Abstract
Cadophora luteo-olivacea has been reported as an emerging postharvest pathogen of pome fruit and kiwifruit with the potential to have a significant economic impact. To date, the biology and epidemiology of C. luteo-olivacea has been poorly investigated. Therefore, the present study aims to gain knowledge on C. luteo-olivacea biology, by analyzing the parameters that can influence the fungal growth and virulence and by getting information about the sensitivity to fungicides. A mycelial growth study at different temperatures was conducted with five C. luteo-olivacea isolates belonging to different host plants (apple and kiwifruit). The optimum fungal growth was observed in a temperature range between 20 °C and 25 °C, with C. luteo-olivacea isolates from kiwifruit that resulted in faster growth than apple isolates. The pathogenicity of C. luteo-olivacea isolates was evaluated on detached apple and kiwifruit twigs, on 'Golden Delicious' and 'Fuji' apples, and on 'Hayward' and 'Sungold' kiwifruit, stored both at 0 °C and 20 °C. The pathogenicity on fruit and on woody twigs was variable, depending on the host cultivar, with a minor effect related to the fungal isolate. Moreover, the efficacy of ten different plant protection products (PPPs) against the conidial germination of the isolates was determined. Few PPPs were found to be effective (e.g. fludioxonil, dithianon, and cyprodinil) against C. luteo-olivacea. These results represent a starting point for further research on the biology and epidemiology of C. luteo-olivacea and the development of effective management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change and the widespread global commercialization of plant materials and products are contributing factors to the outbreak of emerging or re-emerging fungal pathogens (Engering et al., 2013). In recent years, Cadophora luteo-olivacea (J.F.H. Beyma) T.C. Harr. and McNew, a fungus usually associated with trunk diseases of woody crops (Gramaje et al., 2011; Diaz et al., 2021), has been reported as an emerging postharvest pathogen of pome fruits (Malus x domestica Borkh) and kiwifruit (Actinidia spp.) (Amaral Carneiro et al., 2022; Di Francesco et al., 2022; Grantina-Ievina, 2015; Wenneker et al., 2016).
There is increasing evidence that C. luteo-olivacea is a pathogen characterized by a long quiescent period on fruit infected in the field (Di Francesco et al., 2021, 2022, 2023), followed by a transition to necrotrophic colonization, usually after several months of cold storage (Köhl et al., 2018; Di Francesco et al., 2019). It has to be noted, that this kind of infections can arise from bloom to maturity at different phenological stages of fruit development (Harteveld et al., 2014; Nemsa et al., 2012; Di Francesco et al., 2023). Nevertheless, the increasing disease incidence observed on fruit represents a critical problem during the postharvest storage with economically significant losses reported during the shelf-life of the produce (Spadaro et al., 2011; Wenneker & Thomma, 2020). Cold storage represents a crucial phase for the control of postharvest pathogens because of the lack of effective fungicides and the few active ingredients allowed in this phase of the production chain (Romanazzi et al., 2016). C. luteo-olivacea is the causal agent of side rot and skin pitting of apple and kiwifruit, respectively. On pome fruits, C. luteo-olivacea induces dark brown circular to oval lesions that commonly appear after at least three months of cold storage (Spadaro et al., 2011; Sugar, 2014; Wenneker et al., 2016). Instead, symptoms on kiwifruit develop after four months of cold storage as circular-oval skin depressions under which little brown spots are visible (Di Francesco et al., 2023). In both cases, on apple and kiwifruit, the symptomatic tissues have a spongy and dry appearance. The infection biology of C. luteo-olivacea may contribute to a variable incidence of the pathogen over time, which is furthermore influenced by environmental conditions (Spadaro et al., 2010). The epidemiology of C. luteo-olivacea is still poorly investigated, which makes the management of the disease in the field even more challenging. In order to gain knowledge on the biology of C. luteo-olivacea and its sensitivity to fungicides, the present study was set up to: i) explore the pathogenicity of five fungal isolates during cold-storage and shelf-life on different apple and kiwifruit cultivars; ii) investigate the impact of different temperatures, ranging between 0 °C to 35 °C, on the mycelial growth; iii) investigate the vegetative compatibility and the possibility of coexistence between C. luteo-olivacea isolates belonging to different hosts; iv) investigate the effect of different fungicides on the conidial germination of the pathogen.
Materials and methods
Characterization of fungal isolates
The isolates of C. luteo-olivacea used in this study were isolated from cold stored symptomatic apple and kiwifruit collected in different Italian packing houses at different times (Table 1).
