Abstract
Botrytis cinerea is a common pathogenic agent, causing a significant reduction in tomato quality and yield. In this study, the potential of two biocontrol fungal agents, Trichoderma harzianum and Clonostachys rosea to prevent and alleviate B. cinerea infection was assessed. To this end, the fungicidal effects of both biocontrol fungi were evaluated on ten common phytopathogens, including B. cinerea. Then, various ratios of T. harzianum and C. rosea mixtures were assessed to find the ratio that best promotes the growth of tomato seedlings and prevents the establishment of B. cinerea. The results showed that T. harzianum and C. rosea had significant fungicidal effects on all tested phytopathogens. Also, both biocontrol agents reduced the establishment of B. cinerea, in vitro assays, indicating they had significant fungicidal effects against this phytopathogen. However, when applied as a biofungicide to tomato plants, a combination of both biocontrol agents gave better control of B. cinerea. Investigating the molecular basis of resistance to B. cinerea induced by the mixture of T. harzianum and C. rosea revealed that the salicylic acid signaling pathway plays an important role in modulating the antioxidant enzyme activity. Taken together, the results of this research confirmed that both biocontrol fungal agents could induce the mechanism of B. cinerea resistance in tomato plants and shed light on the regulatory pathways associated with the resistance mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Lycopersicon esculentum Mill.), is a plant member of the Solanaceae family and is native to South America (Guillemaud et al., 2015). Tomato is one of the most widely cultivated crops in the world due to its unique flavor and rich nutritional values (Cheng et al., 2022). However, large areas of tomato production are prone to plant diseases, with Botrytis cinerea (gray mold) being a major cause of postharvest rot in tomatoes globally (Meng et al., 2022).
Botrytis cinerea releases a large number of spores on infected tissues, causing the pathogen to spread quickly to unaffected tissues (Wang et al., 2010). Botrytis cinerea can infect over 200 plant species (Williamson et al., 2007), and it has become a major limiting factor in tomato production (Cantu et al., 2009). The application of chemical fungicides is the most common method to prevent and control B. cinerea in agricultural production (Gullino et al., 1991). While chemical fungicides can provide good control of B. cinerea, long-term chemical fungicide applications can lead to fungicide resistance development and environmental pollution (Zubrod et al., 2019). Incorporating biological fungicides into disease management programs is one option to tackle the issues caused by the application of chemical fungicides.
Several studies have reported Trichoderma and Clonostachys as effective biological agents against B. cinerea (Cota et al., 2008; Meng et al., 2022; Saraiva et al., 2015; Silva et al., 2020). For instance, T. harzianum can reduce B. cinerea in tomato, lettuce and tobacco (De Meyer et al., 1998). Additionally, it was noted that C. rosea inhibited the establishment of B. cinerea and reduced the fruit decay rate (Peng & Sutton, 1990). Trichoderma harzianum belongs to the semi-fungal subphylum, Pezizomycotina and is a key soil antifungal strain (Otieno et al., 2003). Trichoderma harzianum has been used as a biocontrol agent against over 20 plant fungal diseases such as soybean stem rot, caused by Sclerotinia sclerotiorum and tomato fusarium wilt caused by Fusarium oxysporum f. sp. lycopersici (El Komy et al., 2015; Zhang et al., 2016). In addition to antifungal activity, T. harzianum was found to improve seed vigour, seedling emergence times, and the rate of photosynthesis (Fiorini et al., 2016; Vukelić et al., 2021). Currently, several commercially T. harzianum-based fungicides (e.g., Shibeijian, Trichodex and Topshield) are available that can control various plant diseases.
Clonostachys rosea is also a fungal biocontrol agent that is widely present in soils (Meng et al., 2022; Sun et al., 2020). Clonostachys rosea can effectively control various plant diseases, caused by Fusarium species, S. sclerotiorum and B. cinerea (Cota et al., 2009; Meng et al., 2022; Nobre et al., 2005), and prevents the establishment of Aphanomyces Euteiches Dreehsler in pea (Xue, 2003). Similar to T. harzianum, it has been shown that C. rosea can promote plant growth (Han et al., 2022).
It has been noted that a combination of biocontrol agents exerts more effective control against plant diseases than applying a single biocontrol agent (Alamri, 2014). For instance, a mixture of Trichoderma viride, Pseudomonas fluorescens and Bacillus subtilis gave better suppression of collar and root rot disease in physic nut (Latha et al., 2011), suggesting that using a mixture of strains can improve the efficacy of pathogen control (Burgess et al., 1999). Krauss et al. (2013) noted that a combination of T. harzianum and C. rosea could be used as a mixed biocontrol agent against B. cinerea; however, they noted that while there is competition between the two strains when used in mixtures, the antagonism between C. rosea and Trichoderma can be overcome by adjusting the mixing ratio of both biocontrol agents (Hoopen et al., 2010).
Salicylic acid (SA) and jasmonic acid (JA) signaling pathways are primary resistance signaling pathways in plants (Zhao et al., 2018). The SA signaling pathway plays an important role in the plant's immune response to phytopathogens, and its mechanism of inducing resistance is of great significance for crop protection (Vlot et al., 2009). Exogenous SA treatment can control the occurrence of navel orange canker by inducing defense mechanisms, such as elevating H2O2 levels and promoting stomatal closure (Wang & Liu, 2012). In plants, the nonexpressor of pathogenesis-related genes 1 (NPR1) gene as a major regulator of SA signaling, interacts with transcription factors such as TGA in the nucleus to promote the production of genes, inducing plant disease resistance (Kinkema et al., 2000). The JA signaling pathways also has a direct or indirect function in plant resistance signal transduction (Kunkel & Brooks, 2002). Both SA and JA signaling pathways can work synergistically/antagonistically to promote disease resistance mechanisms in plants. For example, it has been shown that T. longibrachiatum H9 induced cucumber resistance to B. cinerea by activating both JA and SA signaling pathways (Yuan et al., 2019).
In our preliminary investigation, we noted that mixtures of T. harzianum and C. rosea had a better growth promotive effect on tomato plants than single-bioagent treatment. These results implied that some chemical substances might have been produced during the co-existence of T. harzianum and C. rosea. However, these results raised the question of whether combining both biocontrol agents can also improve disease control in tomato plants. In this research, we developed a combined biofungicide composed of T. harzianum and C. rosea and evaluated its impact on tomato growth and B. cinerea control.
