Abstract
Ascochyta blight epidemics have been observed in many countries since the early 1900s but studies on an interaction between the amount of inoculum, environmental factors and the spatiotemporal development of Ascochyta blight are rare due to the historic emphasis on developing resistant cultivars and chemical control of the disease. I used generalised linear mixed models to investigate key environmental factors affecting the spatiotemporal development of Ascochyta blight from primary infection foci. Briefly, four replicate plots (20 m × 20 m) of a susceptible chickpea cultivar were planted at two different locations (Billa Billa and Tosari) in Queensland, Australia. Four naturally infested stubble pieces were placed at the centre of each newly emerged chickpea plot 14 days after sowing. The number of infected plants was counted in 1 m2 observation quadrats at the distances of 3, 6 and 9 m in a concentric arrangement. The number of infected plants increased with each assessment date, approaching 100% plant infections at the time of final assessment. The rate of disease progress was significantly faster at Tosari. The rate of disease progress significantly decreased as the distance from the primary infection foci increased. There was a significant positive effect of an optimum temperature, increasing rainfall and omni-directional wind. The influence of wind speed was not significant. The finding that single infection foci were enough to spread disease across whole plots indicate that limited inoculum is not a barrier in the development of an epiphytotic under conducive conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ascochyta blight caused by Ascochyta rabiei (syn. Phoma rabiei) is a devastating disease that causes significant losses in chickpea production in almost all countries where chickpea is grown (Nene, 1982; Pande et al., 2005). Chickpea (Cicer arietinum) is the second most important legume crop globally, known for providing dietary fibre, iron, protein and vitamins (Wallace et al., 2016). Australia is the second largest chickpea producer in the world (FAOSTAT, 2018). Several diseases are known to affect chickpea production in Australia, but Ascochyta blight is the most serious (Khan, 1999). Ascochyta rabiei survives on infected seed, infested stubble and volunteer chickpea plants. The survival period on infested stubble is up to 2 years when infested stubble are at the soil surface, but the pathogen loses viability in up to 5 months when infested stubble are 5–40 cm deep in soil (Kaiser, 1973). Infested stubble and volunteer chickpea plants serve as the main source of inoculum for disease initiation, as 100% control of seed borne Ascochyta blight infections can be achieved via chemical treatments (Kevin Moore, personal communication). Very low levels of infested stubble, serving as a primary inoculum, can cause serious damage in chickpea (Kaiser, 1992). Disease transmission from infected seed is non-systemic, i.e., contact must be made between emerging growing points and lesions on seed coat or cotyledon (Dey & Singh, 1994).
Conidia are the only spore type involved in the disease cycle in Australia (Bar et al., 2020; Leo et al., 2016), therefore both primary and secondary infections can be attributed to conidial infections. Conidia have been known to primarily dispersed by rain splash to nearby plants (Kimber, 2002). Primary infections resulting from splash dispersed conidia typically result in distinct foci randomly scattered in the field (Kimber et al., 2007). Further infections and disease development are favoured by temperatures 5–30 °C (optimum 20 °C), relative humidity >95% (Nene, 1982), and wetness period of 10 h or more (Khan, 1999). Rain is the most important factor for an epiphytotic, as the impact energy of rain is required to disperse conidia from pycnidia, dew alone will not cause a significant spread (Moore et al., 2016). There is no resistant variety against Ascochyta blight in Australia, all varieties are susceptible to moderately susceptible (https://www.nvtonline.com.au/).
Ascochyta blight epidemics have been observed in many countries since the early 1900s. An example is a severe Ascochyta blight epidemic across south-eastern Australia in 1992 (Khan, 1999), where the chickpea industry has not yet fully recovered. Historically, much emphasis has been placed on developing resistant cultivars and chemical control of Ascochyta blight, but there is limited information on when, where and why epidemics occur. That is, gaps exist in understanding of the likely interaction between weather variables, the amount of inoculum, and the host-pathogen interactions leading to epidemics (Pande et al., 2005). In addition, much attention has been given to the sexual spores (ascospores) of A. rabiei due to its long distance (hundreds of metres to kilometres) spread by wind (Kaiser & Küsmenoglu, 1997; Trapero-Casas & Kaiser, 1992). Conidia have received little attention due to the presumed assumption that conidia spread over short distances (~1 m) via rain splash (Kimber, 2002) and up to 10 m in the presence of strong wind and rain (Kimber et al., 2007). However, recent findings have shown that the role of conidia in the epidemiology of A. rabiei has been seriously underestimated, and that conidia spread over much longer distances, i.e., at least 70 m (Khaliq et al., 2020a).