Symptomatic fruit portions (3 × 3 × 3 mm) were transferred onto Potato Dextrose Agar (PDA, 39 g/L of distilled water) (Sigma, St. Louis, MO, USA) at 20 °C for 10 days, subsequently, the isolates, were purified, and single spores were grown on new PDA plates. The isolates 19-DSS-BS-3–012, 18-DSS-CAFA-2–001, 19-DSS-KA-4–060 obtained from symptomatic apples belong to the mycological collection of University of Bolzano (Amaral Carneiro et al., 2022). The isolates CAD20 and CAD21 derived from symptomatic kiwifruits, belong to the mycological collection of Di4A-University of Udine (Di Francesco et al., 2023). Morphological differences among isolates were determined on PDA and Oatmeal Agar (OA) (60 g oatmeal, 12.5 g agar technical per 1 L of distilled water) (Sigma, St. Louis, MO, USA). Inoculated dishes were incubated at 20 ºC for two weeks. Five replicates of each isolate were grown on either culture medium. Each isolate was assigned to a specific morphological group according to the classification of Gramaje et al. (2011).
Plant materials and plant protection products
Fruits of apple cultivars ‘Golden Delicious’ and ‘Fuji’ were harvested at commercial maturity in the experimental orchard of the University of Udine (46°01′56.4"N13°13′23.6"E). Kiwifruit of both ‘Hayward’ and ‘Sungold’ cultivars were harvested in orchards located in Sedegliano (Udine, Italy, 46°02′19.08″N, 12°57′33.66″E) at commercial maturity. Fruits were selected to be homogeneous in size and injury-free, and immediately processed. One-year-old lignified twigs of a length of 50 cm of ‘Golden Delicious’ apple and ‘Hayward’ kiwifruit plants were collected, and stored at 5 °C for one month before use. Ten commercial plant protection products (PPPs), belonging to different fungicide classes, were selected (Table 2). The PPPs were used at different concentrations against pathogen conidial germination to establish the EC50 values.
The effect of temperature on fungal growth
To investigate the impact of different temperatures on the mycelial growth of C. luteo-olivacea isolates, mycelial plugs (6 mm Ø) from the active edges of 10 days-old fungal colonies were inoculated on PDA and incubated at different temperatures ranging between 0 °C and 35 °C. Colony growth was measured 14 days post inoculation (dpi) at 10, 15, 20, 25, 30, and 35 °C and after 21 and 30 dpi at 5 °C and 0 °C, respectively. The diameter of radial growth was measured at two perpendicular axes and reported as mm/day of growth. Sample unit for each isolate and temperature was represented by five Petri dishes and the experiment was conducted twice.
Vegetative compatibility
The vegetative compatibility between C. luteo-olivacea isolates was checked on two different agar media: PDA and OA. Fungal mycelial plugs (4 mm Ø) were cut from the edge of active colonies and placed on each medium following a pattern whereby all plugs were spaced 1 cm apart and paired among themselves. After 14 days of incubation at 20 °C, the vegetative compatibility/incompatibility was macroscopically detected by barrage formation. The sample unit was represented by three Petri dishes. The experiment was conducted twice.
Pathogenicity test on detached fruits
Apple (‘Golden Delicious’ and ‘Fuji’) and kiwifruits (‘Hayward’ and ‘Sungold’’) were surface-sterilized by immersion for 1 min in 1% sodium hypochlorite solution and washed twice in sterile water. Fruits were wounded once by a sterile needle (2 × 2 × 2 mm) at the equatorial region and inoculated with 20 µL of C. luteo-olivacea spore suspensions (1 × 105 conidia/mL). The inoculum was prepared by using sterile water and by scraping sporulating fungal colonies of 10 days, adjusting to the final concentration by using a hemocytometer. Fruit wounds inoculated with sterile water were used as negative control. A batch of fruits was stored at 20 °C and 80% of relative humidity (R.H.) for four weeks, another batch was stored for four months at 0 °C, R.H. 90%. The sample unit was represented by 30 fruits per isolate at each storage condition.
The disease severity was assessed as lesion diameter (mm) with a caliper on apple and kiwifruit and recorded at different storage temperatures 0 °C and 20 °C after 4 months and 1 month, respectively. The experiment was performed twice.
Pathogenicity tests of detached twigs
Thirty twigs of both ‘Golden Delicious’ and ‘Hayward’ cultivars were cut in 10 cm segments, sterilized with 90% ethanol, rinsed with tap water, surface disinfected with 1% hypochlorite, rinsed with tap water and air dried. Three little portions of the bark were lifted by using a sterile scalpel and inoculated with 10 µL of spore suspension (1 × 105 conidia/mL) of each isolate. Wounds were covered with Parafilm® (Pechiney Plastic Packaging, USA) and the twigs were inserted in glass dishes containing sterile filter paper on the bottom, soaked daily with 1 mL of sterile water. Twigs inoculated with sterile water were used as negative controls. Twigs were incubated at room temperature (20 °C), with 12-h dark and 12-h light at 70% of RH for 1 month, and then the lesions were macroscopically evaluated. The pathogens were reisolated from the necrotic lesions on PDA and morphologically (conidia and mycelium) compared with the original strains. Symptoms images were taken by a digital camera (Canon E0S 3 Digital) and lesion color intensity was analyzed by using ImageJ software (1.8.0) to evaluate the severity intensity index (SI). The SI was rated on a scale from 0 to 100, where values close to zero denoted darker lesion and higher disease severity index and values close to 100, clearer tissue and lower disease severity index. The experiment was repeated twice.
