Abstract
Insect feeding induces physiological and biochemical changes in host plants. Indeed, symptom severity often depends on insect density. Recently, the carrot psyllid, Bactericera trigonica, has negatively affected carrot production in Mediterranean countries. The present study was conducted to evaluate effects of psyllid nymphal density on photosynthetic pigments and biochemical parameters of carrot leaves. Also, changes to physico-chemical parameters of carrot juice in response to insect feeding were quantified. A significant decrease in leaf weight, total number of leaves, root weight, chlorophyll (a + b) and carotenoid content was recorded in response to elevated infestations of B. trigonica. A positive correlation was observed between psyllid feeding and proline, total phenolic and flavonoid contents of leaves. A significant increase of titratable acidity was also observed in carrot juice obtained from infested plants, while total soluble solids decreased as number of attacking insects increased. Results indicate that the stress response of carrot plants to psyllid infestation depended on nymphal density.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psyllid species are small insects that feed on plants by ingesting phloem sap. Psyllid species are vectors of economically important plant diseases, including many newly emerging ones. The three ‘Candidatus Liberibacter’ species associated with huanglongbing (HLB) citrus disease are transmitted by Diaphorina citri Kuwayama, Trioza erytreae Del Guercio, and Cacopsylla citrisuga. Candidatus Liberibacter solanacearum (CaLsol) is transmitted by Bactericera cockereli (Šulc) to potato and tomato plants (Secor et al., 2009). In northern Europe this disease was observed in carrot fields and transmitted by Trioza apicalis Foerster (Nissinen et al., 2014). Recently, a ‘Candidatus Liberibacter’ species was associated with vegetative disorders in carrot fields in Tunisia and transmitted by Bactericera trigonica Hodkinson (Ben Othmen et al., 2018; Ben et al., 2019). This psyllid causes considerable damage to carrots (Daucus carota L) through injection of toxic saliva during feeding. Severe carrot psyllid attacks can lead to serious yield loss. Because effective management of psyllids is lacking worldwide, it is necessary to determine the impact of psyllid feeding on the physiology of their host plants.
The chlorophyll content of plant tissues is one of the main parameters impacted by interactions between host plants and insects. Indeed, chlorophyll levels change in response to a wide variety of stresses, including biotic stresses such as insect feeding and pathogen infection (Goławska et al., 2010; Heng-Moss et al., 2003; Ni et al., 2001, 2002). However, previous studies on Bactericera trigonica on carrots mainly focused on the control and biology of the pest. Thus, little is known about how psyllid damage may affect quantity and quality of carrot yields. Previous studies emphasized that carrot psyllid feeding significantly affects total sugars and phenolic content of taproots of carrot plants attacked by T. apicalis (Nissinen et al., 2012). The aims of the present study are to: i) investigate how nymphal density affects carrot yield; ii) quantify variation in foliar pigments, phenolic, flavonoids and proline levels in leaf tissues in response to increasing nymphal density; and iii) assess whether psyllid feeding affects the sugar content of carrot roots.
Materials and methods
Plant material and sample processing
The study was carried out in a carrot field located in the South Kairouan delegation “Zaafrana area” (35°32′31.21"N, 10°04′30.48"E). The field was planted with “Arbi Zaafrana” hybrids. The following symptoms were frequently observed in this field: general stunting, yellowing and curling of leaves. These symptoms were associated with the presence of a B. trigonica population. After collecting symptomatic plants, every five plants with the same nymph density were grouped together; in total, six groups of different psyllid nymph density were collected. The plant sampling was done according to the degree of damage sustained by each plant. Plant samples were immediately transported to the laboratory. The control group was represented by five plants not colonized by psyllid nymphs. To assess leaf damage, leaf pigments contents were analyzed using a spectrophotometer (ThermoSpectronic Heλios γ, Cambridge, England).
Carrot yield
For each carrot plant, roots were lifted, washed, and weighed individually.
