Abstract
The aim of this study was to characterize the sediment samples from vicinity of a landfill in Qayen City, Iran. The samples were obtained from four different sampling stations. Sequential extraction was performed via a four-step procedure defined to evaluate the distribution of the element fraction in various samples. In the stations 3 and 4, Cd was found in large quantities during the first extraction F1, accounting for 40.4 and 38.7 %, respectively. Pb was primarily presented in F2 of station 1 (approximately 44.80 %), station 2 (approximately 41.8 %), and station 4 (approximately 37.7 %). Moreover, principal component analysis showed that heavy metal fraction in the sediment samples can be explained by two principal components (PCs). PC1 represented Cd, Cr, Ni, and Zn, while PC2 represented Pb and Cu. Pearson correlation coefficient indicated significant correlations in Cu-Pb, Zn-Cu, and Cr-Zn pairings. The present study concluded that the spatial distributions of sediment heavy metals were influenced by MSW landfill.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Generally, the disposal of municipal solid waste is a major concern throughout the world. However, many landfills are not properly managed and pose a serious threat to the environment. Landfill leachate leads to contamination of water, soil, and sediment (Kjeldsen 1993; Sayadi and Sayyed 2011). Although organic compounds are the principal contaminants in leachate-contaminated environments, additionally, inorganic compounds including metals (such as Cd, Ni, Pb, Cu, Zn, and Cr) also commonly leach from the landfills (Christensen et al. 2001). Heavy metal pollution as a serious and widespread environmental problem is due to the toxic, persistent, and nonbiodegradable and bioaccumulation properties of these contaminants (Yuan et al. 2004). A study conducted in soil at eastern part of Iran (Birjand City) showed the highest concentration of Pb (166.64 mg/kg), Zn (346.50 mg/kg), and Cd (8.57 mg/kg) which are greater than the threshold values, indicating that the environment is contaminated (Sayadi and Rezaei 2014). The surface sediments have become a feeding source for biological life, a transporting agent for pollutants, and an ultimate sink for organic and inorganic settling matters (Algan et al. 2009). The reliable concentration determination of various trace metal chemical forms in sediments is one of the most challenging problems faced by environmentalists. Speciation and determination studies of trace metals have been widely carried out in sediments (Mendil et al. 2010; Oyeyiola et al. 2011; Sayadi et al. 2008, 2010). Various studies have reported the enrichment of heavy metals in the streams sediments influenced by a sanitary landfill (Mantei and Coonrod 1989; Olivares-Rieumont et al. 2007). Similarly, heavy metal quantities have been reported in the sediments of rivers affected by leachate landfill (Mantei and Sappington 1994).
Modified Community Bureau of Reference (BCR) sequential extraction procedure provides useful information for migration assessment in the landfill since the amounts of metals mobilizable under different changes in environmental conditions can be determined. This should be effectively considered if the contaminated sediment is to be dredged (Velimirović et al. 2010). The BCR method is faster, simpler, and validated for routine laboratory unlike the Tessier scheme. Consequently, the three-step or modified four-step BCR procedure is a good approach to partition and predict the mobilization and dynamic processes of metals in sediments affected by contamination. A partitioning study showed that more easily mobilized forms of toxic metals were dominant (Kazi et al. 2005). The study in the vicinity of an old unmonitored municipal landfill in Prague, Czech Republic, showed the environment is polluted by toxic metals and spontaneous precipitation of metal-bearing calcite exhibiting significant concentrations of Fe, Mn, Mg, Sr, Ba, Pb, Zn, and Ni and binding to Fe(III) oxyhydroxides like Pb, Zn, Cu, and Ni and also preferential bonding to sediment organic matter like Cu (Ettler et al. 2005). Fractions and bioavailability of sediment heavy metals have rarely been investigated in the areas with rapid landfill intensification in Iran. In addition, few studies have discussed the relationships between river sediment heavy metal bioavailability and landfill. Therefore, the objectives of this research were to (I) analyze fraction concentrations of heavy metals, (II) investigate the relationships between different fractions of sediment heavy metals, and (III) explore the controlling factor of sediment heavy metals within the study area.