Materials and methods
Microorganisms
Clonostachys rosea (CR) and T. harzianum (TH) strains were isolated from rhizospheric soil samples collected from a farm at Northeast Agricultural University, following the method outlined by Warcup (1950). The isolated C. rosea and T. harzianum strains were maintained on potato dextrose agar (PDA) cubes, sealed with paraffin wax, and stored at -80 °C under storage numbers CGMCC No.1977 and CGMCC No.12165, respectively. The strains were re-streaked onto fresh media every six months to retain active growth. Ten phytopathogenic fungi: 1) B. cinerea (OR137135; leaves; Lycopersicon esculentum Mill.); 2) Fusarium verticillioides (OR137142; corn ears; Zea mays L.); 3) Rhizoctonia solani (OR137143; leaves; Oryza sativa L.); 4) Colletotrichum sp. (OR137138; leaves; Cucumis sativus L.); 5) Fusarium equiseti (OR137139; stems; Zea mays L.); 6) Fusarium solani (OR137141; stems; Solanum melongena L.); 7) Fusarium oxysporum (OR137140; leaves; Citrullus vulgaris Schrad.); 8) Cladosporium fulvum (OR137137; leaves; Lycopersicon esculentum Mill.); 9) Sphaerotheca fuliginea (OR131269; leaves; Cucumis sativus L.); and 10) Cercospora beticola (OR137136; leaves; Beta vulgaris L.) were also isolated from infected plants, such as the leaves, roots, or fruits, using the method outlined by Ambikapathy et al. (2023). All isolated pathogenic agents were sent to Huada Gene Technology Co., Ltd. (Shenzhen, China) for identification. The sequences have been deposited with the National Center for Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov/).
Culture observation and molecular identification of T. harzianum and C. rosea strains
Trichoderma harzianum and C. rosea were inoculated from laboratory stocks onto sterilized PDA (Basu et al., 2015). To monitor colony morphology, both fungal strains were incubated under constant temperature (28℃) for 5 days. For this, small mycelium samples were intermittently removed with sterile needles, smeared onto slides and the morphology of the mycelium was examined using a microscope (YS100, Hitachi Co. Ltd., Japan). To validate the taxonomy of T. harzianum and C. rosea, mycelia were collected from PDA plates using a sterile hole-puncher (5 mm). Subsequently, the collected mycelia were ground into powder using liquid nitrogen. Genomic DNA was extracted using the method described by Zhang et al. (1996). Polymerase chain reaction (PCR) was performed to amplify the 18S rRNA regions. The gene regions studied are the binding internally transcribed spacer (ITS) and translation extension factor 1α (TEF1). The primers used for the ITS amplification were ITS1F (forward primer): 5'-CTTGGTCATTTAGAGGAAGTAA-3' and ITS4 (reverse primer): 5'-TCCTCCGCTTATTGATATGC-3' (Gardes & Bruns, 1993). The primers used for the TEF1 amplification were elongation-1F (forward primer): 5'-CATCGAGAAGTTCGAGAAGG; elongation-1R: (reverse primer) 5'-GCCATCCTTGGGAGATACCAGC (Hyun et al., 2009).
The PCR reaction (30 µl) contained 17.8 µl of molecular water, 3.0 µl of buffer (0.1 mol/L Tris–Cl), 2.0 µl of dNTPs (10 μmol/L), 3.0 µl of each forward (10 μmol/L) and reverse primers, 1.0 µl of DNA template and 0.2 µl of the enzyme. The PCR thermocycling program included initial denaturation at 95℃ for 5 min (1 cycle), 35 cycles of denaturation at 95℃ for 60 s, annealing at 55℃ for 30 s and extension at 72℃ for 60 s, followed by one final cycle of extension at 72℃ for 10 min. The PCR products were sent to Huada Gene Technology Services Co. Ltd., (Shenzhen, China) for sequencing. Sequencing was performed using the same forward and reverse primers outlined above. The sequence results were analyzed, aligned, and compared using the method described by Ghanizadeh et al. (2020), and phylogenetic analysis of DNA joint sequences associated with the ITS and TEF1 regions was performed to identify T. harzianum and C. rosea using MEGA 5.0.
The in vitro effects of T. harzianum and C. rosea on ten common phytopathogens
The isolated phytopathogenic fungi described above were cultured on PDA and kept in a constant temperature incubator (28℃) (LKYC-1102C, Lifu Technology Co. Ltd., Ningbo, China) for 8 days. Then, fresh mycelia from the phytopathogen cultures were obtained using a sterile hole-puncher (5 mm). The collected mycelia were placed on the centre of PDA media in a Petri dish containing the hyphae of T. harzianum or C. rosea. There were three replicates (i.e. Petri dishes) for each phytopathogen and biocontrol fungi combination (experimental unit). Petri dishes with phytopathogens only (no biocontrol fungi) were used as a control. At 10 and 20 days after incubation (DAI), the inhibition rates of T. harzianum and C. rosea were estimated using the following equation (Yu et al., 2011):
The effects of T. harzianum and C. rosea inoculants on B. cinerea
The effect of T. harzianum and C. rosea inoculants on the establishment of B. cinerea was assessed. For each treatment group, disease-free tomato seeds (Dongnong 713, a tomato F1 hybrid variety, developed by the Tomato Research Institute of Northeast Agricultural University in China) were immersed in 5 mL of sterile water and subsequently, placed on filter papers in sterile 90-mm Petri dishes (100 seeds per Petri dish) at room temperature. The filter papers were kept moist by adding 1 ml of sterile water daily until tomato seeds germinated. Germinated tomato seeds were planted in pots (5 × 8 cm size) filled with 200 g of media (peat moss: vermiculite = 3:1 sterilized for 30 min at 121℃ and 103 kPa) and kept in an incubator (HPG-280HX, Yongda Chemical Reagent Co. Ltd., Tianjin, China) at 28℃. Eventually, the tomato plants (four-leaf stage) were sub-irrigated with one of the treatment groups, namely, No.1 = T. harzianum, No.2 = C. rosea, No.3 = T. harzianum:C. rosea at a 2:3 ratio, No.4 = Shibeijian, a commercial biofungicide (active ingredient: T. harzianum chlamydospore) and No.5 = water (control treatment). The spores' suspension concentration of T. harzianum and C. rosea was 106 cfu/mL. Each treatment group was repeated three times, and each replicate consisted of 50 tomato seedlings. The tomato leaves were sprayed with a suspension containing B. cinerea spores (106 cfu/mL) 24 h after treatment (HAT). First, fourth and eighth days after the treatment (DAT), plant height (cm), incidence rate and disease index were measured to evaluate the effect of biocontrol fungi on tomato and B. cinerea. The activities of three key enzymes associated with plant disease defense system were also determined at 1, 4 and 8 DAT. The amount of proline (PRO) was measured using the method described by Bates et al. (1973). The activity of the antioxidant enzymes peroxidase (POD) and superoxide dismutase (SOD) was assessed following the method described by Radić et al. (2006) and Yu and Rengel (1999), respectively.
