification was reduced, especially in terms of concentration data. For loading, the volume of water seemed to be the most important factor, thereby having significant stratification in almost every tidal condition. Other bodies of water probably experience similar degrees of FIB stratification in the absence of mixing. For example, studies in a Lake Geneva bay demonstrated that FIB concentrations during stratification were greater in water depths to 25 m relative to surface concentrations (Poté et al. 2009). During the stratified condition, FIB associated with a wastewater treatment plant effluent were trapped beneath the thermocline while surface FIB presumably were degraded by ultraviolet light and microorganism predation. However, during an extensive study of FIB tidal dynamics in two marinas in Newport Bay, California, Jeong et al. (2005) found that FIB concentrations were essentially similar between samples collected at the surface and at 1 m depth, indicating good mixing. It is possible that tidal currents in the marinas resulted in surface mixing, unlike the condition measured in the BWER where water is very still during slack-water flood periods, and the water column becomes stratified. Similarly, in a study by Lewis et al. (2013), northern California estuaries that were closed to the ocean by sandbars tended to have FIB concentration gradients associated both with depth and distance from the estuary mouth.
Often the loading and concentration patterns tracked with each other and with increased turbidity. Specific examples were seen during spikes in low tides (e.g., July) that tracked with turbidity. Overall, these results support the findings of previous studies in the BWER (Dorsey et al. 2010), nearby Del Rey Lagoon (Dorsey et al. 2013) and the Talbert Marsh salt marsh to the south in Huntington Beach (Grant et al. 2001; Boehm et al. 2002; Jeong et al. 2008) with regard to the complex nature of FIB, while adding new components of stratification assessments and understanding loading patterns within a wetland water column.
Although both FIB groups exhibited stratification, often the results were less prominent for the enterococci group than the E. coli, and occasionally different patterns emerged (e.g., concentrations of enterococci were less stratified during the July survey). Often, the concentrations for enterococci were quite small (i.e., ≤10 MPN/100 mL), leading to less stratification overall due to a smaller range and less variation. The variability between the two FIB groups may be explained by differing rates of deactivation in the water column over time, i.e., different rates of survival and/or resistance to ultraviolet light radiation. Conflicting results have been reported on the deactivation rate of enterococci versus E. coli, with some studies finding significantly faster deactivation of enterococci (e.g., Sinton et al. 2002; Noble et al. 2004) while E. coli had a faster rate in others (e.g., Bordalo et al. 2002). These studies suggest that deactivation may be system-specific and based on varying environmental conditions. In the case of the BWER, it may be that the variation in deactivation rates caused E. coli to become more stratified in the water column, especially given that previous studies suggest depth and attenuation to be factors in deactivation (Maïga et al. 2009). Since higher overall FIB concentrations were found closer to the surface of the water column, it is possible that enterococci were deactivated more quickly, causing less appearance of stratification for that FIB group within the water column.
Wetlands as a FIB source or sink?
Ballona Creek, an urban runoff-dominated channel, is a significant source of FIB to the estuary and hence, the wetlands (Dorsey and Lindaman 2004; Stein and Tiefenthaler 2005; Dorsey 2006; Tiefenthaler et al. 2009; Brown et al. 2013). The wetlands generally act as a sink for FIB, since concentrations of these bacteria diminished during high tide periods in the wetlands; estimated loads during ebb flows were very small compared to the flood tides. Overall concentrations in the estuary were typically greater than those in the wetlands, sometimes by several orders of magnitude, suggesting that even the spikes in FIB concentrations ebbing from the wetlands were diluting the estuary to the extent that a small volume of outflowing wetland water could dilute the much larger, contaminated volume of the Ballona Creek estuary. This consequence shows a significant ecosystem service, water purification, for the BWER, even in its degraded state.
