Abstract
In this study, from 2015 to 2020, the occurrence and distribution of fungal diseases in the middle and late stages of sugarcane growth, and the resistance of sugarcane varieties were investigated and identified in low latitude plateaus of Yunnan Province. Yield, sucrose content and loss rates of sugarcane were measured and analyzed during the mature period. The results showed that there were three epidemic fungal diseases in the middle and late periods of sugarcane growth, including pokkah boeng, brown stripe and rust diseases. Mixed infections were common. The pathogens of pokkah boeng were Fusarium verticillioides and F. proliferatum, and the dominant species was F. verticillioides. The pathogen of brown stripe was Bipolaris setariae; the pathogens of rust were Puccinia kuehnii and P. melanocephala, and the dominant species was P. melanocephala. Among the 34 main cultivated varieties tested, there were 18 (52.9%), 24 (70.6%), and 25 (73.5%) were highly to moderately resistant to pokkah boeng, brown stripe, and rust, respectively. Among the 60 new varieties tested, there were 35 (58.3%), 32 (53.3%), and 41 (68.3%) were highly to moderately resistant to these three diseases, respectively. Among them, 14 new varieties were highly resistant and recommended for rational use. The average relative yield loss rates due to pokkah boeng, brown stripe and rust were 38.43%, 25.6% and 24.9%, and the average sugar content decreased by 3.54%, 2.82%, and 3.11%, respectively. The results improve our knowledge of sugarcane diseases in low latitude plateaus, and provided guidance for the effective control of the diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grain, cotton, oilseed and sugarcane are main agricultural products and of national importance in China. The development of sugar is a matter of national food safety and a means to increase the farmer’s income. Yunnan is the second largest sugarcane growing area in China, which is located in a low latitude plateau; sugarcane is planted between 21°8′-29°15′ north latitude and 97°31′-106°11′ east longitude, with the climate and environment of the sugarcane planting area being complicated and variable (Zhang et al., 2013). In addition, sugarcane is a ratoon crop with asexual propagation, with long-term continuous cropping, resulting in the accumulation and aggravation of sugarcane diseases. Moreover, mixed infection of many pathogens make sugarcane diseases spread rapidly, and their damage to sugarcane is extremely serious (Huang et al., 2018; Huang & Li, 2016).
Since 2015, due to rain and high humidity coupled with large-scale planting of susceptible varieties, a large-scale outbreak of fungal diseases in the middle and late stages of sugarcane growth occurred in the low latitude plateaus of Yunnan Province, resulting in serious cane yield loss and sugar reduction (Li et al., 2017; Shan et al., 2015). In most sugarcane planting areas, large numbers of stalks of susceptible varieties die because disease control is not in place at the righ growth stages. Disease incidence was between 60 and 100%, cane yields decreasedby 30–48%, and sucrose content decreased by 2–4%, causing huge economic losses (Li et al., 2017; Shan et al., 2018). Therefore, the accurate diagnosis and control of epidemic fungal diseases in the middle and late stages of sugarcane growth has become an important task for high quality development of the sugarcane industry.
Epidemic fungal diseases in the middle and late stages of sugarcane growth mainly include pokkah boeng, brown stripe and rust. Pokkah boeng is a worldwide fungal disease caused by Fusarium spp., which was first discovered in Java in 1890. It is widely distributed in sugarcane growing countries in the world, and it often causes varying degrees of economic losses (Ricaud et al., 1989). Pokkah boeng occurs in all sugarcane growing areas in China. It occurred only sporadically before the 1970s, and caused no threat to sugarcane production. Damaging epidemics occurred in the 1980s in Guangxi and Guangdong. The main varieties ‘Guitang 11’, ‘Yuetang 57–423’ and ‘Yuetang 54–176’ were highly susceptible to disease, resulting in incidence of 30–50%, with the highest value over 80%. Cane yield and Brix (sugar content) decreased by 14% and 7%, respectively, which caused serious economic losses to the local area (Huang et al., 1990; Liu et al., 1991). Brown stripe is an important leaf disease of sugarcane. It was first found in Cuba in 1924, and to date, more than 20 sugarcane growing countries have reported this disease (Ricaud et al., 1989). It has been reported in all the sugarcane growing areas of China. The incidence of brown stripe was more than 80% in severely affected fields and was described as resembling ‘fire burning’. Yield losses are generally in the 18–35% range, but can reach 40% in seriously affected areas, resulting in 15–30% sucrose yield losses (Huang et al., 2018). Rust mainly includes brown rust caused by Puccinia melanocephala Syd. and orange rust caused by Puccinia kuehnii Butler (Ricaud et al., 1989). Since the first report of brown rust in Java in 1980, this disease has rapidly spread, with outbreaks occurring in most cane-growing countries (Egan, 1980; Comstock et al., 1992). Orange rust has a relatively limited distribution, found in India, Australia, the United States and parts of Latin America (Egan, 1980; Flores et al., 2009). In mainland China, brown rust is the main rust type. It was first recorded in Gengma, Yunnan Province in 1982 (Ruan et al., 1983), with subsequent reports in Fujian, Guangdong, Sichuan, Jiangxi, Guangxi and Hainan (Huang & Li, 1998; Wei et al., 2010).