Quantitative information on the spatiotemporal development from primary infection foci, and determining a relationship between the amount of initial inoculum and subsequent epiphytotic is crucial in understanding of epidemics (Kimber et al., 2007). However, fungal populations are difficult to quantify under field conditions, as visual assessment of the percent plant area covered by lesions can be quite subjective, and hence may not be a very reliable method of inoculum assessment (Van Maanen & Xu, 2003). The number of fungal colonies or pustules can be quantified accurately under a microscope on a small scale, but this is impractical on a large scale under field conditions (Van Maanen & Xu, 2003). Calculating disease incidence (percentage of plants with disease symptoms) is an excellent method of assessing fungal populations under field conditions (Madden & Hughes, 1995; Xu & Ridout, 1998), but it maybe impractical in large scale field studies. An alternative could be counting the number of infected plants in observation quadrats. Calculating spatial and temporal disease progress in relation to weather factors is of fundamental importance in epidemiology for understanding spatiotemporal dynamics of a disease and for evaluating disease treatments (Xu & Ridout, 1998). The number of infected plants is plotted against time to give a disease progress curve (rate), which is used to compare disease outbreaks at different times, at different locations or under different disease management practices (Madden & Hughes, 1995; Xu & Ridout, 1998). Disease progress rates are also interpreted in relation to biological processes, such as sporulation and infection, under different environmental conditions with the aim of identifying similarities and differences between individual disease epidemics; and thus identifying key factors responsible for epidemics (i.e., comparative epidemiology) (Xu & Ridout, 1998).
Disease progress in space and time is a function of an interaction among host, pathogen and the environment (Madden et al., 2007). Determining the relationship between environmental factors and disease development is therefore the key component (often the only component) of disease forecasting systems (Van Maanen & Xu, 2003). Despite infested stubble being the primary source of disease initiation, and the fact that a very small amount of infested stubble can lead to severe yield losses, little information exists on the effect of different environmental factors on the spatiotemporal development of Ascochyta blight from infested stubble under field conditions. Studies on the spatiotemporal development of Ascochyta blight from primary infection foci are rare (Coventry, 2012; Kimber, 2002), and those studies mainly focussed on the distance disease spread from primary foci, rather than understanding an interaction between environmental factors and disease progress in space and time.
To address this key gap in the epidemiology of Ascochyta blight, the present study was conducted to determine the spatiotemporal development of Ascochyta blight from primary infection foci , and to investigate the key environmental factors playing a role in disease progress in space and time. Generalised linear mixed models (GLMMs) were used to describe the effect of environmental factors and location on disease progress in space and time. Since rain is essential to spread splash dispersed pathogens spores, it is hypothesized that the spatiotemporal development of Ascochyta blight will have a linear positive relationship with rain. It is also hypothesized that single infection foci will be enough to spread disease across whole plots under conducive environmental conditions.
Methods
Study area and field sites management
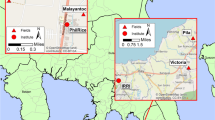
The spatiotemporal spread of Ascochyta blight from primary infection foci was determined in a susceptible chickpea cultivar (Kyabra) at two different locations: Billa Billa (−28.1011505, 150.3307084) and Tosari (−27.856248, 151.447391), Queensland, Australia. The trial site at both locations was surrounded by wheat crop and neither had chickpea planted on them for at least four years. Additionally, no disease had been observed in Queensland for the last three years (2017–2019) due to a severe drought in the region. PreDicta®B tests were conducted to ensure both trial sites were free of A. rabiei inoculum. Briefly, a bulk soil and surface residues sample (6 kg comprised of 12 × 500 g soil to a depth of 10–20 cm and surface residue) was collected from four replicate plots (3 × 500 g soil and surface residue per replicate plot from three different locations) at each location. The bulk samples were mixed thoroughly, and a 500 g representative sub-sample for each location was sent to the South Australian Research and Development Institute (SARDI) for a PreDicta®B test to investigate the presence of A. rabiei inoculum. No A. rabiei DNA was detected in PreDicta®B test, confirming trial sites were free of A. rabiei inoculum.
The trial at Billa Billa was planted on 4 June 2020 and the one at Tosari was planted on 27 June 2020. At each location, four replicate plots of Kyabra (disease-free seed treated with a Thiram based dressing) were sown in a 20 m × 20 m plot using a 25 cm row spacing and a seeding rate of 40 seeds per m2. There was a 4.5 m gap between each replicate plot, which was sown to wheat buffer to prevent disease spread among chickpea plots. Additionally, a 4.5 m wide buffer of wheat was sown around all four replicate plots to serve as a barrier.