Materials and methods
Sediment samples were collected from four different sites: S1 (before the landfill area), S2 (surrounding the landfill area), S3 (after the landfill area), and S4 (those surrounding the pound were formed by the rivers coming from the landfill area) (Fig. 1). The sediments were air-dried in clean air, grounded using clean mortar and pestle, sieved through less than 63-μm mesh for heavy metal determinations, homogenized, and later stored in previously acid-soaked sample bottles. Analysis of the heavy metal concentration to less than 63-μm sediment fraction is recommended as these particles contain significant organic matter and metals in sediments (Soares and Boaventura 1999; Olivares-Rieumont et al. 2005) and sieving does not affect the metal concentration (Soares and Boaventura 1999).
Sequential extraction test and determination of heavy metals
The total concentrations of metals, viz. Cr, Ni, Pb, Cu, Zn, and Cd in the river sediment samples, were determined by the modified sequential extraction described by QUWV AUVIL. Sequential extraction was performed by four-step procedure defined to investigate the distribution of the element fraction in various sediment samples. During the extraction, metals were classified into acid-soluble/exchangeable fraction (F1), reducible Fe-Mn oxides and hydroxide fraction (F2), oxidizable organic matter bound fraction (F3), and residual fraction (F4), respectively. The detailed procedure is described as follows (Purushothaman and Chakrapani 2007; Yao 2008; Qiao et al. 2013):
-
F1 (acid-soluble/exchangeable fraction)
Sediment sample (1 g) was introduced in 20 ml of 11 M HOAc and shaken for 16 h at room temperature. The solution and solid phases were separated by centrifugation at 6000 rpm for 20 min. Subsequently, the suspension was filtered through a 0.45-μm membrane filter and the solid residues were preserved for the subsequent extractions.
-
F2 (reducible Fe-Mn oxides and hydroxide fraction)
The residue from F1 was leached with 20 ml of 0.1 M NH2OH·HCl (adjusted to pH 2 with nitric acid) for 16 h. The extraction procedure was similar to that of F1.
-
F3 (oxidizable organic matter bound fraction)
The residue from F2 was mixed with 5 ml of hydrogen peroxide (30 %) and digested at room temperature for 1 h with occasional shaking. A second 5-ml aliquot of hydrogen peroxide was introduced into and digested at 85 °C (water bath) for 1 h. The contents were evaporated to a small volume (1–2 ml). Twenty-five milliliters of ammonium acetate (0.1 ml/l, adjusted to pH 2 with nitric acid) was added to the cool and moist residue. The sample was then shaken and centrifuged, and the extract was separated as described in F1.
-
F4 (residual fraction)
The residue from F3 was digested with 5 ml HNO3 in acid. The contents were heated on a hot plate and evaporated to almost dryness. After cooling, the residues were dissolved in 5 % (v/v) HNO3. The resultant solutions were subsequently used to determine the heavy metals. The concentration of Cr, Ni, Pb, Cu, Zn and Cd in different fractions and the resultant solutions obtained in the different fractions were determined using a Contra AA 700 flame atomic adsorption spectrophotometer with detection limits (mg/l) for Cr (1.7), Ni (2), Pb (3.5), Cu (1), Zn (0.3) and Cd (0.5). Tests on each sample were conducted in triplicate, and average values of results were reported.
Results
Physicochemical properties of sediment
Table 1 presents the properties of the sediment samples, indicating relatively high values with pH ranging from 6.94 to 8.09. The organic carbon concentrations ranged from 9.2 to 16.1, 2.13 to 3.84 % for total N, 1.60 to 3.41 % for total P, and 0.64 to 1.92 % for total K, respectively.
