Abstract
The increasing incidence of pokkah boeng has been observed throughout the sugarcane-producing areas in the Philippines. Sugarcane plants exhibiting characteristic symptoms of the disease were collected from different areas in the three major geographical regions: Luzon, Visayas and Mindanao. Symptoms surveyed in Visayas and Mindanao fields were more severe and diverse than those observed in Luzon. A total of 306 Fusarium spp. were isolated from these diseased plant samples on potato dextrose medium and water agar medium, and molecular identification using the genus-specific primer pair (ITS-Fu-f/r) and translation elongation factor (TEF)-1α gene sequencing were performed. Chlorosis after 30 days of inoculation and leaf necrosis after 45 days of inoculation in sugarcane seedlings (VMC 86-550) was observed, wherein 37% (112 out of 306) isolates were proven pathogenic. Traditional taxonomic characterization of the 112 pathogenic isolates identified them as F. sacchari, F. proliferatum, F. verticillioides, F.subglutinans, F. graminareum and F. incarnatum. This was further supported by molecular identification of the representative 56 isolates based on TEF-1α sequences as F. sacchari (66%), F. proliferatum (16%), F. incarnatum (7%), F. verticillioides (5%) and F. subglutinans (5%). Furthermore, these fungal pathogens were established as closely related species regardless of geographical origin based on the established clustering pattern in the constructed phylogenetic tree. These findings are important information on the etiology of pokkah boeng in the Philippines, and in formulating studies for control measures of the pathogen to prevent the disease from becoming an epidemic which may cause extensive loss to the sugarcane industry in the future. This is the first report on characterizing the pathogenic Fusarium spp. on sugarcane pokkah boeng in the Philippines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pokkah boeng is becoming a disease of quarantine importance in the Philippines. Until recently, it was not considered a major threat to the sugarcane industry, but favorable environmental conditions such as high humidity and rainy seasons (Vishwakarma et al. 2013), in which susceptible varieties are exposed to, can cause significant yield losses. Symptoms of pokkah boeng are mainly divided into three stages. First stage is when chlorosis develops on the base of the young leaves accompanied by leaf shortening, wrinkling and leaf twisting. The second is the top-rot phase, an advanced stage wherein distortion of the apical shoot, necrosis and death of the entire growing spindle of the plant occurs. Finally, the knife-cut phase, considered as the most serious stage, is characterized by internal or external cut-like lesions in the stalk due to the penetration of infection inside the growing plant (Vishwakarma et al. 2013, 2016; Martin et al. 1989; Viswanathan et al. 2017). In this aspect, pokkah boeng can cause immense loss to the industry, specifically when severe stages of the disease emerge and spread across large masses of sugarcane fields.
In terms of impact to the industry worldwide, pokkah boeng has been attributed to affected susceptible varieties. This disease has been recorded in various sugarcane-producing countries such as India (Singh et al. 2006; Viswanathan and Rao 2011; Viswanathan et al. 2017; Vishwakarma et al. 2013), China (Lin et al. 2015; Hilton et al. 2017), Malaysia (Siti Nordahliawate et al. 2008) and Iran (Mohammadi et al. 2012), among others. Disease outbreak was also noted in 1989 and 2005 in Guangxi, China (Zhang et al. 2015). In the Philippines, damage and yield loss due to pokkah boeng have not previously been properly documented. However, due to changing environmental conditions combined with the country’s tropical climate, increased disease incidence in susceptible commercial varieties is anticipated without effective management strategies.
Moreover, prior to understanding the impact of the disease to the industry, starting preliminary studies focusing on the occurrence of pokkah boeng in sugarcane-growing areas in the Philippines is essential in assessing the extent of damage it may cause, and in formulating methods on how to control the disease. Identification of Fusarium species causing pokkah boeng in the Philippines is also an important area of study since the established causal pathogens that belong to the Liseola section are capable of causing various diseases in important agricultural crops such as sugarcane, sorghum and maize and possess mycotoxins which can be harmful to both human and animal health (Hilton et al. 2017).
Fusarium spp. causing pokkah boeng in other countries are F. verticillioides, F. proliferatum, F. subglutinans and F. sacchari (Viswanathan et al. 2017; Lin et al. 2014, 2015; Khani et al. 2013; Siti Nordahliawate et al. 2008; Mohammadi et al. 2012; Hilton et al. 2017). In the Philippines, this has yet to be established. Hence, this study was conducted to generate information on the occurrence, pathogenicity and cultural characters of the Fusarium isolates causing pokkah boeng in sugarcane-growing areas of Luzon, Visayas and Mindanao through field survey, traditional taxonomic characterization, polymerase chain reaction (PCR) assays and gene sequencing.