Damage estimate
The number of symptomatic leaves and the total number of leaves of each sampled carrot plant were determined. If the oldest leaves were yellowish in color these were not considered as damaged but a result of normal senescence. The fresh weight of total carrot leaves was measured for each plant.
RT-PCR analysis
To confirm that psyllids were responsible for changes observed in carrot plants, a real-time PCR assay was performed according to Li et al. (2006) using a Light Cycler 480 (Roche, Switzerland) to detect the presence of CaLsol. The reaction mix consisted of 1X Quantimix (Biotools, Spain), 0.24 μM of each primer, 0.12 μM of TaqMan probe and 3 μl of the template (purified DNA or direct extraction from the spot) in a final volume of 12 μl. The real-time PCR amplification protocol included the following steps: 95 °C for 20 s followed by 45 cycles of 95 °C for 1 s and 58 °C for 40 s. Primers and probe sequences were, respectively: LsoF, 5’-GTCGAGCGCTTATTTTTAAT AGGA-3’; HLBr, 5’-GCGTTATCCCGTAGAAAAAGG TAG-3’; and HLBp, 5’FAM-AGACGGGTGAGTAA CGCG-3’BHQ. The positive results (Ct values ≤ 40 and exponential curve) and the negative results were also checked by the real-time PCR protocol of Teresani et al. (2014). In this case, the reaction mix consisted of 1X Quantmix (Biotools, Spain), 0.5 μM of each primer, 0.16 μM TaqMan probe and 3 μl of the template. The amplification protocol consisted of 95 °C for 10 min followed by 45 cycles of 95 °C for 15 s and 60 °C for 1 min. Primers and probe sequences were the following: CaLsppF, 5’GCAGGCCTAACACATGCAAGT3’; CaLsppR, 5’GCACACGTTTCCATGCGTTAT-3’, and the specific TaqMan probe CaLsolP, 5` FAM-AGCGCTTA TTTTTAATAGGAGCGGCAGACG-3` TAMRA (Teresani et al., 2014).
Titratable acidity (TA), pH, total soluble solids (TSS)
Carrot roots were crushed using a juicer. Juice pH was measured using a pH-meter (Jenway). The titratable acidity was determined by acid–based potentiometry (0.1 mol L−1 NaOH up to pH 8.1), expressed as g L−1. The TSS content was recorded using a refractometer (Atago) at 20 °C with values being expressed as °Brix.
Extraction and measurement of chlorophylls a, b and carotenoids
The determination of chlorophyll was made according to Holden (1965). 0.1 g of leaves (± 5 leaves) were ground in a mortar with 10 ml of 80% acetone (CH3COCH3). The extract was then filtered through a 5 µm diameter millipore filter. After 72 h in the dark at a temperature of 4 °C, the obtained filtrate was used to measure chlorophyll a, chlorophyll b and carotenoids using a spectrophotometer (ThermoSpectronic Heλios γ, Cambridge, England). Chlorophyll a and chlorophyll b were measured at 645 and 663 nm and carotenoids at 470 nm. Each sample measurement was performed in three replicates and the pigment content was calculated from the following equations:
With: A: absorbance, V: extraction volume (10 ml), W: sample mass, e: dilution factor.
Determination of proline content
From each sample, 100 mg of leaves cut into small pieces were placed in test tubes containing 2 ml Methanol (40%). The mixture was heated to boiling in a water bath at 85 °C for 60 min. After cooling samples, 1 ml of the extract was removed from each sample and placed into a new tube to which was added: 1 ml of acetic acid, 1 ml of a solution containing 120 ml H2O, 300 ml acetic acid, 80 ml ortho-phosphoric acid "H3PO4, density 1.7" and 25 mg of ninhydrin. The mixture was heated to boiling for 30 min, till the solution turned to red. After cooling, 5 ml of toluene was added to each tube with stirring for two phases, then the upper phase was removed to which a pinch of Na2SO4 was added to remove water and the optical density was measured by a spectrophotometer at 528 nm (Bagues et al., 2017).