Total concentration of heavy metals in sediment
Heavy metal concentrations of sediment are presented in Table 2. The Cd content varied from 0.97 to 10.97 mg/kg with an average of 6.629 mg/kg in the sediment samples. As shown in Table 2, the Cd content is higher than the world average sediment (0.3 mg/kg), so it can be suggested that the Cd concentrations are releasing from anthropogenic activities. Similar high level of Cd was reported in the Izmit Bay, Turkey (Pekey 2006). The Ni content of the samples varied from 16.64 to 63.54 mg/kg with an average of 48.29 mg/kg in the sediment samples. The related Ni concentration was found in San Francisco Bay (van Geen and Luoma 1993), while the reported Ni concentration in the Tilehbon River sediments was 108.5 mg/kg (Goorzadi et al. 2009). The Ni content is lower than the world average sediment (68 mg/kg). The Pb content of the samples varied from 39.45 to 76.44 mg/kg with an average of 53.39 mg/kg in the sediment samples. The comparable Pb concentration was indicated in Yamuna River in India and Shantou in China (Jain 2004; Qiao et al. 2013). The Pb content is much higher than the world average sediment (20 mg/kg) which suggests that the samples are highly affected by anthropogenic activities. Cu content varied from 20.77 to 71.75 mg/kg with an average of 44.69 mg/kg in sediment samples. Nevertheless, high Cu content level (60.6–139 mg/kg) was found in Izmit Bay, Turkey (Pekey 2006). S4 and S2 presented higher Cu concentration than the world average sediment (45 mg/kg). Zn content varied from 50.44 to 159.93 mg/kg with an average of 109.88 mg/kg in sediment samples. As shown in Table 2, the identical Zn concentration was found in Shantou River, China (Qiao et al. 2013). Zn content in S2, S3, and S4 is higher than the world average sediment (95 mg/kg). Cr content varied from 62.99 to 166.55 mg/kg with an average of 123.85 mg/kg in the sediment samples. The similar Cr concentration was found in the Tilehbon River (152.5 mg/kg) (Goorzadi et al. 2009). The Cr content in S2, S3, and S4 was higher than the world average sediment (90 mg/kg).
Discussion
Total Cd, Ni, Pb, Cu, Zn, and Cr contents as well as the control standards for pollutions in sediment rivers are listed in Table 2. Generally, the sediment samples had higher concentrations of Cd, Pb, Cu, Zn, and Cr, but relatively low concentration of Ni at the different stations. A comparison of sediment metal concentrations with permissible values indicated that the concentrations of Cd and Pb in all sampling stations and Cu, Zn, and Cr in all sites except S1 exceeded the permissible values (Turkian and Wedepohl 1961). Even it was interesting to note that with a few exceptions, the concentrations of heavy metals in the study area were always lower than the Canada standards (Fuentes et al. 2004).
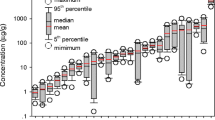
BCR procedure could be useful as a potential method to determine if the heavy metals can be removed by remediation techniques or predict removal efficiency (Mulligan et al. 2001). Cd, Ni, Pb, Cu, Zn, and Cr fractions were determined by the BCR procedure in the sediment and presented as the percentage of total concentrations in sediment, as shown in Fig. 2. The results of each heavy metal fraction in the sediment samples are listed in Table 3.
Cd distribution in various fractions showed different patterns for each sediment sample. Particularly, in the samples S3 and S4, Cd was found in large quantities during the first extraction F1, accounting for 40.4 and 38.7 %, respectively. These results are consistent with the findings of Dongting Lake, central China (Yao 2008), and Kongsfjorden, Svalbard (Grotti et al. 2013). However, Cd was hardly detected in the reducible fraction, inferring that cadmium bound to Fe and Mn oxides in S3 and S4 samples was low. Over 30 % of the total Cd was distributed in the fraction F2 and F3 in the sediment samples S3 and S4, showing high bioavailability in an environment when sediment conditions changed. Similar studies assessing the metal pollution in the sediments of Yamuna River (India), highly polluted by contaminants contained in domestic and industrial effluents, showed that Cd which had a high percentage in the exchangeable fraction presented an optimal risk to the environment (Jain 2004).
In the samples S2 and S4, Cu was found in large quantities during the second extraction (F2), accounting for 41.8 and 37.4 %, respectively. These results are consistent with the findings of Alvarez et al. (2002) and Huang et al. (2013). However, in S3, F3 was predominant, with Cu as high as 38.8 %. Cu in the natural soils is primarily associated with the residual fraction and secondarily with the organic/sulfide fraction (Wong et al. 2002).