To assess the effect of biocontrol fungi mixtures on B. cinerea control in tomato plants, the incidence rate and disease index were calculated according to the following equations:
(disease series: Level 0, no necrotic spots; Level 1, 3 necrotic spots on a single leaf. Level 3, 4–6 necrotic spots on a single leaf; Level 5, 7–10 necrotic spots on a single leaf; Level 7, 11–20 necrotic patches on a single leaf, with some of them densely packed into patches. Level 9, single leaf with dense necrotic spots, covering more than 1/4 of the leaf area) (Chiang et al., 2017). All analyses were performed in triplicate.
To assess the chlorophyll fluorescence of tomato plants treated with either of the five treatment groups, plants were grown from seeds using the above-mentioned method. When tomato plants reached the 4–5 leaf stage, they were bagged for 30 min (dark adaptation) prior to measuring the chlorophyll fluorescence using an imaging fluorimeter (MAXI, Zealquest Scientific Technology Co., Ltd., Harbin, China). The initial fluorescence (Fo) was measured following irradiation with visible light (10 μmol/M2/S) for 0.8 s. The maximum fluorescence (Fm) was obtained following irradiation with the saturated pulse light (8000 μmol/M2/S) for 0.8 s. The maximum photochemical quantum yield of photosystem II (Fv / Fm = (Fm-Fo) / Fm) was automatically recorded by the fluorimeter, and the corresponding image was generated.
Effects of T. harzianum and C. rosea on salicylic acid and jasmonic acid signaling pathways
To understand the effects of T. harzianum and C. rosea on the signaling pathways of SA and JA in tomatoes, the content of both signaling molecules and the expression of the key genes involved in their signaling pathways were assessed. The content of SA and JA in tomato leaves was measured at 1, 4, 6, 8 and 10 DAT using SA ELISA Kit (Aidi Biotech Co. Ltd., Wuhan, China) and JA ELISA Kit (Aidi Biotech Co. Ltd., Wuhan, China), respectively, following the manufacturer's instructions.
The key genes associated with the SA and JA signaling pathways were identified using the KEGG pathway database (https://www.kegg.jp/pathway/ath04075). To evaluate the expression of the key genes, trizol reagent was used to extract total RNA from tomato leaves at 1, 4, 8 DAT. The extracted RNA was reverse-transcribed into cDNA using a kit (R101-01, Vazyme Co., Nanjing, China) following the manufacturer's instructions. The cDNA was used for a qRT PCR analysis to assess the expression of the key genes using a commercial kit (Q421-02, Vazyme Co., Nanjing, China). The qRT PCR reaction contained Mix ChamQ SYBR Color qPCR Master Mix (10.0 µl), forward and reverse primers (0.4 µl), 1.0μL of template cDNA and 8.2 µl of ddH2O. The qRT PCR analysis was performed using the conditions outlined by Miao et al. (2022). The forward/reverse primers designed for the genes associated with the SA and JA signaling pathways are listed in Table S1. The expression level of the key genes was correlated to the physiological indices measured in tomato plants treated with the T. harzianum and C. rosea mix (No.3) and the control (No.5) treatment groups. The content of JA and SA, and the expression of the key genes associated with the JA and SA signaling pathways were assessed in triplicate samples.
Statistical analyses
All experiments were established using a completely randomized design with three replicates. Data were tested for normal distribution and variance equality before being subjected to variance analysis (ANOVA). Means were separated using Fisher's protected test at a 5% level of probability. All statistical analyses were conducted using SPSS 20.0 and Origin 2022 software.
Results
Trichoderma harzianum and C. rosea identification and sequence analysis (18S rRNA)
The colony morphology of T. harzianum and C. rosea after activation and rejuvenation is illustrated in Fig. 1a and c. Initially, the mycelia of T. harzianum were fine, with no changes in color up to 3 days after inoculation. As culturing progressed, the white flocculent mycelia gradually changed into green at 4 days after inoculation, and eventually at 8 days after inoculation, the mycelia turned dark green. Clonostachys rosea colonies were white, and the mycelia were initially longer and whiter. After a prolonged culture period, the mycelia gradually shortened and turned yellow at approximately 7 days after inoculation.
Amplification of ITS and TEF1 sequences from T. harzianum and C. rosea yielded products of about 500 bp and 300 bp, respectively, as estimated by agarose gel electrophoresis (Fig. S1). Phylogenetic trees of T. harzianum and C. rosea were constructed based on the ITS and TEF1 sequences (Fig. 1b and d). The strain of T. harzianum used in this research showed an overall 98% homology with the previously published T. harzianum sequences (MK322671.1; OM471990.1), indicating that our strain had ITS and TEF1 region sequences identical to those of the previously studied strain. The C. rosea strain from this research had 98% homology to the ITS and TEF1 sequences of the previously studied strains (OQ933422.1; LT220777.1), suggesting that our strain was conspecific with C. rosea.
The inhibitory effects of the two biocontrol fungi against ten common phytopathogenic fungi
Both biocontrol fungi exhibited significant inhibitory effects against all ten phytopathogens investigated in this research. As shown in Table 1, T. harzianum had the greatest (86.74%) and lowest (61.87%) inhibitory effects against R. solani and F. oxysporum, respectively. The inhibition rate of T. harzianum against B. cinerea was 85.47%, while an inhibition rate of 85.79% was recorded against F. solani for T. harzianum. The inhibition rate of T. harzianum against all phytopathogens, except F. equiseti and C. fulvum was over 80%. The inhibitory effects of T. harzianum against all ten phytopathogens were slightly lower at 10 DAI than at 20 DAI, but the difference was not significant.
The inhibition rate of C. rosea against all ten phytopathogens was greater at 20 DAI. Among all ten phytopathogens tested in this research, C. rosea exhibited the greatest inhibition rate against Colletotrichum sp. (77.12%). The lowest inhibition rate of C. rosea was recorded for F. solani at 10 (53.87%) and 20 (56.83%) DAI. The inhibition rates of C. rosea against B. cinerea were 74.93% and 78.27% at 10 and 20 DAI, respectively.
The effects of T. harzianum and C. rosea biological agents on the control of B. cinerea
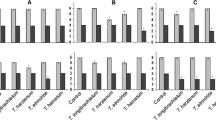
The control efficacy of all treatment groups against B. cinerea infection in tomato plants and the related physiological indices were investigated (Fig. 2). The results showed that the mixture of T. harzianum and C. rosea (No.3 treatment group) greatly promoted plant height by 8 DAT, and the treated plants were 10.1 cm taller than those of the control group (Fig. 2a). Furthermore, the lowest incidence rate and disease index values were recorded for the tomato seedlings treated with the No. 3 treatment group (Fig. 2b, c). Compared to the control group, the incidence rate and disease index in tomato plants treated with the No.3 treatment group reduced by 80.14% and 73.84%, respectively, by 8 DAT. Furthermore, the results indicated that the mixture of T. harzianum and C. rosea gave the best control against B. cinerea and even a single bioagent (No.1 and No.2) performed better than the commercial biofungicide, Shibeijian (No. 4).