Determination of total phenolic content
The total phenolic content was determined using the Folin–Ciocalteu method (Aryal et al., 2019). Briefly, 1 mL of methalonic extract solution was mixed with 2.5 mL of 10% (w/v) Folin–Ciocalteu reagent. After 5 min, 2.0 mL of Na2CO3 (75%) were subsequently added to the mixture and incubated at 50 °C for 10 min with intermittent agitation. The absorbance was measured utilizing a UV spectrophotometer at 765 nm. The results (mean ± standard error) were expressed as mg/g of gallic acid equivalents (mg GAE/g).
Determination of flavonoid content
The flavonoid contents were measured as described by Aryal et al. (2019). An aliquot of 1 mL of extract solution was mixed with 0.2 mL of 10% (w/v) AlCl3 solution in methanol, 0.2 mL 1 M potassium acetate and 5.6 mL distilled water. The mixture was incubated for 30 min and the absorbance was measured at 415 nm. The results were expressed as mg quercetin equivalent/g.
Statistical analyses
Statistical analyses were performed using one-way analysis of variance (ANOVA), and the significant differences between means were determined by Duncan’s multiple range test using SPSS 20 software. Significance was defined at P < 0.05.
Results
CaLsol incidence
The real-time PCR assays were not able to confirm presence of CaLsol bacteria in any of the tested carrot plants.
Leaf weight, total number of leaves, number of curled leaves, root weight
The weight of fresh leaves decreased with nymphal density. A decrease of over 62% of leaf weight was observed when psyllid density exceeded eight nymphs per plant compared to controls. Results also showed that total number of leaves was significantly affected by psyllid nymph feeding and decreased with nymphal density. Also, the number of leaves with curling symptoms significantly increased with nymphal density. However, when the number of nymphs exceeded 8 nymphs per plant, there was no additional increase in number of symptomatic leaves with nymphal density (Table 1).
The root weight of the psyllid-exposed carrots significantly decreased as psyllid density increased. The psyllid nymphs greatly reduced the root fresh weight when their density exceeded eight per plant. A density of 15 nymphs per plant caused a decrease of over 85% of taproot weight (Table 1).
Changes in foliar pigments
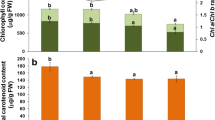
Significant differences were recorded for pigment contents of carrot leaves infested with different densities of B. trigonica nymphs (Table 2). The chlorophyll a (Ch a) content was significantly lower in infested carrot leaves than that in control plants, with Ch a content decreasing as nymphal density increased. Also, a decrease of chlorophyll b (Ch b) content was recorded as psyllid nymph density increased. Chlorophyll b content varied from 10.04 mg/g FW in controls to 6.62 mg/g FW in plants infested by eight nymphs and 3.43 mg/g FW in plants infested with 15 nymphs. Psyllid nymph density also had a significant effect on total carotenoid content. The highest content (891.37 mg/g FW) was registered in control plants. However, a decrease of 3.46-fold of total carotenoid content was observed for plants infested with 15 nymphs compared to controls.
Total phenolic content, total flavonoid content, proline content
Significant differences in total phenolic, flavonoid and proline contents were recorded between infested and non-infested leaves (Table 3).
Total phenolic contents (TPC) ranged from 13.97 mg/100 g F.W for control leaves to 70.60 mg/100 g FW in leaves infested with 15 B. trigonica nymphs. The TPC content showed a consistent increase in response to insect damage. Also, the total flavonoid content increased with the rate of B. trigonica leaf infection. The TFC content varied from 1.06 mg/100 g for control leaves to 21.44 mg/100 g for leaves infested with 15 nymphs. Regarding the proline content, a higher concentration was observed in infested leaves than in control leaves, with the increase proportional to nymphal density It varied from 104.04 mg/100 g in control leaves to 521.87 mg/100 g in leaves infested with eight nymphs and 1274.18 mg/100 g in leaves infested with 15 nymphs.