Few studies exist on pathogen species, damage losses and causal factors of epidemic fungal diseases in the middle and late stages of sugarcane growth in low latitude plateaus. This paper is intended to fill this knowledge gap.
Materials and methods
Disease investigation and sample collection
During the disease development period from july to october in 2015–2020, the occurrence and distribution of epidemic fungal diseases in the middle and late stages of sugarcane growth in the low latitude plateaus of Yunnan Province were sampled and disease symptoms recorded.
For pokkah boeng disease, a three points (three lines) method was used for sampling. For each point (line), 100 plants were surveyed; thus a total of 300 plants were surveyed. Incidence was calculated from:
Incidence (%) = (the number of diseased plants / total number of surveyed plants) × 100%.
For brown stripe and rust disease, a three points (three lines) method was used for sampling. For each point (line), 20 plants were surveyed; thus a total of 60 plants were surveyed. The infection status and infected area (%) of all fully developed leaves was assessed visually. The disease grade was classified according to Yang et al. (2010) and Comstock et al. (1992) as follows: Grade 1, no symptoms; Grade 3, disease lesions in 0–25% of the leaves; Grade 5, disease lesions in 26–50% of the leaves; Grade 7, disease lesions in 51–75% of the leaves; and Grade 9, disease lesions in 76–100% of the leaves.
Disease index = Ʃ[the number of diseased plants at all grades × corresponding grade value] / [total number of plants × highest grade value] × 100.
Pathogen identification
DNA extraction
Total DNA was extracted from leaf tissue (0.2 g) using the Easy Pure™ plant Genomic DNA Kit (TransGen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s protocol. The quality of the extracted DNA was assessed using an AG22331 protein and nucleic acid analyzer (Eppendorf, Germany). Then, the DNA samples were stored at −20 °C.
Molecular detection of sugarcane pokkah boeng pathogens
The specific primers for Fusarium verticillioides and F. proliferatum, denoted Fv-F4/Fv-R4 and Fp-F3/Fp-R3, were designed based on the internal transcribed spacer (ITS) of rDNA (rDNA-ITS), and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Their sequences are as follows: Fv-F4: 5’-TCGGGGCCGGCTTGCCGC-3′; Fv-R4: 5’-TACAACTCCCAAACCCCTGTGAACATAC-3′; Fp-F3: 5’-GTTTTACTACTACGCTATGGAAGCT-3′; Fp-R3: 5’-CGAGTTTACAACTCCCAAACCCCT-3′. The size of the expected amplified products was 400 bp. PCR amplification was carried out in a 25 μL reaction mixture containing 5.5 μL ddH2O, 12.5 μL 2 × Easy Taq PCR SuperMix (TransGen Biotech Co., Ltd., Beijing, China), 2.0 μL total DNA template and 2.5 μL of each primer (10 μmol/L). The reaction was heated to 95 °C for 5 min, followed by 30 cycles of denaturation for 30 s at 94 °C, annealing for 15 s at 63 °C, and extension for 30 s at 72 °C, with a final extension for 10 min at 72 °C. PCR products were analyzed by electrophoresis on a 1.5% agarose gel. Positive clones were identified by PCR and sent to BGI Sequencing Co. Ltd. (Beijing, China) for sequencing. After performing a BLAST search in GenBank, sequence similarity analysis was performed with DNAMAN, version 6.0. Based on the alignment results, rDNA-ITS sequences of sugarcane pokkah boeng pathogens were downloaded from GenBank to construct a phylogenetic tree by using neighbor-joining and Kimura 2-parameter model as implemented in the genetic analysis software MEGA, version 6.0 (Tamura et al., 2013). Bootstrap analysis (1000 replicates) was used to estimate stability, and Sporisorium scitamineum (GenBank accession number: EF185083) was included as outgroup.
