Abstract
Bauxite and iron ore mining is the major contributor to metal pollution in Tasik Chini, Malaysia. Deforestation of the protected zone of reserve forest exacerbates the problem. The current study is to understand the speciation of metals spatially in sediment to analyse the risk associated in terms of its mobility and bioavailability. The samples of sediment are collected from Sungai Jemberau, Laut Jemberau, and Laut Gumum of Tasik Chini. Four samplings were conducted for a year, by collecting the surface sediment. Sequential extraction method was followed for speciation of sediment and classified it into exchangeable, reducible, Fe–Mn oxides, organic and residual fractions. The results were also analyzed using principal component analysis (PCA) and cluster analysis (CA). The result reveals that Fe, Al, Mn, Zn, and Pb are the primary constituents of sediment contributing to about 98% of residual fraction. Co, Cd, Cr, As, and Ni are found in trace metal concentration and are identified to be mainly released from anthropogenic sources nearby. Although the individual proportion is less than major metals in exchangeable and carbonate fraction, they possess geochemically significant concentration above the permissible limit. More than 70–80% of all its total concentration proportion is hence found in mobile and bioavailable state. These possess toxic and have chronic effects to aquatic life and public health even in trace elemental concentration. Hence, these metals are the most toxic and bioavailable metals pausing risk for aquatic and public health. PCA analysis highlights that the enrichment of heavy metals in bioavailable fraction is mostly contributed from anthropogenic sources. The same results are emphasized by cluster analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic sediments are an important site of deposition and chemical transformation of compounds entering the system. Metals can be bound to sediments in different chemical forms, which are susceptible to slightest changes in the water quality (Arnason and Fletcher 2003). Under certain forms and conditions, these metals easily migrate to water column and can be bioavailable to aquatic organisms. Hence, metals in aquatic systems have always been a major concern due to its complex chemical interactions with susceptible environmental changes. Metals can transform into various metal species under change in pH, redox, temperature, etc. (Mason 2013; Pokhrel and Dubey 2013). Speciation or the chemical form of metals is strongly depended on its association with sediments, and it defines the fate, transport, mobility, and toxicity of the metals in the aquatic environment. Therefore, it is not only a threat due to its elevated metal concentration but also the physio-chemical form (species) of its parent entity (Mason 2013; Duruibe et al. 2007).
Release of toxic metals to freshwater system is of considerable environmental and social concern at the second largest freshwater lake, Lake Chini, Malaysia. Unregulated surface mining of iron ore and bauxite in Bukit Ketaya, causes erosion and sedimentation to the lake (Reyhani and Reza 2013). The caustic red sludge and toxic mine tailings spread downstream with wind, while other mine tailings are washed down the hill directly into Lake Chini or are seeped into the aquifer. It ultimately pollutes the lake and its feeder rivers such as Sugai Jemberau (Islam et al. 2015). It has reduced the natural depth of lake to a significant extent (Soosal and Sivalingam 2011). Tasik Chini, being a hotspot for commercial fishes, it is presumed that higher metal contamination in lake has adverse impact to the public health of larger population and environment. Exposure studies have proved that long term exposure to even low concentration toxic chemicals cause cancers, fertility problems and other illnesses including diabetes and obesity (EPA 2011). Hence, it is an imperative need to eliminate or reduce exposure to these heavy metals to the minimum.
Most of the previous studies have focused on identifying the total concentration of metals present and its impact on specific flora and fauna. Yet, question still remained on specific metal speciation and its influencing physical and chemical factors in Lake Chini. The identification of the specific metal species could address the mobility, risk of toxicity, and bioavailability of metals to aquatic species. The objective of the present study was to study dominant metal speciation in sediments and its spatial variation with season.