The trials were sown and managed by Queensland Department of Agriculture and Fisheries (https://www.daf.qld.gov.au/). No fungicides were applied during the trial. Agronomic practices, such as fertilization, application of herbicides and rhizobium inoculation for root nodulation were applied as recommended to chickpea growers.
Determination of spatiotemporal dynamics
Four naturally infested stubble pieces (each stubble piece was 6–8 cm in length with approximately 80% of area covered by characteristic Ascochyta blight lesions) were placed, in a vertical fashion, at the centre of each newly emerged chickpea plots approximately 14 days after sowing and the locations were marked with coloured flags. Infested stubble pieces were sourced in the previous year from volunteer chickpea plants in a wheat paddock located in New South Wales. The total number of plants in each observation quadrat was counted when placing inoculum to get an estimate of the total number of plants present in each observation quadrat. The spatiotemporal development from primary infection foci was then investigated in selected 1 m2 observation quadrats by visual inspection for the presence of characteristics Ascochyta blight lesions on individual seedlings.
To investigate the spatiotemporal development of Ascochyta blight, each plot was subdivided into a total of 24 observation quadrats at the distances of 3, 6 and 9 m (there were 8 observation quadrats at each distance), marked out at each corner with white pin markers, in a concentric arrangement around the primary infection foci (Fig. 1). The quadrats were labelled as shown in Fig. 1. The number of infected plants were counted in each quadrat at fortnightly intervals (the final assessment at Tosari was conducted a week apart because the disease had spread across whole plots) from the date infested stubble were introduced into the plots (Table S1). The number of infected plants was first counted in the central quadrat ‘F’ (corresponding to 0 m distance for all directions), and then at the distances of 9, 6, and 3 m from the primary infection foci in the directions N, NE, E, SE, S, SW, W and NW, respectively (Fig. 1). Disease assessments were conducted in a structured way, beginning at the perimeter and moving towards the central quadrat ‘F’ to reduce the chances of mechanical spread of the inoculum. The number of infected plants observed on each assessment date gave an estimate of disease progress in time, and the distance (in metres) disease spread from the primary infection foci gave an estimate of disease progress in space. At the end of the experiment, whole plots were visually inspected for the presence of characteristics Ascochyta blight lesions.
Quadrats selected (represented by shaded cells) to investigate the spatiotemporal development of Ascochyta blight in all directions of the inoculated central quadrat (F). The number of infected plants were counted at the distances of 3, 6 and 9 m, in a concentric arrangement, around the inoculated central quadrat at fortnightly intervals
Boots were sprayed with 70% ethanol at the start and end of each disease assessment. Extreme care was taken not to step inside the observation quadrats. For the entire duration of the experiment, meteorological data were recorded by automated weather stations (with GoanaAg, https://goapp.goannatelemetry.com.au/#components/weatherstationnowview/view.html?LocationID=13996 at Tosari; and with OZ forecast, https://ozforecast.com.au/cgi-bin/weatherstation.cgi?station=11324 at Billa Billa). The weather stations were located directly beside the experimental plots at both locations. The data collected were comprised of date and time, daily average air temperature (°C), total daily rain (mm), daily average relative humidity (%), daily average wind speed (m s−1) and daily average wind direction (°).
Statistical analyses
The influence of total rain, distance from the primary infection foci, average air temperature, average relative humidity, average wind direction, average wind speed, and location on the spatiotemporal development of A. rabiei was investigated by fitting generalised linear mixed models using the `glmmTMB` function of the ‘glmmTMB’ package (Brooks et al., 2017) in R programming language (Core Team, 2021).
A quasipoisson family was used because preliminary analyses showed the data to highly overdispersed due to the absence of disease in the sampled quadrats at the beginning of the season (especially at the increasing distances from primary infection foci). The overdispersion was tested using Bolker’s custom function ‘overdisp_fun` (Bolker, 2021). The logarithm of the observation quadrat was included as an offset term (offset are used in GLMMs to account for variation in plot sizes or exposure times) indicating the number of infected plants in each observation quadrat were proportional to the total number of plants present in that quadrat, which also allowed to model the rate of disease progress. Since disease progress was quantified as a time series, different assessment dates were included as a random effect to account for temporal autocorrelation and the lack of biological independence (i.e., the number of infected plants at a later assessment date depended on the number of infected present at the former assessment date).
The best fitted model was selected using Akaike’s Information Criterion (AIC) (Akaike, 1974).
