Abstract
Background
OSCA (hyperosmolality-gated calcium-permeable channel) is a calcium permeable cation channel protein that plays an important role in regulating plant signal transduction. It is involved in sensing changes in extracellular osmotic potential and an increase in Ca2+ concentration. S. habrochaites is a good genetic material for crop improvement against cold, late blight, planthopper and other diseases. Till date, there is no report on OSCA in S. habrochaites. Thus, in this study, we performed a genome-wide screen to identify OSCA genes in S. habrochaites and characterized their responses to biotic and abiotic stresses.
Results
A total of 11 ShOSCA genes distributed on 8 chromosomes were identified. Subcellular localization analysis showed that all members of ShOSCA localized on the plasma membrane and contained multiple stress-related cis acting elements. We observed that genome-wide duplication (WGD) occurred in the genetic evolution of ShOSCA5 (Solhab04g250600) and ShOSCA11 (Solhab12g051500). In addition, repeat events play an important role in the expansion of OSCA gene family. OSCA gene family of S. habrochaites used the time lines of expression studies by qRT-PCR, do indicate OSCAs responded to biotic stress (Botrytis cinerea) and abiotic stress (drought, low temperature and abscisic acid (ABA)). Among them, the expression of ShOSCAs changed significantly under four stresses. The resistance of silencing ShOSCA3 plants to the four stresses was reduced.
Conclusion
This study identified the OSCA gene family of S. habrochaites for the first time and analyzed ShOSCA3 has stronger resistance to low temperature, ABA and Botrytis cinerea stress. This study provides a theoretical basis for clarifying the biological function of OSCA, and lays a foundation for tomato crop improvement.
Similar content being viewed by others
Background
Tomato (Solanum lycopersicum) is an important cash crop and plays an important role in the global vegetable industry [1]. However, environmental stresses like drought, heat, cold and biotic stresses caused by pathogenic microbes and insects are the significant constraints of tomato production, ultimately leading to a severe negative impact on yield [2]. Therefore, more excellent materials and genes need to be identified to cultivate excellent varieties. S. habrochaites is a wild relative of cultivated tomato belonging to Solanaceae and Solanum family. It has many excellent agronomic traits, such as cold tolerance, disease resistance and resistance to many other stresses [3]. Therefore, as superior stress resistant materials, S. habrochaites often play an essential role in improving the quality characteristics of tomatoes.
As a second messenger, calcium ion (Ca2+) participates in regulating the growth and development of plants through sensing external environmental stresses. In living organisms, the core of Ca2+ based signal transduction is the concentration of free Ca2+ in the cytosol ([Ca2+] cyt). In non-stimulated or resting cells, the capacity of [Ca2+] cyt is in the range of 10− 7 M. However, when stimulated, it increased about 10 times to a low level- μM level [4]. One hypothesis is that the mechanism leading to the increase of [Ca2+] cyt is the “opening mechanism” [5, 6]. In order to operate Ca2+ as an effective signal system, it is necessary to restore [Ca2+] cyt to its pre stimulation level, which is realized through the so-called “shutdown mechanism”. The “closure mechanism” covers a series of membrane proteins that move Ca2+ along the concentration gradient into or out of the cell’s internal storage areas (such as vacuoles). The result of the switching mechanism is the formation or shaping of Ca2+ signal. When plants are directly under biotic stress or abiotic stress, they can respond by forming Ca2+ signal [7]. Signals from various stimuli can also activate different Ca2+ channels to form Ca2+ signals with certain temporal and spatial characteristics, so as to ensure high specificity between stimuli and responses [8]. Known calcium channels in plants include cyclic nucleotide gated channels (CNGCs) [9], two pore channels (TPCS) [10], mechanosensitive channels (MCAS), glutamate receptor channels (GLRS) [11], possibly through Orai channels [12] (at least in plants outside Angiosperm Flora) and recently discovered decreased high osmotic pressure induced Ca2+ increased permeability (OSCAs).
Plasma membrane protein OSCA1 is a hypertonic calcium receptor, encoding a protein of 772 amino acids and contains 9 transmembrane domains [13]. OSCA in A.thalliana has 15 family members, which are divided into four subfamilies. It contains homologues in other plant species and eukaryotes [14]. Multiple sequence alignment analysis showed that OSCA gene family contained a conserved DUF221 domain. DUF221 represents seven transmembrane domains of calcium dependent channels, which are shown in Inter Pro to function as calcium channels in osmotic induction [15]. The detection of OSCA1 in transgenic A.thalliana showed that the gene is expressed in leaves, guard cells and roots [14]. In addition, some studies have found that OSCA responds to osmotic stress and acts as an ion channel for mechanical stimulation. OSCA1.2 can be activated by mechanical force transmitted through the membrane. Its 11 transmembrane helices form a homodimer. The cytoplasmic domain contains an RNA recognition motif and two unique long helices anchored in the lipid bilayer. OSCA1.2 is very similar to mammalian TMEM16 in structure, but there is no homology in sequence [16,17,18]. The structure of OSCA1.1 and OSCA1.3 were observed by low temperature electron microscope. It is found that the activation of OSCA ion channel will change the conformation of M0 and M6 in the transmembrane helix, straighten M6 and bend M0, resulting in the increase of cross-sectional area of transmembrane region and the opening of ion channel [19]. Therefore, OSCA is a mechanically sensitive Ca2+ channel.