Lake Chini
Lake Chini is the second largest freshwater lake in Malaysia (3° 22′ 30" to 3° 28′ 00" N and 102° 52′ 40" to 102° 58′ 10" E). It is located in the state of Pahang, east of Peninsular Malaysia (Islam et al. 2015; Toriman et al. 2009). Lake Chini is the pioneering UNESCO biosphere reserve to be conserved for its cultural and biological richness (Soosal and Sivalingam 2011). Lake Chini comprises of a network of 12 small lakes (Gasim et al. 2008) and the area ranges from 150 ha (dry season) to 300 ha (wet season) depending on the season. The lake is flown out to the nearest river, Sugai Chini (Sg. Chini) (Ahmad et al. 2008; Toriman et al. 2009). The lake serves as an important breeding and spawning ground for fishes as it connects to Pahang River through Chini River. It is not only a livelihood for the native indigenous people nearby but also an important food source (Ahmad and Othman 2010). Excessive erosion of soil from nearby mining sites increases the sedimentation, thereby turbidity and conductivity. It has a counter effect on the physiology and behavior (spawning and breeding) of fish (Low et al. 2016). Therefore, high bioavailable metal concentration released would be harmful for the health of aquatic species in the freshwater.
Materials and methods
Study area
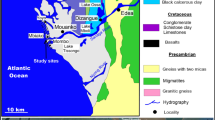
The speciation of metals was studied in surface sediments in Laut Jemberau, Laut Gumum, and Sugai Jemberau of Lake Chini catchment area. Considering the geographical area, the land alteration, active mining of iron ore and bauxite in the close vicinity of Laut Jemberau, and its reach towards the whole Lake Chini are the main factors to particularly concentrate on Laut Jemberau. In Jemberau region of Lake Chini, the forest has been cut down for bauxite and iron ore mining in the soil. The surface mining in the soil has loosened the surface soil in the region, and hence, the loosened soil is being directly washed into the Sugai Jemberau (Feeder River) and Laut Jemberau (part of the lake close to mining site) along with wind and rain. The reactivation of mining activity in the region has altered the Sugai Jemberau flow, resulting in the drying up of part of the river. The alteration of river flow also has affected the hydrological regime of the river and the lake (Sharip and Jusoh 2010). The logging of trees has increased the soil erosion and sedimentation in the region. Earlier, Laut Jemberau used to be the one of the deepest lakes in the Lake Chini catchment (3 to 5 m). Now, it is only 1–3 m deep during rainy season.
Sample collection and procedure
Standard methods were followed to determine the chemical speciation of metals in the surface sediment from Laut Jemberau near the mining sites. Part of the objective was to conduct a risk assessment study of surface sediment due to surrounding mining activities. The surface sediment samples were collected from 0–20 cm depth using grab sampler. A total of 12 samples were prepared from 4 different seasons. The period of study was 1 year, four samplings with an interval of 3 months; sampling 1 (S1)—Sept 15; sampling 2 (S2)—Jan 16; sampling 3 (S3)—Apr 16; sampling 4 (S4)—Jul 16. Sampling sites were chosen to understand the spatial variation or mobility of metal contamination (Fig. 1). These samples were kept in air-tight sealed plastic bags and refrigerated until further analysis. Dried in oven at (70° C) and sieved (> 63 μm) due to the strong affinity of metals to fine-grained particles, and samples were used for the tests. Different types and species of metals have various strength and affinity towards its ligands, hence has different leaching behaviors (Xiong et al. 2014).
Sequential extraction procedure
Five step modified Tessier et al. sequential extraction method was applied on dried samples to determine the speciation of metals present in surface sediment. The procedure developed by Tessier et al. in 1979 for sequential extraction is widely used method for chemical speciation of metals in sediments. The speciation is done in five fractions: (i) exchangeable form, (ii) bound to carbonates, (iii) bound to Fe–Mn oxides, (iv) bound to organics, (v) residual form. The exchangeable fraction was extracted by adding 8 ml of 1 M MgCl2 (pH adjusted to 7) to 1 g of dry sediment and agitated for an hour. The residue of fraction I was extracted with 8 ml of 1 M NaOAc (pH adjusted to 5 with acetic acid) and was mixed continuously for 5 hours at room temperature, to get the metals bound to carbonates. To extract the metals bound to Fe–Mn oxides, the residue of fraction II was added with 20 ml of 0.04 M NH2OH. HCl in 25% (v/v) acetic acid and heated to 96° C, with an occasional agitation for 6 hours. Then, to sequestrate fraction IV (bound to organics) the residue of fraction III was extracted with 3 ml of 0.02 M HNO3 and 5 ml of 30% (v/v) H2O2 adjusted to pH 2 and heated to 85° C for 2 hours; 3 ml of 30% of H2O2 (pH 2) and heat for 3 hours was added. Later, 5 ml of 3.2 M NH4OAc in 20% (v/v) HNO3 was added. To get the residual form, the residue of fraction IV was dried at 105° C. Later, it was acid digested with 5 ml of conc. nitric acid (HNO3) (70% w/w), 10 ml of hydrofluoric acid (HF, 40% w/w), and 10 ml of per chloric acid (HClO4, 60% w/w) (Roberts et al. 2005). The metal concentration in each sequestrated solution was analyzed using ICP-MS (Yuan et al. 2004).