Materials and Methods
Survey and Sample Collection
From August 2014 to September 2016, a total of 23 sugarcane fields in seven sugarcane-growing provinces in the Philippines (Fig. 1) under various climatic conditions were surveyed for pokkah boeng disease occurrence. Areas selected for sample collection were local farmers’ fields and varietal field trial sites wherein the quadrat sampling method was used per field. Infected sugarcane exhibiting symptoms of the top-rot phase leaves manifesting twisting, wrinkling, stunting, chlorosis near the base, reddish discoloration and stalks with cut-like lesions were collected and pooled during harvest per site.
Isolation and Purification of Fusarium spp
Fusarium spp. cultures were isolated following the method by Hsuan et al. (2010). Single sample was obtained per plant. Upon collection, infected apical shoot/leaves/stalks were sectioned into approximately 1 cm2 and were surface-sterilized using 10% NaClO solution for 1–3 min, followed by washing with sterile distilled water three times at 1-minute interval. Plant tissues were blot-dried using sterilized Whatman® No. 1 filter paper (Cat. No. 1001-110), then plated on potato dextrose agar (PDA) medium. After incubation at 25 °C for 5–7 d, plates were observed for fungal growth. Isolates with bacterial and fungal contamination were purified by growing overnight in water agar (WA) and transferred to PDA.
Purified Fusarium spp. single-spore subcultures were obtained using the standard protocol (Leslie and Summerell 2006) with the following modifications (Hsuan et al. 2010; Lin et al. 2015). Conidia from a 7-day-old fungal colony were dislodged from PDA and transferred to WA. Plates were incubated in the dark at 25 °C for 18 h to permit conidial germination. Germlings identified by examination through a dissecting microscope (40×) were excised from the medium using a flame-sterilized nichrome needle and transferred to new PDA plates for further analysis.
Pathogenicity Test
One-month-old sugarcane plants cv. VMC 86-550 were inoculated with conidial suspensions adjusted to 106 CFU/ml using a haemocytometer. Suspensions were prepared by adding sterile distilled water in 5–7 d old cultures, followed by thorough agitation. Inoculation was conducted by microinjecting the prepared fungal suspension about 4–5 ml per plant in three young spindle leaves using a 26-gauge needle 1 cc sterile syringe. A healthy, 1-month-old cv. VMC 86-550 was used as the control, which was injected with sterile distilled water. The inoculated plants were maintained under controlled greenhouse conditions and monitored every 10 d for symptom expression and disease development for a period of 60 d. Sugarcane seedlings that manifested pokkah boeng symptoms were noted, and the fungus was re-isolated using PDA to prove Koch’s postulates. The identities of the re-isolated fungi were confirmed by cultural characterization and detection by PCR assay.
Morphological Characterization of Fusarium spp
Cultural characterization of the isolates was studied starting 5 DAI by microscopic and direct examination. Microscopic examination was done using CX23 upright microscope (Olympus) with AM422X Dino-Eye eyepiece camera (Dino-Lite) (40×; 100×, OIL), and the spore size was measured using ImageJ software (Abramoff et al. 2004). Morphological characterization was done following the Fusarium Laboratory Manual (Leslie and Summerell 2006). Colony morphology, pigmentation, density, presence/absence of chlamydospores, micro/macroconidia, clustering, chain formation and septation were noted from the cultures.
Molecular Identification of Fusarium spp. Through PCR Assays and Gene Sequencing DNA Extraction
A CTAB extraction protocol for total fungal DNA extraction (Doyle and Doyle 1987; Cullings 1992) was adapted with the following modifications. Five-day single-spore subculture in agar block was excised and transferred to potato dextrose water (PDW). Cultures were allowed to grow for 7 d at 25 °C. Mycelia were harvested and homogenized using sterile mortar and pestle in 2000 µl of 2% CTAB buffer (1 M Tris buffer in pH 8.0, 5 M NaCl, 0.5 M EDTA, CTAB). Solutions were centrifuged at ~ 15,000×g or ~ 16,000×g, depending on the required step. Isopropanol and 70% ethanol were removed from each of the tubes by pipetting out the solution. DNA pellets were air-dried and re-suspended in 100 µl 1X TE buffer (1 M Tris buffer, pH 8.0, 0.5 M EDTA). DNA quality was checked in 2% agarose gel electrophoresis, and the quantity was assessed in A260/A280 using a spectrophotometer (Epoch™ Microplate Spectrophotometer, BioTek).