Physiological indicators of tomato seedlings and enzyme activity in tomato leaves at 1, 4 and 8 days after treatment. (a) plant height, (b) incidence rate, (c) ddisease index, (d) proline (PRO), € peroxidase (POD), (f) superoxide dismutase (SOD), (g) plant morphology and (h) chlorophyll fluorescence images. Vertical bars represent the mean ± standard deviation. No.1–5 treatment groups, i.e., No. 1 = T. harzianum (TH), No. 2 = C. rosea (CR), No. 3 = TH + CR mixture, and No. 4 = Shibeijian (biofungicide) No. 5 = water (control)
The activity of enzymes in tomato leaves treated with the biocontrol agent is illustrated in Fig. 2d-f. The results showed that the level of PRO had an overall increasing trend (Fig. 2d). In addition, at 8 DAT, the level of PRO in plants treated with the NO.1, NO.2 and NO.3 treatment groups was higher than those treated with the Shibeijian group (No. 4) or the control group (No. 5). The level of POD activity in all treatment groups had an increasing trend up to 4 DAT (Fig. 3e), with plants treated with the No.3 treatment group had 28.85% and 40.99% greater POD activity than those treated with the No.4 treatment group and control group, respectively. Overall, the level of SOD activity in tomato seedlings treated with the No. 3 treatment group was higher than those treated with other treatment groups (Fig. 2f).
The signaling pathways of salicylic acid (SA) and jasmonic acid (JA) in tomato plants after treatment. (a) SA content, (b) JA content, (c) the SA and JA signaling pathway diagram, (d) the heatmap of genes associated with the SA and JA signaling pathways, (e) correlation analysis of plant disease resistance indices and the SA and JA signaling pathways` genes. The treatment groups in the horizontal axes are: No. 1 = T. harzianum (TH), No. 2 = C. rosea (CR), No. 3 = TH + CR mixture, No. 4 = Shibeijian (biofungicide) and No. 5 = water (control). Vertical bars represent the mean ± standard deviation
The chlorophyll fluorescence images from tomato seedlings treated with different treatment groups are illustrated in Fig. 2g and h. The blue color indicates excited states of pigments in the photosystem II reaction centers in leaves, suggesting a higher rate of photosynthesis in plants. The leaves of tomato seedlings treated with the No.3 treatment group were bluer than the control and had an Fv/Fm value of 0.746, indicating that photosynthesis was enhanced in those plants. Among the treatments, the lowest Fv/Fm values were recorded for the control group (0.672).
Effects of T. harzianum and C. rosea on salicylic acid and jasmonic acid disease resistance signaling pathways
The effect of different treatment groups on SA and JA content, and signaling pathways are shown in Fig. 3. There was a generally increasing trend in the content of SA for all treatments, though the SA content was different among treatments throughout the study (Fig. 3a). The results showed that the content of SA in tomato plants treated with the No.3 treatment group was the greatest up to 8 DAT, when it reached a maximum value of 403 ng/g FW, which was 42.79% greater than that of the control group.
In contrast to SA, a downward trend was recorded for the JA content in tomato plants for all treatment groups with increasing time, and there was a small difference among all treatment groups at each time course (Fig. 3b). The signaling pathway diagrams of SA and JA, and the expression levels of the key genes associated with both compounds are shown in Fig. 3c and d, respectively. As shown in Fig. 3d, the expression levels of the SlNPR1, SlPR1, SlJAZ1, SlMYC2 and SlCOI1 genes increased significantly at 4 DAT, in plants treated with the No.1, No.2 and No.3, treatment groups. Particularly, at 4 DAT, a greater level of expression was recorded for the SlNPR1, SlPR1 and SlMYC2 genes in plants treated with the No.3 treatment group. At 8 DAT, all genes, but the SlJAZ1 and SlMYC2 genes were highly over-expressed in plants treated with the No.1, No.2 and No.3, treatment groups. It was also noted that in plants treated with the No.3 treatment group, the SA content was strongly correlated with the PRO content and POD activity, while it was negatively correlated with SOD activity (Fig. 3e). Furthermore, the SlNPR1 and SlPR1 genes strongly correlated with plant defense-related physiological indices (red mark in Fig. 3e). In particular, the SlNPR1 and SlPR1 genes were positively correlated with the activity of POD and SOD, and PRO content in the plants treated with the No.3 treatment group, and the correlation value was higher than those in the control group (No. 5). The content of JA was found to be negatively correlated with PRO content in the plants treated with the No.3 treatment group; however, a strong positive correlation was recorded between the SlJAZ1 gene and the activity of POD and SOD.
Discussion
In this research, the antifungal activity of T. harzianum and C. rosea for B. cinerea management in tomatoes was investigated. Initially, both T. harzianum and C. rosea were isolated from soil samples, and their taxonomy was confirmed using morphological (microscopic) and genetic (18S rRNA) markers. Both techniques are commonly used in fungal identification (Anderson et al., 2003). Both T. harzianum and C. rosea showed broad-spectrum antifungal activity against ten common plant diseases, though the degree of inhibition was different among the tested phytopathogens. These results indicate that both biocontrol fungi can be used as a commercial biofungicide.
The inoculum ratio of fungal agents in combined biofungicides is critical in the efficacy of biocontrol, with suboptimal inoculation ratios resulting in antagonistic interactions between biocontrol agents (Hoopen et al., 2010). For instance, Krauss et al. (2013) noted that increasing the concentrations of C. rosea in a mixture of C. rosea and T. harzianum dramatically decreased the parasitic growth of T. harzianum. They suggested that the higher growth rate of T. harzianum resulted in more physical contact between hyphae and more exposure to mycoparasitism, which rendered T. harzianum more susceptible to hyphal damage. A screening approach using various ratios of biocontrol agents can aid the development of compatible combined biofungicides (Hoopen et al., 2010). In this regard, our study evaluated the effect of different biological agents on plant growth and B. cinerea control in tomatoes. Among the various microbial agents tested (data not shown), the T. harzianum and C. rosea mixture significantly promoted the growth of tomato plants and gave the best control against B. cinerea in tomato seedlings.