Risk Assessment Code
In order to estimate the environmental risk involved with the contamination of metal tailings in the region, the samples were studied and classified according to a Risk Assessment Code (RAC). It is a method to classify the risk associated with the chemical speciation of metals (Zhu et al. 2012). Metals bound to different phases have various binding strength, hence, behave differently in the aquatic ecosystem. The strength of metals bound to different geochemical phases indicates its mobility, bioavailability, and other associated risks at each stage to the surrounding aquatic ecosystem (Saleem et al. 2015; Reyhani and Reza 2013). Risk Assessment Code (RAC) was calculated with the percent associated with the first two geochemical fractions (F1 exchangeable metals and F2 bound to carbonates) in Tessier et al. procedure (Zimmerman and Weindorf 2010). In these stages, the metals are weakly bound to the solid phases. Hence, these loosely bound metals are more bioavailable to the aquatic organisms.
Multivariate statistical analyses
The experimental data were analyzed by Principal Component Analysis (PCA) and Cluster Analysis (CA). PCA is a multivariate data analysis method that identifies patterns in a data and simplifies a large dataset to a small set of new variables using correlation structure. The new set of few variables is set to have maximum information from the original dataset highlighting their similarities and differences. As it is difficult to find patterns in the large set of data, PCA is a powerful tool to reduce the number of dimensions without losing much of its information (Smith 2002). Hence, this study is using PCA for produce new set of variables to reflect significant information about speciation and seasonal variation of metal species.
When the dataset has more dimensions, it is difficult to statistically calculate the spread and see the variance and its correlation with covariance. In such cases, PCA is used to derive simpler dataset with principal components. In PCA, a dataset of interrelated or correlated p variables (also known as analytical constituents) to several degrees are reduced to new dataset of p new orthogonal variables are called principal components (PCs). PCA has principal components (PC) which is a linear function. It contains original variables in such a way that their sums of variances are equal to that of the original variables (Olsen et al. 2012).
Cluster analysis
Categorizing similar objects into groups that are dissimilar to other objects forms a cluster. It is a useful technique to group similar data objects or variables in a dataset (Saraçli et al. 2013). It identifies similarities and differences of an object within a group to another, and clusters similar object data into one group. The greater the similarity or homogeneity, it groups to one cluster. The foundation of the clustering method lies in finding similarities or dissimilarities or distances (Euclidean distance, squared Euclidean) between objects when it forms clusters. Based on single dimension or multiple dimensions, set rules serve as criteria to group objects. The hierarchical cluster is displayed in dendrogram structures. It has clusters and sub-clusters and order.
If for a data set of (Xi) is clustered to a dendrogram, where it is grouped into a hierarchical tree, then the Euclidean distance is measured as follows:
whereas, the dendrogrammatic distance between the model points Ti and Tj would be t (i, j). It is the height of the dendrogrammic node where the points join.
where c is the cophenetic correlation c and x is the average of x (i, j) and t is the average of t (i,j). Statistically, it measures how well a dendrogram still maintains the pair wise distances of the original unmodeled dataset points (Saraçli et al. 2013; Anderberg 2014).