Ni was found in large quantities during the first extraction (F1) of acid-soluble/exchangeable fraction. Quantities varied from 15.9 % in S1 to 28.1 % in S4. Ni was widely distributed in the four fractions. The sum of the first two fractions ranged from 34.4 % in sediment S1 to 51.1 % in sediment S4. However, in the last two samples, Ni was mainly associated to the oxidizable fraction (48.9 and 65.6 % extraction, respectively). This high mobility of Ni was also suggested by Fuentes et al. (2004) and Liu and Sun (2013) in the speciation of heavy metals in different sediment samples.
A high proportion of metal Zn was extracted from fractions F1 (21.12 % in S3 to 35.83 % in S2), F2 (6.45 % in S3 to 38.33 % in S4), and F3 (18.74 % in S2 to 38.5 % in S3), respectively. Thus, Zn appeared to have high bioavailability and ecotoxicity potential. The present result reflects the findings of Alvarez et al. (2002). The highest value for Zn was observed in the oxidizable fraction in the sediment samples of S4. Moreover, the chemical speciation data showed that Zn in an unstable form bound to carbonates and Fe/Mn oxides. Therefore, it can be safely concluded that Zn exchanged easily.
Surprisingly, the distribution of Pb in S1, S2, and S4 stations showed a strong affinity with the reducible fraction that is potentially bioavailable. Pb was primarily present in F2 of S1 (approximately 44.80 %), S2 (approximately 41.8 %), and S4 (approximately 37.7 %). Our results are consistent with the findings of Dongting Lake, China (Yao 2008), and Shantou Bay, China (Qiao et al. 2013). Likewise, in the paddy sediments, a large fraction of Pb was bound in Fe-Mn oxide phase, and the second most important fraction was the residual phase (Wong et al. 2002). However, in S3, F4 was predominant, with Pb accounting for 32.4 %. The importance of soil organic matter in limiting Pb bioavailability has also been demonstrated. Under reducing conditions, the decomposition of Fe and Mn oxides can result in the remobilization of Fe3+ and Mn4+ into the aquatic systems (Boughriet et al. 2006), and Pb in the reducible fraction F2 turned unstable and had the potential to be transferred to available forms of the redox potential (Guven and Akinci 2008).
Cr was principally distributed between F3 and F4 (F3 and F4 accounted for over 60 %, except S4), as mentioned in the literature (Alvarez et al. 2002; Liu and Sun 2013). The results showed that although most of the Cr in S1, S2, and S3 in the tributary sediment samples are retained in the residual fraction (F4 and F3), Cr was mainly associated with the residual fraction. Our results are in concurrence with the findings of the Izmir Inner Bay, Turkey (Guven and Akinci 2013). Nevertheless, in S4, F2 was predominant, with Cr accounting for 34.9 %.
The orders of heavy metals in F1 were Zn > Cd > Cu > Cr > Pb > Ni in S1, Zn > Cr > Pb > Cd > Cu > Ni in S2, Cd > Cr > Pb > Cu > Zn > Ni in S3, and Cd > Ni > Zn > Cr > Cu > Pb in S4, respectively. The dominant proportion of Cd, Zn, and Pb was found in the soluble fraction indicating their binding to carbonates. Therefore, it can be concluded that the sediments containing high percentages of Cd and Zn could be potentially hazardous to the environment. Other studies also reported cadmium and zinc association with labile fractions in sediments (Garcia de Oliveira et al. 2008; Yao 2008; Alshemmari et al. 2012).
In F2 (reducible fraction), the largest amount of Pb and Cu is associated with iron and manganese oxide fractions in S1, S2, and S3 (40 %). The orders of heavy metals in F2 were Pb > Cu > Cd > Ni > Cr > Zn in S1, Pb > Cu > Ni > Cd > Cr > Zn in S2, Pb > Ni > Cd > Cu > Cr > Zn in S3, and Cu > Zn > Pb > Cr > Ni > Cd in S4, respectively. In the reducible fraction, the Pb and Cu content associated with Fe and Mn hydrous oxides was high. The results obtained for Pb and Cu were similar to those reported by the district (Yu et al. 2010; Nemati et al. 2011). Indeed, most of the Pb is present in the reducible fraction. This is in agreement with results reported in the study of Morillo et al. (2004) who reported that Fe and Mn hydrous oxides are important scavengers of Pb in sediments. The heavy metals might be present as metal oxides, but it is more likely that the metals are adsorbed in or onto the large amounts of iron oxide present in the sediment (Øyoar et al. 2008).
