Abstract
European Canker and Neonectria fruit rot (NFR), caused by Neonectria ditissima, has become a major problem for the production of apples (Malus domestica) in Brazil. This study characterized environmental factors affecting in vitro growth and germination of Neonectria ditissima as well as infection, colonization and reproduction, ex vivo, on ‘Gala’ and ‘Eva’ fruit. Temperatures between 17 to 20 °C were optimum for mycelial growth, and minimal growth occurred at 35 °C. Micro/macroconidia sporulation ratio was lower at 10 °C for most of the isolates tested, and no macroconidia were produced at 30 °C. More than 70% of conidia germinated after 12 h of exposure to constant humidity incubation and a temperature of 25 °C. Germination rate as high as 90% occurred after 48 h regardless of the temperature. Incubation of 2 h with high humidity is not sufficient for germination of 10% of conidia at any temperature between 10 to 30 °C. The cultivars Eva and Gala did not differ in relation to incubation period. A minimum of 25 days was necessary for sporulation on fruit incubated at 16.8–21.7 °C. Unwounded fruit did not present symptoms even 40 days after inoculation on intact epidermis, demonstrating the need of wounds for NFR development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of apples (Malus domestica Borkh) is constrained by several factors including fungal diseases that are damaging to the crop. Among them, European Canker, caused by Neonectria ditissima (Tul. & C. Tul.) Samuels & Rossman (syn. Nectria galligena Bres), anamorph Cylindrocarpon heteronema (Berk. & Broome) Wollenw., is a disease of worldwide occurrence and damaging to apple production in the most important production regions of Europe, North America, Chile, Australia, New Zealand, Japan and South Africa (Beresford and Kim 2011). In Brazil, the disease was officially reported to occur in the southernmost states Rio Grande do Sul and Santa Catarina in 2012, and five years later, the disease was detected in two municipalities of Paraná state. Currently, the pathogen is classified as an A2 quarantine pest in Brazil, which means that the organism is already present in the country but exhibits localized distribution and is targeted by an official control program (Brasil 2013).
N. ditissima causes canker-like lesions in twigs and trunks, while in fruit it causes a symptom of soft rot, which may be pre-harvest (in the orchard) or post-harvest (during storage) (Xu and Robinson 2010). The disease can cause serious damage due to fruit rot in orchards with high inoculum on twigs and trunks. However, even though the infection of woody tissues has generally been well elucidated, few studies have investigated the infection of fruit by the fungus, and most of the existing studies were performed by Swinburne in the 1970s (Brown and Swinburne 1971; Swinburne 1971, 1973, 1974, 1975) using apple cultivars that are not grown in Brazil. In addition, studies on the behavior of the pathogen in fruit under the climatic conditions of the southern region of Brazil are lacking. In this region the disease occurs at more severe levels when compared with other countries, probably due to the more favorable climatic conditions or to the greater amount of inoculum in orchards, in addition to a lack of experience in the disease management (Alves and Czermainski 2015).
The literature is inconsistent regarding the name of the disease affecting fruit. When the symptoms occur during the pre-harvest period, the disease is often called ‘eye rot’, ‘stylar-end rot’, ‘dry eye rot’ or ‘calyx rot’, which are generic terms used for symptoms that are caused by many fungal pathogens in apple (Weber and Dralle 2013). Furthermore, during the post-harvest period, Brown and Swinburne (1971), Swinburne (1974), and Noble and Drisdale (1983) referred to the disease as ‘Nectria galligena apple rot’; Xu and Robinson (2010) named the disease as ‘Nectria rot’. Sutton et al. (2014) used the term ‘Neonectria galligena fruit rot’ and Maxin et al. (2014) used the term ‘Neonectria rot’. In the present study, the disease is referred to as ‘Neonectria fruit rot’ (NFR), and we propose this name to be used as a reference for the disease.