Results and discussion
The sediment samples from Sungai Jemberau, near to mining site exhibit reddish clayey sediment with acidic pH. The dominance of reddish color in sediment showed the presence of iron oxide in the sediment. It is attributed to the mine tailings and soil eroded from uphill. In Laut Jemberau, the dominant color in sediment is grey that indicates the presence of organic matter in the sample (Moritsuka et al. 2014). Except in the center of Laut Jemberau, all other sampling sites of Laut Jemberau and Laut Gumum portray acidic pH. The acidity of sediment suggests easily dissolubility of metals in the sediment (Islam et al. 2015) (Table 1).
Speciation of metals in sediments
Following the sequential extraction procedure, the concentration of sediment samples is classified as—exchangeable, reducible, oxides, organic, and residual. Speciation conducted indicated that the concentration of each metal extracted from each fraction varied widely. Figure 2 shows the distribution of metals in each fraction; great spatial spread is displayed according to its binding capacity towards certain ligands in the sediments (Saleem et al. 2015; Passos et al. 2011).
Exchangeable fraction
The exchangeable fraction of metals in sediments is presented in Fig. 2. Fe and Mn showed higher concentration in all sampling sites especially sites near to mining site. Above 5% of the total concentration of Fe and Mn are attributed to exchangeable fractions with a concentration. About 25% of Pb was attributed to unstable mobile and bioavailable state of Pb due to its presence in exchangeable (13.7% of total Pb concentration; 1.69 ± 0.13 mg/l). Generally, the concentration of Co was found to be in trace amount level in relative to other metals. Though in trace amount, it is found in all sampling stations in Sungai Jemberau, Laut Jemberau, and Laut Gumum. The spatial spread of Co was higher with less concentration in farther sites from mining sites. As it is mainly released from anthropogenic sources, it is found highest in exchangeable fraction (50.8% of Co concentration, 0.50 ± 0.26 mg/l). Similarly, Cd showed instable and is in bioavailable state and exchangeable that is contributed for about 45.2% of total Cd concentration (0.12 ± 0.09 mg/l). Furthermore, unstable mobile Cr, As, and Ni were mainly released from anthropogenic sources, which is indicated by its exchangeable fraction (0.15 ± 0.08 mg/l, (0.33 ± 0.19 mg/l and 0.04 ± 0.017 mg/l) respectively. Though in proportion, it is less in exchangeable fraction, the exchangeable fraction of Cr, As, and Ni contributed to its 12.2%, 28.8%, and 24.3% of its total concentration.
A clear spatial variation in concentration was visible in exchange fraction distribution. The concentration of metals was higher in stations near to mining site. First of all, the higher concentration of exchangeable fraction in the lake indicates the metal contamination and the risk associated with it in Lake Chini. By acting a sink and source of metals, sediments accumulate metals from both soil and water. As exchangeable fraction represented only the concentration from non-lithogenic (anthropogenic) sources, obvious contamination is visible from Fig. 2. Factors such as particle size of sediments (dominance of clayey particles) and slightly acidic to neutral pH presence favor the accumulation or weak adsorption of metals onto sediment particles (Ferronato 2013). They are easily soluble which makes them readily mobile and bioavailable for aquatic uptake (Morrison 2011).
Carbonate fraction
In Fig. 3, reducible fraction accounted for higher Fe concentration though it only belongs to 2% of its total concentration (4.06 ± 1.57 mg/l). Other prominent metals such as Al accounted for 14% of total reducible fraction which is 1.94 ± 0.023 mg/l, Mn belongs to 31.5% (4.18 ± 2.18 mg/l), and Zn for 10% (1.38 ± 0.56 mg/l). Exception to the exchangeable fraction, Pb (10% 1.41 ± 0.45 mg/l) was also a common leachable metal that is locked up in carbonate matrix. Especially in sites near to the mining hill, Pb and Zn showed special affinity to carbonate. Though considering in the context of total reducible proportion, Co, Cd, Cr, As, and Ni contributed less than 2%, these metals contribute considerably to its total concentration, and it is still above the permissible limit suggested by NLWQS Malaysia.