To understand the disease resistance mechanisms induced by T. harzianum and C. rosea, we investigated the antioxidant enzyme activity in the treated plants and their correlations with the SA and JA disease resistance signaling pathways. Antioxidant enzymes are crucial in conferring tolerance against biotic and abiotic stresses in plants (Liu et al., 2013, 2020). The activity of antioxidant enzymes reflects the physiological state of plants (Li et al., 2023; Zhang & Feng, 2018). Hence, evaluating the activity of antioxidant enzymes can be used as an index to compare the effects of T. harzianum and C. rosea treatments on tomato seedlings, with high levels of enzyme activity implying that the biofungicide treatment provided effective control against phytopathogenic agents (Zhang & Feng, 2018). This research revealed that the protection conferred by the T. harzianum and C. rosea mixture against B. cinerea in tomato plants could be associated with activating antioxidant enzymes such as POD, PRO and SOD. In agreement with our results, Zehra et al. (2017) showed that the application of T. harzianum enhanced the tolerance of tomato plants against Fusarium wilt disease by inducing antioxidant defense systems. Zheng et al. (2018) also noted that C. rosea improved the tolerance to B. cinerea in tomato plants by activating of antioxidant enzymes, such as SOD. Antioxidant enzymes can enhance the efficiency of biofungicides by improving the response of the plant immune system rather than by killing the fungi (Meng et al., 2022).
Disease resistance mechanisms in plants can be broadly categorized into: 1- systemic acquired resistance (SAR) induced by pathogenic microorganisms and 2- the induced systemic resistance (ISR) promoted by microorganisms with biological control function (Cordier et al., 1998; Durrant & Dong, 2004; Nawrocka & Małolepsza, 2013). In both mechanisms, the induction of disease-resistance related genes in plants promotes the production of pathogenic-related proteins (PR proteins), increases the accumulation of phenolic substances and induces the activity of plant defense enzymes, enabling the plants to withstand the infection of pathogens (Pieterse et al., 2001; van Loon et al., 1998). However, ISR and SAR mechanisms do not use the same signaling pathways to promote disease resistance in plants. For instance, in Arabidopsis thaliana, it was found that the ISR mechanism triggered by non-pathogenic rhizobacterial strain Pseudomonas fluorescens was induced through the jasmonic acid /ethylene signaling pathway, while the SAR mechanism was induced through the SA signaling pathway (Conrath et al., 2002). However, it has been shown that some biological control microorganisms can increase disease resistance in plants by increasing the expression of the SA signaling pathway (Jiang et al., 2018). Therefore, it is crucial to comprehend the pathways associated with plant disease resistance mechanism induced by microorganisms.
In previous research, Wang et al. (2019) confirmed that C. rosea can improve B. cinerea resistance in tomatoes through the JA and SA signaling pathways. Therefore, we explored if T. harzianum and C. rosea could also enhance the plant immune response through both signaling pathways. This research showed that the expression level of the NPRl gene was greater in plants treated with the T. harzianum and C. rosea mixture. The NPRl gene regulates the cross-talk between SA and JA signaling pathways (Pieterse & Van Loon, 2004). Hence, it plays a role in inducing disease resistance mechanisms in plants. Similarly, Zhang et al. (2010) concluded that the overexpression of the NPR1 gene primarily induced citrus canker resistance. In this research, it was also noted that the expression levels of SlNPR1 and SlPR1, two key genes modulating the SA signaling pathway, were significantly greater in tomato plants treated with the T. harzianum and C. rosea mixture. Enhancing the content of SA can induce disease resistance mechanism in plants through promoting the activity of antioxidant enzymes. For example, it has been shown that exogenous treatment of SA reduced improved resistance to Penicillium expansum by stimulating the activity of SOD and POD enzymes in sweet cherry fruit (Chan & Tian, 2006).
The JA signaling pathway can also play a role in inducing disease resistance and enhancing defense mechanisms in plants (Li et al., 2023; Zhao et al., 2003). However, this research showed that the SA signaling pathway played a greater role in regulating resistance to B. cinerea than the JA signaling pathway, in tomato plants treated with the T. harzianum and C. rosea mixture. Similarly, it has been shown that Paenibacillus alvei K165 promoted Verticillium dahliae resistance in A. thaliana by enhancing the defense mechanisms modulated by the SA signaling pathway (Tjamos et al., 2005).
Biofungicides can trigger pathogen-associated genes or plant-resistance proteins to activate plant defense against phytopathogens (Luo et al., 2018). Hence, applying biofungicide before plants are infected or at the early stage of infection can improve the control against phytopathogens by bolstering the immune system of plants (Meng et al., 2022; Zheng et al., 2018). Enhanced control against phytopathogens in plants can also be triggered by biocontrol fungal genes that promote disease resistance in the host plants (Islam & Sherif, 2020). Thus, future studies will investigate the genes associated with resistance to B. cinerea triggered by the T. harzianum and C. rosea treatment.