In F3 (oxidizable fraction), the result showed that the distributions of metals were somewhat different for each station: Ni > Zn > Cd > Cu > Cr > Pb in S1, Ni > Cd > Cr > Zn > Pb > Cu in S2, Ni > Cu > Zn > Cr > Pb > Cd in S3, and Pb > Cd > Cr > Ni > Zn > Cu in S4, respectively. In the oxidizable fraction, the Ni content associated with organic matter was very high, accompanied by high amounts of organic matter. Other studies also reported Ni association with labile fractions in sediments (Nemati et al. 2011; Alvarez et al. 2002). However, nickel is a common pollutant resulting from various industrial activities like mining and refining of Ni ore, electroplating, production of Ni-Cd batteries, waste incineration and others, domestic wastewater, landfill, and to a lesser, from natural weathering (Sayyed and Sayadi 2011; Sayadi and Sayyed 2011). The presence of a nonpoint source in the nearby landfill area along with leached landfill could account for the high Ni contents in the oxidizable fraction.
In F4 (reducible), the result showed that the distribution of metals were somewhat different for each station: Cr > Zn > Cu > Cd > Ni > Pb in S1, Cr > Zn > Cd > Cu > Ni > Pb in S2, Zn > Pb > Cr > Cd > Ni > Cu in S3, and Ni > Cu > Cd > Pb > Cr > Zn in S4, respectively. In the residual fraction, the Cr and Zn content associated with crystal lattices was high. These results indicate that Cr has the strongest association to the crystalline structures of sediments (Cuong and Obbard 2006). In the present study, similar results (except S4) for the speciation of Cr were observed by Huang et al. (2013). In 2013, the Cr association with liable fractions in sediment was reported by Zhang et al. (2013).
Principal component analysis
Principal component analysis (PCA) reduced the six heavy metal variables mapped in the PRE to two principal components using a varimax rotated method for comparison with the work of Zhou et al. (2004). Principal component analysis was performed on the metal concentration data from four fractions at four different sampling sites. Table 4 shows that the two factor principal components for six variables in sediments were represented by four cases corresponding to eigenvalues >1 and cumulative variance of 77.52 %, which was sufficient to provide information for the data structure. The first factor (PC1) represented Cd, Ni, Zn, and Cr, while factor 2 (PC2) represented Pb and Cu. These two principal factors explained 77.52 % of the total variance: factor 1 (48.61 %) and factor 2 (28.91 %). Moreover, the principal component analysis loading plot (Fig. 3) showed that these metals were classified into group 1 (Cd, Ni, Zn, and Cr) and group 2 (Pb and Cu) according to the similarities in behavior and metal distributions in sediments.
Pearson correlation coefficient
In order to measure the strength of correlation between variables, the Pearson correlation was not only performed for four different sites but also between averages from four fractions of varied heavy metals using SPSS 17 software. Table 5 shows the correlation among Cd, Ni, Pb, Cu, Zn, and Cr in the sediment. The pairs of Cu-Pb, Zn-Cu, and Cr-Zn showed very high correlations at (0.01) confidence level. High correlations between specific heavy metals in the sediments may reflect similar levels of contamination and/or release from the same sources of pollution (Li et al. 2009). Yi and associates (in 2011) showed a positively strong correlation between Pb-Cu, Cu-Zn, and Cr-Zn in sediments of the Yangtze River basin. Similarly, a positively strong correlation between Pb and Cu in the sediment river basin (Portugal) was reported (Soares and Boaventura 1999).