Genus-Specific PCR Assay
Genomic DNA of Fusarium spp. was subjected to PCR amplification using the genus-specific ITS-Fu/f/ITS-Fu-r (Abd-Elsalam et al. 2003) (Table 1). PCR cocktails were prepared in sterile microcentrifuge tubes with a final volume of 15 µl consisting of diethyl pyrocarbonate (DEPC) water, 1X PCR buffer, 2.5 mM MgCl2, 0.2 mM dNTP, 0.2 µM of each primer, 1 U/µl Taq DNA polymerase and 50 ng template DNA. All cocktails were assembled on ice and loaded on a preheated (95 °C) thermal cycler. The temperature profile comprises the following: initial step at 94 °C for 5 min, followed by 1 min at 94 °C, 1 min at 52 °C, 2 min at 72 °C, all in 35 cycles, with a final extension step at 72 °C for 5 min. PCR products were electrophoresed in 1.5% agarose gel and stained using GelRed (Biotium Inc.).
Molecular Identification by TEF-1α Sequencing
Amplification of the partial sequence of the translation elongation factor (TEF-1α) region was done using the EF1 and EF2 primer pair (Fourie et al. 2009) (Table 1) with the following cycling conditions: 94° for 2 min; followed by 34 amplification cycles consisting of 94 °C for 45 s, 60 °C for 45 s and 72 °C for 90 s; and a final extension step of 72 °C for 5 min. PCR products were sent to AIT Biotech Pte Ltd (Singapore) for purification and standard (Sanger) sequencing. Sequences were assembled and manually edited in Geneious version R9.1 (http://www.geneious.com, Kearse et al. 2012). Similarity searches and alignments comparing the Fusarium spp. sequences generated in this study with deposited sequences in the GenBank were performed in Nucleotide Basic Local Alignment Search (BLASTn) Tool. Phylogenetic tree consisting of 56 representative isolates and one outgroup isolate was constructed in the Geneious tree builder (http://www.geneious.com, Kearse et al. 2012) using the neighbor-joining method [NJ] of reconstruction. The Tamura-Nei genetic distance model was used to generate rooted NJ trees with a total of 1000 bootstrap replicates performed for node support evaluation of the constructed trees.
Results
Geographic Distribution and Field Symptom Variability of Pokkah Boeng
A total of 306 fungal colonies were isolated through sampling of various sugarcane fields in Luzon, Visayas and Mindanao. One isolate was acquired per plant sample. Isolates were ascertained as Fusarium spp. based on the cultural and morphological characters of the fungi obtained from symptomatic plant parts. The host cultivars were commercial sugarcane varieties locally grown by farmers and in field trials. Luzon was represented by 101 isolates, whereas 153 isolates were from Visayas. Samples from Mindanao yielded a total of 52 fungal isolates (Table 2).
Various symptoms of sugarcane pokkah boeng were noted during the field surveys. In Luzon, pokkah boeng infected plants from Batangas, Laguna, Pampanga and Tarlac were observed to exhibit symptoms differing from leaf wrinkling and chlorosis of the young leaves to reddish streaks on the leaves. From the municipalities of Negros Occidental in the Visayas, symptoms noted include “top-rot” (distortion of the apical shoot), twisting and/or wrinkling of the infected leaves, leaf chlorosis, reddish streaks with necrosis of leaves and the “knife-cut” phase (internal and external cut-like lesions of the stalk). In Cebu, Visayas, leaf chlorosis and wrinkling were spotted on the affected varieties. In localities of Bukidnon in Mindanao, infected plants manifested the top-rot phase, curling of the leaves to leaf chlorosis, leaf wrinkling/twisting with or without reddish streaks (Fig. 2).
Pokkah boeng disease symptom variability in the Philippines. a Wrinkling of affected leaves with reddish streaks, as observed in Laguna. b.1 Leaf wrinkling and b.2 leaf chlorosis, as observed in Batangas. c.1 “Top-rot” phase c.2 “knife-cut” phase and c.3 leaf wrinkling with chlorosis, as observed in La Carlota, Negros Occidental. d.1 Leaf chlorosis d.2 “top-rot” phase and d.3 leaf necrosis with wrinkling, as observed in Talisay, Negros Occidental. e.1 Leaf chlorosis with wrinkling e.2 Leaf necrosis and e.3 “top-rot” phase, as observed in Bukidnon
Pathogenicity, Morphological and Cultural Characterization
Our results revealed that 112 Fusarium spp. isolates were pathogenic. Inoculation of one-month-old cv. VMC 86-550 plants maintained under controlled conditions yielded symptoms of leaf chlorosis and leaf necrosis. Chlorosis was first noted within 30–35 days after inoculation (DAI), while leaf necrosis started to appear by 45 DAI on plants which initially showed chlorosis, indicating advanced symptom expression. The control plants remained asymptomatic.