Studies have shown that 10 members of OSCA gene family in rice respond to drought, salt, ABA and osmotic stress, and the function of four of these in response to osmotic stress has been characterized. During seed water absorption, the functions of 6 OSCAs genes were related to osmotic stress, and 3 OSCAs were strongly related to circadian rhythm [20]. Twelve OSCA gene family members were identified in maize, including ZmOSCA4.1 gene, which response to drought stress [21]. In G. hirsutum, G. arboretum and G.raimondii, 35, 21 and 22 gene family members were identified, respectively, including GhOSCA1.1 a key gene involved in drought and salt tolerance [22]. Recent studies have shown that OSCA responds to plant immune response. In A.thalliana PTI immune response, when plasma membrane related cytoplasmic kinase BIK1 is activated, it will be phosphorylated by OSCA1.3. When the channel of OSCA1.3 is opened, Ca2+ in cytoplasm increases, forming osmotic potential and regulating stomatal closure of guard cells [23].
No OSCA family member in S. habrochaites has been characterized till date. Thus, in order to discover the function and genetic evolution of OSCA gene family in S. habrochaites, we identified OSCA genes in S. habrochaites by bioinformatics analysis. Further analysis was conducted to identify their molecular characteristics, gene evolution, conserved motif, cis element, protein structure, etc. Their gene expression under biotic (Botrytis cinerea) and abiotic stresses (drought, low temperature and ABA) were identified by qRT-PCR, and the function of ShOSCA3 was validated by VIGS. This study provides an experimental description of the biological function of OSCA gene in response to stress and lays a foundation for tomato crop improvement.
Results
Identification of OSCA gene family members in S. habrochaites
Using the whole genome sequence of S. habrochaites and the conservative structure DUF221 of OSCA gene family, 11 members of OSCA gene family were identified by the bioinformatics method and named according to their position on the chromosome (Table S1). These genes were distributed on 8 chromosomes, respectively (Fig. 1a). Their physical and chemical properties, such as gene length, amino acid length, isoelectric point size and molecular weight, were obtained using the ExPASY website. The gene length ranges from 2142 bp - 2889 bp, and the number of amino acids ranges from 707 aa - 962 aa (Table S1). The isoelectric points of 10 members of the OSCA family of S. habrochaites are relatively similar and greater than 7, ranging from 8.58 to 9.39, except that ShOSCA3 is 6.40. In silico subcellular localization was performed with CELLOv2, and all identified OSCA members were located on the plasma membrane, suggesting that OSCA genes function in roles related to the plasma membrane.
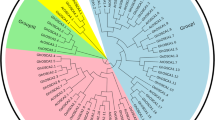
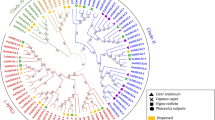
Chromosome locations and phylogenetic analysis of OSCA gene family. a Chromosome locations of OSCA gene family in Solanum habrochaites. The chromosome numbers are indicated at the top of each chromosome. Only chromosomes contained OSCA genes are represented in this figure. Those genes were named according to their position on the chromosome. b Phylogenetic relationships of OSCA protein among A.thalliana (Arabidopsis thaliana L.), S.lycopersicum (Solanum lycopersicum), S.habrochaites (Solanum habrochaites), O.sativa (Oryza sativa L.), Z.mays (Zea mays L.). The phylogenetic tree was built by MEGA10 with bootstrap of 1000. Symbols with different colors represent different species
Evolutionary relationship among OSCA members was identified with phylogenetic trees consisting of a total of 61 protein sequences from S. habrochaites, A.thalliana (Arabidopsis thaliana L.), S.lycopersicum (Solanum lycopersicum), O.sativa (Oryza sativa L.), Z.mays (Zea mays L.). As shown in Fig. 1b, the OSCA genes in the five species were classified into four major groups (Groups 1, 2, 3, and 4). Group I contains the most OSCA gene members, including 10 members of A.thalliana and 7 members of the S. habrochaites. Groups 2 and 3 consist of two OSCA gene, while group 4 include two OSCA genes, OsOSCA2.3 and ZmOSCA2.3.
Cis acting element analysis
Cis acting elements located upstream of the transcription initiation codon do not code for proteins, but can affect gene expression. 1500 bp upstream of the start codon of OSCA genes were obtained with UGENE software, and cis acting elements related to stress contained in them were identified and analyzed with Plant CARE software (Table S2). 82% of the members of OSCA gene family in S. habrochaites have cis acting element ABRE involving abscisic acid reaction and CGTCA motif involving MeJA reaction. 36% of gene family members have cis acting element TCA involved in salicylic acid reaction. 27% of the gene family members have cis acting element TC-rich which is involved in stress response, cis acting element LTR involved in low temperature response and cis acting element W-box involved in salt response.
Analysis of conserved motifs and gene structure of OSCA gene family in S. habrochaites
We analyzed OSCA gene members in S. habrochaites by meme software, and predicted 20 conserved motifs (Fig. 2a). Proteins with similar genetic relationship have similar conserved motifs, indicating that their structure is highly conserved. For example, the conserved motifs and permutation structures of ShOSCA1, ShOSCA2, ShOSCA5 and ShOSCA11; ShOSCA4, ShOSCA7, ShOSCA6, ShOSCA10 and ShOSCA8 are highly similar. GSDS tool was used to analyze the structure of OSCA gene in S. habrochaites. We found that most members of this family are intron enriched genes. Except for ShOSCA3, there are multiple introns in other genes (Fig. 2b).
Architecture of conserved protein motifs and gene structure of OSCA gene family. a The motif composition of OSCA genes. The protein sequences of 11 genes contain 20 conserved motifs, and the same conserved motifs between proteins are represented by the same color; b Exon-intron structure of OSCA genes. Blue boxes indicate untranslated region (UTR), yellow boxes indicate exons, black lines represent introns
Protein structure analysis and function prediction of OSCA gene family in S. habrochaites
Regions with the same domain usually have similar functions. Some differences in domains among members of OSCA genes in S. habrochaites may lead to distinct response to stresses. We analyzed the folding mode through analysis of the secondary structure and predicted function through the tertiary structure of the proteins. The results showed that 11 family members contained a large number of α- Spirals and irregular curls, with only a small amount of β-corner (Table S3). The content of α- helix and irregular curl is as high as 94% in these OSCA proteins. In addition to ShOSCA1, ShOSCA3, ShOSCA8 and ShOSCA11, the other 7 members α- helix content reached more than 50%. Their tertiary structure model was constructed with the Phyre2.0 software, revealing that these genes have highly similar protein structure (Fig. S1).