Data were processed and visualised in the R programming language using the contributed packages ‘tidyverse’ (Wickham et al., 2019), ‘lubridate’ (Grolemund & Wickham, 2011) and ‘clifro’ (Seers & Shears, 2015). Mean wind speed was calculated using a ‘circular.averaging’ function from ‘SDMTools’ (VanDerWal et al., 2014).
Results
Weather conditions over the growing season
At Billa Billa, a total of 86.6 mm rain was received from the date of inoculation until the date of final assessment, i.e., 17 June 2020–27 October 2020. Daily average temperature during each assessment date (assessment number) ranged from 10.5–21.7 °C, daily average wind speed ranged from 1.5–2.8 m/s and daily average relative humidity ranged from 39.1–85.9% (Table 1).
At Tosari, a total of 132.2 mm rain was received from the date of inoculation until the date of final assessment, i.e., 15 July 2020–5 November 2020. Daily average temperature during each assessment date ranged from 11.8–21.3 °C, daily average wind speed ranged from 2.5–3.5 m/s and daily average relative humidity ranged from 55.1–68.6% (Table 1).
Spatiotemporal dynamics
At Billa Billa, Ascochyta blight symptoms were observed on two seedlings 29 days after inoculation, in the inoculated central quadrat, in one of the four replicated plots. The number of infected plants and the distance disease spread increased gradually in patches closer to the inoculated central quadrat with each assessment date (assessment number) until the end of the growing season when heavy rains rapidly spread disease uniformly across whole plots leading to 100% plant infections (i.e., all plants in the plots were infected) (Fig. 2). Disease spread uniformly across whole plots by assessment 10. Wind was blowing in all directions (Fig. S1); however, the predominant daily average wind direction over the entire growing season was towards the SW (i.e., wind was mostly coming from the NE) (Fig. 3).
Wind roses showing daily average wind speed and direction over the chickpea growing season. The notation ‘[]’ represents a ‘closed interval’ indicating an interval is inclusive of both lower and upper values, whereas the notation ‘(]’ represent ‘half open interval’ indicating an interval is exclusive of the lower value but inclusive of the upper value
At Tosari, Ascochyta blight symptoms were observed 15 days after inoculation on 16 seedlings in three of the four replicated plots (symptoms were observed in the inoculated central quadrats only). Similar to the Billa Billa trial site, the number of infected plants and the distance disease spread increased in patches, closer to the inoculated central quadrat, with each assessment date until the end of the growing season when heavy rains spread disease uniformly across whole plot leading to 100% plant infections (Fig. 2). Disease spread uniformly across whole plots by assessment 8. The rate of disease progress was significantly (p < 0.001) faster at Tosari where a total of 113 days were taken to reach 100% plant infections across all four replicated plots compared to that of Billa Billa where 132 days were taken to reach 100% plant infection across all four replicated plots. In contrast to Billa Billa, wind was not blowing in all directions (Fig. S1). Wind was mostly blowing from E, SE, SW, W directions (Fig. 3).
The generalised linear mixed model fitted to determine the influence of distance from the inoculated central quadrat on disease progress in time and space was significant (p < 0.001), with a significant increase in disease progress rate closer to the inoculated central quadrats compared to the outer quadrats towards the plot edges (Fig. 4).
Influence of environmental factors on spatiotemporal dynamics
The increasing amount of rain (p < 0.001) and the tendency of the wind to blow in all directions (p < 0.01) had a significant positive effect on the spatial and temporal disease progress of Ascochyta blight (Fig. 4). Similarly, the influence of average temperature on the spatial and temporal disease progress was significant (p < 0.001), with a positive effect of optimum temperature (20 °C ± 2 °C) most of the of the growing season (Table 1). Average wind speed (p < 0.05) had not significant effect on the spatial and temporal disease progress. The effect of average relative humidity alone was not significant (p > 0.05), but there was a significant (p < 0.05) negative effect of an interaction between average relative humidity and wind direction. That is, higher disease progress rates were observed when wind tend to blow in all directions and relative humidity was low (Fig. 4).
The best fitted model had a deviance explained value of 5974, AIC value of 5994 and a BIC value of 6049.
Discussion
This study successfully described the spatiotemporal development of Ascochyta blight from primary infection foci at two different locations and improved our understanding on how environmental factors affect the spatiotemporal development of the disease. Hypotheses proposed were supported by findings. That is, a single infection focus was enough to spread disease across whole plot. Of the different weather variables affecting the spatiotemporal development of Ascochyta blight, rain had the most prominent effect.