pH, titratable acidity (TA), total soluble solids (TSS)
The pH value of carrot juices made from infested and control plants ranged from 6.36 to 6.93. The juice pH decreased significantly with psyllid density. The lowest pH value was observed in carrot juice from plants infested with 15 nymphs. The titratable acidity showed a significant variation between infested and non-infested plants (Table 4). A significant increase of TA values was associated with the increase of psyllid nymph density. The values ranged from 0.03 for controls to 0.19 in the most infested plants (15 nymphs). Bactericera trigonica infestation also significantly affected total soluble solids (TSS) values in carrot juice (Table 4). The TSS values ranged from 3.67°Brix to 7.90°Brix. The control plants had higher total soluble solids content than infested plants. A decrease of TSS values was noted with the increase of insect damage. The most exposed carrot plants (15 nymphs) had the lowest total soluble solids content.
Discussion
Bactericera trigonica is the most abundant insect species in carrot fields (occurring from crop emergence to harvest). This psyllid causes direct damage to carrot (Ben Othmen et al., 2018).
In this study, a significant decrease of root fresh weight was observed in response to increasing psyllid density. Feeding also significantly affected leaf fresh weight. Number of curled leaves increased with number of nymphs suggesting that leaf-curling is caused by psyllid feeding. Previous studies reported also that nymphs feeding at high density leads to a dramatic reduction in yield and leaf curling (Nissinen et al., 2007, 2012).
Changes in chlorophyll (Cha and Chb) and carotenoid contents in carrot leaves were associated with psyllid density. These contents decreased as number of nymphs increased. Similar results from other studies have revealed a significant reduction in total chlorophyll and carotenoid content in response to herbivore attack (Blanchfield et al., 2005, 2007; Kumar & Sharma, 2014). Grape leaves infested by phylloxera (Daktulospharia vitifoliae Fitch) had significantly lower contents of total chlorophyll and carotenoid than those in the control (Blanchfield et al., 2005, 2007). Huang et al. (2014) also reported that relative chlorophyll loss on Brassicaceous plant species was related to the feeding damage caused by Bagrada hilaris Burmeister. However, as psyllids are phloem feeders they are often thought to cause less severe damage to leaves then other groups of herbivores. Nevertheless, it was shown that Diaphorina citri (Kuwayama) and Aonidella orientalis (Newstead) are able to decrease the total chlorophyll and carotenoid contents in citrus and guava leaves, respectively (Killiny and Nehela, 2017; Kumar & Sharm, 2014). Dai et al. (2009) also reported that Hypericum sampsoni Hance plants synthesized less chlorophyll pigment in leaves infested with Thrips tabaci (Linderman) compared to control leaves. In fact, chlorophyll degradation is a complex phenomenon that is often linked to insect feeding damage to plants (Ni et al., 2001). Therefore, these changes have often been regarded as a relatively late mechanism of photosynthetic adaptation (Anderson et al., 1995). Golan et al. (2014) reported that Coccus hesperidum L. feeding induced a stress response in host plants, represented by a decrease in chlorophyll and carotenoid content and photosynthesis in response to increasing insect density.
In this study, psyllid infestation affected the total phenolic content of carrot leaves, which increased in response to B. trigonica feeding. These results are consistent with those reported by Talcott and Howard (1999) and Nissinen et al. (2012). Often, plants may respond to pathogen and insect infestations by producing greater levels of secondary metabolites such as certain phenolics and terpenoids (Wallis et al., 2008). Plant phenols constitute one of the most common and widespread groups of defensive compounds, which play a major role in host plant resistance (HPR) against herbivores including insects (Sharma, 2008; Usha et al., 2010; War et al., 2011). Previous studies also reported that increased levels of polyphenolic molecules in the leaves increase defense response or resistance in several plants (Felton & Duffey, 1990; Miles & Oertli, 1993). Lattanzio et al. (2009) revealed that polyphenolic molecules in plants have been reported to fluctuate in response to insect damage. A defense mechanism occurs after pathogen or insect damage and may involve the activation of phenylalanine ammonia-lyase (PAL) (Felton et al., 1999). This enzyme acts as a catalyst in phenylpropanoid biosynthesis. Similar results revealed that PAL activity correlated with elevated concentration of phenols was strongly elevated in Chrysanthemum during the early period (0.5 to 6 h) after aphid infestation (He et al., 2011) and in kale after P. brassicae herbivory (Ibrahim et al., 2018).