Efficacy of plant protection products (PPPs) on conidial germination of C. luteo-olivacea
The sensitivity of C. luteo-olivacea to a selection of commercial PPPs, usually used in apple and kiwifruit orchards, was assessed. PDA medium was amended with each formulated commercial product at different concentrations by evaluating the inhibitory effect on each isolate conidial germination.
Starting from the recommended application dose of each PPP (Table 2), other 4 doses (2x, 0.5x, 0.25x, 0.12x) were used to amend liquid PDA medium. One hundred µL of spore suspensions (1 × 103 conidia/mL) of each isolate were spread on amended media and incubated at 20 °C for seven days. The medium without any addition of PPPs was used as control. The sample unit was represented by five dishes for each combination of isolate × PPP dose. The experiment was conducted twice.
Statistical analysis
Data were subjected to one-way ANOVA analysis. The separation of means was performed with a Tukey’s test (α < 0.05) by using the software MiniTab.16. For the in vivo assays, data were submitted to two-way ANOVA analysis. Least significant difference (LSD) at P < 0.05 was used to separate means of significant ANOVA factors (cultivar, isolate, temperature). The EC50 value of each PPP was calculated using the probit analysis applied to the percentage of inhibition of conidial germination (Lesaffre & Molenberghs, 1991).
Results
Isolates morphological characteristics
Considering the morphological characteristics of the fungal colonies, isolates 19-DSS-BS-3–012, 18-DSS-CAFA-2–00, 19-DSS-KA-4–060 could be assigned to group 4, while CAD20 and CAD21 to groups 2 and 3, respectively (Table 3). Cadophora luteo-olivacea colonies on PDA varied in color from white to grey-olivaceous and the mycelium resulted flat, felty, and cottony in the center with an even edge. Instead, fungal colonies grown on OA varied in color, from olivaceous-buff to greenish olivaceous. Cadophora luteo-olivacea isolates, 18-DSS-CAFA-2–001, 19-DSS-BS-3–012, 19-DSS-KA-4–060 and CAD21, produced yellow pigmentation on OA as shown in Fig. 1. Isolate CAD21 produced yellow pigmentation also on PDA.
The effect of temperature on radial growth of C. luteo-olivacea
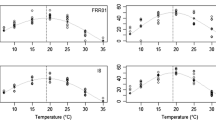
The effect of temperature on mycelial growth of C. luteo-olivacea isolates was assessed considering the temperature range between 0 °C and 35 °C. The optimum growth temperature for the five C. luteo-olivacea isolates was observed in a range of 20 °C to 25 °C with 2.52 ± 0.11 mm/day on average of growth (Fig. 2A). The colony growth of the isolates significantly decreased both below 15 °C and over 30 °C. Indeed, at 10 °C and 5 °C, the fungal growth was of 1.66 ± 0.08 and 0.62 ± 0.02 mm/day, respectively. Interestingly, at 0 °C mycelial growth was observed in a range of 0.25 to 0.46 mm/day. At 35 °C no fungal growth was observed. Moreover, the fungal development at cold storage temperature was further monitored. After two- and three-months C. luteo-olivacea colonies reached a diameter on average of 27.4 ± 1.4 and 40 ± 1.9 mm, respectively (data not shown). By grouping the fungal isolates according to their host source, significant differences between groups were observed at 10 °C, 20 °C, 25 °C, and 30 °C, with kiwifruit isolates showing a faster growth compared to the isolates derived from apple (Fig. 2B).
Temperature influence on Cadophora luteo-olivacea isolates growth. The five Cadophora luteo-olivacea isolates were grown on Potato dextrose agar. Colony growth (mm/d) was measured 14 days post inoculation at 10, 15, 20, 25, 30, and 35 °C and after 21 and 30 days at 5 °C and 0 °C, respectively. (A) Mean value (± standard error) of fungal growth calculated among the five isolates per temperature. Different letters indicate significant differences according to Tukey’s test (α = 0.05). (B) Mean value (± standard error) of fungal growth calculated among the C. luteo-olivacea isolates from kiwifruit (light grey) and the apple fruit subgroup (dark grey). Asterisks indicate values that differ significantly in the pairwise comparison of kiwi-and apple fruit isolates subgroup
Vegetative compatibility
All C. luteo-olivacea isolates were paired with each other in a vegetative compatibility test conducted on PDA and OA media. Table 4 reports the results of the pairings among the isolates on OA, the only of the two media that resulted in the production of clear barrages between the tested isolates (Fig. 3). Four pairings were compatible (C). Vegetative compatibility among all the three C. luteo-olivacea isolates from apple was observed. Isolate CAD21 from kiwifruit resulted incompatible (I) with all the other tested isolates. Conversely, the kiwifruit isolate CAD20 resulted compatible only with the apple isolate 19-DSS-KA-4–060.