Conclusions
The results of this research revealed that applying the mixture of T. harzianum and C. rosea provided significantly enhanced resistance to B. cinerea in tomato plants under our experimental conditions. According to the correlation analysis of plant physiological parameters and resistance pathway indices, the fungal mixture induced the SA signaling pathway to promote resistance to B. cinerea in tomato plants.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Alamri, S. A. (2014). The synergistic effect of two formulated biofungicides in the biocontrol of root and bottom rot of lettuce. Biocontrol Science, 19(4), 189–197. https://doi.org/10.4265/bio.19.189
Ambikapathy, V., Sengottaian, N., Shijila Rani, A. S., Babu, S., & Anbukumaran, A. (2023). Isolation of endophytic fungi from stems, leaves, or roots. In A. Sankaranarayanan, N. Amaresan, & M. K. Dwivedi (Eds.), Endophytic Microbes: Isolation, Identification, and Bioactive Potentials (pp. 27–29). New York: Springer, US. https://doi.org/10.1007/978-1-0716-2827-0_4
Anderson, I. C., Campbell, C. D., & Prosser, J. I. (2003). Potential bias of fungal 18S rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil. Environmental Microbiology, 5(1), 36–47. https://doi.org/10.1046/j.1462-2920.2003.00383.x
Basu, S., Bose, C., Ojha, N., Das, N., Das, J., Pal, M., & Khurana, S. (2015). Evolution of bacterial and fungal growth media. Bioinformation, 11(4), 182–184. https://doi.org/10.6026/97320630011182
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207. https://doi.org/10.1007/BF00018060
Burgess, J. G., Jordan, E. M., Bregu, M., Mearns-Spragg, A., & Boyd, K. G. (1999). Microbial antagonism: A neglected avenue of natural products research. Journal of Biotechnology, 70(1), 27–32. https://doi.org/10.1016/S0168-1656(99)00054-1
Cantu, D., Blanco-Ulate, B., Yang, L., Labavitch, J. M., Bennett, A. B., & Powell, A. L. T. (2009). Ripening-regulated susceptibility of tomato fruit to Botrytis cinerea requires NOR but not RIN or ethylene. Plant Physiology, 150(3), 1434–1449. https://doi.org/10.1104/pp.109.138701
Chan, Z., & Tian, S. (2006). Induction of H2O2-metabolizing enzymes and total protein synthesis by antagonistic yeast and salicylic acid in harvested sweet cherry fruit. Postharvest Biology and Technology, 39(3), 314–320. https://doi.org/10.1016/j.postharvbio.2005.10.009
Cheng, M., Meng, F., Mo, F., Qi, H., Wang, P., Chen, X., Liu, J., Ghanizadeh, H., Zhang, H., & Wang, A. (2022). Slym1 control the color etiolation of leaves by facilitating the decomposition of chlorophyll in tomato. Plant Science, 324, 111457. https://doi.org/10.1016/j.plantsci.2022.111457
Chiang, K. S., Liu, H. I., & Bock, C. H. (2017). A discussion on disease severity index values. Part I: warning on inherent errors and suggestions to maximise accuracy. Annals of Applied Biology, 171(2), 139–154. https://doi.org/10.1111/aab.12362
Conrath, U., Pieterse, C. M. J., & Mauch-Mani, B. (2002). Priming in plant–pathogen interactions. Trends in Plant Science, 7(5), 210–216. https://doi.org/10.1016/S1360-1385(02)02244-6
Cordier, C., Pozo, M. J., Barea, J. M., Gianinazzi, S., & Gianinazzi-Pearson, V. (1998). Cell defense responses qssociated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. Molecular Plant-Microbe Interactions, 11(10), 1017–1028. https://doi.org/10.1094/MPMI.1998.11.10.1017
Cota, L. V., Maffia, L. A., & Mizubuti, E. S. G. (2008). Brazilian isolates of Clonostachys rosea: Colonization under different temperature and moisture conditions and temporal dynamics on strawberry leaves. Letters in Applied Microbiology, 46(3), 312–317. https://doi.org/10.1111/j.1472-765X.2007.02312.x
Cota, L. V., Maffia, L. A., Mizubuti, E. S. G., & Macedo, P. E. F. (2009). Biological control by Clonostachys rosea as a key component in the integrated management of strawberry gray mold. Biological Control, 50(3), 222–230. https://doi.org/10.1016/j.biocontrol.2009.04.017
De Meyer, G., Bigirimana, J., Elad, Y., & Höfte, M. (1998). Induced systemic resistance in Trichoderma harzianum T39 biocontrol of Botrytis cinerea. European Journal of Plant Pathology, 104(3), 279–286. https://doi.org/10.1023/A:1008628806616
Durrant, W. E., & Dong, X. (2004). Systemic acquired resistance. Annual Review of Phytopathology, 42(1), 185–209. https://doi.org/10.1146/annurev.phyto.42.040803.140421
El Komy, M. H., Saleh, A. A., Eranthodi, A., & Molan, Y. Y. (2015). Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato fusarium wilt. Plant Pathology Journal, 31(1), 50–60. https://doi.org/10.5423/ppj.Oa.09.2014.0087
Fiorini, L., Guglielminetti, L., Mariotti, L., Curadi, M., Picciarelli, P., Scartazza, A., Sarrocco, S., & Vannacci, G. (2016). Trichoderma harzianum T6776 modulates a complex metabolic network to stimulate tomato cv. Micro-Tom Growth. Plant and Soil, 400(1/2), 351–366.
Gardes, M., & Bruns, T. D. (1993). ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology, 2(2), 113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Ghanizadeh, H., Mesarich, C. H., & Harrington, K. C. (2020). Molecular characteristics of the first case of haloxyfop-resistant Poa annua. Scientific Reports, 10(1), 4231. https://doi.org/10.1038/s41598-020-61104-0
Guillemaud, T., Blin, A., Le Goff, I., Desneux, N., Reyes, M., Tabone, E., Tsagkarakou, A., Niño, L., & Lombaert, E. (2015). The tomato borer, Tuta absoluta, invading the Mediterranean Basin, originates from a single introduction from Central Chile. Scientific Reports, 5(1), 8371. https://doi.org/10.1038/srep08371
Gullino, M. L., Aloi, C., & Garibaldi, A. (1991). Integrated control of grey mould of tomato. Integrated Control of Grey Mould of Tomato, 14(5), 211–215.
Han, Z., Ghanizadeh, H., Zhang, H., Li, X., Li, T., Wang, Q., Liu, J., & Wang, A. (2022). Clonostachys rosea promotes root growth in tomato by secreting auxin produced through the tryptamine pathway. Journal of Fungi, 8(11), 1166.
Hyun, J. W., Yi, S. H., MacKenzie, S. J., Timmer, L. W., Kim, K. S., Kang, S. K., Kwon, H. M., & Lim, H. C. (2009). Pathotypes and genetic relationship of worldwide collections of Elsinoë spp. causing scab diseases of citrus. Phytopathology, 99(6), 721–728. https://doi.org/10.1094/PHYTO-99-6-0721
Islam, M. T., & Sherif, S. M. (2020). RNAi-based biofungicides as a promising next-generation strategy for controlling devastating gray mold diseases. International Journal of Molecular Sciences, 21(6), 2072. https://doi.org/10.3390/ijms21062072
Jiang, C.-H., Liao, M.-J., Wang, H.-K., Zheng, M.-Z., Xu, J.-J., & Guo, J.-H. (2018). Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biological Control, 126, 147–157. https://doi.org/10.1016/j.biocontrol.2018.07.017
Kinkema, M., Fan, W., & Dong, X. (2000). Nuclear localization of NPR1 is required for activation of PR gene expression. The Plant Cell, 12(12), 2339–2350.
Krauss, U., ten Hoopen, M., Rees, R., Stirrup, T., Argyle, T., George, A., Arroyo, C., Corrales, E., & Casanoves, F. (2013). Mycoparasitism by Clonostachys byssicola and Clonostachys rosea on Trichoderma spp. from cocoa (Theobroma cacao) and implication for the design of mixed biocontrol agents. Biological Control, 67(3), 317–327. https://doi.org/10.1016/j.biocontrol.2013.09.011
Kunkel, B. N., & Brooks, D. M. (2002). Cross talk between signaling pathways in pathogen defense. Current Opinion in Plant Biology, 5(4), 325–331.