‘Gala’ and its clones are widely cultivated in the world and represent ~65% of apple production in Brazil, which was estimated at 1.30 million tons in 2017 (FAOSTAT 2014). Meanwhile, ‘Eva’ apple, which was developed by the Instituto Agronômico do Paraná (Iapar) as a cross between ‘Anna’ and ‘Gala’, is being grown at a large scale in Paraná state, owing to its high productivity, low chill requirements, and early production, when compared with ‘Gala’ and ‘Fuji’ apples, and adaptation to the tropical and subtropical climates that are predominant in Brazil (Hauagge and Tsuneta 1999). Another reason for the expansion of ‘Eva’ apple is the low susceptibility of the cultivar to Glomerella leaf spot (Colletotrichum spp.), a yield-limiting disease of ‘Gala’ apples grown at regions of tropical and subtropical climates. The cultivar is also gaining market due to its color, taste, and wide acceptance by consumers (Oliveira et al. 2011).
Even though previous studies have reported the susceptibility of ‘Gala’ apples to European Canker (Alves and Nunes 2017) and NFR (Xu and Robinson 2010), the monocyclic components of fruit rot in this cultivar are yet to be investigated. For example, there is no information in the literature regarding the susceptibility of ‘Eva’ fruit to the pathogen or regarding the pathogen’s incubation and latent periods.
The infectivity-related physiological traits of the pathogen, such as mycelial growth, sporulation at different temperatures, and production of macroconidia and microconidia (Weber 2014) have not been extensively studied in isolates from Brazil. Those studies are important to better understand the behavior of the pathogen under different environmental conditions. Latorre et al. (2002) reported that the in vitro germination of the species’ conidia occurred at 6–32 °C, provided that sufficient moisture conditions were maintained, but no information about conidia germination of Brazilian isolates at different temperatures and incubation periods are available.
The objectives of the present study were to evaluate i) the effect of temperature on mycelial growth and sporulation of N. ditissima, ii) the effect of temperature and incubation period on the germination of N. ditissima conidia, and iii) the susceptibility of wounded and unwounded ‘Eva’ and ‘Gala’ fruit to N. ditissima and the incubation and latent periods of NFR on both cultivars.
Material and methods
Isolates
Four isolates of N. ditissima were used in this study. These were collected in 2012 (Codes 32 and I8, from Embrapa collection, Genbank accession numbers MK564529 and MK564532, respectively) and 2016 (Codes FRR01 and EMB, Genbank accession numbers MK564531 and MK564530, respectively). They were isolated from symptomatic twigs of apple trees cv. Gala from commercial and experimental orchards of the city of Vacaria, in Rio Grande do Sul state, southern Brazil.
Twigs were cut into pieces of about 10 cm, and had their cortex removed with sterilized blade. The area of transition of the healthy and the darkened diseased tissue was cut into pieces of about 5 mm and these were disinfested by immersion in 70% alcohol solution for 1 min, followed by a 3-min immersion in sodium hypochlorite 1% solution and three rinses in sterilized distilled water. The pieces were then transferred to Petri dishes containing water agar (WA) medium and incubated at 20 °C in the dark. When colonies started to form, mycelial plugs were transferred to Petri dishes with potato dextrose agar (PDA) medium and incubated for 15 days at 20 °C in the dark. Isolates were confirmed as pathogenic to wounded ‘Gala’ apple fruit, as this is a known susceptible cultivar to NFR (data not shown). After growth, the fungi were removed from the culture medium, preserved in 2-mL microtubes containing silica and kept at 4 °C.
Effect of temperature on mycelial growth and sporulation
Mycelial growth was measured on PDA plates, with three replicates. The colony diameter was measured after the fungi had been incubated at different temperatures (7, 10, 15, 20, 25, 30 and 35 °C) for 14 days in Biochemical Oxygen Demand growth chambers (BOD) with 12-h photoperiod to assess radial growth. A beta-generalized model (Bassanezzi et al., 1998) was fitted to mycelial growth data under different temperature and is given by:
where, Yopt refers to the value of colony diameter (mm) at the optimum temperature; T is the temperature (°C); Tmin, Topt and Tmax the minimum, optimum and maximum temperatures, respectively; B3 represents the amplitude of the curve in its asymptotic range. The analyses were performed using the R software (R Core Team 2016).