Total flavonoid contents were higher in infested leaves compared to non-infested ones. Beyond their well-known antioxidant properties, flavonoids also play an important role in insect–plant interactions (Kovalikova et al., 2019). They are generally involved in plant resistance to insects. In the present study, carrot plants responded to B. trigonica feeding by increasing flavonoid production. The onset of heavy infestations was observed in conjunction with higher levels of flavonoids.
An increase of proline content was also noted under the stress of psyllid feeding. A positive correlation was recorded between proline content and number of psyllid nymphs.
This may indicate that proline accumulation is a defensive response to insect herbivory as proline is a tissue-repairing metabolite. Similar results have been reported in eucalypt leaves infested with xylem-feeding insects (Khattab, 2005). Proline is a universal osmolyte accumulated in response to several stresses (Öncel et al., 1996) and may have a role in plant defense reactions (Ding et al., 2000; Stevenson et al., 2009). The excessive proline accumulation in proportion to number of attacking insects suggest that proline estimates may be used to determine the extent of herbivory in carrot and other plants. Such increases in total phenols, flavonoids and proline contents are considered elements of induced resistance in hosts against herbivory.
Changes of physico-chemical properties in carrot juices under insect stress were also determined. A decrease of juice pH was observed.
However, increasing psyllid density led to an increase in titratable acidity. Similarly, psyllid feeding was shown to increase orange juice acidity as reported by Plotto et al. (2010).
Total soluble solids (TSS) decreased as nymph density increased. This decrease may be due to a reduction in the surface area of the leaf exposed to light due to the damage associated with increased insect pest populations (Zubair et al., 2015). Zubair et al. (2017) reported that carbohydrates and total soluble solids also decreased due to stress induced by insect attack, which led to the reduction of prospective growth and ultimate juice content in citrus fruits. Thus, the reduction in sugar content caused by psyllid attack indicates increased respiration and carbohydrate consumption due to stress and wound-healing activity by the plant. This is confirmed by results from other studies of psyllid-exposed carrots (Nissinen et al., 2012).
Conclusion
Psyllid infestation causeds physiological and biochemical changes to carrot plants. A decrease in the photosynthetic pigments (chlorophyll a, chlorophyll b and carotenoid content) was associated with psyllid nymph feeding. Proline content increased in response to B. trigonica infestation. Infested leaves also showed higher phenol and flavonoid contents. Feeding by nymphs of B. trigonica also caused changes to parameters associated with carrot juice quality such as pH, TSS and titratable acidity. These changes could be considered as a result of the host’s defense response.
All the tested carrot samples were negative for the presence of “Ca. L. solanacearum”. Thus, the obtained results were mainly caused by nymph feeding. However, further studies are required to examine how carrot host chemistry can be affected by ‘Ca. L. solanacearum’ which may contribute to developing a clear picture of carrot host responses to ‘Ca. L. solanacearum’ or psyllids.
References
Anderson, J. M., Chow, W. S., & Park, Y. I. (1995). The grand design of photosynthesis: Acclimation of the photosynthetic apparatus to environmental cues. Photosynthesis Research, 46, 129–139.
Aryal, S., Baniya, M. K., Danekhu, K., Kunwar, P., Gurung, R., & Koirala, N. (2019). Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants. J., 8, 96.