Of these pathogenic isolates, out of the 44 isolates from Visayas morphologically identified as F. verticillioides (17), F. proliferatum (8), F. sacchari (14) and F. graminareum (5), that were able to incite chlorosis, 8 isolates of F. verticillioides and 4 isolates of F. proliferatum were able to advance and caused necrosis on inoculated susceptible plants by 45 DAI. Also, the 30 isolates from Luzon, characterized morphologically as F. verticillioides (9), F. proliferatum (8), F. incarnatum (6) and F. sacchari (7), expressed chlorosis on the inoculated plants, wherein F. verticillioides (5) and F. proliferatum (7) incurred necrosis by 45-50 DAI. The 38 isolates from Mindanao comprised of F. verticillioides (19), F. proliferatum (5), F. subglutinans (4) and F. sacchari (10) also expressed chlorosis by 32 DAI wherein F. verticillioides (9) and F. sacchari (4) progressed to necrosis by 47 DAI.
Phenotypic observations of the pathogenic isolates grown in PDA were aligned with the key characteristics of F. proliferatum, F. sacchari, F. subglutinans, F. verticillioides, F. graminareum and F. incarnatum as classified by Leslie and Summerell (2006).
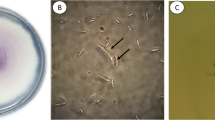
Cultures identified as F. graminareum have dense mycelia which are white to pale orange or yellow in color and develop red pigments with age in PDA media (Fig. 3a). Morphological characteristics such as macroconidia which possessed 5–6 septate, moderately slender, lunate, curved to almost straight, with prominent foot-shaped basal cell, and a relatively constricted to tapered-shaped apical cell are present (Fig. 3b). Globular chlamydospores which occurred as single structures and in clusters were also observed and were often found with macroconidia (Fig. 3c) (Leslie and Summerell 2006). Measured spore size of the isolates ranged from 21.36–27.05 × 2.20–3.15 µm.
Morphological characters of the Fusarium spp. isolated from sugarcane pokkah boeng. a–cF. graminareum in PDA; slightly curved macroconidia with 5–6 septate; globule chlamydospores in pairs. d–eF. proliferatum in PDA; falcate macroconidia with 3 septate. f–gF. sacchari in PDA; microconidia with 1–2 septate h–jF. verticillioides in PDA; relatively straight macroconidia with 3 septate; microconidia in clusters and monophialides. k–lF. subglutinans in PDA; microconidia in clusters. Macroconidia = 2600x
Other isolates were also observed to exhibit morphological characters specific to species such as F. proliferatum and F. sacchari. Cultures of F. proliferatum possessed profuse white aerial mycelium, which mature to purple-violet in media with age (Fig. 3d). Overall pigmentation varied from colorless to closely black. Microconidia which are abundant in aerial mycelium are non-septated. Macroconidia are slender and moderately straight and possess 3-5 septate, with curved apical cell (Fig. 3e). Chlamydospore formation was absent (Leslie and Summerell 2006). Measured microconidial sizes ranged from 4.48–7.76 µm × 1.21–1.96 µm. For F. sacchari, cultures were observed to have relatively abundant mycelia which are pale to violet in color with age. Formation of violet pigmentation in media was also noted (Fig. 3f). Macroconidia are moderately slender, slightly curved, possess 3-septate and with sickle-shaped apical cell. Microconidia/mesoconidia are oval, slender and present as 0–3 septated structures (Leslie and Summerell 2006) (Fig. 3g). Measured microconidial/mesoconidial sizes ranged from 4.14–10.41 µm × 1.77–2.52 µm.
Likewise, phenotypic characteristics of F. verticillioides and F. subglutinans were also identified among the fungal isolates. Cultures of F. verticillioides have initially white mycelia which form pink to violet pigments upon maturity (Fig. 3h). Relatively, very few macroconidia which are long and slender in size, with 3–5 septate, straight to slightly falcate were observed (Fig. 3i). Moreover, microconidia which are oval to club-shaped with a flattened base, present in long chains, and non-septated that may originate from monophialides which present as V-shaped pairs comparable to a “rabbit ear” appearance were noted (Leslie and Summerell 2006) (Fig. 3j). These microconidia possessed spore sizes ranging 3.89–5.72 µm × 1.18–23.74 µm. Conversely, F. subglutinans isolates have copious mycelial growth in media in initially white to violet color with age (Fig. 3k). Overall pigmentation may become colorless to dark purple. Microconidia are oval and non-septated (Fig. 3l). Macroconidia were also present but were few in number and difficult to discern. Chlamydospores are absent (Leslie and Summerell 2006). Measured microconidial sizes ranged from 3.92–6.45 µm × 1.34–14.67 µm.