Planting trials on different dates at two different locations allowed a reasonable comparison of the effect of environmental factors and location on the spatiotemporal development of Ascochyta blight. At both locations, the number of infected plants increased with each assessment date approaching 100% plant infections at the time of final assessment. However, the rate of disease progress was significantly faster at Tosari where it took 113 days to reach 100% plant infections compared to that of Billa Billa where it took 132 days to reach 100% plant infections. Average temperature, average relative humidity and average wind speed values were very similar at both locations. The rapid disease progress at Tosari is likely due to different amount, frequency and distribution of rain events between the two locations. At Tosari, 70.4 mm rain occurred within 45 days of inoculation with a total of 132.2 mm rain from the date of inoculation until 100% plant infections were observed, whereas only 7.8 mm rain occurred within 45 days of inoculation at Billa Billa with a total of 86 mm rain from the date of inoculation until 100% plant infections were observed. Rain early in the season at Tosari led to greater inoculum production and subsequent spread from the beginning of the season, resulting in a relatively rapid disease progress. This finding indicates that rain has a major influence on the spatiotemporal development of Ascochyta blight, and is consistent with the findings from the previous research showing that rain is the major factor for an epiphytotic (Coventry, 2012; Kimber et al., 2007). It has been reported that a monthly rain of at least 40 mm and an average monthly temperature of at least 8 °C are required to initiate Ascochyta blight epidemic (Ketelaer et al., 1988). My results are fully consistent with these studies as disease was largely sporadic most of the growing season, but heavy rains later in the season (rain events during assessment 9–10 at Billa Billa and assessment 8 at Tosari) uniformly spread disease across whole plots at both locations.
The influence of average wind speed on the spatial and temporal disease progress was not significant. The non-significant effect of average wind speed on the distance Ascochyta blight conidia travel from the source of infection has been established (Khaliq et al., 2020b). This study suggests that wind speed might have a little influence on within paddock disease spread too. The non-significant effect of wind speed can be attributed the absence of the sexual spores (ascospores) of A. rabiei in Australia, which are primarily dispersed by wind up to hundreds of metres to kilometres (Kaiser & Küsmenoglu, 1997; Trapero-Casas & Kaiser, 1992). Only asexual spores (conidia) are found in Australia. The asexual spores are primarily spread by a rain splash (Khan, 1999). The rate of spatial and temporal disease progress significantly increased as the average temperature values increased from 10.5 to 21.7 °C. This is not surprising as Ascochyta blight development is favoured by temperature range between 5 and 30 °C, with optimum temperature being 20 °C (Khan, 1999). Average relative humidity had no significant effect on the spatial and temporal disease progress. This can be explained by the fact that average relative humidity values were below optimum in the current study (Table 1). The optimum humidity for Ascochyta blight development is over 95% (Nene, 1982). Ascochyta blight development on infested stubble was found to be very low at lower relative humidity (e.g., 86%) irrespective of temperature (Navas-Cortés et al., 1998). Moreover, A. rabiei requires the impact energy of rainfall to disperse conidia from pycnidia, highly relative humidity or heavy dews will not cause a significant spread (Moore et al., 2016).
The spatial and temporal disease progress increased as wind tended to blow in all directions, which suggest that splash dispersed conidia tend to get uniformly distributed by omni-directional wind. The directional effect of wind has been reported in the previous studies, where disease was more severe in the downwind direction of the primary infection foci (Coventry, 2012; Kimber et al., 2007). The model failed to converge when the effect of quadrat direction was included in the model (suggesting that there is not enough data to explain the variation). Based on visual observations, disease was more severe in quadrats located in the downwind directions (E, N, NE) at Tosari (wind was mostly blowing from E, SE, SW, W). Conversely, omni-directional wind at Billa Billa resulted in rather uniform disease spread patterns and there was no obvious disease spread in a particular direction (based on visual assessment). It should be noted that the downwind spread patterns were more pronounced early in the season when there was limited inoculum and rain. With the onset of heavy rain towards the end of the season, the directional spread was not pronounced, and disease spread uniformly across whole plots.
There was a significant negative effect of an interaction between relative humidity and wind direction on the spatial and temporal disease progress. That is, higher disease progress rates were observed when wind tended to blow in all directions and relative humidity was low. However, the negative interaction was weakly significant (p < 0.05). Disease progress rates were unlikely to have been affected by this negative interaction because the effect of relative humidity alone was not significant. There was a moderate positive correlation (R = 0.5) between wind direction and relative humidity, so wind direction probably favoured relative humidity by moving water vapour around (this study doesn’t provide an evidence of this, as correlation does not mean causation). It is plausible that disease progress rates were higher when wind omni-directional and other factors, especially rain as there was a very weak correlation (R = 0.28) between rain and relative humidity, favoured disease development. It just happened that relative humidity was low when wind was omni-directional and other factors favoured disease development.