Molecular detection of sugarcane brown stripe pathogens
Two primer pairs of ITS1 (5’-TCCGTAGGTGAACCTGCGG-3′) / ITS4 (5’-TCCTCCGCTTATTGATATGC-3′) (White et al., 1990) and gpd1(5’-CAACGGCTTCGGTCGCATTG-3′)/ gpd2(5’-GCCAAGCAGTTGGTTGTGC-3′) (Berbee et al., 1999) were used for PCR amplification of ITS and glyceraldehydes-3-phoaphate dehydrogenase (GDPH) gene sequences respectively. The PCR amplification mixture (25 μL) contained 8.0 μL ddH2O, 12.5 μL 2 × Easy Taq PCR SuperMix (TransGen Biotech Co., Ltd., Beijing, China), 2.5 μL total DNA template and 1.0 μL of each primer (10 μmol/L). The reaction of ITS and GDPH were heated to 94 °C for 3 min, followed by 35 cycles of denaturation for 30 s at 94 °C, annealing for 45 s at 55 °C, and extension for 45 s at 72 °C, with a final extension for 10 min at 72 °C. Electrophoresis on a 1.5% agarose gel was used to analyze PCR products. Positive clones were identified by PCR and sent to BGI Sequencing Co. Ltd. (Beijing, China) for sequencing. Sequencing results were searched by BLAST in NCBI, compared with the known sequences in NCBI, and then analyzed by MEGA 6.0 software (Tamura et al., 2013). The phylogenetic tree was constructed by Maximum Likelihood method, and 1000 replicates of Bootstrap tests were carried out to determine its classification status.
Molecular detection of sugarcane rust pathogens
Rust-specific primers Rust2inv (5′- GATGAAGAACACAGTGAAA-3′) and LR6 (5’-CGCCAGTTCTGCTTACC-3′) described by Aime (2006) were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The size of the expected amplified products was 1500 bp. PCR amplification was carried out in a 25 μL reaction mixture containing 10.0 μL ddH2O, 12.5 μL 2 × Easy Taq PCR SuperMix (TransGen Biotech Co., Ltd., Beijing, China), 0.5 μL total DNA template and 1.0 μL of each primer (20 μg/μL). The reaction was as follows: 2 min at 94 °C, 40 cycles of 30 s at 94 °C, 1 min at 57 °C, 1.5 min at 72 °C, and a final extension for 7 min at 72 °C. PCR products were analyzed by electrophoresis on a 1.5% agarose gel. PCR products were excised from the gel and purified using an Axy Prep DNA Gel Purification Kit (Axygen Biotechnology, Hangzhou, China). DNA fragments were ligated into the plasmid vector pMD18-T (Takara, Dalian, China), and the recombinant plasmids were introduced into Escherichia coli strain DH5ɑ (Sangon, Shanghai, China) by transformation. Six positive clones from each sample were selected and the inserts were sequenced at BGI Sequencing (Beijing) Co. Ltd. After performing a BLAST search in GenBank, the obtained sequences were analyzed by DNAMAN version 6.0 (Lynnon Biosoft, U.S.A) and MEGA 6.0 software (Tamura et al., 2013) to construct the phylogenetic tree and determine their classification status.
Determination of cane yield and sugar loss
During the mature harvest period, the cane yield from damaged and undamaged areas was harvested and weighed using a three point sampling method encompassing 66 m2 each. After cutting and weighing, the relative yield loss was calculated as follows:
Relative yield loss (%) = [(measured yield in undamaged area – measured yield in damaged area) / measured yield in undamaged area] × 100%.