Isolates identified morphologically as F. incarnatum possessed aerially dense mycelial growth in PDA. Oval-shaped microconidia, with 1-2 septate and polyphialides present as “rabbit ear” appearance, were noted. Few slender, curved-shaped macroconidia with 3–5 septate were also observed (Leslie and Summerell 2006).
Molecular Identification and Phylogenetic Analysis of Fusarium Isolates
Using the genus-specific primers ITS-Fu-f/r which targets the 18S rDNA (Kaur et al. 2015), the genomic DNA of the 112 pathogenic fungal isolates collected from various sugarcane areas in the Philippines yielded PCR products of ~ 389 bp size, confirming the identity of Fusarium spp. as shown in Fig. 4a. We then selected 56 representative isolates and one F. oxysporum f. sp. cubense isolated from banana fusarium wilt as outgroup for TEF-1α gene sequencing. Amplified PCR products generated ~ 650 bp size of the TEF-1α gene fragment (Fig. 4b) further validating the specificity of the EF1/EF2 primer pair. The TEF-1α sequences of the 56 Fusarium spp. isolates, along with the primer sequences, were aligned for comparison and species identification in the BLASTn search utility. Identities of each fungal isolates were established by selection of the most closely related sequences deposited in the Genbank with the highest percentage of sequence homology with the isolate. Based on high degree of nucleotide sequence similarity (94–100%), we characterized the 56 representative isolates as F. sacchari (66%), F. proliferatum (16%), F. incarnatum (7%), F. verticillioides (5%) and F. subglutinans (5%). Furthermore, the results of the TEF-1α sequence comparison affirmed our initial identification of the 56 isolates using morphological and cultural analyses. Multiple sequence alignment of the 56 Fusarium spp. also displayed high degree of similarity as compared to divergence with the outgroup F. oxypsorum f. sp. cubense (data not shown) indicating the relatedness of these species.
a Agarose gel of amplified PCR products using the genus-specific primer ITS-Fu-f/r at 389 bp of representative Fusarium spp. Lanes 1–4 represented Luzon isolates (1,2-Tarlac; 3-Laguna; 4-Batangas). Lanes 5–9 represented Visayas isolates (5,6,7-Negros Occidental; 8,9-Cebu). Lanes 10–13 represented Bukidnon, Mindanao isolates. b Agarose gel of amplified PCR fragments using the EF1/EF2 primer pair at 650 bp of three representative Fusarium isolates. Lane 1: Fusarium sacchari. Lane 2: Fusarium proliferatum. Lane 3: Fusarium proliferatum. Lane NC: negative control
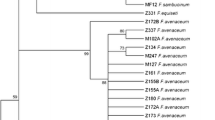
The cladogram (Fig. 5) constructed at 0.5 similarity coefficient revealed that the isolates were majorly clustered based on species identity regardless of the geographical origin or place of collection. Phylogenetic analyses of the TEF-1α sequences separated the isolates into three clades (A, B and C) and five lineages (I–V). Clade A comprised of the outgroup isolate (F. oxysporum f. sp. cubense), while Clade B consisted of lineages II, III and IV, whereas Clade C included lineage V. Four of the isolates identified as F. proliferatum sharing 100% similarity were grouped together. At 89.9% similarity, all the 37 isolates of F. sacchari assembled together and delineated as a major cluster including three isolates of F. subglutinans and five isolates of F. proliferatum. The outgroup F. oxysporum f. sp. cubense deviated as a distinct clade. Using 1000 bootstrap replicate values, the TEF-1α sequences of the isolates in this study assembled according to the same species signifying species-dependent separation and polymorphisms on the clustering of closely related species.