To measure sporulation, conidia from each isolate previously incubated at different temperatures were collected by adding 5 mL of sterile distilled water and then gently scraping the mycelial surface with a plastic Drigalsky spatula, with a final volume of 5 mL for each conidial suspension in sterile water, and the total conidial production (macro and microconidia counted separately) in the suspension was counted with a hemacytometer with a light microscope (Olympus®). From the total number of conidia and the area of the colony from which they were taken, obtained by the formula of the area of a circle, the amount of conidia produced per square millimeter of colony was calculated. Data was expressed as n° of conidia. mm−2. All the replicate plates were examined for each isolate. The experiments were completely randomized in a 4 × 7 factorial scheme, consisting of four isolates at seven temperatures. They were conducted twice and the data from the two experiments were combined for the analysis.
Effect of temperature and humidity on conidia germination
For this experiment, only the ‘EMB’ isolate was used. To measure germination frequency at different temperatures of 7, 10, 15, 20, 25, 30 and 35 °C and incubation periods with humidity of 3, 4, 6, 8, 9, 12, 24, 30 and 48 h, four 40 μL aliquots of suspension at the concentration of 105 macroconidia mL−1 were deposited on a glass Petri dish (15 × 90 mm). The plates were placed in plastic boxes (Gerbox) disinfested with alcohol 70%, containing a cotton soaked in sterile distilled water to maintain humidity. Gerbox containing the plates were wrapped in aluminum foil to keep them in the dark, and then stored in BOD growth chambers at different temperatures. After the time intervals of the incubation periods, 20 μL of lactophenol with cotton blue dye was added to each drop to stop the germination process. The percentage of conidia that germinated was determined by examining 100 conidia at each of the four aliquots in the Petri dish with a light microscope (Olympus®), and the results for each repetition were the mean of the four aliquots. Conidia with a germ tube length at least twice its length were considered germinated. The experiment was conducted in a completely randomized factorial design consisting of 7 temperatures and 9 constant humidity incubation periods with five replicates. The test was conducted twice. For the estimation of the equations the temperature data between 7 and 35 °C were used. The response surface graph was performed using the SigmaPlot 11.0 software (Systat Software, Inc.).
A polynomial model was fitted to germination data affected by temperature and wetness period, and is given by
where, Y is the germination (%); b1 is the intercept; T is the temperature (°C) and; and WP is the wetness period (h). The analyses were performed using the R software (R Core Team 2016).
Effect of temperature and fruit wound presence on infection by Neonectria ditissima
The ‘EMB’ isolate was used in this experiment. Detached mature fruit of Gala and Eva cultivars were disinfested by immersion in 70% alcohol for 1 min, immersion in 1% sodium hypochlorite and rinsed three times in distilled water. Then, they were dried on paper towel and placed in disinfested transparent plastic pots with moistened cotton to maintain humidity. Subsequently, two experiments were conducted. In one trial, fruit were inoculated in 5 mm deep wounds, made with autoclaved toothpicks on top of the fruit. In the other assay, fruit were inoculated without wound.
The inoculated conidial suspension was prepared from seven days old colonies of N. ditissima growth in PDA medium, by adding 2 mL of sterilized distilled water and scraped with a sterilized Drigalsky handle. The suspension from the plate was filtered on sterile gauze. The suspension was shaken, and the conidia concentration was measured in a hemacytometer under light microscope (Olympus®) and adjusted to the concentration of 106 conidia mL−1. After preparation of the suspension, aliquots of 30 μL were immediately inoculated into the fruit with the aid of a pipette. Aliquots of 30 μL of the suspension were deposited on plates containing 10 mL of water-agar (WA) medium, to evaluate the germinative capacity of the isolate at each simulated temperature.
The inoculated fruit were then kept in Conviron® growth chambers, which have been programmed with average temperatures from 2010 to 2016 apple harvest season (December to February) of the states of Paraná (Curitiba), Santa Catarina (São Joaquim) and Rio Grande do Sul (Bom Jesus) (Table 1). The region of Curitiba in Paraná was chosen to understand the behavior of the pathogen in fruit in this region, which has not yet reported the disease. Temperatures were defined throughout the day according to the availability of data provided by the Instituto Nacional de Pesquisas Espaciais (INPE).
The experiments were conducted in a completely randomized factorial design: two cultivars and seven temperatures. Each repetition consisted of ten fruit. The whole experiment was repeated once.