The higher degree of association of Fe, Mn, and Cr in carbonate fraction showed its higher affinity to form precipitate with carbonates (Sundaray 2011). It also indicated the accumulation of metals from anthropogenic sources in sediments. A slightly higher proportion of reducible fraction is exhibited than exchangeable showed dominance of carbonate fraction over exchangeable phase in sediments. pH is one of the controlling factors metals bound in carbonates, acidic changes in sediments make these accumulated metals more mobile and bioavailable (Hong et al. 2011).
Oxide fraction
The geochemical fraction, Fe and Mn oxides, are the acid reducible fractions (Fig. 4). The metal binding strength to oxides differ from the earlier geochemical fractions. Mn, Fe, Al, and Zn are bound to oxides, and all other metals are geochemically insignificant (< 1% of total concentration of oxide fraction). The figure displayed a consistent proportion of these metals in the sampling stations regardless of sampling periods. Fe accounted for more than 40% (32.38 ± 4.60 mg/l) to oxide fraction whereas Al contributed to 20% (16.39 ± 5.02 mg/l), Mn to 36.5% (29.30 ± 7.03 mg/l), and Zn to 2% (2.00 ± 0.75 mg/l).
The concentration of oxide fraction of all the metals is not significantly varying in different stations. Considerably higher concentration is portrayed by all metals in oxide fraction. It is due to the higher scavenging affinity to Fe–Mn oxide ligands. Unlike exchangeable and carbonate fraction, metals locked up in oxide fraction of sediment are relatively unavailable. Due to the strong ionic bonding between ligands and metals, slight changes in water or sediment quality parameters do not affect the bonding and its release. The metals locked up in Fe–Mn oxide stage can only be mobilized under strongly acidic or reducing conditions. Metals such as Fe–Mn hydroxides of Fe, Al, and Mn are redox-sensitive and act as major sinks in sediments during oxidizing conditions. With regards to mobility and bioavailability, the oxide fraction is more stable than exchangeable and carbonate fraction (Arenas-Lago et al. 2014). Hence, in Lake Chini, the oxide fraction puts less risk than exchangeable and carbonate fraction.
Organic fraction
In organic fraction, the most prominent metal is Al (Fig. 5). Al has special affinity towards organic matter in the sediment; it could be understood from the higher proportion of Al in organic fraction (32% 15.63 ± 0.19 mg/l). The less variation in standard deviation shows its consistency in all the samples analyzed. The proportion of Fe and Mn is usually higher concentration near to mining activity and decreases as it reaches the center of the lake. Mn is other metal that shows a consistent binding with organic matter; it accounts for 29.85% with an average of 14. 48 ± 3.27 mg/l. Similar to oxide fraction, other remaining metals have less than 1% contribution to organic fraction, except for Cr. The organic fraction also has a higher proportion of Cr (0.32 ± 0.31 mg/l). Other metals are under permissible limit and are in stable state.
Organically bound metals also have higher concentration in sediment of Lake Chini. The higher content in organic matter in the sediment is a prominent sink for metals in the lake. The organic bound metals are considered relatively stable phase as it only releases its metals upon degradation of organic matter under strongly oxidizing conditions (Ashraf et al. 2012). Only under strong oxidation and decomposition of organic matter, these metals will be released to mobile state. However, lower DO or dissolved oxygen presence in these sampling stations lowers the possibility of decomposition of organic matter, thereby the release of metals. They are effectively immobilized and not bioavailable (Hong et al. 2011). Substantial amount of metal in organic matter in sediment hence trap excess metals in Lake Chini lowering the risk paused by the contamination of metals. Besides the concentration of metal, bioavailability and solubility of metals in sediment are depended on properties like sediment pH and organic matter (Vogel-Mikuš et al. 2005). Due to higher organic content in the lake (especially Laut Jemberau), more concentration of trace metals such as Pb and Zn are locked up in organic matter in sediment in relative to soil phase. Hence, change in pH pauses risk on metals adsorbed onto organic fraction (Ikem et al. 2003). The remaining metals are found in either exchangeable, reducible, or oxide forms that are very susceptible to slight water quality changes such as lowering pH and temperature (Ebrahimpour and Idris 2008). Hence, the metals adsorbed onto sediment particles will be dissolved into water increasing the water contamination (Ikem et al. 2003; Li et al. 2013). Thereby, their mobility and bioavailability increase risks the aquatic life.