Phylogenetic analysis of 56 Fusarium spp. isolated from sugarcane pokkah boeng using the partial sequence of the translation elongation factor (TEF-1α) gene region. Analysis was conducted by maximum likelihood method using 1000 bootstrap replicates in Geneious version R9.1 (Kearse et al. 2012). Trees are drawn to scale and analyzed using neighbor-joining method in Nei-Tamura genetic distances
Discussion
Occurrence and severity of sugarcane pokkah boeng are affected by various predisposing factors such as varietal susceptibility, environmental conditions, farming practices, temperature and plant stress (Vishwakarma et al. 2013). In terms of field symptom variability, it was observed that symptoms expressed by infected sugarcane during field sampling in Visayas and Mindanao were more advanced and diverse as compared to symptoms manifested by diseased plants in the fields surveyed in Luzon. The Negros Island in the Visayas, which is accounted for about 55% of the total sugarcane production nationwide, practices homogenous farming and monocrop cultures (Padilla-Fernandez and Nuthall 2009); thus, the probability of spreading the pathogen and incurring the advanced stages of the disease is high.
Disease incidence was also observed to be higher when the humidity is high with cloudy-to-rainy weather (Vishwakarma et al. 2013). The Philippines is a tropical country with dry and wet seasons which are conditions auspicious for the spread of the pathogen. For instance, in the province of Negros Occidental in the Visayas, the rainy season starts from June to November, while the dry season begins from late December to May (Padilla-Fernandez and Nuthall 2009). Disease incidence per area can also vary depending on the conditions present in a particular area. This is more evident in Visayas and Mindanao, wherein pokkah boeng symptoms observed in the fields were more severe compared to the symptoms present on sugarcane in the sampled fields in Luzon. Luzon experiences more droughts than Visayas and Mindanao, and Visayas and Mindanao were projected to have more rainfall than Luzon (Israel 2012), conditions that may augment pathogen spread and increase disease incidence.
Our results confirmed that sugarcane pokkah boeng in the Philippines is caused by the Fusarium species complex, supporting the studies reported in other countries (Lin et al. 2014, 2015; Khani et al. 2013; Siti Nordahliawate et al. 2008; Mohammadi et al. 2012; Hilton et al. 2017; Viswanathan et al. 2017). This was further validated by molecular detection of the pathogenic and morphologically characterized Fusarium isolates and by BLASTn search of the translation elongation factor (TEF-1α) region sequences (Fourie et al. 2009).
Molecular identification using TEF-1α sequences confirmed that F. sacchari was the most predominant pathogen-causing pokkah boeng in Luzon followed by F. proliferatum, F. incarnatum, F. verticillioides and by F. subglutinans. In Negros Occidental in the Visayas, the most commonly isolated pathogens were F. sacchari and F. proliferatum. Similarly, in Mindanao, F. sacchari was the most prevalent, followed by F. proliferatum and by F. verticillioides, F. subglutinans and F. incarnatum. These results were also aligned with the findings of Viswanathan et al. (2014) wherein F. sacchari and F. verticillioides were reported as the main causative pathogens of sugarcane pokkah boeng and wilt in Tamil Nadu, South India.
Sequencing of conserved housekeeping genes such as TEF-1α used in this study enables the reliable classification of the species complex of unidentified Fusarium pathogens. Sequence divergence based on the TEF-1α gene analysis of the 56 Fusarium spp. grouped the isolates as morphologically and phylogenetically interrelated. This pattern of clustering supported by database sequences was aligned with the findings of Fourie et al. (2009) wherein F. proliferatum, F. subglutinans and F. verticillioides are grouped as related species which are phylogenetically different from one group composed of F. avenaceum, F. tricinctum and F. torulosum or the other grouping of F. redolens and F. hostae. However, based from the results of this study, there is no direct correlation between the morphological and molecular grouping of the identified Fusarium spp. causing pokkah boeng in the Philippines.
In this study, it was also interesting to note that the species causing the disease can be associated with the types of symptoms manifested. Advanced symptom such as the “top-rot” and the typical pokkah boeng symptoms such as leaf wrinkling and curling, necrosis and chlorosis were correlated with F. verticillioides. This fungal pathogen can be easily dispersed by means of its airborne conidia and is capable of multiplying rapidly especially during rainy season that is coupled with low relative humidity (Cumagun 2007). Furthermore, its prevalence in Mindanao can be attributed to its ubiquitous nature since it is also present in corn-growing fields with ear rot disease (Pascual et al. 2016) which may be situated near commercial sugarcane areas. For F. sacchari isolates, these were mostly procured from diseased plants expressing leaf chlorosis, red streaking and stalk rot. In the case of F. proliferatum, this pathogen was mostly obtained from infected plants manifesting leaf chlorosis, leaf curling, twisting and wrinkling and the “top-rot” phase. Moreover, diseased plant samples in which F.graminareum, F. incarnatum and F. subglutinans were isolated were from earlier and milder stages of pokkah boeng. These observations were supported by various researches, such as in one study wherein F. verticillioides was isolated from diseased sugarcane which have crumpled and twisted leaves (Lin et al. 2015). In India, F. verticillioides was established to cause foliar infection such as malformation and twisted top especially during monsoon seasons (Viswanathan et al. 2014). Moreover, F. verticillioides and F. proliferatum were identified on inoculated sugarcane seedlings which expressed symptoms of chlorosis and lens or rhomboid-shaped holes on the leaves (Lin et al. 2014), whereas F. sacchari was reported as a root/stalk pathogen (Viswanathan et al. 2014).