Conclusion
Fraction concentrations of heavy metals in sediments were investigated to present their bioavailability using the BCR sequential extraction procedure. Cd, Cu, Ni, Zn, and Pb were mainly presented in the reducible fraction and oxidizable fraction, while Cr was mostly presented in their residual fractions. Cd distribution in various fractions showed different patterns for each sediment sample. Particularly, in the samples S3 and S4, Cd was found in large quantities during the first extraction F1, accounting for 40.4 and 38.7 %, respectively. In the samples S2 and S4, Cu was found in large quantities during the second extraction (F2), accounting for 41.8 and 37.4 %, respectively. The distribution of Pb in S1, S2, and S4 stations showed a strong affinity with the reducible fraction that is potentially bioavailable. Pb was primarily present in F2 of S1 (approximately 44.80 %), S2 (approximately 41.8 %), and S4 (approximately 37.7 %). Furthermore, principal component analysis indicated that heavy metal fraction in the sediment samples can be explained by two principal components. PC1 represented Cd, Cr, Ni, and Zn, while PC2 represented Pb and Cu, respectively. Finally, the Pearson correlation coefficient exhibited significant correlations in Cu-Pb, Zn-Cu, and Cr-Zn pairings. Thus, it can be safely concluded that the spatial distributions of heavy metals were effectually influenced by MSW landfill and sediment physicochemical properties.
References
Algan, O., Balkıs, N., Çağatay, M. N., & Sarı, E. (2009). The sources of metal contents in the shelf sediments from the Marmara Sea, Turkey. Environmental Geology, 46(6–7), 932–950.
Alshemmari, H., Ali, L., Alotaibi, Y. (2012) Trace metal speciation in marine sediments from. Aquatic Ecosystem Health & Management 15(S1)(1463–4988):33–40.
Alvarez, E., Mochón, M. C., Sanchez, J., & Rodríguez, M. T. (2002). Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere, 47(7), 765–775.
Aprile, F., & Bouvy, M. (2008). Distribution and enrichment of heavy metals at the Tapacurá River basin, northeastern Brazil. Brazilian Journal of Aquatic Science and Technology, 12(1), 1–8.
Boughriet, A., Proix, N., Billon, G., Recourt, P., & Ouddane, B. (2006). Environmental impacts of heavy metal discharges from a smelter in Deûle-canal sediments (northern France): concentration levels and chemical fractionation. Water Air and Soil Pollution, 180(1–4), 83–95.
Christensen, T. H., Kjeldsen, P., Bjerg, P. L., Jensen, D. L., Christensen, J. B., Baun, A., et al. (2001). Biogeochemistry of landfill leachate plumes. Applied Geochemistry, 16(7–8), 659–718.
Cuong, D. T., & Obbard, J. P. (2006). Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Applied Geochemistry, 21(8), 1335–1346.
Ettler, V., Matura, M., Mihaljevič, M., & Bezdička, P. (2005). Metal speciation and attenuation in stream waters and sediments contaminated by landfill leachate. Environmental Geology, 49(4), 610–619.
Fuentes, A., Lloréns, M., Sáez, J., Soler, A., Aguilar, M. I., Ortuño, J. F., et al. (2004). Simple and sequential extractions of heavy metals from different sewage sludges. Chemosphere, 54(8), 1039–1047.
Garcia de Oliveira, M. T., Rolim, S. B. A., Mello-Farias, P. C., Meneguzzi, Á., & Lutckmeier, C. (2008). Industrial pollution of environmental compartments in the Sinos River Valley, RS, Brazil: geochemical–biogeochemical characterization and remote sensing. Water Air and Soil Pollution, 192(1–4), 183–198.
Goorzadi, M., Vahabzadeh, G. H., Ghanbarpour, M. R., & Karbassi, A. R. (2009). Assessment of heavy metal pollution in Tilehbon River sediments, Iran. Journal of Applied Sciences, 9(1812–5654), 1190–1193.
Grotti, M., Soggia, F., Ianni, C., Magi, E., & Udisti, R. (2013). Bioavailability of trace elements in surface sediments from Kongsfjorden, Svalbard. Marine Pollution Bulletin, 77(1–2), 367–374.
Guven, D. E., & Akinci, G. (2008). Heavy metals partitioning in the sediments of Izmir Inner Bay. Journal of Environmental Sciences (China), 20(4), 413–418.
Guven, D. E., & Akinci, G. (2013). Effect of sediment size on bioleaching of heavy metals from contaminated sediments of Izmir Inner Bay. Journal of Environmental Sciences, 25(9), 1784–1794.