Residual fraction
The residual fraction exhibited an interesting pattern (Fig. 6). Fe and Al are prominent metals which are present in the residual state of Bukit Ketaya soil. So, its presence in sediment was expected to be higher, and it concurred with the resulting value. About 68% (133.36 ± 150.06 mg/l) of total concentration is attributed to Fe. The spatial variation does not follow any trend and exhibits consistent concentration. Zn is the third most prominent metal found in Bukit Ketaya soil residual fraction. The primary constituents of sediments or lithogenic metals are retained in the residual phase. As it is strongly bound in the crystal lattices of silicates, it is only leached through weathering or decomposition which is much longer than the life period. The metals represented in this phase have geogenic origin and indicate that the sediment is unpolluted due to its presence (Davutluoglu et al. 2010).
RAC of Metals in Sediments
The Risk Assessment Code of sediments in Sungai Jemberau, Laut Jemberau, and Laut Gumum is illustrated in Fig. 7. The RAC of sediment is scattered ranging from low ecological risk to very high ecological factor. It indicated the different accumulation mechanism of metals in sediment medium. In the mining regions, As, Cd, Co, Pb, and Ni fall in the category of very high risk. These toxic metals could be hence released to the overlying water under slight changes in water quality parameters. The metals possessing high ecological risk are Mn, Zn, and Cr. As expected primary constituents such as Al and Fe exhibited low to moderate ecological risk. However, when it comes to center of the lake, same elements have more than 50% contributing to very high risk indicating its rapid mobility from mining stations to other parts of the lake. Only Cd possesses very high risk whereas Co, Ni, and Pb exhibit high risk in sediments. The risk assessment of metals in sediment also highlighted the fact that metals are not permanently trapped inside the sediments. Especially, the highest risk-possessing metals are crucial due to its instability to transform to dissolve state in the overlying water being more bioavailable and toxic.
Principal component analysis and cluster analysis
From PCA analyses in Fig. 8, the PC1 represent 59.7% of the total variance, in which it has more number of strong variables explaining the relationship. The dominant variables Fe, Pb, Al, Zn, and Cr indicate similar speciation fractionation in sediment. All these metals had higher residual concentration in terms of concentration in sediment. PC2 accounts for 19.8% of total variance with higher dominance of Co, As, Cd, and Ni. The higher magnitude of these metals indicates its input through anthropogenic source as it is attributed mostly in exchangeable and reducible fractions. Negative-loading score could be due to its very different pattern of binding, mainly with Fe–Mn hydroxides and organic fraction.
The dendrogram of cluster analysis in Fig. 9 well-differentiated clusters in two main clusters. Cluster I has Fe and Al and cluster II with sub-cluster groups. However, the Euclidean distances between clusters are very less in sediment. In cluster 1, Fe and Al are the prominent metals present in lithogenic metals. These metals are bound mainly in the residual fraction that has no influence on its mobility and bioavailability. The abundance of Fe and Al in sediment and dissimilarity from other group of metals have caused them to cluster together in cluster I. Cluster II is a collection of subclustered groups of metals that are mainly released to environment from anthropogenic sources. Sub-clustering in cluster II is formed by a group of metals possessing similar properties. Though from similar source, Mn is mainly bound in the Fe–Mn hydroxides and organic fraction resulting in its separate clustering from the other groups. Similar input, abundance, and binding pattern have brought Pb and Zn together in the same group of cluster Pb and Zn, it is clubbed together whereas the presence in exchangeable and reducible accounts for a different cluster group in the case of Co, As, Cd, Cr, and Ni (Wuana and Okieimen 2011). Sub-grouping within the cluster mainly explains its source of release but also explains their similar characteristics such as bioavailability and binding behavior.