Vegetative compatibility test of Cadophora luteo-olivacea isolates on A) Oatmeal agar and C) Potato dextrose agar. The inoculated plates were incubated for 14 days at 20 °C. Scheme of the isolates combination B): CAD20 (A); CAD21 (B); 19-DSS-BS-3–012 (C), 19-DSS-KA-4–060 (D) and 18-DSS-CAFA-2–001 (E)
Pathogenicity tests on detached fruits and twigs
Fungal isolates successfully infected both apple and kiwifruit. The lesions slowly developed on both fruit species and at the two different tested storage temperatures (0 °C and 20 °C).
Taken together, ‘Golden Delicious’ displayed a higher susceptibility compared to ‘Fuji’ apples, with lesion diameters of 5.9 ± 0.41 mm and 3.3 ± 0.31 mm, respectively (Table 5a). Isolate 18-DSS-CAFA-2–001 resulted in the greatest lesion diameter on both ‘Golden Delicious’ and ‘Fuji’ apples. The storage conditions did not significantly impact the activity of the isolates and the lesion diameter, even if it must be considered that the incubation time was different. Indeed, at cold storage temperature after four months of incubation reached the value of 4.6 mm, approximately the same value was obtained after one month at shelf-life temperature. Regarding kiwifruit, only the cultivar represented a significant discriminating parameter. ‘Hayward’ was susceptible, whereas ‘Sungold’ showed a high resistance to skin pitting disease, with lesion diameters of 3.5 ± 0.32 mm and 0.0 ± 0.0 mm, respectively. In kiwifruit, CAD20 and CAD21 produced lesion diameters slightly larger than the isolates from apple (Table 5b). On the fruits used as the negative control, no rotten tissue appeared.
The pathogenicity of C. luteo-olivacea isolates on twigs of the apple cultivar ‘Golden Delicious’ was observed. Specifically, all the inoculated isolates produced visible necrosis on twigs. The isolates 18-DSS-CAFA-2–001 and 19-DSS-BS-3–012 displayed a higher SI with respect to the untreated control (Table 6 and Fig. 4). Interestingly, no visible symptoms were detected on the twigs of the kiwifruit cultivar ‘Hayward’.
Pathogenicity of Cadophora luteo-olivacea isolates on apple twigs. The twigs were artificially wounded and inoculated with the spore suspension (1 × 104 conidia/mL) of each C. luteo-olivacea isolate: 18-DSS-CAFA-2–001 (B), 19-DSS-BS-3–012 (C), 19-DSS-KA-4–060 (D), CAD21 (E), CAD20 (F). Twigs inoculated with sterile water were used as negative control (A). Necroses were assessed after 30 days at 20 °C
Efficacy of plant protection products (PPPs) on the inhibition of conidial germination of C. luteo-olivacea
The derived EC50 values for conidial germination inhibition of the tested C. luteo-olivacea isolates ranged between 0.0001 and > 10 g/L of PPP. Results reported in Table 7 show that Geoxe (Fludioxonil), 3logy (Thymol, Geraniol, Eugenol), Kuki (Dithianon), Syllit (Dodine), and Chorus (Cyprodinil) were the most effective PPPs on conidial growth inhibition, showing the total suppression of all C. luteo-olivacea isolates at low concentrations (< 0.0001 g/L).
Poltiglia dispress (Copper sulfate), Nando (Fluazinam), and Cantus (Boscalid) displayed the lowest antifungal effect against the tested isolates of C. luteo-olivacea. For these PPPs, EC50 ranged from 2.37 to > 10.0 g/L: values above the recommended application dosages provided on the official labels (Table 7). The isolates CAD20 and 18-DSS-CAFA-2–001 were found to have a more pronounced resistance against the tested PPPs, while CAD21 and 19-DSS-BS-3–012 were observed to be more sensitive (Table 7).
Discussion
The present study aimed to characterize selected isolates of C. luteo-olivacea to induce rot on different cultivars of apple and kiwifruit, and to cause lesions on lignified branch tissue. The results indicate that the pathogenicity of different isolates on fruits was highly influenced by the host and intrinsic characteristics of each isolate.