At both locations, the rate of spatial and temporal disease progress significantly decreased as the distance from the primary infection foci increased. Such successive Ascochyta blight spread, i.e., first close to the foci then progressing across whole plot over time has long been known as patches of disease (Cubero, 1984). This is expected of splash dispersed pathogens, as plants close to primary infection foci are more exposed to inoculum load due to localised splash dispersal and thus become infected quickly (Coventry, 2012). Briefly, pycnidia imbibe moisture during rain events and hydrostatic pressure forces conidia in pycnidia to exude through the ostiole in a cirrhus. When raindrops strike the cirrhus, the kinetic energy breaks up the spore mass and spread conidia to nearby healthy plants (Coventry, 2012; Kaiser, 1992; Nene & Reddy, 1987). The detached conidia can also be ‘washed off’ from upper leaves to lower leaves in a wash off effect or transported across a field by wind-driven rain (Coventry, 2012). Similar dispersal patterns have been reported for other splash dispersed pathogens, such as Colletotrichum acutatum (Yang et al., 1990), Parastagonospora nodorum (previously known as Septoria nodorum) (Griffiths & Ao, 1976) and Ascochyta fabae (Pedersen et al., 1994).
Although Ascochyta blight spread was patchy most of the growing season, heavy rains towards the end of the season uniformly spread disease from one quadrat to the next in a continuous fashion. The uniform disease spread towards the end of the season was partly facilitated by the thinner canopy allowing conidia to land farther from the source of infection. Disease spread early in the season is primarily due to splash dispersal until the plant canopy becomes too dense (especially at mid-season) and forms a physical barrier to slow down splash dispersal (Coventry, 2012). Along with splash dispersal, disease spread later in the season is further facilitated by wind-driven rain when canopy is thinner and inoculum is plentiful (Coventry, 2012). Chickpea plant resistance against Ascochyta blight decreases with crop maturity (Nene & Reddy, 1987), which may also have contributed to rapid and uniform disease spread towards the end of the growing season. Moreover, Ascochyta blight infected tissues have the tendency to break off and fall on the ground. These infected tissues are spread to uninfected areas of the plot by wind, which quickly become infected after a rain event and result in multiple disease foci that merge as the disease progresses. The uniform and rapid spread is further facilitated by the short life cycle of A. rabiei with symptoms appearing within 5–6 days after infection in cool and moist conditions (Khan, 1999). The rapid production and spread of spores from infected to healthy plants is the primary driver of epidemics in polycyclic diseases (Madden et al., 2007). The uniform and rapid disease spread pattern is especially pronounced in susceptible chickpea cultivars (Coventry, 2012), but moderate resistance to Ascochyta blight can be overcome when chickpea plants are exposed to intermediate to high disease pressure under conducive (cool and wet) weather conditions (Chongo et al., 2003; Coventry, 2012).
Study locations in the present study experienced severe drought for almost three years preceding our trial with no disease being observed over the drought period. Nonetheless, the onset of rains spread disease across whole plots from single infection foci, which supports the findings from the previous studies that a very limited amount of primary infection (less than 1%) can have devastating effects on susceptible cultivars under cool and wet conditions (Kaiser, 1992). The confirmation that rain is the key driver of Ascochyta blight epidemics has led to the practice of delaying sowing dates, in order to reduce the crop exposure to the number of rain events to decrease losses from Ascochyta blight, in many chickpea growing areas (Pande et al., 2005). However, plots in a relatively high rainfall area (Tosari) were sown 23 days later than those sown at a relatively low rainfall area (Billa Billa). It took 113 days to reach 100% plant infections at Tosari compared to that of Billa Billa where it took 132 days to reach 100% plant infections. This indicates that delaying sowing dates alone will be not be enough to reduce yield losses, especially when limited inoculum is not a constraint to disease spread across whole plots, therefore fungicide applications will be necessary. Strategies (e.g., crop rotation) to reduce initial inoculum will be essential to reduce disease severity. A study at a larger scale, including replicating trials in more diverse environments with cultivars of variable resistance, and relating these factors to yield losses, will further increase our understanding of the main processes involved in the spatial and temporal progress of the disease.
References
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 716–723.
Bar, I., Sambasivam, P. T., Davidson, J., Caceres, L. M. F., Lee, R. C., Hobson, K., et al. (2020). Current population structure and pathogenicity patterns of Ascochyta rabiei in Australia. bioRxiv. https://doi.org/10.1101/2020.12.21.423875.
Brooks, M. E., Kristensen, K., Van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal, 9(2), 378–400.