During the mature harvest period, damaged and undamaged areas were selected to sampling at three points. Ten healthy plants and 10 diseased plants were randomly selected at each point. Two rotatory analysis methods established by the National Sugar Industry Standardization and Quality Detection Center were used to determine quality indexes including juice yield (%), sucrose content (%), gravity purity (%) and reduction in sugar content (%). A fully automatic sugar analysis system, Rudolph, Autopol 880 + J257 (United States), was used. The loss of each index was calculated as follows:
Field resistance investigation of different sugarcane varieties
From 2015 to 2020, during the disease development period from July to October, the incidence of diseases in newly planted and ratoon cane of representative main cultivated varieties and new varieties, was investigated.
For pokkah boeng disease, according to the method described plant disease grade was investigated and calculated. Grades 1, 2, 3, 4, and 5 represented highly resistant, resistant, moderately resistant, susceptible, and highly susceptible, respectively. The corresponding ranges of disease incidence for each grade were 0.0%, 0.1–10.0%, 10.1–20.0%, 20.1–40.0%, and 40.1–100.0%, for Grades 1, 2, 3, 4, and 5, respectively.
For brown stripe and rust disease, the three points method was used to evaluate field resistance. The disease incidence of 100 millable plants was investigated at each point. Therefore, a total of 300 plants were tested per variety. The disease status and proportion of area infected in fully developed leaves were assessed by visual inspection. Field resistance to brown stripe was rated on a scale of grade 1 to 6 as follows: Grade 1, highly resistant (HR), no symptoms; Grade 2, resistant (R), a diseased leaf area < 10%; Grade 3, moderately resistant (MR), 11–25% diseased leaf area; Grade 4, moderately susceptible (MS), 26–40% diseased leaf area; Grade 5, susceptible (S), 41–65% diseased leaf area; Grade 6, highly susceptible (HS), 66–100% diseased leaf area (Yang et al., 2010). Field resistance to rust was evaluated using a grading system from 1 to 9, according to the evaluation method described by Costet et al. (2012).
Results
Types and distribution of fungal diseases
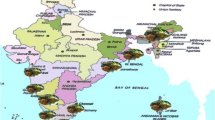
Based on the investigation and analysis of disease occurrence in the main planting areas, there were three epidemic fungal diseases in the middle and late stages of sugarcane growth, including pokkah boeng, brown stripe and rust. Moreover, they were widely distributed in planting areas in the low latitude plateau of Yunnan Province (Table 1). Moreover, in five sugarcane planting areas including Gengma, Shuangjiang, Zhenkang, Menghai, Menglian, these three diseases co-occured caused the most extensive damage (Fig. 1). Among these three diseases, the outbreak trend of pokkah boeng was obvious, and the incidence of susceptible varieties was severe, with the disease incidence as high as 81.2% on average (Table 2).
Pathogen species identified
Molecular identification results showed that F. verticillioides and F. proliferatum were the pathogens causing pokkah boeng disease and was present as a mixed infection. The obtained sequences of F. verticillioides (GenBank accession number: MZ126549-MZ126555) and F. proliferatum (GenBank accession number: MZ102259-MZ102265) from seven mixed infection samples shared 98.6–100% and 100% nucleotide sequence identity with F. verticillioides isolate 20 (GenBank accession number: KU508286) and F. proliferatum strain Dehong (GenBank accession number: KJ629482), respectively. The phylogenetic tree indicated that the pathogens causing pokkah boeng in Yunnan Province were mainly divided into the F. verticillioides group and the F. proliferatum group. In the F. verticillioides group, ROC 25 (Lancang, Yunnan) and Funong 10–1405 (Mile, Yunnan) were clustered into an independent branch; the remaining five mixed infection samples were clustered with different geographical F. verticillioides isolates, and had close genetic relationship with F. oxysporum Guangxi isolate. All of the F. proliferatum isolates from different geographic sources were clustered together into one group.
For brown stripe disease, the molecular identification results showed that Bipolari setariae was the pathogen. The ITS sequences (GenBank accession number: MW466590-MW466591) obtained in this study shared 99.47% and 100% nucleotide sequence identity with B. setariae model strain CBS 141.31 (GenBank accession number: EF452444) and strain CBSHN01 (GenBank accession number: GU290228), respectively. The GPDH sequences (GenBank accession number: MW473721-MW473722) obtained in this study shared 99.83% and 100% nucleotide sequence identity with B. setariae model strain CBS 141.31 (GenBank accession number: EF452444) and strain CPC28802 (GenBank accession number: MF490833), respectively. A phylogenetic tree constructed based on ITS and GPDH gene sequences found that strains of BS1 and BS2 were clustered into the same branch with B. setariae.