In fact, C. luteo-olivacea isolates showed differences of virulence by considering fruit infection and wood colonization. Regarding the latter, only twigs obtained from apple trees that were artificially inoculated with conidial suspension of C. luteo-olivacea showed necrosis, highlighting the ability of the fungus to penetrate via wounds into lignified tissue. In contrast, no necroses were observed on kiwifruit twigs in the current experiment, even though C. luteo-olivacea and Cadophora melinii were isolated from the wood of kiwifruit trees affected by trunk hypertrophy and longitudinal bark crack (Prodi et al., 2008). The observed necroses on apple twigs could serve as an overwintering site for C. luteo-olivacea, as suggested in the case of other pre- and postharvest pathogens, like Neonectria ditissima, Colletotrichum acutatum, and Colletotrichum gloeosporioides (Shuttleworth, 2021; Wenneker & Thomma, 2020). Considering the fruit, the apple cultivar ‘Golden Delicious’ showed a higher susceptibility to side rot displaying lesion diameters 1.7-fold larger with respect to ‘Fuji’. Spotts et al. (1999) reported that the main commercial apple cultivars displayed a different degree of susceptibility to fungal pathogens. It has been reported that in Europe several late-harvest apple cultivars, such as ‘Golden Delicious’ and ‘Fuji’, are particularly susceptible to fungal diseases like bull’s eye rot and bitter rot (Neri et al., 2009; Velho et al., 2015). However, the limited information available about the relative resistance of apple cultivars toward the main postharvest diseases are often contradictory, due to the lack of a standardized pathogenicity test design and different plant material (e.g., origin of the fruits from different management practices and/or fruit ripeness degree) (Konstantinou et al., 2011; Spotts et al., 1999).
Looking at kiwifruit, ‘Sungold’ was found to be resistant to the tested isolates of C. luteo-olivacea. Conversely, skin pitting symptoms were detected in yellow-fleshed cultivar ‘Hort16A’ by Manning et al. (2003). Whereas a high susceptibility was confirmed for ‘Hayward’ as reported by Spadaro et al. (2010) and Di Francesco et al. (2022). The effect of host genotype on disease resistance was also reported considering Botrytis cinerea infection. ‘Hort16A’ showed a greater resistance compared to ‘Hayward’, both in terms of disease severity and incidence (Wurms, 2005).
The genetic differences among strains belonging to the same species can represent another important factor, that could influence their virulence and aggressiveness on a specific host. Diversity within species is the result of continuous processes of mutation and subsequent selection (Van Rossum et al., 2020). In our study, the tested isolates showed morphological variation, low differences in aggressiveness (except for the isolate 18-DSS-CAFA-2–001), marked vegetative compatibility and the ability to infect different hosts (cross-infection ability). Even though, the relevance of the morphological categories is questionable (Gramaje et al., 2011), and the genetic base of these differences and possible relation between morphotype and virulence has to further investigate (Kowalski & Cramer, 2020). The cross-infection ability represents a very important topic for understanding pathogens dissemination and evolution (Leslie, 1993). To better characterize C. luteo-olivacea isolates, a vegetative compatibility test was performed. A high level of hyphal anastomosis was detected between the isolates belonging from the same isolation host except for kiwifruit isolates that resulted incompatible within each other. Nevertheless, the co-existence of different isolates, also belonging to different hosts (e.g., CAD20 and 19-DSS-KA-0–060, isolated from kiwifruit and apple fruit, respectively) may have implications to be considered for establishing effective control strategies. In fact, different isolates may manifest different sensitivity to different fungicides.
Cadophora luteo-olivacea is often considered a pathogen of minor importance and therefore limited information about control strategies are currently available (Di Francesco et al., 2021). Some previous studies investigated the use of different biocontrol agents, basic substances, and physical treatments to control C. luteo-olivacea in the field (Di Francesco et al., 2021, 2022, 2023). No studies conducted in vitro and in vivo, so far, were focused on the effectiveness of common fungicides used in different agricultural systems (organic, conventional, and integrated) to potentially control C. luteo-olivacea. The most effective active substances inhibiting the conidial germination of the isolates were fludioxonil, dithianon, dodine, and cyprodinil, which represent the most important fungicides in apple crop protection (Ticha et al., 2008). These active substances are mainly applied in the field to control Venturia spp. but were reported to act versus a plethora of pathogens of pome fruit like Alternaria spp., B. cinerea, Gloeosporium spp., and Penicillium spp. (Table 2). Interestingly, fluodixonil was reported to be effective in controlling Phlyctema vagabunda on apple as postharvest treatment (Lolas et al., 2015; Russouw et al., 2021). In fact, the use of fludioxonil is also admitted for postharvest applications. However, the concern for its potential impact on human health has recently emerged (Brandhorst & Klein, 2019). The preharvest application of dodine, showed variable results in controlling storage fruit rot (Minář, 2006, Ticha et al., 2008), even if it was recently reported as a promising active substance for controlling the emergent white haze disease, caused by the epiphytic fungus Tilletiopsis spp. on apple in Northern Italy (Angeli et al., 2022). Promising results were revealed for a product based on three plant terpenes (eugenol, thymol, geraniol), which is authorized to be used in organic agriculture to control gray mold on kiwifruit. The commercial product allowed in organic agriculture and based on geraniol, thymol and geraniol showed similar efficacy in inhibiting the conidial germination as some of the most effective synthetic fungicides.