The incidence was evaluated daily, from the appearance of the first lesion until 40 days after inoculation. The distribution of the incubation period in time of the cultivars Eva and Gala was also analyzed by plotting the accumulated frequencies. The non-parametric Kolmogorov-Smirnov test compared the distributions of the accumulated frequencies of the data over time and provided the maximum difference between the distributions. Differences in time to onset of symptoms (incubation period) were evaluated using the Welch t-test at 5% significance (R Core Team 2016).
The latent period was also evaluated daily from the time of inoculation until 40 days after inoculation by monitoring sporulation on the fruit epidermis. The sporulating fruit had their surface scraped and the structures were observed at light optical microscope (400x) to confirm as N. ditissima conidia. The data were summarized as percentage of fruit with pathogen structures and was not subjected to statistical tests.
Results
Effect of temperature on mycelial growth and spore production
The growth of N. ditissima colonies at different temperatures is shown in Fig. 1. The optimal growth temperatures of all isolates ranged from 17 to 20 °C. The minimum temperatures at which colony growth could occur were between 0 and 2.5 °C, whereas the maximum temperatures at which colony growth could occur were between 35 and 40 °C (Fig. 1).
The average production of micro- and macroconidia by 25-days old colonies of the four N. ditissima isolates is shown in Fig. 2. Microconidia production was greater than macroconidia at any of the tested temperatures. The data about the number of spores produced is presented as average number of macro- and microconidia per mm2 of colony because great variation was observed between replicates of the same isolate (Fig. 2). Data from colonies incubated at 7 and 35 °C are not shown due to the low sporulation rates observed at 10 and 30 °C.
At 15–30 °C, the production of microconidia was superior to that of macroconidia at any temperature. Indeed, the microconidia/macroconidia ratio at these temperatures was greater than 1 regardless of which isolate was evaluated (Table 2). No macroconidia production was observed at 30 °C for any of the isolates.
Effect of temperature and incubation period on conidial germination
After 12 h of incubation at high humidity, >70% of the conidia germinated at 25 °C (Fig. 3), and after 48 h, >90% of the conidia germinated, regardless of temperature. After 8 h of incubation, germination was greater at 20 °C than at 25 °C, exceeding 50%. With 4 h of incubation, germination at 25 °C was >10%, whereas <10% of the conidia were able to germinate after only 2 h. At 35 °C, germination was drastically reduced, regardless of incubation period.
Susceptibility of detached ‘gala’ and ‘Eva’ apples to NFR
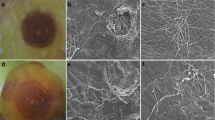
Unwounded inoculated apples showed no symptoms of NFR during the 40-d evaluation period. The behavior of the disease regarding symptoms onset in wounded ‘Gala’ and ‘Eva’ fruit was similar, starting at 15 d after inoculation, and no difference was observed between the time of disease onset for both cultivars in the accumulated frequency (P = 0.317) (Fig. 4). Because the latent period was not reached for any of the tested treatments (there were less than 50% of the fruit with signs of the disease), the sporulation results are shown as percent of fruit with signs (Table 3). Sporulating fruit exhibited white to yellow structures on the surface (Fig. 5a), and the structures were primarily composed of macroconidia, with very few microconidia (Fig. 5b).
Discussion
The four N. ditissima isolates collected from Vacaria (Rio Grande do Sul) behaved similarly regarding physiological traits, which were favored by temperatures between 10 to 25 °C. The incubation period with constant humidity and the temperature can influence epidemics of European canker and NFR. ‘Eva’ apples, which are produced at a large scale in the state of Paraná, were as susceptible to the disease as ‘Gala’ apples.
Even at 7 and 35 °C, the four isolates exhibited germination rates of >40% when incubated under continuous humidity for at least 24 h. This result contrasts with those of Latorre et al. (2002), who reported minimal (<10%) in vitro germination of conidia at 6 and 32 °C, even with a 24-h incubation period. The same authors also reported that the optimal temperature for conidial germination was between 20 and 25 °C. However, at these temperatures, the authors observed germination rates of >80% within 24 h of incubation, whereas lower rates were observed in the present study. This difference may be due to the fact that the isolates were originated from different locations, and/or related to the addition of dextrose (1.5%) to the media by Latorre et al. (2002), which could have been used as a source of nutrients by the conidia. The addition of nutrients to media have already been reported to influence conidia germination. For example, conidial suspensions incubated in apple sap amended water agar (ASAWA) medium was reported to enhance germination, when compared with conidial suspensions incubated in water agar alone (Campbell et al. 2018).