Latha, P., Anand, T., Prakasam, V., Jonathan, E. I., Paramathma, M., & Samiyappan, R. (2011). Combining Pseudomonas, Bacillus and Trichoderma strains with organic amendments and micronutrient to enhance suppression of collar and root rot disease in physic nut. Applied Soil Ecology, 49, 215–223. https://doi.org/10.1016/j.apsoil.2011.05.003
Li, X., Ghanizadeh, H., Han, Z., Li, T., Li, Y., Dou, Z., Qiu, Y., Chen, X., Zhang, Y., Liu, J., & Wang, A. (2023). Development and characterization of yeast-incorporated coating films for improving the postharvest shelf-life of snap beans. Postharvest Biology and Technology, 197, 112215. https://doi.org/10.1016/j.postharvbio.2022.112215
Liu, J., Wu, Y.-C., Kan, J., Wang, Y., & Jin, C.-H. (2013). Changes in reactive oxygen species production and antioxidant enzyme activity of Agaricus bisporus harvested at different stages of maturity. Journal of the Science of Food and Agriculture, 93(9), 2201–2206. https://doi.org/10.1002/jsfa.6027
Liu, J., Li, Z., Ghanizadeh, H., Kerckhoffs, H., Sofkova-Bobcheva, S., Wu, W., Wang, X., Liu, Y., Li, X., Zhao, H., Chen, X., Zhang, Y., & Wang, A. (2020). Comparative genomic and physiological analyses of a superoxide dismutase mimetic (SODm-123) for its ability to respond to oxidative stress in tomato plants. Journal of Agricultural and Food Chemistry, 68(47), 13608–13619. https://doi.org/10.1021/acs.jafc.0c04618
Luo, Y., Liang, J., Zeng, G., Chen, M., Mo, D., Li, G., & Zhang, D. (2018). Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Management, 71, 109–114.
Meng, F., Lv, R., Cheng, M., Mo, F., Zhang, N., Qi, H., Liu, J., Chen, X., Liu, Y., Ghanizadeh, H., & Wang, A. (2022). Insights into the molecular basis of biocontrol of Botrytis cinerea by Clonostachys rosea in tomato. Scientia Horticulturae, 291, 110547. https://doi.org/10.1016/j.scienta.2021.110547
Miao, S., Li, F., Han, Y., Yao, Z., Xu, Z., Chen, X., Liu, J., Zhang, Y., & Wang, A. (2022). Identification of OSCA gene family in Solanum habrochaites and its function analysis under stress. BMC Genomics, 23(1), 547. https://doi.org/10.1186/s12864-022-08675-6
Nawrocka, J., & Małolepsza, U. (2013). Diversity in plant systemic resistance induced by Trichoderma. Biological Control, 67(2), 149–156. https://doi.org/10.1016/j.biocontrol.2013.07.005
Nobre, S. A. M., Maffia, L. A., Mizubuti, E. S. G., Cota, L. V., & Dias, A. P. S. (2005). Selection of Clonostachys rosea isolates from Brazilian ecosystems effective in controlling Botrytis cinerea. Biological Control, 34(2), 132–143. https://doi.org/10.1016/j.biocontrol.2005.04.011
Otieno, W., Jeger, M., & Termorshuizen, A. (2003). Effect of infesting soil with Trichoderma harzianum and amendment with coffee pulp on survival of Armillaria. Biological Control, 26(3), 293–301. https://doi.org/10.1016/S1049-9644(02)00168-8
Peng, G., & Sutton, J. C. (1990). Biological methods to control grey mould of strawberry. Paper presented at the Brighton Crop Protection Conference, Pests and Diseases, Thornton Heath, Council Farnham.
Pieterse, C. M., & Van Loon, L. (2004). NPR1: The spider in the web of induced resistance signaling pathways. Current Opinion in Plant Biology, 7(4), 456–464.
Pieterse, C. M. J., Van Pelt, J. A., Van Wees, S. C. M., Ton, J., Léon-Kloosterziel, K. M., Keurentjes, J. J. B., Verhagen, B. W. M., Knoester, M., Van der Sluis, I., Bakker, P. A. H. M., & Van Loon, L. C. (2001). Rhizobacteria-mediated induced systemic resistance: Triggering, signalling and expression. European Journal of Plant Pathology, 107(1), 51–61. https://doi.org/10.1023/A:1008747926678
Radić, S., Radić-Stojković, M., & Pevalek-Kozlina, B. (2006). Influence of NaCl and mannitol on peroxidase activity and lipid peroxidation in Centaurea ragusina L. roots and shoots. Journal of Plant Physiology, 163(12), 1284–1292. https://doi.org/10.1016/j.jplph.2005.08.019
Saraiva, R. M., Czymmek, K. J., Borges, Á. V., Caires, N. P., & Maffia, L. A. (2015). Confocal microscopy study to understand Clonostachys rosea and Botrytis cinerea interactions in tomato plants. Biocontrol Science and Technology, 25(1), 56–71. https://doi.org/10.1080/09583157.2014.948382
Silva, P. R. A., Araujo, L., Resende, R. S., Maffia, L. A., Einhardt, A. M., Oliveira, H. R., Alves, Y. H., Silva, L. F., & Rodrigues, F. Á. (2020). Silicon, Clonostachys rosea, and their interaction for gray mold management in cucumber. Journal of Plant Pathology, 102(4), 1257–1262. https://doi.org/10.1007/s42161-020-00643-x
Sun, Z. B., Li, S. D., Ren, Q., Xu, J. L., Lu, X., & Sun, M. H. (2020). Biology and applications of Clonostachys rosea. Journal of Applied Microbiology, 129(3), 486–495. https://doi.org/10.1111/jam.14625
ten Hoopen, G. M., George, A., Martinez, A., Stirrup, T., Flood, J., & Krauss, U. (2010). Compatibility between Clonostachys isolates with a view to mixed inocula for biocontrol. Mycologia, 102(5), 1204–1215.
Tjamos, S. E., Flemetakis, E., Paplomatas, E. J., & Katinakis, P. (2005). Induction of resistance to Verticillium dahliae in Arabidopsis thaliana by the biocontrol agent K-165 and pathogenesis-related proteins gene expression. Molecular Plant-Microbe Interactions, 18(6), 555–561.
van Loon, L. C., Bakker, P. A. H. M., & Pieterse, C. M. J. (1998). Systemic resistance induced by rhizosphere bacteria. Annual Review of Phytopathology, 36(1), 453–483. https://doi.org/10.1146/annurev.phyto.36.1.453
Vlot, A. C., Dempsey, D. M. A., & Klessig, D. F. (2009). Salicylic acid, a multifaceted hormone to combat disease. Annual Review of Phytopathology, 47, 177–206.