The less effective active compounds against C. luteo-olivacea conidial germination were boscalid, fludioxonil, orange oil, fluazinam, and copper sulfate. Field experiments reported that boscalid did not provide a consistent significant reduction of many apple rots (Everett & Timudo-Torrevilla, 2007). The only reduction of rot incidence and severity were achieved probably for the persistence of this fungicide, able to prevent some later infections (Everett & Timudo-Torrevilla, 2007). Copper sulfate resulted non-effective against other apple pathogens, such as C. acutatum (Everett et al., 2015). This PPP has the potential to damage the fruit skin and to promote pathogen entry (Everett et al., 2015). Moreover, Cadophora spp. has been reported to be tolerant to several heavy metals (Karunasekera & Daniel, 2013; Likar & Regvar, 2013). For instance, the gene expression of Cadophora finlandica encoding for several extracellular proteins and transporters was observed in response to cadmium exposition (Gorfer et al., 2009).
Conclusions
In conclusion, the findings of the current study provided new information on C. luteo-olivacea isolate diversity, mostly in terms of virulence and efficacy of several fungicides. With regard to this, more research into fungal biology and epidemiology is required to better understand the relationships among fungal virulence, latency, and cross-infection ability in relation to climate change, in order to reduce the risk of C. luteo-olivacea incidence in postharvest, and to determine the best time to implement sustainable disease management strategies.
Data availability
Data supporting this research is available upon reasonable request from the last author.
References
Amaral Carneiro, G., Walcher, M., & Baric, S. (2022). Cadophora luteo-olivacea isolated from apple (Malus domestica) fruit with post-harvest side rot symptoms in northern Italy. European Journal of Plant Pathology, 62, 247–255. https://doi.org/10.1007/s10658-021-02388-4
Angeli, D., Turrini, L., Zeni, F., Longa, C. M. O., Gualandri, V., & Roman, T. (2022). Controlling white haze disease under in vitro controlled conditions. In VI International Symposium on Postharvest Pathology: Innovation and Advanced Technologies for Managing Postharvest Pathogens, 1363, 95–100.
Brandhorst, T. T., & Klein, B. S. (2019). Uncertainty surrounding the mechanism and safety of the post-harvest fungicide fludioxonil. Food Chemical Toxicology, 123, 561–565. https://doi.org/10.1016/j.fct.2018.11.037
Di Francesco, A., Cameldi, I., Neri, F., Barbanti, L., Folchi, A., Spadoni, A., & Baraldi, E. (2019). Effect of apple cultivars and storage periods on the virulence of Neofabraea spp. Plant Pathology, 68, 1525–1532. https://doi.org/10.1111/ppa.13074
Di Francesco, A., Di Foggia, M., Vittoria, A., & Baraldi, E. (2021). Post-harvest non-conventional and traditional methods to control Cadophora luteo-olivacea: Skin pitting agent of Actinidia chinensis var. deliciosa (A. Chev.). Horticulturae, 7, 169. https://doi.org/10.3390/horticulturae7070169
Di Francesco, A., Di Foggia, M., Baldo, D., Ratti, C., & Baraldi, E. (2022). Preliminary results on Cadophora luteo-olivacea pathogenicity aspects on kiwifruit. European Journal of Plant Pathology, 163, 997–1001. https://doi.org/10.1007/s10658-022-02518-6
Di Francesco, A., Jabeen, F., Di Foggia, M., Zanon, C., Cignola, R., Sadallah, A., & Martini, M. (2023). Study of the efficacy of bacterial antagonists against Cadophora luteo-olivacea of kiwifruit. Biological Control, 180, 105199. https://doi.org/10.1016/j.biocontrol.2023.105199
Díaz, J. A., Garza-García, J. J., Zamudio-Ojeda, A., León-Morales, J. M., López-Velázquez, J. C., & García-Morales, S. (2021). Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. Journal of the Science of Food and Agricultrure, 101, 1270–1287. https://doi.org/10.1002/jsfa.10767