Apple fruit is stored for months under low-temperature conditions (Petri et al. 2011), and contact between fruit with sporulating lesions and wounded healthy fruit has the potential to increase the frequency of NFR in cold storage. As indicated by the results of the present study, although not at temperatures as low as those of storage, the pathogen is still able to germinate at low temperatures. In addition, the in vitro tests of the present study demonstrated that sporulation can occur at low temperatures and that sporulation under such conditions occurs mainly in the form of macroconidia, which, according to Zeller (1926), are the main infective spore type under field conditions. The sporulation and spore germination of N. ditissima at cold-chamber temperatures should be studied further, and future studies should also investigate the presence and viability of its spores in apple transport boxes to better guide producers and to prevent the entry of the pathogen into regions where it is yet to occur.
The greater production of macroconidia in vitro was induced by temperatures of <15 °C, whereas higher temperatures favored the predominance of microconidia, which are less commonly found in field infections. Temperatures of ≥30 °C were limiting to macroconidia production, as well as to the growth of N. ditissima mycelia. This indicates the pathogen adaptation to lower temperatures, which are characteristic of apple-producing regions that are affected by the diseases caused by N. ditissima. Except at 10 °C, microconidia production was consistently higher than macroconidia production in the PDA medium. The rate of production of microconidia in relation to macroconidia in culture medium has been studied by Scheper et al. (2014), who reported that most N. ditissima isolates are unable to produce conidia in most culture media and that these isolates generally produce microconidia rather than macroconidia, which are typically found in nature. The authors, who did not include PDA medium in their study, recommend the use of either Matsushima synthetic medium (MM) or ASAWA medium to favor macroconidia production. However, regarding conidia germination, the isolates cultured and maintained in PDA culture medium in the present study behaved similarly to those used by Latorre et al. (2002), which were grown in MM medium. Since the epidemiological role of microconidia is uncertain (Weber 2014), more detailed studies of the conidia produced by N. ditissima in other culture media and the differences between the physiological traits of macro- and microconidia should be conducted. Such information would be useful for producing either macro- or microconidia-predominant inoculum to determine whether these types of conidia have different infective capacities in woody tissues and fruit.
The inoculation of wounded and unwounded mature fruit was performed using N. ditissima isolates from twigs. This is important since the main source of inoculum for fruit infections are sporulating lesions on woody tissues, as indicated by correlations between canker lesion incidence and NFR severity during both pre-harvest (Weber and Dralle 2013) and post-harvest (Weber 2014) periods. In the present study, the inoculations were performed on detached fruit, since in our region (Curitiba, Paraná state), which is quarantined for N. ditissima, inoculation of fruit in the field is prohibited. However, the susceptibility of mature fruit to NFR does not vary greatly from 5 weeks pre-harvest until harvest (Xu and Robinson 2010), which ensures that, by inoculating mature fruit, the results of the present study are applicable to field conditions during the 5 weeks before harvest.
The present study demonstrated that ‘Eva’ and ‘Gala’ fruit possess similar susceptibilities to NFR. Therefore, control measures are necessary for both cultivars. Ripe apple fruit are more susceptible to N. ditissima infection (Xu and Robinson 2010), due to the lower production of benzoic acid, which is an important compound related to disease resistance during pre-harvest periods (Brown and Swinburne 1971; Seng et al. 1985). Apple fruit decreases in acidity and an increase in pH after harvest, which causes dissociation of the benzoic acid molecule (Swinburne 1971), and, therefore, reduces fruit defense against N. ditissima. This may explain the appearance of symptoms in inoculated wounded fruit after more than two weeks of incubation.