For rust disease, the molecular identification results showed that the pathogen present in four rust samples from Haiyin 1, collected in Menghai city, Yunnan Province, was P. kuehnii Butler. Their nucleotide sequences (GenBank accession number: KP201840- KP201843) shared more than 99.9% sequence identity with some reported sequences of P. kuehnii from GenBank (GenBank accession number: GU058021, GQ283004- GQ283009), and they were clustered into one branch in the phylogenetic tree. The pathogen present in 53 rust samples collected in Baoshan, Lincang, Xishuangbanna, Puer and Wenshan city was P. melanocephala. Their nucleotide sequences (GenBank accession number: KP201824- KP201839, KU886334-KU886368, and KU 896993-KU 896994) shared more than 99.8% sequence identity with some reported sequences of P. melanocephala from GenBank (GenBank accession number: GU058001 and JX036025), and they were clustered into one branch in the phylogenetic tree.
Damage and losses
The occurrence, cane yield and quality results of the three fungal diseases are shown in Table 2. As can be seen from Table 2, the degree of damage caused by the three diseases in different sugarcane areas was different, and the yield loss and quality were different with varied degrees of damage. The sucrose content, Brix and gravity purity of damaged plants were significantly lower than those of healthy plants, while the reduced sugar content of damaged plants was significantly higher than that of healthy plants.
Field resistance of different sugarcane varieties
The level of resistance of the 34 main varieties and the 60 new varieties for the three diseases are given in Table 3. Table 3 shows that 10 main varieties including ‘Yuetang 83–88’, ‘Chuantang 61–408’, ‘Guitang 21’, ‘Yunyin 10’, ‘Liucheng 03–182’, ‘Yunyin 58’, ‘Yuetang 79–177’, ‘Yunzhe 05–51’, ‘Guitang 36’ and ‘ROC 16’, had excellent resistance to these three diseases.
Table 3 also shows that13 new varieties including ‘Guitang 11–1076’, ‘Mintang 12–1404’, ‘Funong 09–2201’, ‘Funong 09–71111’, ‘Guitang 06–1492’, ‘Guitang 08–1180’, ‘Yunzhe 11–1074’ and ‘Guitang 06–2081’ and ‘Guitang 08–1589’ had excellent resistance to these three diseases.
Discussion
Yunnan is the second largest sugarcane growing area in China, which is located in the low latitude plateau (97°31′E-106°11′E, 21°8’N-29°15’N).. In recent years disease incidence and severity increased in the middle and late stage of sugarcane growth, resulting in cane yield loss and reduction of sugar production (Huang et al., 2018; Huang & Li, 2016). In this paper, we investigated the fungal diseases in the middle and late period of sugarcane growth and quantified yield loss. Moreover we characterized the level of disease resistance of a range of varieties. Survey results showed that there were three fungal diseases, involved, pokkah boeng, brown stripe and rust. Mixed infection of two or three diseases was commonRust occurred from June to September, pokkah boeng occurred from July to December, and brown stripe occurred from August to December. Pokkah boeng and brown stripe both occurred in 40 sugarcane growing area. Rust occurred in 28 sugarcane growing area. In the the areas with most extensive damage, all three diseases occurred (Gengma, Shuangjiang, Zhenkang, Menghai and Menglian).
There are many Fusarium species which can cause pokkah boeng disease, and seven species have been reported, including F. sacchari, F. verticillioides, F. proliferarum, F. subglutinans, F. andiyazi, F. incarnatum and F. oxysporum (Khani et al., 2013; Mohammadi et al., 2012; Samaco & Dela Cueva, 2019). There are differences in the pathogen population and dominant species of pokkah boeng disease in different countries and sugarcane areas. In Malaysia, F. sacchari, F. proliferatum and F. subglutinans were identified as the causal pathogens (Siti Nordahliawate et al., 2008). In Iran, F. verticillioides, F. proliferatum, and F. subglutinans were the causal pathogens (Khani et al., 2013; Mohammadi et al., 2012). In South Africa, F. sacchari, F. proliferatum and F. andiyazi were the causal pathogens (Govender et al., 2010). In Philippines, F. sacchari, F. proliferatum, F. incarnatum, F. verticillioides and F. subglutinans were the causal pathogens (Samaco & Dela Cueva, 2019). In this study, it was found that F. verticillioides and F. proliferatum were the pathogens of pokkah boeng disease in the low latitude plateau of Yunnan Province, which was consistent with the research results of Lin et al. (2014) and Guo et al. (2019).