Conclusion
The comprehensive study conducted for a year to understand the spatial variation of metal accumulation in Jemberau and Gumum identified noticeable spatial spread of contamination by all metals, and it also varies seasonally from one sampling period to another. Higher concentration of dissolved metals is found in the close vicinity of mining areas. It was found out that Fe, Al, Mn, Zn, and Pb are the primary constituents of metals naturally in the Malacca soil series found in Bukit Ketaya, Lake Chini. The higher concentration of metals in exchangeable and reducible fractions is reflected in the dissolved concentration in the lake. Other metals like Co, Cd, Cr, As, and Ni are not naturally found in the sediment. More than 70–80% of all its total concentration proportion is hence found in the mobile and bioavailable state that are very susceptible to slight physical and chemical changes of environmental matrices. They possess geochemically significant concentration above permissible limit suggested by NLWQS Malaysia. Hence, these metals are the most toxic and bioavailable metals causing risk for aquatic and public health.
References
Ahmad A, Othman M (2010) Heavy Metal concentration in sediments and fishes from Lake Chini, Pahang. Malaysia. J Biol Sci 10(2):93–100
Ahmad AK, Othman MS, Mushrifah I (2008) The heavy metals temporal variability in fish of Tasik Chini, Peninsular Malaysia. Proceedings of Taal 2007: The 12th World Lake Conference. 2131-2135
Anderberg MR (2014) Cluster analysis for applications: probability and mathematical statistics: a series of monographs and textbooks. Academic Press, p 19
Arenas-Lago D, Andrade ML, Lago-Vila M, Rodríguez-Seijo A, Vega FA (2014) Sequential extraction of heavy metals in soils from a copper mine: distribution in geochemical fractions. Geoderma 230:108–118
Arnason JG, Fletcher BA (2003) A 40+ year record of Cd, Hg, Pb and U deposition in sediments of Patroon Reservoir, Albany County, NY, USA. Environ Pollut 123:383–391
Ashraf MA, Maah MJ, Yusoff I (2012) Chemical speciation and potential mobility of heavy metals in the soil of former tin mining catchment. Sci World J 2012:1–11
Davutluoglu OI, Seckın G, Kalat DG, Yılmaz T, Ersu CB (2010) Speciation and ımplications of heavy metal content in surface sediments of Akyatan Lagoon–Turkey. Desalination 260(1):199–210
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2(5):112–118
Ebrahimpour M, Idris M (2008) Heavy metal concentrations in water and sediments in Tasik Chini, a freshwater lake, Malaysia. Environ Monit Assess 141(3):297–307
EPA (2011) Increase in cancers and fertility problems may be caused by household chemicals and pharmaceuticals. EPA
Ferronato C (2013) Speciation of heavy metals at water-sediment interface. EQA Int J Environ Qual 10(10):51–64
Hong YS, Kerry AK, Danny DR (2011) Effects of cyclic changes in pH and salinity on metals release from sediments. Environ Toxicol Chem 30(8):1775–1784
Ikem A, Egiebor NO, Nyavor K (2003) Trace elements in water, fish, and sediment from Tuskegee Lake, Southeastern USA. Water Air Soil Pollut 149:51–75
Islam MS, Ahmed MK, Raknuzzaman M, Habibullah-Al-Mamun M, Islam MK (2015) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol Indic 1(48):282–291
Gasim MB, Toriman ME, Abas A, Islam MS, Chek TC (2008) Water quality of several feeder rivers between two seasons in Tasik Chini, Pahang. Sains Malays. 37(4):313–321
Li H, Shi A, Li M, Zhang X (2013) Effect of pH, temperature, dissolved oxygen, and flow rate of overlying water on heavy metals release from storm sewer sediments. J Chem 2013:1–11
Low KH, Koki IB, Juahir H, Azid A, Behkami S, Ikram R (2016) Evaluation of water quality variation in lakes, rivers, and ex-mining ponds in Malaysia (review). Desalin Water Treat 57(58):28215–28239
Mason RP (2013) Trace metals in aquatic systems. Wiley-Blackwell, Chichester
Moritsuka N, Matsuoka K, Katsura K, Sano S, Yanai J (2014) Soil color analysis for statistically estimating total carbon, total nitrogen and active iron contents in Japanese agricultural soils. J Soil Sci Plant Nutr 60(4):475–485
Morrison GMP (2011) Urban storm water pollution- heavy metal speciation studies of natural waters: a review. Chalmers tekniska hogskola. Goteborg 2:84.