For further studies and directions, developing control measures on eliminating the disease from commercial sugarcane farms through the implementation of fungicide sensitivity tests is recommended as well as determining whether mycotoxins are produced in sugarcane tissues with pokkah boeng disease infected by Fusarium spp. Likewise, the possibility of Fusarium stalk and systemic infection in sugarcane infected with pokkah boeng should also be explored. More attention must be given to this disease to prevent its spread and effect on the yield of sugarcane. Sugarcane varieties, both commercial and germplasm collections, must be evaluated for resistance to pokkah boeng disease.
References
Abd-Elsalam, K.A., I.N. Aly, M.A. Abdel-Satar, M.S. Khalil, and J.A. Verreet. 2003. PCR identification of Fusarium genus based on nuclear ribosomal-DNA sequence data. African Journal of Biotechnology 2 (4): 82–85.
Abramoff, M.D., P.J. Magalhes, and S.J. Ram. 2004. Image processing with ImageJ. Biophotonics International 11 (7): 36–42.
Cullings, K.W. 1992. Design and testing of a plant-specific PCR primer for ecological and evolutionary studies. Molecular Ecology 1: 233–240.
Cumagun, C.J.R. 2007. Population genetic analysis of plant pathogenic fungi with emphasis on Fusarium species. The Philippine Agricultural Scientist 90 (3): 244–256.
Doyle, J.J., and J.L. Doyle. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin 19: 11–15.
Fourie, G., E.T. Steenkamp, T.R. Gordon, and A. Viljoen. 2009. Evolutionary relationships among Fusarium oxysporum f. sp. cubense vegetative compatibility groups. Applied and Environmental Microbiology 75 (14): 4770–4781.
Hilton, A., H. Zhang, W. Yu, and W.B. Shim. 2017. Identification and characterization of pathogenic and endophytic fungal species associated with Pokkah Boeng Disease of Sugarcane. Plant Pathology Journal 33 (3): 238–248.
Hsuan, H.M., L. Zakaria, and B. Salleh. 2010. Characterization of Fusarium isolates from rice, sugarcane and maize using RFLP-IGS. Journal of Plant Protection Research 50 (4): 409–411.
Israel, D.C. 2012. Typhoons, floods and droughts: Regional occurrence and value of damages to rice farming in the Philippines. Philippine Institute for Developmental Studies Policy Notes (2012–15):1–6.
Kaur, A., V.K. Sharma, A. Sirari, J. Kaur, G. Singh, and P. Kumar. 2015. Variability in Fusarium oxysporum f. sp. ciceris causing wilt in chickpea. African Journal of Microbiology Research 9 (15): 1089–1097.
Kearse, M., R. Moir, A. Wilson, S. Stones-Havas, M. Cheung, S. Sturrock, S. Buxton, A. Cooper, S. Markowitz, C. Duran, T. Thierer, B. Ashton, P. Mentjies, and A. Drummond. 2012. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28 (12): 1647–1649.
Khani, K.T., A. Alizadeh, R.F. Nejad, and A.S. Tehrani. 2013. Pathogenicity of Fusarium proliferatum, a new causal agent of pokkah boeng in sugarcane. Proceedings of International Society of Sugar Cane 1–5.
Leslie, J.F., and B.A. Summerell. 2006. The Fusarium laboratory manual. Ames, IA: Blackwell Publishing.
Lin, Z., Y. Zhang, Y. Que, R. Chen, B. Chen, and M. Zhang. 2015. Characterization of Fusarium verticilloides isolates from pokkah boeng on sugarcane and the disease incidence in field. Journal of Microbiology & Experimentation 2 (5): 1–7.
Lin, Z., S. Xu, Y. Que, J. Wang, J.C. Comstock, J. Wei, P.H. McCord, B. Chen, R. Chen, and M. Zhang. 2014. Species-specific detection and identification of Fusarium species complex, the causal agent of sugarcane pokkah boeng in China. PLoS ONE 9 (8): 1–12.