Synteny analysis and Ka / Ks ratio of OSCA gene pairs
Gene replication plays an important role in genome evolution. It can accumulate evolutionary raw materials in the process of replication to promote plant evolution [24]. In particular, genome-wide duplication (WGD) and tandem duplication (TD) have been found to be important for the expansion of gene families [25]. As shown in Fig. 3a, ShOSCA5 (Solhab04g250600) and ShOSCA11 (Solhab12g051500) had WGD in genetic evolution. To further infer the phylogenetic mechanism among OSCA family members, we constructed the comparative syntenic maps of three OSCA gene families, namely, the synteny analysis among A.thalliana, S. habrochaites and S.lycopersicum (Fig. 3b). A total of 8 OSCA genes in S.lycopersicum showed synteny with A.thalliana, 6 OSCA genes in S. habrochaites showed synteny with A.thalliana, and 12 OSCA genes in S.lycopersicum showed synteny with 11 OSCA genes in S. habrochaites. Some OSCA genes were associated with at least two syntentic gene pairs (OSCA genes between S. habrochaites and A.thalliana), such as ShOSCA2, ShOSCA11 and SlOSCA3. In order to better understand the evolutionary constraints acting on OSCA gene family, the Ka / Ks ratio of OSCA gene pairs and divergence time was calculated (Fig. 3c, d). The Ka / Ks of most direct homologous OSCA gene pairs is < 1, indicating that tomato OSCA genes may have experienced strong purification and selection pressure in the process of evolution (Fig. 3c). Four pairs, Solhab08g070100.1 (ShOSCA8) versus AT1G32090.1 (AtOSCA1.8), Solyc02g081030.2 (SlOSCA3) versus AT1G62320.3 (AtOSCA1.4), Solyc02g088300.2 (SlOSCA5) versus AT1G69450.3 (AtOSCA2.4), and Solyc08g076310.2 (SlOSCA10) versus AT1G32090.1 (AtOSCA1.8) Ks values could not be calculated, indicating that there might be more sequence divergence between these genes.
Synteny analyses and Ka/Ks ratio of OSCA gene pairs. a Syntenic analysis of OSCA gene family in S.habrochaites genome. The gray line represents the syntenic blocks in the S.habrochaites genome, and the red line highlights represents the OSCA syntenic gene pair; b Syntenic analysis of OSCA gene family among genomes. Gray lines in the background represent collinear blocks within the genome of S. habrochaites and other plants, while red lines highlight represents the collinearity of OSCA gene pairs. The species were A. thaliana, S. lycopersicum and S. habrochaites respectively; c Ks and Ka/Ks values of orthologous OSCA gene pairs between any two of the A. thalliana, S.lycopersicum and S.habrochaites; d Divergence time estimation of orthologous OSCA gene pairs between any two of the A. thalliana, S.lycopersicum and S.habrochaites
Expression analysis of OSCA gene family in S. habrochaites under stress
We hypothesized that the expression of ShOSCAs genes might respond to a series of plant stress because bioinformatic analysis revealed the presence of stress-associated cis-acting elements in their promoter. Therefore, to further analyze the function of OSCA, the gene expression under Botrytis cinerea and drought, low temperature and ABA stresses was evaluated by qRT-PCR. We set the expression of 0 h as 1 and compared the expression at other time points. As shown in Fig. 4, under drought stress, the expression levels of ShOSCA1, ShOSCA3, ShOSCA6 and ShOSCA9 were the highest at 0.5, 3, 9 and 12 h after treatment. Among them, the expression of ShOSCA8 was significantly up-regulated at 3 and 12 h in response to low temperature stress. The expression of ShOSCA10 reached the maximum at 0.5 h after ABA treatment. Under Botrytis cinerea stress, the gene expression of ShOSCA3 was significantly up-regulated at 9 and 12 h. As shown in Fig. 4, the expression of ShOSCA3 was significantly up-regulated under four stress treatments, indicating that ShOSCA3 responds to Botrytis cinerea drought, low temperature and ABA stresses.
Functional characterization of ShOSCA3
We used functional analysis by VIGS to characterize the function of ShOSCA3. Firstly, we constructed VIGS vector PTRV2-ShOSCA3 and transferred the silencing vector PTRV2-PDS (phytoene dehydrogenase), an indicator gene of the VIGS system into S. habrochaites by Agrobacterium infection. After 17 days of tomato inoculation, ShOSCA3 silenced plants began to turn white from the veins in new leaves (Fig. S2, a, b, c), and the silencing efficiency of ShOSCA3 was confirmed by qRT-PCR (Fig. S2, d). When the plant is damaged by stress, a large amount of ROS is produced, which will poison the cells, causing membrane damage and cell death. Therefore, the oxidative damage degree of ShOSCA3 silenced plants and control plants was evaluated by measuring the contents of superoxide dismutase (SOD), peroxidase (POD), polyphenol oxidase (PPO) and ascorbic acid peroxidase (APX) under Botrytis cineria, low temperature, drought and ABA stress.