Engering, A., Hogerwerf, L., & Slingenbergh, J. (2013). Pathogen–host–environment interplay and disease emergence. Emerging Microbes & Infections, 2, 1–7. https://doi.org/10.1038/emi.2013.5
Everett, K. R., Pushparajah, I. P. S., Taylor, J. T., Timudo-Torrevilla, O. E., Spiers, T. M., & Chee, A. A. (2015). Evaluation of fungicides for control of bitter and sprinkler rots on apple fruit. New Zealand Plant Protection, 68, 264–274. https://doi.org/10.30843/nzpp.2015.68.5801
Everett, K. R., & Timudo-Torrevilla, O. E. (2007). In vitro fungicide testing for control of avocado fruit rots. New Zealand Plant Protection, 60. https://doi.org/10.30843/nzpp.2007.60.4632
Gorfer, M., Persak, H., Berger, H., Brynda, S., Bandian, D., & Strauss, J. (2009). Identification of heavy metal regulated genes from the root associated ascomycete Cadophora finlandica using a genomic microarray. Mycological Research, 113, 1377–1388. https://doi.org/10.1016/j.mycres.2009.09.005
Gramaje, D., Mostert, L., & Armengol, J. (2011). Characterization of Cadophora luteo-olivacea and C. melinii isolates obtained from grapevines and environmental samples from grapevine nurseries in Spain. Phytopathologia Mediterranea, 50, S112-S126. https://www.jstor.org/stable/26458715
Grantina-Ievina, L. (2015). Fungi causing storage rot of apple fruit in integrated pest management system and their sensitivity to fungicides. Rural Sustainaibility Research, 34, 2–11. https://doi.org/10.1515/plua-2015-0007
Harteveld, D. O. C., Akinsanmi, O. A., Chandra, K., & Drenth, A. (2014). Timing of infection and development of Alternaria diseases in the canopy of apple trees. Plant Disease, 98, 401–408. https://doi.org/10.1094/PDIS-06-13-0676-RE
Karunasekera, H., & Daniel, G. (2013). Molecular identification and phylogenic analysis by sequencing the rDNA of copper-tolerant soft-rot Phialophora spp. International Biodeterioration and Biodegradation, 82, 45–52. https://doi.org/10.1016/j.ibiod.2013.01.019
Kohl, J., Wenneker, M., Groenenboom-de Haas, B. H., Anbergen, R., Goossen-van de Geijn, H. M., Lombaers-van der Plas, C. H., Pinto, F. A. M., & Kastelein, P. (2018). Dynamics of post-harvest pathogens Neofabraea spp. and Cadophora spp. in plant residues in Dutch apple and pear orchards. Plant Pathology, 67, 1264–1277. https://doi.org/10.1111/ppa.12854
Konstantinou, S., Karaoglanidis, G. S., Bardas, G. A., Minas, I. S., Doukas, E., & Markoglou, A. N. (2011). Postharvest fruit rots of apple in Greece: Pathogen incidence and relationships between fruit quality parameters, cultivar susceptibility, and patulin production. Plant Disease, 95, 666–672. https://doi.org/10.1094/PDIS-11-10-0856
Kowalski, C. H., & Cramer, R. A. (2020). If looks could kill: Fungal macroscopic morphology and virulence. PLoS Pathogens., 16, e1008612. https://doi.org/10.1371/journal.ppat.1008612
Lesaffre, E., & Molenberghs, G. (1991). Multivariate probit analysis: A neglected procedure in medical statistics. Statistics in Medicine, 10, 1391–1403. https://doi.org/10.1002/sim.4780100907
Leslie, J. F. (1993). Fungal vegetative compatibility. Annual Review of Phytopathology, 31, 127–150. https://doi.org/10.1146/annurev.py.31.090193.001015
Likar, M., & Regvar, M. (2013). Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L.. Plant and soil, 370, 593–604. https://www.jstor.org/stable/42952691
Lolas, M., Díaz, G., Mendez, R., Cáceres, M., & Neubauer, L. (2015). Evaluation of the efficacy of fungicide fludioxonil in the postharvest control of bull's eye rot (Neofabraea alba) in Chile. In III International Symposium on Postharvest Pathology: Using Science to Increase Food Availability 1144 (pp. 461–464).