Martin, J.P., H. Handojo, and C.A. Wismer. 1989. Pokkah boeng. In: Diseases of sugarcane: Major diseases, pp. 157–165. Netherlands: Elsevier Science Publishers.
Mohammadi, A., R.F. Nejad, and N.N. Mofrad. 2012. Fusarium verticilloides from sugarcane, vegetative compatibility groups and pathogenicity. Plant Protection Science 48 (2): 80–84.
Padilla-Fernandez, M.D., and P.L. Nuthall. 2009. Technical efficiency in the production of sugarcane in Central Negros area, Philippines: An application of data envelopment analysis. Journal of ISSAAS 15 (1): 77–90.
Pascual, C.B., A.K. Barcos, J.L. Mandap, and E.M. Ocampo. 2016. Fumonisin-producing Fusarium species causing ear rot of corn in the Philippines. Philippine Journal of Crop Science 41 (1): 12–21.
Singh, A., S.S. Chauhan, A. Singh, and S.B. Singh. 2006. Deterioration in sugarcane due to pokkah boeng disease. Sugar Tech 8 (2&3): 187–190.
Siti Nordahliawate, M.S., M.Z. Nur Ain Izzati, A.R. Azmi, and B. Salleh. 2008. Distribution, morphological characterization and pathogenicity of Fusarium sacchari associated with pokkah boeng disease of sugarcane in Peninsular Malaysia. Pertanika Journal of Tropical Agricultural Science 31 (2): 279–286.
Vishwakarma, S.K., P. Kumar, A. Nigam, A. Singh, and A. Kumar. 2013. Pokkah boeng: An emerging disease of sugarcane. Journal of Plant Pathology & Microbiology 4 (3): 1–5.
Vishwakarma, S.K., A. Nigam, and A. Singh. 2016. Molecular phylogenetic analysis of Fusarium isolates causing pokkah boeng disease in sugarcane based on RAPD marker. International Journal of Agricultural Science and Research 6 (3): 177–186.
Viswanathan, R., and G.P. Rao. 2011. Disease scenario and management of major sugarcane diseases in India. Sugar Tech 13 (4): 336–353.
Viswanathan, R., P. Malathi, A. Annadurai, C. Naveen Prasanth, and M. Scindiya. 2014. Sudden occurrence of wilt and pokkah boeng in sugarcane and status of resistance in the parental clones in national hybridization garden to these diseases. Journal of Sugarcane Research 4 (1): 62–81.
Viswanathan, R., C.G. Balaji, R. Selvakumar, P. Malathi, A. Ramesh Sundar, C. Naveen Prasanth, M.L. Chhabra, and B. Parameswari. 2017. Epidemiology of Fusarium diseases in sugarcane: A new discovery of same Fusarium sacchari causing two distinct diseases, wilt and pokkah boeng. Sugar Tech 19 (6): 638–646.
Zhang, C., J. Wang, H. Tao, X. Dang, Y. Wang, M. Chen, Z. Zhai, W. Yu, L. Xu, W.B. Shim, G. Lu, and Z. Wang. 2015. FvBck1, a component of cell wall integrity MAP kinase pathway, required for virulence and oxidative stress response in sugarcane Pokkah Boeng pathogen. Frontiers in Microbiology 6 (1096): 1–12.
Acknowledgements
This study has been funded by the Philippine Sugar Research Foundation, Inc. (PHILSURIN). The authors would also like to acknowledge the following institutions and persons for their invaluable technical contributions in the formulation of this study: Sugar Regulatory Administration (SRA), BUSCO Sugar Milling Corporation, Bureau of Plant Industry—National Plant Quarantine Service (BPI-NPQS), Ms. Rizalina L. Tiongco, Ms. Rosalyn T. Luzaran, Ms. Chona Untal, Ms. Fatima Florie May Silva, Ms. Aira Waje, Mr. Eddie M. Bueta. The research grant was received from the Philippine Sugar Research Institute Foundation, Inc. and the organization declares no conflict of interest in the subject matter or materials present in the manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human or Animal Participants
This study does not involve human or animal participants
Rights and permissions
About this article
Cite this article
Samaco, M.A., dela Cueva, F.M. Molecular Characterization of Fusarium spp. Associated with Sugarcane Pokkah Boeng from the Philippines Using Partial Translation Elongation Factor-1α (TEF-1α) Gene Sequences. Sugar Tech 21, 619–630 (2019). https://doi.org/10.1007/s12355-018-0662-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-018-0662-7