Many wetland functions have been well documented at reducing concentrations of FIB in the water column, including ultraviolet light radiation (Ki et al. 2007; Mill et al. 2006), uptake by biological organisms (Surbeck et al. 2010), tidal dilution (Mill et al. 2006), and nutrient reductions (Surbeck et al. 2010). This study supports those conclusions and those of Dorsey et al. (2010) at the BWER, reaffirming a similar conclusion that the wetlands are acting as a sink for FIB. Loading comparisons of the flood and ebb tides using the adjusted mean ebb loading suggested that the wetlands were functioning overall as a sink. While the FIB spikes do occur with the ebb flow suspended sediments, as seen more significantly in the March survey, these are not contributing significantly to the loading of the estuary. Many of these FIB reduction functions are likely occurring within the tidal channels and most likely in adjacent acres of tidally inundated marsh plain. Similar processes probably are occurring in the adjacent estuary, to some extent.
Multivariate analyses
Multivariate analyses can often play key roles in differentiating broader patterns in biological data, which is especially important in assessing complicated and highly variable patterns, such as the relationship between FIB and various chemical and physical water quality parameters. For this study, the two factors represented nearly 60 % of the variation among the chemical–physical–biological parameters measured. In the case of the FIB groups, while some of the water quality characteristics were associated, neither FIB group truly associated with any individual parameter. This result was surprising, especially for turbidity, where elevated levels from suspended sediments have been previously correlated with elevated FIB concentrations (e.g., Mill et al. 2006; Dorsey et al. 2010; Dorsey et al. 2013). Many of the physical factors did not seem to individually play a significant role in the stratification of the water column; however, overarching patterns such as turbidity and dissolved oxygen both conversely related to salinity did occur. Also surprisingly, the fresher water inputs from Ballona Creek were not always correlated with higher densities of FIB groups, although there were individual instances of that occurring, specifically associated with freshwater lenses within the upper strata during slack-water high periods. The association of E. coli with depth was indicative of the patterns of stratification seen in the ANOVA results. The more complex nature of the enterococci patterned with multiple factors such as a converse relationship to temperature and loosely conversely related to turbidity and oxygen may mean that individual water quality parameters alone were not enough to determine causes of FIB loading for enterococci.
Additional factors not measured in this study, such as light attenuation or exposure time to ultraviolet radiation, may have contributed more significantly to the FIB patterns, as demonstrated by Noble et al. (2004). Previous studies suggest that temperature is a proxy for solar radiation (e.g., Mill et al. 2006), and while loose correlations with temperature existed within our data, no clear pattern was evident. Because patterns differed for each of the two FIB groups, these biological constituents are even more highly variable than previously thought. Additional research on biological factors such as predation by bacteriophages or other organisms may provide supplemental insight into these complex relationships. As previously discussed, differing deactivation rates may also have played a significant role in the differences in FIB group patterns.
Sampling protocol implications
Surface water concentrations are the basis of water quality standards internationally. Acknowledging the significant differences in FIB found throughout the water column during times of less mixing has implications for the assessments of water quality and compliance monitoring. The results suggest that timing of water quality sampling, especially in tidal waters, may be even more important than previously assumed. Integrating vertical sampling over the entire water column at one or several points would allow for a higher confidence in the precision of FIB estimates at the site, as surface concentration data alone may not be an accurate reflection of FIB density within the water column. Achieving more precise measurements of FIB concentrations, especially in recreational waters, will better serve the public since elevated FIB levels can be accompanied by pathogens (Wu et al. 2011; Dorsey et al. 2013) although viral pathogens tend to correlate poorly with these bacterial indicators (Jiang et al. 2001; Griffin et al. 2003).
In a similar manner, the FIB loading data offered a more thorough understanding of the tidal dynamics of the system. This is best illustrated by the concentration data showing a high MPN/100 mL spike at low tide, which would infer a spike to the estuary but in fact represents significantly less loading to the system given the smaller volumes of water in the channel at low tide.
Future work should be conducted to analyze stratification and loading in the adjacent estuary to have a similar data set for comparison and to determine the percent contribution from the wetlands. These additional analyses would more completely simulate a full-tidal estuarine system and potential contamination of adjacent recreational ocean beaches.