There are relatively few reports on the pathogens causing brown stripe disease. Qian et al. (2015) reported that the pathogen of brown stripe disease in Hainan was Bipolaris. Only Raza et al. (2019) found B. setariae on sugarcane leaf lesions in Guangxi and Guangdong, as we also found in the low latitude plateau of Yunnan Province.
In this study, P. kuehnii that causes orange rust disease was found in Menghai city and P. melanocephala that causes brown rust was confirmed to be the dominant species in the low latitude plateau of Yunnan Province. P. kuehnii is a pathogen that develops epidemics infrequently but does causes high economic losses once it breaks out. The economic losses caused by orange rust exceed $1.77 billion (USD) in Australia and $400 million (USD) in the state of Florida, USA, annually (Braithwaite et al., 2009; Dixon et al., 2010). Even though organge rust poses high risks, research on the identification and evaluation of sugarcane germplasm and cultivars for rust resistance in China have only focused on brown rust (Li et al., 2005, 2007, 2008, 2016). In the future, efforts should be focused on adjusting the strategy of breeding sugarcane with rust resistant cultivars, and increasing the evaluation and screening of sugarcane sources that are resistant to orange rust.
Our research showed that the main popularized varieties which were planted in large scale, were highly susceptible to diseases. Their susceptibility is one of the main reasons for the outbreak of epidemic fungal diseases in recent years. Wang et al. (2016) discovered that the highly susceptible varieties ‘Yuetang 93–159’, ‘Liucheng 03–1137’ and ‘Guitang 42’ exhibited resistance in Guangxi, bu. ourstudy indicates they are highly susceptible to all three diseases. Whether this difference is related to the pathogen population, environmental condisions or even the identification methods in various places needs further studies. Fourteen elite varieties, bred in recent years, exhibited high levels of disease resistance. It is suggested that the major varieties that were susceptible including, Table 3, should be not be grown in areas with high disease pressure.The new resistance varieties, Table 3,should be distributed to achieve effective control the diseases.
References
Aime, M. C. (2006). Toward resolving family-level relationships in rust fungi (Uredinales). Mycoscience, 47, 112–122.
Berbee, M. L., Pirseyedi, M., & Hubbard, S. (1999). Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3- phosphate dehydrogenase gene sequences. Mycologia, 91, 964–977.
Braithwaite, K. S., Croft, B. J., Magarey, R. C., & Scharaschkin, T. (2009). Phylogenetic placement of the sugarcane orange rust pathogen Puccinia kuehnii in a historical and regional context. Australasian Plant Pathology, 38, 380–388.
Comstock, J. C., Shine, J. M., & Raid, R. N. (1992). Effect of early rust infection on subsequent sugarcane growth. Sugar Cane, 4, 7–9.
Costet, L., Le Cunff, L., Royaert, S., Raboin, L. M., Hervouet, C., Toubi, L., Telismart, H., Garsmeur, O., Rousselle, Y., Pauquet, J., Nibouche, S., Glaszmann, J. C., Hoarau, J. Y., & D’Hont, A. (2012). Haplotype structure around Bru1 reveals a narrow genetic basis for brown rust resistance in modern sugarcane cultivars. Theoretical & Applied Genetics, 125, 825–836.
Dixon, L. J., Castlebury, L. A., Aime, M. C., Glynn, N. C., & Comstock, J. C. (2010). Phylogenetic relationships of sugarcane rust fungi. Mycological Progress, 9(4), 459–468.
Egan, B. T. (1980). A review of the world distribution of Puccinia spp. attacking sugarcane. Plant Pathology, 17, 1373–1381.
Flores, R. C., Loyo, J. R., Ojeda, R. A., Rangel, O. C. A., Cerón, F. A., Márquez, W., Guerra-Moreno, A. S., Hernandez-Ibarra, H. M., González, R. E., Castlebury, L. A., Dixon, L. J., Glynn, N. C., Comstock, J. C., Flynn, J., & Amador, J. (2009). First report of orange rust of sugarcane caused by Puccinia kuehnii in Mexico, El Salvador and Panama. Plant Disese, 93, 1347.