Bagues, M., Souli, I., Boussora, F., Lachiheb, B., & Nagaz, K. (2017). Response of two barley accessions “Ardhaoui” to deficit irrigation using saline water in southern Tunisia. J. New. Sci., 37, 2013–2023.
Ben Othmen, S., Morán, F.E., Navarro, I., Barbé, S., Martínez, M.C., Marco-Noales, E., Chermiti, B., López, M.M., 2018. ‘Candidatus Liberibacter solanacearum’ haplotypes D and E in carrot plants and seeds in Tunisia. J. Plant. Pathol. 1–11.
Ben Othmen S., Abbes K., El Imem M., Marroquíns C., Ouvrard D., Rapisarda C., Chermiti B., 2019. Bactericera trigonica and B. nigricornis (Hemiptera: Psylloidea) in Tunisia as potential vectors of “Candidatus Liberibacter solanacearum” on Apiaceae. Oriental Insects, 53 (4): 497–509, https://doi.org/10.1080/00305316.2018.1536003.
Blanchfield, A. L., Powell, K. S., & Robinson, S. A. (2007). Preliminary Investigations of Pigment Responses to Phylloxera Infestation. Acta Horticulture, 733, 123–133.
Blanchfield, A. L., Robinson, S. A., Renzullo, L. J., & Powell, K. S. (2005). Phylloxera-infested grapevines have reduced chlorophyll and increased photoprotective pigment content- can leaf pigment composition aid pest detection? Functional Plant Biology, 33, 507–514.
Dai, Y., Shao, M., Hannaway, D., Wang, L., Liang, J., Hu, L., & Lu, H. (2009). Effect of Thrips tabaci on anatomical features. photosynthetic characteristics and chlorophyll fluorescence of Hypericum sampsonii leaves. Crop Protection, 28, 327–332.
Ding, H., Lamb, R. J., & Ames, N. (2000). Inducible production of phenolic acids in wheat and antibiotic resistance to Sitodiplosis mosellana (Diptera: Cecidomyiidae). Journal of Chemical Ecology, 26, 969–985.
Felton, G.W., Bi, J.L., Mathews, M.C., Murphy, J.B., Korth, K., Wesley, S.V., Lamb, C., Dixon, R.A., 1999. Cross-talk between the signal pathways for pathogen-induced systematic acquired resistance and grazing-induced insect resistance. In: Insect-plant interactions and induced plant defence, Novartis Foundation Symposium 223. Wiley, Chichester, UK, pp. 166±171
Felton, G. W., & Duffey, S. S. (1990). Inactivation of Baculovirus by quinones formed in insect damaged plant tissue. Journal of Chemical Ecology, 16, 1221–1236.
Golan, K., Rubinowska, K., & Kmiec, ´K., Kot, I., Go´rska-Drabik, E., Łagowska, B., Michałek, W. (2014). Impact of scale insect infestation on the content of photosynthetic pigments and chlorophyll fluorescence in two host plant species. Arth. Plant. Int., 9(1), 55–65.
Goławska, S., Krzyżanowski, R., & Łukasik, I. (2010). Relationship between infestation and chlorophyll content in Fabaceae species. Acta Biol Crac Ser Bot., 52, 76–80.
He, J., Chen, F., Chen, S., Lv, G., Deng, Y., Fang, W., Liu, Z., Guan, Z., & He, C. (2011). Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. Journal of Plant Physiology, 168, 687–693.
Heng-Moss, T. M., Ni, X., Macedo, T., Markwell, J. P., Baxendale, F. P., Quisenberry, S. S., & Tolmay, V. (2003). Comparison of chlorophyll and carotenoid concentrations among Russian wheat aphid (Homoptera: Aphididae)-infested wheat isolines. Journal of Economic Entomology, 96, 475–481.
Holden, M., 1965. Chlorophylls, p. 461–488. In T. W. Goodwin red.], Chemistry and biochemistry of plant pigments. Academic, New York
Huang, T. I., Reed, D. A., Perring, T. M., & Palumbo, J. C. (2014). Feeding damage by Bagrada hilaris (Hemiptera: Pentatomidae) and impact on growth and chlorophyll content of Brassicaceous plant species. Arthropod-Plant Interact., 8, 89–100.