Olsen RL, Rick WC, Jim CL (2012) Water quality sample collection, data treatment and results presentation for principal components analysis–literature review and Illinois River watershed case study. Water Res 46(9):3110–3122
Passos EA, Alves JPH, Garcia CAB, Costa ACS (2011) Metal fractionation in sediments of the Sergipe River, northeast Brazil. J Braz Chem Soc 22
Pokhrel LR, Dubey B (2013) Global scenarios of metal mining, environmental repercussions, public policies, and sustainability: a review. Crit Rev Environ Sci Technol 43(21):2352–2388. https://doi.org/10.1080/10643389.2012.672086
Reyhani P, Reza AM (2013) Assessment of heavy metals contamination in surface water of the upstream Sardabrud River, North Iran. Life Sci 10(7):884–892
Roberts D, Maarten N, Donald LS (2005) Speciation of metals in soils. Soil Sci Soc Am J 8:619–654
Saleem M, Iqbal J, Shah MH (2015) Geochemical speciation, anthropogenic contamination, risk assessment and source identification of selected metals in freshwater sediments – a case study from Mangla Lake, Pakistan. Environ Nanotechnol Monit Manag 4:27–36. https://doi.org/10.1016/j.enmm.2015.02.002
Saraçli S, Nurhan D, İsmet D (2013) Comparison of hierarchical cluster analysis methods by cophenetic correlation. J Inequal Appl 2013(1):203
Sharip Z, Jusoh J (2010) Integrated lake basin management and its importance for Lake Chini and other lakes in Malaysia. Lakes Reserv Res Manag 15:41–51
Smith L (2002) A tutorial on principal components analysis. Cornell University, USA. 51(52): 65
Soosal V, Sivalingam A (2011) Tasik Chini - a national heritage in Peril. Transp Int Malaysia 22(2):1–2
Sundaray SK (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: Mahanadi basin, India. J Hazard Mater 186(2):1837–1846
Tessier A, Campbell PG, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851
Toriman ME, Kamarudin MKA, Idris M, Jamil NR, Gazim MB, Aziz NAA (2009) Sediment concentration and load analyses at Chini River, Pekan, Pahang, Malaysia. EESRJ 1(2):43–50
Vogel-Mikuš K, Damjana D, Marjana R (2005) Zn, Cd and Pb accumulation and arbuscular mycorrhizal colonization of pennycress Thlaspi praecox Wulf. (Brassicaceae) from the vicinity of a lead mine and smelter in Slovenia. Environ Pollut 133(2):233–242
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol 2011:1–20
Xiong Y, Zhu F, Zhao L, Zhang Z (2014) Heavy metal speciation in various types of fly ash from municipal solid waste incinerator. J Mater Cycles Waste J 16(4):608–615
Yuan C, Shi J, Liu HB, Liu J, Liang L, Jiang G (2004) Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environ Int 30:769–783
Zhu H, Yuan X, Zeng G, Jiang M, Liang J, Zhang C, Yin J, Liu Z, Jiang H (2012) Ecological risk assessment of heavy metals in sediments of Xiawan Port based on modified potential ecological risk index. Trans Nonferrous Metals Soc China 22:1470–1477
Zimmerman AJ, Weindorf DC (2010) Heavy metal and trace metal analysis in soil by sequential extraction: a review of procedures. Int J Anal Chem 2010:1–7
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krishnankutty, N., Idris, M., Hamzah, F.M. et al. The chemical form and spatial variation of metals from sediment of Jemberau mining region of Tasik Chini, Malaysia. Environ Sci Pollut Res 26, 25046–25056 (2019). https://doi.org/10.1007/s11356-019-05680-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05680-3