Govender, P., Mcfarlane, S. A., & Rutherford, R. S. (2010). Fusarium species causing pokkah boeng and their effect on Eldanasaccharina Walker (Lepidoptera: Pyralidae). Proc S Afr Sug Technol Ass, 83, 267–270.
Guo, Q., Ma, W. Q., Chen, H. S., Bi, D. J., Shi, Z. S., Peng, C., Qin, C. X., He, H. L., & Tang, L. Q. (2019). Isolation identification and biological characteristics of the pathogen causing sugarcane pokkah boeng in Chongzuo, Guangxi. Journal of Southern Agriculture, 50(8), 1728–1734.
Huang, H. N., Deng, G. A., Yuan, Z. M., & Chen, Y. C. (1990). Survey of the incidence and damage of Pokkah boeng in Dongguan sugar factory area. Sugarcane and Canesugar, 1, 20–23.
Huang, Y. K., & Li, W. F. (1998). Epidemic and control strategies of sugarcane rust disease in Yunnan sugarcane field. Plant Protection Technology Extension, 18, 22–23.
Huang, Y. K., & Li, W. F. (2016). Colored atlas of control on diseases, insect pests and weeds of modern sugarcane (pp. 103–114). China Agriculture Press.
Huang, Y. K., Li, W. F., Zhang, R. Y., Wang, X. Y., Shan, H. L., Li, J., Yin, J., Luo, Z. M., & Cang, X. Y. (2018). Color illustration of diagnosis and control for modern sugarcane diseases, pests, and weeds (pp. 11–20). Springer Nature Singapore Pte Ltd.
Khani, K. T., Alizadeh, A., Nejad, R. F., & Tehrani, A. S. (2013). Pathogenicity of fusarium proliferatum, a new causal agent of pokkah boeng in sugarcane. Proceeding of the International Society of Sugar Cane Technologists, 28, 1–5.
Li, W. F., Cai, Q., Huang, Y. K., Fan, Y. H., & Ma, L. (2005). Identification of fine sugarcane wild germplasm resources resistant to Puccinia erianthi. Plant Protection, 31(2), 51–53.
Li, W. F., Cai, Q., Huang, Y. K., Fan, Y. H., & Ma, L. (2008). Identification of sugarcane cultivated original species resistance to Puccinia erianthi. Journal of Yunnan Agricultural University (Natural Science), 22(1), 25–28.
Li, W. F., Cai, Q., Huang, Y. K., Fan, Y. H., & Wang, L. P. (2007). Identification of fine sugarcane parent and innovative gerplasma to resistant Puccinia erianthi. Sugar Crops of China, 4, 10–12.
Li, W. F., Shan, H. L., Huang, Y. K., Zhang, R. Y., Cang, X. Y., Yin, J., Wang, X. Y., & Luo, Z. M. (2017). The occurrence epidemic dynamics, control thoughts and countermeasures of important sugarcane diseases in raininess and high humidity season. Sugar Crops of China, 39(2), 75–77.
Li, W. F., Wang, X. Y., Huang, Y. K., Zhang, R. Y., Shan, H. L., Luo, Z. M., & Yin, J. (2016). Identification of resistance to brown rust and molecular detection of Bru 1 gene in 101 main sugarcane breeding parents in China. Acta Agronomica Sinica, 42, 1411–1416.
Lin, Z. Y., Xu, S. Q., Que, Y. X., Wang, J. H., Comstoch, J. C., Wei, J. J., McCord, P. H., Chen, B. S., Chen, R. K., & Zhang, M. Q. (2014). Species-specific detection and identification of fusarium species complex, the causal agent of sugarcane pokkah boeng in China. PLoS One, 9.
Liu, M. L., Huang, D. F., Li, D. J., & Zhang, C. C. (1991). A preliminary report on the research about the occurrence and control of sugarcane pokkah boeng disease in Guangxi. Guangxi Agricultural Science, 4, 175–178.
Mohammadi, A., Nejad, R. F., & Mofrad, N. N. (2012). Fusarium verticillioides from sugarcane, vegetative compatibility groups and pathogenicity. Plant Protection Sciences, 48(3), 80–84.