Ibrahim, S., Mir, G. M., Rouf, A., War, A. R., & Hussain, B. (2018). Herbivore and phytohormone induced defensive response in kale against cabbage butterfly, Pieris brassicae Linn. J. Asia-Pac. Entomol., 21, 367–373.
Khattab, H. I. (2005). Responses of Eucalypt trees to the insect feeding (gall forming psyllid). International J. Agric. Biol., 7, 979–984.
Kovalikova, Z., Kubes, J., Skalicky, M., Kuchtickova, N., Maskova, L., Tuma, J., Vachova, P., & Hejnak, V. (2019). Changes in content of polyphenols and ascorbic acid in leaves of white cabbage after pest infestation. Molecules, 24, 2622.
Kumar, H., & Sharma, S. (2014). Determination of chlorophyll and carotenoid loss in Dalbergia sissoo caused by Aonidiella orientalis (Newstead) [Homoptera: Coccoidea: Diaspididae]. J. Entomol. Zool. Stud, 2, 104–106.
Lattanzio, V., Kroon, P. A., Quideau, S., & Treutter, D. (2009). Plant phenolics- secondary metabolites with diverse functions. Recent. Adv Polyphenol. Res., 1, 1–35.
Li, W., Hartung, J. S., & Levy, L. (2006). Quantitative real-time PCR for detection and identification of ‘Candidatus Liberibacter’ species associated with citrus huanglongbing. Journal of Microbiol Methods, 66, 104–115.
Miles, P. W., & Oertli, J. J. (1993). The significance of antioxidants in the aphid-plant interaction: The redox hypothesis. Entomologia Experimentalis Et Applicata, 67, 275–283.
Killiny, N., & NehelaOne Target, Y. (2017). Two Mechanisms: The Impact of “Candidatus Liberibacter asiaticus” and Its Vector. Diaphorina citri. on Citrus Leaf Pigments Mol. Plant-Microbe Interact., 30, 543–556.
Ni, X., Quisenberry, S. S., Markwell, J., Heng-Moss, T., Hgley, L., Baxendale, F., Sarath, G., & Klucas, R. (2001). In vitro enzymatic chlorophyll catabolism in wheat elicited by cereal aphid feeding. Entomologia Experimentalis Et Applicata, 101, 159–166.
Ni, X., Quisenberry, S. S., Heng-Moss, T., Markwell, J., Higley, L., Baxendale, F., Sarath, G., & Klucas, R. (2002). Dynamic change in photosynthetic pigments and chlorophyll degradation elicited by cereal aphid feeding. Entomologia Experimentalis Et Applicata, 105, 43–53.
Nissinen, A., Haapalainen, M., Jauhiainen, L., Lindman, M., & Pirhonen, M. (2014). Different symptoms in carrots caused by male and female carrot psyllid feeding and infection by ’Candidatus Liberibacter solanacearum’. Plant Pathology Journal, 63, 812–820.
Nissinen, A., Vanhala, P., Holopainen, J. K., & Tiilikkala, K. (2007). Short feeding period of carrot psyllid (Trioza apicalis) females at early growth stages of carrot reduces yield and causes leaf discolouration. Ent. Exp. Apl., 125, 277–283.
Nissinen, A., Lemmetty, A., Pihlava, J. M., Jauhiainen, L., Munyaneza, J. E., & Vanhala, P. (2012). Effects of carrot psyllid (Trioza apicalis) feeding on carrot yield and content of sugars and phenolic compounds. An.Appl. O Biologico, 161, 68–80.
Öncel, L., Ustun, A.S., Keles, Y., 1996. Proline accumulation in pepper (Capsicum annuum L.) resistant and susceptible to root rot Phytophthora capsici (Leon.). TURK. J. BOT.20, 489–495.