Under Botrytis cinerea stress, as shown in Fig. 5, the SOD content of control plants and ShOSCA3 silenced plants showed a gradually increasing trend, but the SOD content of control plants was higher than that of ShOSCA3 silenced plants. The POD activity of ShOSCA3 silenced plants was higher than that of control plants. The changing trend of PPO content was gradually increasing, but the PPO content of ShOSCA3 silenced plants was higher than that of control. The study of APX content found that the changing trend of ShOSCA3 silenced plants was consistent with that of the control plants, but at 12 h, the changing trend was the opposite, and the APX activity of the control plants was higher.
Effects of Botrytis cinerea stress on SOD, POD, PPO and APX of ShOSCA3 silencing group. Error bars represent the standard deviation of three independent biological replicates. One-way ANOVA test was used for significance analysis. “**” represents a significant difference between the data point and control (p < 0.01, LSD)
After 3 hours of exposure to low temperature, the expression of APX increased sharply. After 12 hours, the content of APX in the control group decreased and was lower than that of ShOSCA3 silenced plants. The results showed that the resistance of ShOSCA3 silenced plants under cold stress was weaker than that of the control plants. The amount of superoxide dismutase (SOD) and peroxidase (POD) in ShOSCA3 silenced plants were lower than those in control plants. After 9 hours of cold stress, the PPO content of ShOSCA3 silenced plants was higher than that of control plants, but the overall trend of PPO content was gradually increased (Fig. 6).
Effects of cold stress on SOD, POD, PPO and APX of ShOSCA3 silencing group. Error bars represent the standard deviation of three independent biological replicates. One-way ANOVA test was used for significance analysis. “**” represents a significant difference between the data point and control (p < 0.01, LSD)
Under drought stress, the SOD content of ShOSCA3 silenced plants increased sharply at 6 h, and the content was lower than that of the POD in ShOSCA3 silenced plants, Control plants increased sharply at 3 h, but the overall trend of POD content in ShOSCA3 silenced plants was higher than that of control plants. The change in PPO content showed a similar trend between the control group and the silenced group. The trend of APX content was the same before 9 h, and then the APX content of ShOSCA3 silenced plants was slightly lower than that of the control (Fig. 7).
Effects of drought stress on SOD, POD, PPO and APX of ShOSCA3 silencing group. Error bars represent the standard deviation of three independent biological replicates. One-way ANOVA test was used for significance analysis. “**” represents a significant difference between the data point and control (p < 0.01, LSD)
As shown in Fig. 8, under ABA stress, the overall trend of SOD content in silenced plants and control plants was the same, and it showed a downward trend at 12 h, but the control group was higher than the silenced group on the whole. The changing trend of POD content in the control and the silencing group was similar, and the POD content increased at 3 h and 6 h. The fluctuation trend of PPO in control group and the transgenic silenced group was similar, but the content of PPO in control group was higher than that in the silencing group. The changing trend of APX content in control and silencing group was similar before 9 h, and the APX content in the silencing group increased after 9 h (Fig. 8).
Effects of ABA stress on SOD, POD, PPO and APX of ShOSCA3 silenced group. Error bars represent the standard deviation of three independent biological replicates. One-way ANOVA test was used for significance analysis. “**” represents a significant difference between the data point and control (p < 0.01, LSD)
Discussion
S. habrochaites is a good genetic material for crop improvement against cold, late blight, planthopper and other diseases [26, 27]. S. habrochaites can be genetically crossed with cultivated tomato only when used as male parent, but the seed setting rate is low [28]. Therefore, it is difficult to transfer the excellent characteristics of S. habrochaites to cultivated varieties. In this study, OSCA genes were identified in S. habrochaites and the role under stress was further evaluated, thus, laying a foundation for the genetic improvement of cultivated tomato.
In this study, a total of 11 OSCA genes were identified in S. habrochaites. Compared with cultivated tomato, S. habrochaites has lost one OSCA gene during evolution, and four in comparison with A.thalliana, but has the same number of OSCA genes as in rice [20]. However, according to the phylogenetic tree constructed, the four species are divided into four branches. This shows that rice, S. habrochaites, S.lycopersicum and A.thalliana have experienced relatively conserved evolution. The occurrence of gene replication events will lead to the contraction and expansion of the gene family and provide complexity for gene function [29]. The collinearity results of A. thaliana, S. lycopersicum and S. habrochaites showed that the amount of replication events occurred in the evolution of OSCA gene.
Conserved genes are more likely to produce functional and lasting duplication. These genes are often involved in signal transduction and regulation, which contributes to their long-term viability in the eukaryotic genome [30, 31]. Such proteins that encode multiple domains and have multiple cis regulatory regions will be preferentially expressed [32]. This study revealed that the promoter regions of OSCA gene family contain cis acting elements associated with many stresses, indicating that they have obtained more diverse functions in gene evolution. In addition to ShOSCA8 and ShOSCA9, members of the OSCA gene family of S. habrochaites contain ABRE cis acting elements, which can reverse activate and produce a large amount of ABA, involved in ABA signal transduction pathway [33, 34]. In addition, OSCA gene family of S. habrochaites also contains cis acting elements CGTCA motif and TGACG motif involved in jasmonic acid signal transduction pathway, and cis acting element TCA of salicylic acid signal transduction pathway. Jasmonic acid and salicylic acid are indispensable in plant disease resistance, but they have a certain antagonistic effect [35, 36]. The OSCA gene family of S. habrochaites contains these cis acting elements, indicating that these genes are likely involved in disease resistance. In addition, ShOSCA4 and ShOSCA9 contain homeopathic elements circadian related to circadian rhythm, while in rice, OsOSCA1.2, OsOSCA2.1 and OsOSCA2.2 also have the same function [20]. The result further shows that OSCA gene is conservative in gene function in the process of evolution, even if gene replication events continue to occur.