In both cultivars, unwounded mature fruit that were inoculated with conidia suspension were not susceptible to infection, which indicated the need for injury. The lack of symptoms, even with artificial inoculation high inoculum concentrations and high humidity must be considered, and practices that reduce the incidence of wounds on fruit are of extreme relevance to NFR control. This result confirms the previous findings of Xu and Robinson (2010), who reported that post-harvest infections occur only in the presence of injuries. Swinburne (1971) also reported that wounds are necessary for disease development in ripe fruit and that germinating N. ditissima conidia did not penetrate the intact surfaces of apples because neither the germ tubes nor hyphae produced appressoria. This behavior has also been reported for certain Colletotrichum isolates that were incapable of infecting unwounded ‘Gala’ or ‘Eva’ apples (Moreira et al. 2018). Therefore, conidia may require a chemical signal that is present in apple pulp to overcome preformed host barriers to appressoria development, i.e., the epidermis and wax layer. Another possibility for the lack of symptoms in unwounded fruit is the formation of quiescent infections that would later develop during post-harvest period or after long-term storage in cold chambers. Prusky (1996) described mechanisms for quiescence in several pathosystems, but this has not yet been studied in N. ditissima. Therefore, additional studies are needed.
Heat treatment has already been investigated for controlling post-harvest rots (Maxin et al. 2014). Similar studies should be performed to investigate the use of heat treatment for controlling NFR in apple fruits from Brazil and for treating quiescent NFR infections.
There is no information in the literature regarding the latent period of NFR. In the present study, 25 d were required for some ‘Eva’ fruit to start presenting sporulation, and 34 d were required for some ‘Gala’ fruit. These results indicate the importance of removing fallen fruit from orchards, even those without symptoms or signs of disease. Indeed, the incubation and latent periods of NFR can be long and fallen fruit can eventually become a source of inoculum to infections in woody tissues of apple trees throughout the cycle. In addition, sporulating fruit can be transported in boxes that are re-used, including in different orchards, a common practice in the production areas of Brazil. Because such boxes can also serve as source of inoculum, the disinfection of transportation boxes is of great importance to managing NFR. In the present study, there was significant variation among the fruit regarding latent period, and no correlations were identified between either cultivar or incubation temperature and sporulation on the fruit epidermis. Additional studies should be performed to elucidate this variation.
The range of temperatures favorable to N. ditissima germination, mycelial growth, and sporulation are typical of the major apple-producing regions in Brazil. It is also alarming that the two most-planted apple cultivars of Brazil are susceptible to NFR when wounded. Cultural methods to reduce initial inoculum (e.g., removal of inoculum sources from orchards and use of healthy planting material) should be put into practice, in order to reduce the use of fungicides in apple protection. The occurrence of abundant sporulation on the surface of apples after the latent period demonstrates that special attention should be given to fruit that is transported or commercialized in areas where the disease is still absent, by monitoring and surveillance.
References
Alves SAM, Czermainski ABC (2015) Controle do Cancro Europeu das Pomáceas com Base no Novo Ciclo Neonectria ditissima – Macieira, nas Condições do Brasil. Comunicado Técnico. Embrapa, n 178
Alves SAM, Nunes CC (2017) Seasonal susceptibility of apple trees to Neonectria ditissima wound infections. New Zealand Plant Protection 70:73–77
Bassanezzi RB, Amorim L, Bergamin Filho A, Hau B (1998) Effects of bean line pattern mosaic virus on the monocyclic components of rust and angular leaf spot of Phaseolus bean at different temperatures. Plant Pathology 47: 289-298
Beresford RM, Kim KS (2011) Identification of regional climatic conditions favorable for development of European canker of apple. Phytopathology 101:135–146
Brasil (2013) Ministério da Agricultura, Pecuária e Abastecimento. Gabinete do Ministro. Instrução Normativa n° 20, de 20 de junho de 2013. Diário Oficial da União, 21 jun. 2013. Seção 1, p. 22. Avaliable in: <http://pesquisa.in.gov.br/imprensa/jsp/visualiza/index.jsp?data=21/06/2013&jornal=1&pagina=22&totalArquivos=168>