Vukelić, I. D., Prokić, L. T., Racić, G. M., Pešić, M. B., Bojović, M. M., Sierka, E. M., Kalaji, H. M., & Panković, D. M. (2021). Effects of Trichoderma harzianum on photosynthetic characteristics and fruit quality of tomato plants. International Journal of Molecular Sciences, 22(13), 6961. https://doi.org/10.3390/ijms22136961
Wang, Y., & Liu, J.-H. (2012). Exogenous treatment with salicylic acid attenuates occurrence of citrus canker in susceptible navel orange (Citrus sinensis Osbeck). Journal of Plant Physiology, 169(12), 1143–1149.
Wang, Y., Yu, T., Xia, J., Yu, D., Wang, J., & Zheng, X. (2010). Biocontrol of postharvest gray mold of cherry tomatoes with the marine yeast Rhodosporidium paludigenum. Biological Control, 53(2), 178–182. https://doi.org/10.1016/j.biocontrol.2010.01.002
Wang, Q., Chen, X., Chai, X., Xue, D., Zheng, W., Shi, Y., & Wang, A. (2019). The involvement of jasmonic acid, ethylene, and salicylic acid in the signaling pathway of Clonostachys rosea-induced resistance to gray mold disease in tomato. Phytopathology, 109(7), 1102–1114.
Warcup, J. H. (1950). The soil-plate method for isolation of fungi from soil. Nature, 166(4211), 117–118. https://doi.org/10.1038/166117b0
Williamson, B., Tudzynski, B., Tudzynski, P., & Van Kan, J. A. L. (2007). Botrytis cinerea: The cause of grey mould disease. Molecular Plant Pathology, 8(5), 561–580. https://doi.org/10.1111/j.1364-3703.2007.00417.x
Xue, A. G. (2003). Biological Control of Pathogens Causing Root Rot Complex in Field Pea Using Clonostachys rosea Strain ACM941. Phytopathology®, 93(3), 329–335. https://doi.org/10.1094/PHYTO.2003.93.3.329
Yu, Q., & Rengel, Z. (1999). Drought and salinity differentially influence activities of superoxide dismutases in narrow-leafed lupins. Plant Science, 142(1), 1–11. https://doi.org/10.1016/S0168-9452(98)00246-5
Yu, X., Ai, C., Xin, L., & Zhou, G. (2011). The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. European Journal of Soil Biology, 47(2), 138–145. https://doi.org/10.1016/j.ejsobi.2010.11.001
Yuan, M., Huang, Y., Ge, W., Jia, Z., Song, S., Zhang, L., & Huang, Y. (2019). Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genomics, 20(1), 1–13.
Zehra, A., Meena, M., Dubey, M. K., Aamir, M., & Upadhyay, R. S. (2017). Synergistic effects of plant defense elicitors and Trichoderma harzianum on enhanced induction of antioxidant defense system in tomato against Fusarium wilt disease. Botanical Studies, 58(1), 44. https://doi.org/10.1186/s40529-017-0198-2
Zhang, L. B., & Feng, M. G. (2018). Antioxidant enzymes and their contributions to biological control potential of fungal insect pathogens. Applied Microbiology and Biotechnology, 102(12), 4995–5004. https://doi.org/10.1007/s00253-018-9033-2
Zhang, D., Yang, Y., Castlebury, L. A., & Cerniglia, C. E. (1996). A method for the large scale isolation of high transformation efficiency fungal genomic DNA. FEMS Microbiology Letters, 145(2), 261–265. https://doi.org/10.1111/j.1574-6968.1996.tb08587.x
Zhang, X., Francis, M. I., Dawson, W. O., Graham, J. H., Orbović, V., Triplett, E. W., & Mou, Z. (2010). Over-expression of the Arabidopsis NPR1 gene in citrus increases resistance to citrus canker. European Journal of Plant Pathology, 128(1), 91–100. https://doi.org/10.1007/s10658-010-9633-x
Zhang, F., Ge, H., Zhang, F., Guo, N., & Wang, Y. (2016). Biocontrol potential of Trichoderma harzianum isolate T-aloe against Sclerotinia sclerotiorum in soybean. Plant Physiology and Biochemistry, 100, 64–74. https://doi.org/10.1016/j.plaphy.2015.12.017
Zhao, Y., Thilmony, R., Bender, C. L., Schaller, A., He, S. Y., & Howe, G. A. (2003). Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. The Plant Journal, 36(4), 485–499. https://doi.org/10.1046/j.1365-313X.2003.01895.x
Zhao, W., Zhou, X., Lei, H., Fan, J., Yang, R., Li, Z., Hu, C., Li, M., Zhao, F., & Wang, S. (2018). Transcriptional evidence for cross talk between JA and ET or SA during root-knot nematode invasion in tomato. Physiological Genomics, 50(3), 197–207.
Zheng, Y., Wang, X., Liu, S., Zhang, K., Cai, Z., Chen, X., Zhang, Y., Liu, J., & Wang, A. (2018). The endochitinase of clonostachys rosea expression in Bacillus amyloliquefaciens enhances the Botrytis cinerea resistance of tomato. International Journal of Molecular Sciences, 19(8), 2221.
Zubrod, J. P., Bundschuh, M., Arts, G., Brühl, C. A., Imfeld, G., Knäbel, A., Payraudeau, S., Rasmussen, J. J., Rohr, J., Scharmüller, A., Smalling, K., Stehle, S., Schulz, R., & Schäfer, R. B. (2019). Fungicides: An overlooked pesticide class? Environmental Science and Technology, 53(7), 3347–3365. https://doi.org/10.1021/acs.est.8b04392
Acknowledgements
This research was funded by the National Natural Science Foundation of China (31872120, 32072588), National Key R&D Program of China (2017YFE0105000), National High-Level Talents Special Support Program (W03020273) to A.W., the China Postdoctoral Science Foundation (2017M621235), the Postdoctoral Science Foundation of Heilongjiang Province (LBH-Z17034), and the "Young Talents" Project of Northeast Agricultural University (17QC26) to J.L.
Author information
Authors and Affiliations
Contributions
Fengshuo Li and Hossein Ghanizadeh: Writing- Reviewing and Editing; Wenwei Song: Picture and Software; Shuang Miao: Formal analysis; Hui Wang: Software; Xiuling Chen: Methodology; Jiayin Liu: Software, Validation, Aoxue Wang: Supervision.
Corresponding authors
Ethics declarations
Ethical approval
Ethics approval was not required for this research.
Conflict of interest
All the authors declare that there is no conflict of interest.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, F., Ghanizadeh, H., Song, W. et al. Combined use of Trichoderma harzianum and Clonostachys rosea to manage Botrytis cinerea infection in tomato plants. Eur J Plant Pathol 167, 637–650 (2023). https://doi.org/10.1007/s10658-023-02732-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02732-w