Manning, M. A., Meier, X., Olsen, T. L., & Johnston, P. R. (2003). Fungi associated with fruit rots of Actinidia chinensis ‘Hort16A’in New Zealand. New Zealand Journal of Crop and Horticulture Science, 31, 315–324. https://doi.org/10.1080/01140671.2003.9514267
Minář, P. (2006). Effect of late summer treatments by strobilurines on storage diseases of apples. Acta Universitatis Agriculturae Et Silviculturae Mendelianae Brunensis, 54, 39–44. https://doi.org/10.11118/actaun200654040039
Nemsa, I., Hernández, M. A., Lacasa, A., Porras, I., García-Lidón, A., Cifuentes, D., & Del Río, J. A. (2012). Pathogenicity of Alternaria alternata on fruits and leaves of ‘Fortune’ mandarin (Citrus clementina × Citrus tangerina). Canadian Journal of Plant Pathology, 34, 195–202. https://doi.org/10.1080/07060661.2012.676570
Neri, F., Mari, M., Brigati, S., & Bertolini, P. (2009). Control of Neofabraea alba by plant volatile compounds and hot water. Postharvest Biology and Technology., 51, 425–430. https://doi.org/10.1016/j.postharvbio.2008.08.006
Prodi, A., Sandalo, S., Tonti, S., Nipoti, P., & Pisi, A. (2008). Phialophora-like fungi associated with kiwifruit elephantiasis. Journal of Plant Pathology, 90, 487–494. https://www.jstor.org/stable/41998543
Romanazzi, G., Smilanick, J. L., Feliziani, E., & Droby, S. (2016). Integrated management of postharvest gray mold on fruit crops. Postharvest Biology and Technology, 113, 69–76. https://doi.org/10.1016/j.postharvbio.2015.11.003
Russouw, A., Meitz-Hopkins, J., Den Breeyen, A., & Lennox, C. (2021). Postharvest applications of fludioxonil and pyrimethanil to control Phlyctema vagabunda on apple in South Africa. Crop Protection, 141, 105451. https://doi.org/10.1016/j.cropro.2020.105451
Shuttleworth, L. A. (2021). Alternative disease management strategies for organic apple production in the United Kingdom. CABI Agriculture and Bioscience, 2, 34.
Spadaro, D., Galliano, A., Pellegrino, C., Gilardi, G., Garibaldi, A., & Gullino, M. L. (2010). Dry matter and mineral composition, together with commercial storage practices, influence the development of skin pitting caused by Cadophora luteo-olivacea on kiwifruit "Hayward". Journal of Plant Pathology, 92, 339–346.
Spadaro, D., Pellegrino, C., Garibaldi, A., & Gullino, M. L. (2011). Development of SCAR primers for the detection of Cadophora luteo-olivacea on kiwifruit and pome fruit and of Cadophora malorum on pome fruit. Phytopathologia Mediterranea, 50, 430–441. https://doi.org/10.14601/Phytopathol_Mediterr-9457
Spotts, R. A., Cervantes, L. A., & Mielke, E. A. (1999). Variability in postharvest decay among apple cultivars. Plant Disease, 83, 1051–1054. https://doi.org/10.1094/PDIS.1999.83.11.1051
Sugar, D. (2014). Side rot. In T. B. Sutton, H. S. Aldwinckle, A. M. Agnello, J. F., & Walgenbach (Eds.), Compendium of apple and pear diseases and pests (2nd ed., pp. 81–82). St. Paul, MN: The American Phytopathological Society.
Ticha, J., Hajslova, J., Jech, M., Honzicek, J., Lacina, O., Kohoutkova, J., & Falta, V. (2008). Changes of pesticide residues in apples during cold storage. Food Control, 19, 247–256. https://doi.org/10.1016/j.foodcont.2007.03.011
Van Rossum, T., Ferretti, P., Maistrenko, O. M., & Bork, P. (2020). Diversity within species: Interpreting strains in microbiomes. Nature Reviews Microbiology, 18, 491–506. https://doi.org/10.1038/s41579-020-0368-1
Velho, A. C., Alaniz, S., Casanova, L., Mondino, P., & Stadnik, M. J. (2015). New insights into the characterization of Colletotrichum species associated with apple diseases in southern Brazil and Uruguay. Fungal Biology., 119, 229–244. https://doi.org/10.1016/j.funbio.2014.12.009
Wenneker, M., & Thomma, B. P. (2020). Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. European Journal of Plant Pathology, 156, 663–681. https://doi.org/10.1007/s10658-020-01935-9
Wenneker, M., Pham, K. T. K., Lemmers, M. E. C., de Boer, F. A., van Leeuwen, P. J., Hollinger, T. C., Groenenboom-de Haas, B. H., & Köhl, J. (2016). First report of Cadophora luteo-olivacea causing side rot on ‘Conference’ pears in the Netherlands. Plant Disease, 100, 10. https://doi.org/10.1094/PDIS-02-16-0212-PDN
Wurms, K. V. (2005). Susceptibility to Botrytis cinerea, and curing-induced responses of lytic enzymes and phenolics in fruit of two kiwifruit (Actinidia) cultivars. New Zealand Journal of Crop and Horticulture Science, 33, 25–34. https://doi.org/10.1080/01140671.2005.9514327
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tomada, S., Staffler, E., Dionis, G. et al. Cadophora luteo-olivacea on apple and kiwifruit: characterization of selected strains and evaluation of fungicides for their control. Eur J Plant Pathol 169, 99–111 (2024). https://doi.org/10.1007/s10658-023-02811-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02811-y