Brown AE, Swinburne TR (1971) Benzoic acid: an antifungal compound formed in Bramley’s seedling apple fruit following infection by Nectria galligena Bres. Physiological Plant Pathology 1:469–475
Campbell RE, Chevalier CE, Touron A, Walter M (2018) The effect of nitrogen source on in vitro growth of Neonectria ditissima (European canker). New Zealand Plant Protection 71:180–188
FAOSTAT (2014) FAO: Food and Agriculture Organization of the United Nations. Available in: <http://www.fao.org.br> Access in: 4 feb 2017
Hauagge R, Tsuneta M (1999) IAPAR 75 - 'Eva', IAPAR 76 - 'Anabela' e IAPAR 77 - 'Carícia' - Novas cultivares de macieira com baixa necessidade em frio. Revista Brasileira de Fruticultura 21:239–242
Latorre B, Rioja M, Lillo C, Muñoz M (2002) The effect of temperature and wetness duration on infection and a warning system for European canker (Nectria galligena) of apple in Chile. Crop Protection 21:285–291
Maxin P, Williams M, Weber RWS (2014) Control of fungal storage rots of apples by hot-water treatments: a northern European perspective. Erwerbs-Obstbau 56:25–34
Moreira RR, Peres NA, May De Mio LL (2018) Colletotrichum acutatum and C. gloeosporioides species complexes associated with apple in Brazil. Plant Disease 103:268–275
Noble JP, Drisdale RB (1983) The role of benzoic acid and phenolic compounds in latency in fruit of two apple cultivars infected with Pezicula malicorticis or Nectria galligena. Physiological Plant Pathology 23:207–216
Oliveira DL, Alvarenga AA, Gonçalves ED, Abrahão E, Zambon CR & Norberto PM (2011) Maçã 'Eva' desponta a produção no estado de Minas Gerais. Belo Horizonte, EPAMIG. 4p. (Circular Técnica, 141)
Petri JL, Leite GB, Couto M, Francescatto P (2011) Avanços na cultura da macieira no Brasil. Revista Brasileira de Fruticultura 33:48–56
Prusky D (1996) Quiescent infections by postharvest pathogens. Annual Review of Phytopathology 34: 413-434
R Core Team (2016) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available in: https://www.R-project.org/
Scheper RWA, Fisher BM, Amponsah NT, Walter M (2014) Effect of culture medium, light and air circulation on sporulation of Neonectria ditissima. New Zealand Plant Protection 67:123–132
Seng JM, Saindrenan P, Bompeix G (1985) Induction of Nectria galligena mutants resistant to benzoic acid and study of their aggressiveness towards immature apples. Journal of General Microbiology 131:1863–1866
Sutton TB, Aldwinckle H, Agnello A, Walgenbach JF (2014) Compendium of apple and pear diseases and pests. American Phytopathological Society, St. Paul, 343pp
Swinburne TR (1971) The infection of apples, cv. Bramley’s seedling, by Nectria galligena Bres. Annals of Applied Biology 68:253–262
Swinburne TR (1973) The resistance of immature Bramley's seedling apples to rotting by Nectria galligena Bres. In Fungal pathogenicity and the Plant's response (ed. R.]. W. Byrde and C. V. Cutting), pp. 365–382. London: Academic Press
Swinburne TR (1974) The effect of store conditions on the rotting of apples, cv. Bramley's seedling, by Nectria galligena. Annals of Applied Biology 78:39–48
Swinburne TR (1975) European canker of apple (Nectria galligena). Review of Plant Pathology 54:787–799
Weber RWS (2014) Biology and control of the apple canker fungus, Neonectria ditissima (syn. N. galligena) from a northwestern European perspective. Erwerbs-Obstbau 56:95–107
Weber RWS, Dralle N (2013) Fungi associated with blossom-end rot of apples in Germany. European Journal of Horticultural Science 78:97–105
Xu X-M, Robinson JD (2010) Effects of fruit maturity and wetness on the infection of apple fruit by Neonectria galligena. Plant Pathology 59:542–547
Zeller SM (1926) European canker of pomaceous fruit trees. Oregon Agricultural College Station Bulletin 222:1–52
Acknowledgements
The authors would like to acknowledge grants provided by the Coordination of Improvement of Higher-Level Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES) under the number 40001016031P6 and Embrapa Uva e Vinho personnel for providing the isolates and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Section Editor: Juan Antonio A. Navas-Cortés
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gelain, J., Alves, S.A.M., Moreira, R.R. et al. Neonectria ditissima physiological traits and susceptibility of ‘Gala’ and ‘Eva’ detached apple fruit. Trop. plant pathol. 45, 25–33 (2020). https://doi.org/10.1007/s40858-019-00314-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-019-00314-y