Chongo, G., Buchwaldt, L., Gossen, B., Lafond, G., May, W., Johnson, E., et al. (2003). Foliar fungicides to manage Ascochyta blight [Ascochyta rabiei] of chickpea in Canada. Canadian Journal of Plant Pathology, 25(2), 135–142.
Coventry, S. A. (2012). Factors affecting short and long distance dispersal of fungal pathogens: Chickpea Ascochyta blight as a model. PhD dissertation. The University of Adelaide, Australia.
Cubero, J. (1984). Ascochyta blight of chickpeas in Spain. In M. C. Saxena & K. B. Singh (Eds.), Ascochyta blight and winter sowing of chickpeas (pp. 273–281). Martinus Nijhoff/Dr. W. Junk Publishers.
Dey, S., & Singh, G. (1994). Seedborne infection of Ascochyta rabiei in chickpea and its transmission to aerial plant parts. Phytoparasitica, 22(1), 31–37.
FAOSTAT (2018). FAOSTAT database. Available at http://www.fao.org/faostat/en/-data/QC.
Gideon, S. (1978). Estimating the dimension of a model. The Annals of Statistics, 6(2), 461–464.
Griffiths, E., & Ao, H. C. (1976). Dispersal of Septoria nodorum spores and spread of glume blotch of wheat in the field. Transactions of the British Mycological Society, 67(3), 413–418.
Grolemund, G., & Wickham, H. (2011). Dates and times made easy with lubridate. Journal of Statistical Software, 40(3), 1–25.
Kaiser, W. (1973). Factors affecting growth, sporulation, pathogenicity, and survival of Ascochyta rabiei. Mycologia, 65(2), 444–457.
Kaiser, W. (1992). Epidemiology of Ascochyta rabiei. In K. Singh & M. Saxena (Eds.), Disease resistance breeding in chickpea (pp. 117–134). ICARDA.
Kaiser, W., & Küsmenoglu, I. (1997). Distribution of mating types and the teleomorph of Ascochyta rabiei on chickpea in Turkey. Plant Disease, 81(11), 1284–1287.
Ketelaer, E., Diekmann, M., & Weltzien, H. (1988). International spread of Ascochyta rabiei in chickpea seeds-an attempt at prognosis. International Chickpea Newsletter(18), 21-23.
Khaliq, I., Fanning, J., Melloy, P., Galloway, J., Moore, K., Burrell, D., & Sparks, A. H. (2020a). The role of conidia in the dispersal of Ascochyta rabiei. European Journal of Plant Pathology, 158, 911–924.
Khaliq, I., Fanning, J., Melloy, P., Galloway, J., Moore, K., Burrell, D., & Sparks, A. H. (2020b). The role of conidia in the dispersal of Ascochyta rabiei. European Journal of Plant Pathology, 158(4), 911–924.
Khan, M. S. A. (1999). Epidemiology of Ascochyta blight of chickpea in Australia. PhD dissertation. The University of Adelaide, Australia.
Kimber, R. (2002). Survival and transmission of Ascochyta rabiei (Ascochyta blight) of chickpea. Honours thesis, University of Adelaide, Australia.,
Kimber, R., Shtienberg, D., Ramsey, M., & Scott, E. (2007). The role of seedling infection in epiphytotics of Ascochyta blight on chickpea. European Journal of Plant Pathology, 117(2), 141–152.
Leo, A. E., Linde, C. C., & Ford, R. (2016). Defence gene expression profiling to Ascochyta rabiei aggressiveness in chickpea. Theoretical and Applied Genetics, 129(7), 1333–1345.
Madden, L., & Hughes, G. (1995). Plant disease incidence: Distributions, heterogeneity, and temporal analysis. Annual Review of Phytopathology, 33(1), 529–564.
Madden, L. V., Hughes, G., & Van Den Bosch, F. (2007). The study of plant disease epidemics. St. American Phytopathological Society.
Moore, K., Hobson, K., Sambasivam, P., Ford, R., Harden, S., Nash, P., et al. (2016). Chickpea Ascochyta - is the pathogen changing and what are the implications for management. Available at https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2015/02/chickpea-ascochyta-is-the-pathogen-changing-and-what-are-the-implications-for-management.
Navas-Cortés, J., Trapero-Casas, A., & Jiménez-Díaz, R. (1998). Influence of relative humidity and temperature on development of Didymella rabiei on chickpea debris. Plant Pathology, 47(1), 57–66.
Nene, Y. (1982). A review of Ascochyta blight of chickpea. International Journal of Pest Management, 28(1), 61–70.