RNA seq data on the response of S. habrochaites to various stresses need to be improved. RNA seq data combined with gene expression pattern analysis can help understand gene function better. According to the expression pattern of OSCA gene in S. habrochaites under low temperature, drought, ABA and Botrytis cinerea stress, it was found that the expression of ShOSCA3 changed significantly under various stresses. The gene structure was analyzed and it was found that the gene had no intron. Genes with few or no introns are thought to be rapidly expressed in plants [37]. The longer the intron, the longer the gene transcription time, and the faster the genes with fewer introns respond to biotic and abiotic stresses. For example, some stress-related gene families, such as Hsp20 family [38], leucine rich repeat family [39] and GRF family [40], also contain a few introns. Therefore, without intron, it may gain the advantage of time in the process of transcription and can quickly respond to all kinds of stress. In addition, corn ZmOSCA4.1 and ShOSCA3 are in the same evolutionary branch, and there is no intron in their gene structure. However, under drought stress, the change of ZmOSCA4.1 is the most significant. The expression of ShOSCA3 also changed significantly under drought stress, which further indicates the conservation of gene function in the process of evolution. In addition, ZmOSCA4 response under other stress still needs to be evaluated.
In order to further analyze the gene function of ShOSCA3, the gene was silenced by VIGS technology. The control plants and ShOSCA3 silenced plants were subjected to low temperature, drought, ABA and Botrytis cinerea stress, and the stress-related physiological indexes were detected. After plants were subjected to biotic and abiotic stress, the balance of reactive oxygen species in plants is distorted and ROS is accumulated in large quantities, resulting in toxic effects on cells [41]. Therefore, detecting the main enzyme activity of the active oxygen scavenging mechanism of ShOSCA3 silenced plant is one of the main methods to detect the damage of plants under stress and analyze the function of the silenced gene. ABA is one of the important endogenous hormones in plants and the critical endogenous messenger in response to a variety of stress [42]. We discovered that the PPO activity of ShOSCA3 silenced plants was lower than that of the control, indicating that the PPO related response mechanism was inhibited under ABA stress [43]. Under ABA treatment, the activities of SOD, POD and APX in control were higher than those in ShOSCA3 silenced plants, indicating that ShOSCA3 silenced plants had the ability to resist the high concentration of ABA stress. Plant disease resistance is closely related to the accumulation of phenylpropane metabolites, the expression of pathogen related protein (PRP), pentose phosphate pathway, glyoxylate cycle and other processes [44]. The key enzymes in disease resistance metabolism include phenylalanine ammonia lyase (PAL), peroxide (POD), polyphenol oxidase (PPO) and so on. The change of its activity is one of the effective indexes to reflect the strength of plant disease resistance. Under the infection of Botrytis cinerea, the PPO enzyme activity in ShOSCA3 silenced plants was higher than that of control plants, and the PPO enzyme activity was also higher than in control plants. This indicates that the plant is more susceptible to disease after silencing ShOSCA3, resulting in the increase of disease resistance related enzyme activity, but it does not affect the response of plant disease resistance, which indicates that ShOSCA3 respond to early plant immune response. We will further understand how ShOSCA3 participates in various stresses and its effect on the change of cytoplasmic Ca2+ concentration.
Conclusion
In this study, 11 OSCA gene family members were identified in S. habrochaites and localized in the cytoplasmic membrane. Phylogenetic results divided these OSCA genes into 4 groups. Structural analysis showed that ShOSCAs is a gene family rich in introns. OSCA gene was subjected to biotic stress (Botrytis cinerea) and abiotic stress (drought, ABA, cold). qRT-PCR results showed that ShOSCA3 responded strongly to multiple stresses. ShOSCA3-silenced plants were subjected to stress treatment and assayed for stress marker enzyme activity, which indicated that ShOSCA3 was more resistant to low temperature, ABA and botrytis stress.
Materials and methods
Plant materials and treatments
S. habrochaites (LA1777) was provided by tomato genetics research center (TGRC, https://tgrc.ucdavis.edu/). The seeds were disinfected with 5% sodium hypochlorite for 15 min, then washed with 75% alcohol once, followed by three times wash with sterile water. Finally, they were placed on wet filter paper and cultured with soil and vermiculite in a ratio 3:1 matrix. The illumination time was 16 h and 8 h night time at 25 °C. Stress treatment was carried out at the five leaves stage. Botrytis cinerea infection: B. cinerea spores was diluted with an appropriate amount of sterile water to make the concentration of spore suspension 107 CFU/mL. Then, the bacterial solution was sprayed on the tomato plants, and cultured in a light incubator with 90% humidity and 20–25 °C, while the control plants were sprayed with water. The sampling time points were 0, 0.5, 3, 6, 9, 12 and 24 h after B. cinerea treatment. Drought stress: the plants were cultured in 20% PEG6000 (1/2 Hoagland nutrient solution) [45], and the sampling time points were 0, 0.5, 3, 6, 9 and 12 h respectively. ABA treatment: 150 μM / mL ABA was added to 1/2 Hoagland nutrient solution. The sampling time points were 0, 0.5, 3, 6, 9 and 12 h, respectively. Cold stress: the plants were placed in a 4 °C incubator (PR205740RCN, Thermo Scientific, USA) for 16 hours of light and 8 hours of darkness. The sampling time points were 0, 0.5, 3, 6, 9, 12 and 24 h, respectively. All treated tissue samples were immediately frozen in liquid nitrogen and stored at − 80 °C. Three plants with the same growth were taken from each treatment for three biological replicates. Three technical replicates were performed for each treatment.