Huang, L., Pu, X., Pan, J., & Wang, B. (2013). Heavy metal pollution status in surface sediments of Swan Lake lagoon and Rongcheng Bay in the northern Yellow Sea. Chemosphere, 93(9), 1957–1964.
Jain, C. K. (2004). Metal fractionation study on bed sediments of River Yamuna, India. Water Research, 38(3), 569–578.
Kazi, T. G., Jamali, M. K., Kazi, G. H., Arain, M. B., Afridi, H. I., & Siddiqui, A. (2005). Evaluating the mobility of toxic metals in untreated industrial wastewater sludge using a BCR sequential extraction procedure and a leaching test. Analytical and Bioanalytical Chemistry, 383(2), 297–304.
Kjeldsen, P. (1993). Groundwater pollution source characterization of an old landfill. Journal of Hydrology, 142(1–4), 349–371.
Li, F., Fan, Z., Xiao, P., Oh, K., Ma, X., & Hou, W. (2009). Contamination, chemical speciation and vertical distribution of heavy metals in soils of an old and large industrial zone in Northeast China. Environmental Geology, 57(8), 1815–1823.
Liu, J., & Sun, S. (2013). Total concentrations and different fractions of heavy metals in sewage sludge from Guangzhou, China. Transactions of Nonferrous Metals Society of China, 23(8), 2397–2407.
Mantei, E., & Coonrod, D. (1989). Heavy metal content in the stream sediments adjacent to a sanitary landfill. Environmental Geology and Water Sciences, 1, 51–58.
Mantei, E. J., & Sappington, E. J. (1994). Heavy metal concentrations in sediments of streams affected by a sanitary landfill: a comparison of metal enrichment in two size sediment fractions. Environmental Geology, 24(4), 287–292.
Mendil, D., Unal, O. F., Tüzen, M., & Soylak, M. (2010). Determination of trace metals in different fish species and sediments from the River Yeşilirmak in Tokat, Turkey. Food and Chemical Toxicology, 48(5), 1383–1392.
Morillo, J., Usero, J., & Gracia, I. (2004). Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere, 55(3), 431–442.
Mulligan, C. N., Yong, R. N., & Gibbs, B. F. (2001). An evaluation of technologies for the heavy metal remediation of dredged sediments. Journal of Hazardous Materials, 85(1–2), 145–163.
Nemati, K., Abu Bakar, N. K., Abas, M. R., & Sobhanzadeh, E. (2011). Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. Journal of Hazardous Materials, 192(1), 402–410.
Olivares-Rieumont, S., de la Rosa, D., Lima, L., Graham, D. W., D’Alessandro, K., Borroto, J., et al. (2005). Assessment of heavy metal levels in Almendares River sediments—Havana City, Cuba. Water Research, 39(16), 3945–3953.
Olivares-Rieumont, S., Lima, L., De la Rosa, D., Graham, D. W., Columbie, I., Santana, J. L., et al. (2007). Water hyacinths (Eichhornia crassipes) as indicators of heavy metal impact of a large landfill on the Almendares River near Havana, Cuba. Bulletin of Environmental Contamination and Toxicology, 79(6), 583–587.
Oyeyiola, A. O., Olayinka, K. O., & Alo, B. I. (2011). Comparison of three sequential extraction protocols for the fractionation of potentially toxic metals in coastal sediments. Environmental Monitoring and Assessment, 172(1–4), 319–327.
Øyoar, J., Gjengedal, E., & Mobbs, H. J. (2008). Trace element exposure in the environment from MSW landfill leachate sediments measured by a sequential extraction technique. Journal of Hazardous Materials, 153(1–2), 751–758.
Pekey, H. (2006). Heavy metal pollution assessment in sediments of the Izmit Bay, Turkey. Environmental Monitoring and Assessment, 123(1–3), 219–231.
Purushothaman, P., & Chakrapani, G. (2007). Heavy metals fractionation in Ganga river sediments, India. Environmental Monitoring and Assessment, 132, 475–489.
Qiao, Y., Yang, Y., Gu, J., & Zhao, J. (2013). Distribution and geochemical speciation of heavy metals in sediments from coastal area suffered rapid urbanization, a case study of Shantou Bay, China. Marine Pollution Bulletin, 68(1–2), 140–146.