References
Alderisio, K. A., & DeLuca, N. (1999). Seasonal enumeration of fecal coliform bacteria from the feces of ring-billed gulls (larus delawarensis) and Canada geese (branta canadensis). Applied and Environmental Microbiology, 65, 5628–5630.
American Public Health Association, American Water Works Association, and Water Environment Federation. (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington: American Public Health Association.
Bay, S., Jones, B.H., and Schiff, K. (1999). Study of the impact of stormwater discharge on the beneficial uses of Santa Monica Bay. Los Angeles, CA: Prepared for the Los Angeles County Department of Public Works.
Boehm, A. B., Grant, S. B., Kim, J. H., Mowbray, S. L., McGee, C. D., Clark, C. D., Foley, D. M., & Wellman, D. E. (2002). Decadal and shorter period variability of surf zone water quality at Huntington beach, California. Environmental Science and Technology, 36, 3885–3892.
Bordalo, A. A., Onrassami, R., & Dechsakulwatana, C. (2002). Survival of faecal indicator bacteria in tropical estuarine waters (Bangpakong river, Thailand). Journal of Applied Microbiology, 93(5), 864–871.
Brown, J. S., Stein, E. D., Ackerman, D., Dorsey, J. H., Lyon, J., & Carter, P. M. (2013). Metals and bacteria partitioning to various size particles in Ballona creek storm water runoff. Environmental Toxicology and Chemistry, 32(2), 320–328.
Dorsey, J.H., and Lindaman, E. (2004). Water quality. In D. McNeill & J. Landry (Eds.), Ballona Creek trail and bikeway: Environmental monitoring and assessment (pp. 62–81). Los Angeles: Baldwin Hills Conservancy. Report submitted to the California State Legislature.
Dorsey, J. H., Carter, P. M., Bergquist, S., & Sagarin, R. (2010). Reduction of fecal indicator bacteria (FIB) in the Ballona Wetlands saltwater marsh (Los Angeles County, California, USA) with implications for restoration actions. Water Research, 44, 4630–4642.
Dorsey, J. H. (2006). Densities of fecal indicator bacteria in tidal waters of the Ballona Wetlands, Los Angeles, County, California. Bulletin of the Southern California Academy of Sciences, 105(2), 59–75.
Dorsey, J. H., Carmona-Galindo, V. D., Leary, C., Huh, J., & Valdez, J. (2013). An assessment of fecal indicator and other bacteria from an urbanized coastal lagoon in the city of Los Angeles, California, USA. Environmental Monitoring and Assessment, 185(3), 2647–2669.
Evanson, M., & Ambrose, R. (2006). Sources and growth dynamics of fecal indicator bacteria in a coastal wetland system and potential impacts to adjacent waters. Water Research, 40, 475–486.
Fries, J. S., Characklis, G. W., & Noble, R. T. (2005). Attachment of fecal indicator bacteria to particles in the Neuse River Estuary, N.C. Journal of Environmental Engineering, 132, 1338–1345.
Fries, J.S., Characklis, G.W., and Noble, R.T. (2008). Sediment-water exchange of Vibrio sp. and fecal indicator bacteria: implications for persistence and transport in the Neuse River Estuary, North Carolina, USA. Water Research, 941-950.
Given, S., Pendleton, L. H., & Boehm, A. B. (2006). Regional public health cost estimates of contaminated coastal waters: a case study of gastroenteritis at Southern California Beaches. Environmental Science & Technology, 40, 4851–4858.
Grant, S. B., Sanders, B. F., Boehm, A. B., Redman, J. A., Kim, J. H., Morse, R. D., Chu, A. K., Gouldin, M., McGee, C. D., Gardiner, N. A., Jones, B. H., Svejkovsky, J., Leipzig, G. V., & Brown, A. (2001). Generation of enterococci bacteria in a coastal saltwater marsh and its impact on surf zone water quality. Environmental Science and Technology, 35(12), 2407–2416.