Bioinformatics analysis of OSCA gene family in S. habrochaites
The whole-genome sequence of S. habrochaites was assessed from the website http://www.solomics.neau.edu.cn. According to the characteristics of OSCA gene family with the conserved structure (DUF221, also known as RSN1_7TM, PfamID: PF02714), which was the screening condition, the hidden Markov model (HMM) file corresponding to DUF221 was downloaded from the Pfam protein family database (http://pfam.sanger.ac.uk/). Then the protein sequence data of S. habrochaites were searched by using the default parameters of HMMER v3.0 [46]. The transcripts were input into CDD and Pfam for to detect the conserved DUF221 domain. Finally, the sequence with the complete DUF221 domain was retained and named according to its position on the chromosome. The molecular weight, isoelectric point and subcellular location of proteins in the identified OSCA gene family were calculated using the ExPASY website (http://web.expasy.org/protparam/).
Phylogenetic relationships analysis and chromosomal localization
The OSCA gene sequences of maize (Zea mays L.) was downloaded from the MaizeGDB database (https://www.maizegdb.org/), and the OSCA gene sequences of rice (Oryza sativa L. ssp. Japonica). A.thalliana was downloaded from the NCBI Conserved Domain Database (www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). For S.lycopersicum, they were retrieved from the Sol Genomic Network (https://solgenomics.net/). We used MEGA7.0 software (neighbor joining adjacency method) to draw the system evolution tree, and the parameters were as follows: Poisson model; Pairwise deletion; the bootstrap method is set to 1000. Based on the nucleotide sequence of the OSCA gene family members obtained above, Mapchart 2.32 software was used to construct the chromosome mapping.
Genic structure and protein structural analysis
The gene sequence 1500 bp upstream of the start codon of OSCA gene family was obtained from the genome sequence of S. habrochaites by UGENE software. The cis acting elements located in the promoters of each members were identified by obtaining the genomic sequence upstream of each gene and analyzed in the Plant CARE software (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Using online program gene structure display server (GSDS: http://gsds.cbi.pku.edu.cn/), the predicted coding sequence of S. habrochaites was compared with the corresponding full-length sequence, and the exon-intron structure diagram was drawn [47]. The secondary structure of the identified protein was simulated with SOPMA software, and then Phyre2.0 software (http://www.sbg.bio.ic.ac.uk/ ≈ phyre /) was.
Synteny analyses
The gene replication events among OSCA members in A.thalliana, S. habrochaites and S.lycopersicum were analyzed by multicollinearity scanning Toolkit (MCScanX), using the default parameters [48, 49]. KaKs_Calculator 2.0 was used to calculate the synonymous (Ks) and non-synonymous (Ka) substitutions for each repeat [50]. The divergence time was calculated by the formula T = Ks/r, where “r “was the divergence rate of the plant nuclear gene. For dicotyledonous plants, the r was taken to be 1.5× 10−8 synonymous substitutions per site per year.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from plant leaves cultured at 25 °C for 3 weeks with Trizol (Invitrogen, USA) reagent, and qRT PCR was performed with Chamq SYBR qPCR Master Mix (without Rox) (Vazyme, Nanjing, China). Sequence of primers used are listed in Table S1. Each reaction contains 10 μL Chamq SYBR qPCR Master Mix (Without Rox) and 0.4 μL forward primer (10 μM), 0.4 μL reverse primer (10 μM) and cDNA samples diluted 10 times with 1.0 μL. The reaction process was as follows: reaction conditions: 95 °C / 30 s; 95 °C / 10 s, 60 °C / 30 s, 40 cycles in total; Then 95 °C / 15 s, 60 °C / 60 s, 95 °C / 15 s. The relative expression level was calculated using the 2-ΔΔct method [51]. Each reaction consisted of three technical replicates, and the housekeeping gene, Actin, was used as the internal reference. Data represent means of three replicates ± standard deviation (SD). Analysis was performed using the Data Processing System, and Turkeys multiple range test were conducted to determine significant differences. P < 0.05 was considered to indicate statistical significance.
Virus-induced gene silencing (VIGS)
The constructed PTRV2-ShOSCA3, PTRV1 and PTRV2 -PDS vectors were transformed into Agrobacterium strain EHA105 using the Agrobacterium tumefaciens transformation method. Cell culture containing the appropriate plasmid was injected into the tomato seedlings through the leaves with a syringe. Infected tomatoes were cultured overnight at 22 °C in the dark, and then cultured in a greenhouse at 21 °C, 30% humidity and 16 h / 8 h photoperiod. After the positive control plants turned completely white, the leave samples were obtained from the test and the control group. The efficiency of gene silencing was evaluated with qRT-PCR.
Enzyme activity detection of SOD, POD, PPO and APX
ShOSCA3 silenced plants were exposed to low temperature (4 °C), drought (20% PEG6000) ABA (150 μM / mL) and Botrytis cinerea treatment. Samples were taken at 0, 3, 6, 9, 12 and 24 h, and enzyme activities were determined using Kit (Solarbio, China). The absorbance values for SOD, POD, PPO and APX were measured at 560 nm, 470 nm, 410 nm and 290 nm, respectively. One-way ANOVA test was used for significance analysis. “**” represents a significant difference between the data point and control (p < 0.01, LSD). All data were expressed as the mean ± SD (stander deviation) after normalization of the three independent experiments.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Klee HJ, Tieman DM. Genetic challenges of flavor improvement in tomato. Trends in Genetics Tig. 2013;29(4):257–62. https://doi.org/10.1016/j.tig.2012.12.003.
Ashrafi-Dehkordi E, Alemzadeh A, Tanaka N, Razi H. Meta-analysis of transcriptomic responses to biotic and abiotic stress in tomato. Peer J. 2018;6:e4631. https://doi.org/10.7717/peerj.4631.
Zhang X, Fowler SG, Cheng H, et al. Freezing sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing tolerant Arabidopsis. Plant J. 2004;39(6):905–19. https://doi.org/10.1111/j.1365-313X.2004.02176.x.
Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. https://doi.org/10.1038/35036035.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, ho-meostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4(7):517–29. https://doi.org/10.1038/nrm1155.
Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–58. https://doi.org/10.1016/j.cell.2007.11.028.
Steinhorst L, Kudla J. Calcium and reactive oxygen species rule the waves of sig -naling. Plant Physiol. 2013;163(2):471–85. https://doi.org/10.1104/pp.113.222950.
Hepler PK. The cytoskeleton and its regulation by calcium and protons. Plant Physiol. 2016;170:3–22. https://doi.org/10.1104/pp.15.01506.
Zelman AK, Dawe A, Gehring C, Berkowitz GA. Evolutionary and structural perspectives of plant cyclic nucleotide-gated cation channels. Front Plant Sci. 2012;3:95–108. https://doi.org/10.3389/fpls.2012.00095.
Galione A, Evans AM, Ma J, Parrington J, Arredouani A, Cheng X, et al. The acid test: the discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca2+ release channels. Pflug Arch Eur J Phy. 2009;458:869–76. https://doi.org/10.1007/s00424-009-0682-y.
Plattner H, Verkhratsky A. The ancient roots of calcium signalling evolutionary tree. Cell Calcium. 2015;57(3):123–32. https://doi.org/10.1016/j.ceca.2014.12.004.
Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338(6112):1308–13. https://doi.org/10.1126/science.1228757.
Batistic O, Kudla J. Analysis of calcium signaling pathways in plants. Biochim Biophys Acta. 2012;1820(8):1283–93. https://doi.org/10.1016/j.bbagen.2011.10.012.
Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, et al. OSCA1 mediates o smotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature. 2014;514(7522):367–71. https://doi.org/10.1038/nature13593.
Hou C, Tian W, Kleist T, He K, Garcia V, Bai F, et al. Duf221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 2014;24(5):632–5. https://doi.org/10.1038/cr.2014.14.
Jojoa-Cruz S, Saotome K, Murthy SE, Tsui CCA, Sansom MS, Patapoutian A, et al. Cryoem structure of the mechanically activated ion channel OSCA1.2. eLife. 2018;7:e41845. https://doi.org/10.7554/eLife.41845.
Liu X, Wang J, Sun L. Structure of the hyperosmolalitygated calcium-permeable channel OSCA1.2. Nat Commun. 2018;9(1):5060. https://doi.org/10.1038/s41467-018-07564-5.
Murthy SE, Dubin AE, Whitwam T, Cruz SJ, Patapoutian A. OSCA/ TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. eLife. 2018;7:e41844. https://doi.org/10.7554/eLife.41844.
Zhang M, Wang D, Kang Y, Wu JX, Yao F, Pan C, et al. Structure of the mechanosensitive OSCA channels. Nat Struct Mol Biol. 2018a;25(9):850–8. https://doi.org/10.1038/s41594-018-0117-6.
Li Y, Yuan F, Wen Z, Li Y, Wang F, Zhu T, et al. Genome-wide survey and expression analysis of the OSCA gene family in rice. BMC Plant Biol. 2015;15(1):261–73. https://doi.org/10.1186/s12870-015-0653-8.
Ding S, Feng X, Du H, Wang H. Genome-wide analysis of maize OSCA family members and their involvement in drought stress. Peer J. 2019;7:e6765. https://doi.org/10.7717/peerj.6765.
Yang X, Yanchao XU, Yang F, Odongo MR, Cai X, Wang X, et al. Genome-wide identification of OSCA gene family and their potential function in the regulation of dehydration and salt stress in gossypium hirsutum. J Cotton Res. 2019;2(2):14–31. https://doi.org/10.1186/s42397-019-0028-z.
Thor K, Jiang S, Michard E, George J, Zipfel C. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature. 2020;585:569–73. https://doi.org/10.1038/s41586-020-2702-1.
Sampedro J, Lee Y, Carey RE, dePamphilis C, Cosgrove DJ. Use of genomic history to improve phylogeny and understanding of births and deaths in a gene family. Plant J. 2010;44(3):409–19. https://doi.org/10.1111/j.1365-313X.2005.02540.x.
Kong H, Landherr LL, Frohlich MW, Leebens-Mack J, Ma H, dePamphilis CW. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: evidence for multiple mechanisms of rapid gene birth. Plant J. 2010;50(5):873–85. https://doi.org/10.1111/j.1365-313X.2007.03097.x.
Brouwer DJ, Clair DA. Fine mapping of three quantitative trait loci for late blight resistance in tomato using near isogenic lines (NILs) and sub-NILs. Theor Appl Genet. 2004;108(4):628–38. https://doi.org/10.1007/s00122-003-1469-8.
Kennedy GG. Tomato, pests, parasitoids and predators: tritrophic interactions involving the genus Lycopersicon. Annu Rev Entomol. 2003;48(1):51–72. https://doi.org/10.1146/annurev.ento.48.091801.112733.
Martin FW. The inheritance of unilateral incompatibility in Lycopersicon Hirsutum. Genetics. 1964;50(3):459–69. https://doi.org/10.1093/genetics/50.3.459.
Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, et al. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473(7345):97–100. https://doi.org/10.1038/nature09916.
Marchler-Bauer A, Anderson JB, Cherukuri PF, DeWeese-Scott C, Geer LY, Gwadz M, et al. CDD: a conserved domain database for protein classification. Nucleic Acids Res. 2005;33:D192–6. https://doi.org/10.1086/423992.
Van de Peer Y, Maere S, Meyer A. The evolutionary significance of ancient genome duplications. Nat Rev Genet. 2009;10(10):725–32. https://doi.org/10.1038/nrg2600.
Davis JC, Petrov DA. Preferential duplication of conserved proteins in eukaryotic genomes. PLoS Biol. 2004;2(3):E55. https://doi.org/10.1371/journal.pbio.0020055.