Plotto, A., Baldwin, E., McCollum, G., Manthey, J., Narciso, J., & Irey, M. (2010). Effect of Liberabacter infection (Huanglong or “Greening Disease”) of citrus on orange juice flavor quality by sensory evaluation. Journal of Food Science, 75, 220–230.
Secor, G. A., Rivera, V. V., Abad, J. A., Lee, I. M., Clover, G. R. G., Liefting, L. W., Li, X., & De Boer, S. H. (2009). Association of ‘Candidatus Liberibacter solanacearum’ with the zebra chip disease of potato established by graft and psyllid transmission. electron microscopy. and PCR. Plant Disease, 93, 574–583.
Sharma, H. C. (2008). Biotechnological Approaches for Pest Management and Ecological Sustainability (p. 526). CRC Press/Taylor & Francis.
Stevenson, P. C., Muyinza, H., Hall, D. R., Porter, E. A., Farman, D., Talwana, H., et al. (2009). Chemical basis for resistance in sweet potato Ipomoea batatas to the sweet potato weevil Cylas puncticollis. Pure and Applied Chemistry, 81, 141–151.
Talcott, S. T., & Howard, L. R. (1999). Chemical and sensory quality of processed carrot puree as influenced by stress induced phenolic compounds. Journal of Agriculture and Food Chemistry, 47, 1362–1366.
Teresani, G. R., Bertolini, E., Alfaro-Fernandez, A., Martinez, C., Tanaka, F. A., Kitajima, E. W., Rosello, M., Sanjuan, S., Ferrandiz, J. C., Lopez, M. M., Cambra, M., & Font, M. I. (2014). Association of ’Candidatus liberibacter solanacearum’ with a vegetative disorder of celery in Spain and development of a real-time pcr method for its detection. Journal of Phytopathology, 104, 804–811.
Usha Rani, P., & Jyothsna, Y. (2010). Biochemical and enzymatic changes in rice as a mechanism of defense. Acta Physiologiae Plantarum, 32, 695–701.
Wallis, C., Eyles, A., Chorbadjian, R., McSpadden-Gardner, B., Hansen, R., Cipollini, D., et al. (2008). Systemic induction of phloem secondary metabolism and its relationship to resistance to a canker pathogen in Austrian pine. New Phytologist, 177, 767–778.
War, A. R., Paulraj, M. G., War, M. Y., & Ignacimuthu, S. (2011). Herbivore- and elicitor-induced resistance in groundnut to Asian armyworm. Spodoptera litura (Fab.) (Lepidoptera: Noctuidae) Plant. Signal. Behav., 6, 1769–1777.
Zubair, M., Balal, R.M, Shahid, M.A., Khan, M.W., Aqueel, M.A., Mustafa, Z, Javaid, M., Sadiq, M.A., Liaqat, M.R., 2017. Nutritional Profile of Fairchild Fruit Infected by Sucking Insect-Pests of Citrus. J. Agric. Sci. 2518–4210.
Zubair, M., Balal, R. M., Aqueel, M. A., Shahid, M. A., Akhtar, G., Akram, A., Khan, M. W., & Sadiq, M. A. (2015). Nutritional Assessment of Kinnow Mandarin Fruit (Citrus reticulata Blanco), infected by Few Sucking Insect-Pests of Citrus. Pak J Nut., 14, 487–491.
Acknowledgements
We gratefully acknowledge the Tunisian Union of Agriculture and Fisheries (UTAP) for facilitating the fieldwork in the governorate of Kairouan.
The authors are grateful to Joshua Gearing for English editing that improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Othmen, S.B., Boussaa, F., Hajji-Hedfi, L. et al. Effects of nymphal density (Bactericera trigonica) and feeding on photosynthetic pigments, proline content and phenolic compounds in carrot plants. Eur J Plant Pathol 163, 51–59 (2022). https://doi.org/10.1007/s10658-021-02456-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02456-9