Greenbaum, A. and Chartier, T.P. (2012). Numerical methods: design, analysis, and computer implementation of algorithms. Princeton University Press, 1st edition.
Griffin, D. W., Donaldson, K. A., Paul, J. H., & Rose, J. B. (2003). Pathogenic human viruses in coastal waters. Clinical Microbiology Reviews, 16(1), 129–143.
Jeong, Y., Grant, S. B., Ritter, S., Pednekar, A., Candelaria, L., & Winant, C. (2005). Identifying pollutant sources in tidally mixed systems: case study of fecal indicator bacteria from marinas in Newport Bay, Southern California. Environmental Science and Technology, 39(23), 9083–9093.
Jeong, Y., Sanders, B. F., McLaughlin, K., & Grant, S. B. (2008). Treatment of dry weather urban runoff in tidal saltwater marshes: a longitudinal study of the Talbert Marsh in southern California. Environmental Science and Technology, 42(10), 3609–3614.
Jiang, S., Noble, R., & Chu, W. (2001). Human adenoviruses and coliphages in urban runoff-impacted coastal waters of southern California. Applied and Environmental Microbiology, 67(1), 179–184.
Johnston, K.K., Del Giudice-Tuttle, E., Medel, I.D., Bergquist, S., Cooper, D.S., Dorsey, J., and Anderson, S. (2011). The Ballona Wetlands ecological reserve baseline assessment program: year one report. Santa Monica Bay Restoration Commission. Prepared for the California State Coastal Conservancy, Los Angeles, California.
Johnston, K.K., Del Giudice-Tuttle, E., Medel, I.D., Piechowski, C.J., Cooper, D.S., Dorsey, J., and Anderson, S. (2012). The Ballona Wetlands ecological reserve baseline assessment program: second year report. Santa Monica Bay Restoration Commission. Prepared for the California State Coastal Conservancy, Los Angeles, California.
Kadlec, R. H., & Knight, R. L. (1996). Treatment wetlands, 1996. Boca Raton, FL: Lewis Publ.
Ki, S. J., Ensari, S., & Kim, J. H. (2007). Solar and tidal modulations of fecal indicator bacteria in coastal waters at Huntington Beach, California. Environmental Management, 39, 867–875.
Kim, J. H., Grant, S. B., McGee, C. D., Sanders, B. F., & Largier, J. L. (2004). Locating sources of surf zone pollution: a mass budget analysis of fecal indicator bacteria at Huntington Beach, California. Environmental Science and Technology, 38(9), 2626–2636.
Lewis, D. J., Atwill, E. R., Pereira, M. G. C., & Bond, R. (2013). Spatial and temporal dynamics of fecal coliform and Escherichia coli associated with suspended solids and water within five northern California estuaries. Journal of Environmental Quality, 42, 229–238.
Maïga, Y., Wethe, J., Denyigba, K., & Ouattara, A. S. (2009). The impact of pond depth and environmental conditions on sunlight inactivation of Escherichia coli and enterococci in wastewater in a warm climate. Canadian Journal of Microbiology, 55(12), 1364–1374.
Mill, A., Schlacher, T., & Katouli, M. (2006). Tidal and longitudinal variation of faecal indicator bacteria in an estuarine creek in south-east Queensland, Australia. Marina Pollution Bulletin, 52, 881–891.
Millennium Ecosystem Assessment. (2005). Ecosystems and human well-being: synthesis. Washington, DC: Island Press.
Mitsch, W. J., & Gosselink, J. G. (2008). Wetlands. New York, New York, USA: Van Nostrand Reinhold.
Noble, R. T., Lee, I. M., & Schiff, K. C. (2004). Inactivation of indicator micro-organisms from various sources of faecal contamination in seawater and freshwater. Journal of Applied Microbiology, 96, 464–472.
Poté, J., Goldscheider, N., Haller, L., Zopfi, J., Khajehnouri, F., & Wildi, W. (2009). Origin and spatial-temporal distribution of faecal bacteria in a bay of Lake Geneva, Switzerland. Environmental Monitoring and Assessment, 154(1–4), 337–348.