Sayadi, M. H., & Rezaei, M. R. (2014). Impact of land use on the distribution of toxic metals in surface soils in Birjand City, Iran. Proceedings of the International Academy of Ecology and Environmental Sciences, 4(1), 18–29.
Sayadi, M. H., & Sayyed, M. R. G. (2011). Comparative assessment of baseline concentration of the heavy metals in the soils of Chitgar Industrial Area Tehran (Iran) with the comprisable reference data. Environmental Earth Sciences, 63(6), 1179–1188. doi:10.1007/s12665-010-0792-z.
Sayadi, M. H., Sayyed, M. R. G., & Saptarshi, P. G. (2008). An assessment of the Chitgar River sediments for the short-term accumulation of the heavy metals from Tehran, Iran. Pollution Research, 27(4), 627–634.
Sayadi, M. H., Sayyed, M. R. G., & Kumar, S. (2010). Short-term accumulative signatures’ of heavy metal in river bed sediments, Tehran Iran. Environmental Monitoring and Assessment, 162, 465–473.
Sayyed, M. R. G., & Sayadi, M. H. (2011). Variations in the heavy metal accumulations within the surface soils from the Chitgar Industrial Area of Tehran (Iran). Proceedings of the International Academy of Ecology and Environmental Sciences, 1(1), 27–37.
Soares, H., & Boaventura, R. (1999). Sediments as monitors of heavy metal contamination in the Ave river basin (Portugal): multivariate analysis of data. Pollution, 105(3), 311–323.
Turkian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the Earth’s crust. Geological Society of America Bulletin, 72, 175–192
van Geen, A., & Luoma, S. N. (1993). Trace metals (Cd, Cu, Ni, and Zn) and nutrients in coastal waters adjacent to San Francisco Bay, California. Estuaries, 16, 559–566.
Velimirović, M. B., Prica, M. D., Dalmacija, B. D., Rončević, S. D., Dalmacija, M. B., Bečelić, M. D., et al. (2010). Characterisation, availability, and risk assessment of the metals in sediment after aging. Water Air & Soil Pollution, 214(1–4), 219–229.
Wong, S., Li, X., Zhang, G., Qi, S., & Min, Y. (2002). Heavy metals in agricultural soils of the Pearl River Delta, South China. Environmental Pollution, 119, 33–44.
Yao, Z. (2008). Comparison between BCR sequential extraction and geo-accumulation method to evaluate metal mobility in sediments of Dongting Lake, central China. Chinese Journal of Oceanology and Limnology, 26(1), 14–22.
Yi, Y., Yang, Z., & Zhang, S. (2011). Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environmental Pollution, 159(10), 2575–2585.
Yu, R., Hu, G., & Wang, L. (2010). Speciation and ecological risk of heavy metals in intertidal sediments of Quanzhou Bay, China. Environmental Monitoring and Assessment, 163(1–4), 241–252.
Yuan, C. G., Shi, J. B., He, B., Liu, J. F., Liang, L. N., & Jiang, G. B. (2004). Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environment International, 30(6), 769–783.
Zhang, R., Zhou, L., Zhang, F., Ding, Y., Gao, J., Chen, J., et al. (2013). Heavy metal pollution and assessment in the tidal flat sediments of Haizhou Bay, China. Marine Pollution Bulletin, 74(1), 403–412.
Zhou, H., Peng, X., & Pan, J. (2004). Distribution, source and enrichment of some chemical elements in sediments of the Pearl River Estuary, China. Continental Shelf Research, 24(16), 1857–1875.
Acknowledgments
This study which was conducted as a research project in 2014 was funded by the Research Council of University of Birjand. The authors genuinely appreciate the authorities of the Research Council and Faculty of Natural Resources and Environment, University of Birjand, for their sincere cooperation. We would also like to thank Dr. Mrs. Mahavash F. Kavian for editing the paper and the anonymous reviewers who helped us to improve our manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sayadi, M.H., Rezaei, M.R. & Rezaei, A. Fraction distribution and bioavailability of sediment heavy metals in the environment surrounding MSW landfill: a case study. Environ Monit Assess 187, 4110 (2015). https://doi.org/10.1007/s10661-014-4110-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4110-1