Qian, S. H., Shen, L. B., Xiong, G. R., Wang, J. G., Feng, C. L., Zhao, T. T., & Zhang, S. Z. (2015). Isolation, identification and laboratory toxicity determination of the pathogen of sugarcane brown stripe. Chinese Journal of Tropical Crops, 36(2), 353–357.
Raza, M., Zhang, Z. F., Hyde, K. D., Diao, Y. Z., & Cai, L. (2019). Culturable plant pathogenic fungi associated with sugarcane in southern China. Fungal Diversity, 99, 1–104.
Ricaud, C., Egan, B. T., Gillaspie, A. G., & Hughes, C. G. (1989). Diseases of sugarcane: Major diseases. Elsevier.
Ruan, X. Y., Yan, F., & Sun, C. J. (1983). Occurrence of Puccinia erianthi on sugarcane in Yunnan province. Acta Mycologica Sinica, 2, 260–261.
Samaco, M. A., & Dela Cueva, F. M. (2019). Molecular characterization of fusarium spp. associated with sugarcane pokkah boeng from the Philippines using partial translation elongation factor-1 (TEF-1) gene sequences. Sugar Tech, 21(4), 619–630.
Shan, H. L., Li, W. F., Huang, Y. K., Wang, X. Y., Zhang, R. Y., Luo, Z. M., & Yin, J. (2015). Epidemic characteristics and control strategies of sugarcane brown stripe disease. Sugar Crops of China, 37(6), 71–73.
Shan, H. L., Li, W. F., Zhang, R. Y., Wang, X. Y., Li, J., Cang, X. Y., Yin, J., Luo, Z. M., & Huang, Y. K. (2018). Analysis on epidemic reason of sugarcane pokahh boeng and its losses on yield and sucrose content. Sugar Crops of China, 40(3), 40–42.
Siti Nordahliawate, M. S., Nur Ain Izzati, M. Z., Azmi, A. R., & Salleh, B. (2008). Distribution, morphological characterisation and pathogenicity of fusarium sacchari associated with pokkah boeng disease of sugarcane in peninsular Malaysia. Pertanika Journal of Tropical Agricultural Sciences, 31(2), 279–286.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Wang, Z. P., Chen, Y., Sun, H. J., Li, Y. J., Duan, W. X., Zhou, Z. G., Zhang, M. Q., & Lin, S. H. (2016). Preliminary evaluation of resistance to pokkah boeng disease of major sugarcane varieties in the field of Guangxi. Chinese Journal of Tropical Crops, 37(5), 952–957.
Wei, J. J., Deng, Z. Y., Huang, W. H., Pan, X. H., Wang, B. H., & Liu, X. J. (2010). Control methods and pathogen biological characteristics of sugarcane rust in Beihai. Journal of Anhui Agricultural Sciences, 38, 14997–11499.
White, T. J., Bruns, T., Lee, S., & Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: A guide to methods and applications (pp. 315–322). New York Academic Press
Yang, Z. L., Zhou, J., & Cheng, S. Y. (2010). Problems in field investigation and improve measures of sugarcane brown stripe disease. China Plant Protection, 30(11), 118–121.
Zhang, Y. B., Deng, J., Cheng, Y., & Long, Y. F. (2013). Study on development and technical strategy of plateau characteristic sugarcane industry in Yunnan (pp. 12–21). China Agriculture Press.
Acknowledgements
This work was supported by China Agriculture Research System of MOF and MARA (CARS-170303), Yunling Industry and Technology Leading Talent Training Program “Prevention and Control of Sugarcane Pests” (2018LJRC56), and Yunnan Province Agriculture Research System (YNGZTX-4-92).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals and informed consent
This research does not involved any animal and/or human trials.
Ethical approval
The authors declare that they have followed the guidelines of Committee on Publication Ethics (COPE) and obeyed all the Ethical Standards requested by EJPP.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, WF., Wang, XY., Shan, HL. et al. Occurrence and damage of epidemic fungal diseases in middle and late stages of sugarcane growth in Yunnan Province of China. Eur J Plant Pathol 164, 353–364 (2022). https://doi.org/10.1007/s10658-022-02566-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-022-02566-y