(PWA) Phillip Williams and Associates, Ltd. (2006) Ballona Wetlands existing conditions report. Report submitted to California Coastal Conservancy. 184 p.
Ricca, D. M., & Cooney, J. J. (1998). Coliphages and indicator bacteria in birds around Boston Harbor. Journal of Industrial Microbiology and Biotechnology, 21, 28–30.
Rifai, H. (2006) Study on the effectiveness of BMPs to control bacteria loads. Final Quarterly Report No. 2. Prepared for Texas Commission on Environmental Quality, P.O. Box 13087, MC-150, Austin, Texas 78711–3087.
Sanders, B. F., Arega, F., & Sutula, M. (2005). Modeling the dry-weather tidal cycling of fecal indicator bacteria in surface waters of an intertidal wetland. Water Research, 39, 3394–3408.
Schueler, T.R., and Holland, H.K. (2000). The practice of watershed protection: techniques for protecting our nation’s streams, lakes, rivers and estuaries. Center for Watershed Protection, 8391 Main St., Ellicott City, MD.
Sinton, L. W., Hall, C. H., Lynch, P. A., & Davies-Colley, R. J. (2002). Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Applied and Environmental Microbiology, 68(3), 1122–1131.
Stein, E. D., & Tiefenthaler, L. L. (2005). Dry-weather metals and bacteria loading in an arid, urban watershed: Ballona Creek, California. Earth and Environmental Science, 164, 367–382.
Stein, E.D., Tiefenthaler, L.L., and Schiff, K.C. (2007) Sources, patterns, and mechanisms of storm water pollutant loading from watersheds and land uses of the greater Los Angeles area, California, USA. Southern California Coastal Water Research Project. Technical Report 510. 103 pp.
Steets, B. M., & Holden, P. A. (2003). A mechanistic model of runoff associated fecal coliform fate and transport through a coastal lagoon. Water Research, 37(3), 589–608.
Surbeck, C. Q., Jiang, S. C., & Grant, S. B. (2010). Ecological control of fecal indicator bacteria in an urban stream. Environmental Science and Technology, 44(2), 631–637.
Surbeck, C.Q., Grant, S.B., Ahn, J.H., Jiang, S., Soeller, S.A., Holden, P.A., Van Der Verfhorst, L.C., & Brooks, D.J. (2008). Urban runoff impact study phase III: land-use and fecal indicator bacteria generation. Final Project Report, National Water Research Institute, Fountain Valley, CA. 139 p.
Tiefenthaler, L. L., Stein, E. D., & Lyon, G. S. (2009). Fecal indicator bacteria (FIB) levels during dry weather from Southern California reference streams. Environmental Monitoring and Assessment, 155, 477–492.
U.S. Environmental Protection Agency (2012). Recreational water quality criteria. U.S. EPA, Office of Water 820-F-12-058. 63 p.
Wu, J., Long, S. C., Das, D., & Dorner, S. M. (2011). Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. Journal of Water and Health, 9(2), 265–278.
Acknowledgments
The authors would like to acknowledge the California State Coastal Conservancy, the Center for Santa Monica Bay Studies, and The Bay Foundation for funding and support and the Department of Civil Engineering and Environmental Science at Loyola Marymount University for laboratory resources. Thanks also to Ivan Medel, Elena Del Giudice-Tuttle, Charles Piechowski, and several student interns for helping to coordinate and implement the field and laboratory sampling and analyses. We could not have completed this project without you. We also thank Ken Schiff (Southern California Coastal Water Research Project) who provided valuable review comments on the initial manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnston, K.K., Dorsey, J.H. & Saez, J.A. Stratification and loading of fecal indicator bacteria (FIB) in a tidally muted urban salt marsh. Environ Monit Assess 187, 58 (2015). https://doi.org/10.1007/s10661-015-4314-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4314-z