Choi H, Hong J, Ha J, Kang J, Kim SY. ABFs, a family of ABA-responsive element binding factors. J Biol Chem. 2000;275(3):1723–30. https://doi.org/10.1074/jbc.275.3.1723.
Fujita Y, Fujita AM, Satoh CR, Maruyama CK, Parvez A, Seki AM. AREB1 is a tr -anscription activator of novel AREB-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell. 2005;17(12):3470–88. https://doi.org/10.1105/tpc.105.035659.
Guo X, Stotz HU. Defense against sclerotinia sclerotiorum in Arabidopsis is dependent on jasmonic acid, salicylic acid, and ethylene signaling. Mol Plant Microbe In. 2007;20(11):1384. https://doi.org/10.1094/MPMI-20-11-1384.
Van der Does D, Leon-Reyes A, Koornneef A, Van Verk MC, Rodenburg N, Pauwels L, et al. Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell. 2013;25(2):744–61. https://doi.org/10.1105/tpc.112.108548.
Jahn L, Mucha S, Bergmann S, Horn C, Staswick P, Steffens B, et al. The clubroot pathogen (Plasmodiophora brassicae) influences auxin signaling to regulate auxin homeostasis in Arabidopsis. Plants. 2013;2(4):726–7492. https://doi.org/10.3390/plants2040726.
Zhao P, Wang D, Wang R, Kong N, Zhang C, Yang C, et al. Genome-wide analysis of the potato Hsp20 gene family: identification, genomic organization and expression profiles in response to heat stress. BMC Genomics. 2018;19(1):61–73. https://doi.org/10.1186/s12864-018-4443-1.
Zhou FL, Guo Y, Qiu LJ. Genome-wide identification and evolutionary analysis of leucine-rich repeat receptor-like protein kinase genes in soybean. BMC Plant Biol. 2016;16:58–71. https://doi.org/10.1186/s12870-016-0744-1.
Sang S, et al. Genome-wide analysis of the GRF family reveals their involvement in abiotic stress response in cassava. Genes. 2018;9(2):110. https://doi.org/10.1186/s12864-018-4443-1.
Moore K, Roberts LJ. Measurement of lipidperoxidation. Free Radic Res. 1998;28(6):659–71. https://doi.org/10.3109/10715769809065821.
Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA perception and signalling. Trends in Plant Sci. 2010;15(7):395–401. https://doi.org/10.1016/j.tplants.2010.04.006.
Cheng HY, Wang Y, Tao X, Fan YF, Dai Y, Yang H. Genomic profiling of exogenous abscisic acid-responsive micrornas in tomato (Solanum lycopersicum). BMC Genomics. 2016;17(1):423. https://doi.org/10.1186/s12864-016-2591-8.
Koike N. Induction of systemic resistance in cucumber against several diseases by plant growth promoting fungi: lignification and superoxide generation. Eur J Plant Pathol. 2001;107(5):523–33. https://doi.org/10.1023/A:1011203826805.
Zhou X, Zheng Y, Cai Z, Wang X, Liu Y, Yu A, et al. Identification and functional analysis of tomato TPR gene family. Int J Mol Sci. 2021;22(2):758. https://doi.org/10.3390/ijms22020758.
Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:29–37. https://doi.org/10.1093/nar/gkr367.
Guo AY, Zhu QH, Chen X, Luo JC. GSDS: a gene structure display server. Yi Chuan. 2007;29(8):1023. https://doi.org/10.1360/yc-007-1023.
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;9:1639–45. https://doi.org/10.1101/gr.092759.109.
Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;7:e49. https://doi.org/10.1093/nar/gkr1293.
Wang D, Zhang Y, Zhang Z, Zhu J, Yu J. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genom Proteom Bioinf. 2010;1:77–80. https://doi.org/10.1016/S1672-0229(10)60008-3.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR. Method. 2002;25(4):402–8. https://doi.org/10.1006/meth.2001.1262.
Acknowledgments
Not applicable.
Plant material declaration
This study deals with plant material compliance with relevant national agencies and international guidelines and legislation.
The plant materials in this study were obtained legally in the public platform TGRC (tomato genetic resource center).
This experiment complies with the IUCN Policy Statement on Research on Endangered Species and the Convention on Trade in Endangered Species of Wild Fauna and Flora.
Funding
This work was mainly funded by the National Natural Science Foundation of China (32002052 and 31872120) and Postdoctoral Foundation of Heilongjiang Province (LBH_Z19114).
We would like to thank the anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Contributions
SM and YZ collected common data sets and performed bioinformatics analysis. FSL and YH were involved in data collection. SM and ZTY performed the experiments. ZQX contributed materials/analysis tools. YZ and SM drafted the manuscript. YZ、XLC and AXW conceived the study and reviewed the manuscript. All of the authors read and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. OSCAs gene family information of S. habrochaites. Table S2. Analysis of cis acting elements of OSCA gene family in S. habrochaites. Table S3. Protein structure of OSCA gene family in S. habrochaites. Table S4. Primers used for qRT-PCR. Fig. S1. Protein tertiary structure of OSCA gene family in Solanum habrochaites. Fig. S2. ShOSCA3 gene expression in silenced plants. a, b, c, plant phenotypes of pTRV2-PDS, pTRV2, pTRV2-ShOSCA3 at 17 days of inoculation of Solanum habrochaites; d, Silencing of ShOSCA3 confirmed by qRT-PCR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Miao, S., Li, F., Han, Y. et al. Identification of OSCA gene family in Solanum habrochaites and its function analysis under stress. BMC Genomics 23, 547 (2022). https://doi.org/10.1186/s12864-022-08675-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-022-08675-6