Nene, Y., & Reddy, M. (1987). Chickpea diseases and their control. In M. Saxena & R. Singh (Eds.), The chickpea (pp. 233–270). CAB International.
Pande, S., Siddique, K., Kishore, G., Bayaa, B., Gaur, P., Gowda, C., et al. (2005). Ascochyta blight of chickpea (Cicer arietinum L.): A review of biology, pathogenicity, and disease management. Australian Journal of Agricultural Research, 56(4), 317–332.
Pedersen, E., Morrall, R., McCartney, H., & Fitt, B. D. (1994). Dispersal of conidia of Ascochyta fabae f. sp. lentis from infected lentil plants by simulated wind and rain. Plant Pathology, 43(1), 50–55.
Core Team, R. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
Seers, B. M., & Shears, N. T. (2015). New Zealand’s climate data in R—An introduction to clifro. The University of Auckland http://stattech.wordpress.fos.auckland.ac.nz/2015-02-new-zealands-climate-data-in-r-an-introduction-to-clifro/.
Trapero-Casas, A., & Kaiser, W. (1992). Development of Didymella rabiei, the teleomorph of Ascochyta rabiei, on chickpea straw. Phytopathology, 82(11), 1261–1266.
Van Maanen, A., & Xu, X.-M. (2003). Modelling plant disease epidemics. European Journal of Plant Pathology, 109(7), 669–682.
Wallace, T. C., Murray, R., & Zelman, K. M. (2016). The nutritional value and health benefits of chickpeas and hummus. Nutrients, 8(12), 766.
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L., François, R., Grolemund, G., Hayes, A., Henry, L., Hester, J., Kuhn, M., Pedersen, T., Miller, E., Bache, S., Müller, K., Ooms, J., Robinson, D., Seidel, D., Spinu, V., Takahashi, K., Vaughan, D., Wilke, C., Woo, K., & Yutani, H. (2019). Welcome to the Tidyverse. Journal of Open Source Software, 4(43), 1686.
Xu, X.-M., & Ridout, M. (1998). Effects of initial epidemic conditions, sporulation rate, and spore dispersal gradient on the spatio-temporal dynamics of plant disease epidemics. Phytopathology, 88(10), 1000–1012.
Yang, X., Madden, L., Wilson, L., & Ellis, M. (1990). Effects of surface topography and rain intensity on splash dispersal of Colletotrichum acutatum. Phytopathology, 80(10), 1115–1120.
Bolker B (2021) GLMM FAQ. Available at https://bbolker.github.io/mixedmodelsmisc/glmmFAQ.html.
Vanderwal J, Falconi L, Januchowski S, Shoo L, Storlie C (2014) Species Distribution Modelling Tools, Tools for processingdata associated with species distribution modelling exercises. CRAN. R-project. org/package= SDMTools
Acknowledgments
This research was supported by Grains Research and Development Corporation (GRDC) through the project USQ1903-003-RTX. The application for the Postdoctoral Fellowship was put together by Adam H. Sparks (Department of Primary Industries and Regional Development) and Ihsanul Khaliq, which was supported by the national modelling project (Disease epidemiology and management tools for Australian grain growers-DAW1810-007RTX) national leader Jean Galloway and the project DSS leader Art Diggle (Department of Primary Industries and Regional Development) along with a valuable feedback on the grant application. I wish to acknowledge Queensland Department of Agriculture and Fisheries (QDAF) and MCA Agronomy Pty Ltd. staff for the trials planting and maintenance. I wish to thank Adam H. Sparks, Jenny Davidson (South Australian Research and Development Institute), Kevin Moore (NSW Department of Primary Industries) and Joshua Fanning (Agriculture Victoria) for their technical input on the experimental design. I also thank Andriamahery Rasolofoharivelo (University of Southern Queensland) and Andrew Erbacher (Queensland Department of Agriculture and Fisheries) for technical support.
Data availability and code availability
All raw and generated data used in the statistical analyses and data visualisation have been made available as a part of a research compendium for reproducibility and FAIR data policy. Please see the fully reproducible and version-controlled code at https://github.com/IhsanKhaliq/spatiotemporaldynamics. The archived code and data are available at https://doi.org/10.5281/zenodo.4563709
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study did not involve working with animals or humans.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
None to disclose.
Supplementary Information
ESM 1
(DOCX 260 kb)
Rights and permissions
About this article
Cite this article
Khaliq, I. Spatiotemporal development of Ascochyta blight in chickpea from primary infection foci: insights from plant, pathogen and the environment interactions to inform an epidemic risk. Eur J Plant Pathol 161, 331–342 (2021). https://doi.org/10.1007/s10658-021-02324-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02324-6