Abstract
Foliar anthracnose is one of the main diseases of onion (Allium cepa L.) under tropical and subtropical conditions. Thus far, only Colletotrichum gloeosporioides has been reported as the causal agent of this disease in Brazil. However, there are no extensive studies characterizing Colletotrichum isolates associated with onion anthracnose in the country. Here, 38 Colletotrichum isolates obtained from onion plants displaying foliar anthracnose across major Brazilian onion-producing regions were characterized using morphometric and molecular information. The Bayesian and Maximum Liklihood methods were used for an initial analysis of the β-tubulin gene (tub2) sequences of all isolates, resulting in the discrimination of nine haplotypes. Three haplotypes grouped with the reference species of the C. acutatum complex and six with the C. gloeosporioides complex. Sequences of either the glyceraldehyde-3-phosphate dehydrogenase (gapdh), actin (act), and calmodulin genes or the intergenic spacer (IGS) region between DNA lyase (apn2) gene and the mating-type mat1–2-1 locus were used to characterize a subset of isolates representing these nine distinct tub2 gene haplotypes. These analyses revealed five anthracnose-inducing Colletotrichum species, including three members of the C. acutatum species complex (C. nymphaeae, C. scovillei, and C. tamarilloi) and two of the C. gloeosporioides species complex (C. fructicola and C. theobromicola). Bioassays confirmed that all these Colletotrichum species are pathogenic to onion, inducing typical anthracnose symptoms on bulbs and leaves. Twenty-six out of 38 isolates were identified as C. theobromicola, indicating this fungus as the prevalent anthracnose pathogen under Brazilian conditions. This Colletotrichum species diversity will affect anthracnose management strategies, including chemical and cultural control as well as the identification and deployment of onion cultivars with species-specific and/or wide-spectrum tolerance/resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foliar anthracnose is one of the major diseases of onions (Allium cepa L.) in tropical and subtropical regions (Maranhão et al. 1997; Massola Jr et al. 2016). This disease induces significant yield and quality losses for both bulb and commercial onion seed production. Thus far, only Colletotrichum gloeosporioides has been reported as the causal agent of this disease in Brazil (Bajungu 1979; Wordell Filho et al. 2006; Vila Nova et al. 2011). The expression of anthracnose symptoms is diverse and highly complex, varying according to the onion cultivar, phenological stage and organ of the plant. In some cases, well-defined, depressed lesions occur on the blades and sheaths of the leaves. These lesions can increase in size and become oval with a predominant pinkish color. The symptoms may evolve to punctuated spots of dark coloration. Damping-off of seedlings can be observed when highly infested seeds are used (Wordell Filho et al. 2006). In some special circumstances, leaf twisting symptoms can be observed giving a serpentine-like aspect to the leaves (Hill 1996). Typical bulb deformations associated with onion anthracnose have been named by Brazilian growers as either “cigar” or “hot dog”, which expression may involve mixed infections with distinct root pathogens (Wordell Filho et al. 2006; Massola Jr et al. 2016). As the disease progresses, dark green to black stroma may develop on the bulbs.
The classical taxonomic approach of Colletotrichum species is based exclusively upon descriptive morphometrical criteria such as conidial shape and size, conidia germination, presence/absence of setae, aspect of the colonies as well as the host range and the type of lesion present in the affected host tissues (Sutton 1980; Cannon et al. 2012). Because morphometric and biological attributes are very often variable and plastic, fungal characterization based solely upon these criteria are not reliable, precluding a correct description of many novel Colletotrichum species. In this context, the phylogenetic analyzes involving the sequence information of distinct genomic regions in combination with morphological and pathogenicity traits can provide more precise descriptions of Colletotrichum species complexes (Cai et al. 2009). This combination of classical and multilocus molecular approaches is allowing the identification of many previously undescribed species within this genus (Weir et al. 2012; Damm et al. 2012; Cannon et al. 2012; Doyle et al. 2013; Hyde et al. 2014; Vieira et al. 2014). The correct identification of Colletotrichum species is essential for a more accurate understanding of the epidemiology and for the development of more efficient control strategies (Phoulivong 2011).
Few studies are available in the literature involving extensive characterization of Colletotrichum isolates associated with onion anthracnose employing multilocus phylogeny. Likewise, only two studies were carried out assessing the molecular diversity of the causal agents of onion anthracnose in Brazil, encompassing a reduced number of isolates and covering a relatively narrow geographical area. One study employed morphological and serological information to characterize six Colletotrichum isolates (Bajungu 1979). A second study employed Random Amplified Polymorphic DNA (RAPD) marker system in combination with genomic information of the rDNA ITS region to investigate the genetic diversity of 14 onion-infecting isolates from Pernambuco State (Vila Nova et al. 2011). In this context, the main objective of the present study was to conduct morphometrical and multilocus analyses of 38 anthracnose-causing Colletotrichum isolates collected in several geographical areas across major onion-producing regions of Brazil. The main objective of this assessment was to acquire a more precise overview about the diversity of the causal agents of this important onion disease under Neotropical conditions.
Material and methods
Colletotrichum isolates associated with onion foliar anthracnose
A collection of 38 monosporic isolates of Colletotrichum was used in the present study. These isolates were obtained from onion plants displaying one or more typical foliar anthracnose symptoms in several onion-producing regions across eight Brazilian States and the Federal District (Fig. 1), from 1998 to 2014. These isolates were preserved in the collection of phytopathogenic fungi and oomycetes of Embrapa Vegetable Crops (Brasília-DF, Brazil) by the Castellani’s method (Castellani 1939) in a cold room at 10 °C in the absence of light. This collection is also cryopreserved at −80 °C. For the present work, isolates were recovered by culturing them in Petri dishes containing PDA (Potato-Dextrose-Agar) medium.
Geographical distribution and cities where the 38 Colletotrichum isolates associated with foliar anthracnose were collected across the onion-producing regions of Brazil. The acronyms of the subset of States where the isolates were collected are: Bahia (BA), the Federal District (DF), Goiás (GO), Minas Gerais (MG), São Paulo (SP) and Rio Grande do Sul (RS)
Fungal DNA extraction
The isolates were first cultivated in PDA medium for one week at 25 °C with a photoperiod of 12 h. After this period, a portion of aerial mycelium was removed from each Petri dish and the genomic DNA was extracted using 2X CTAB buffer and organic solvents with minor adaptations (Boiteux et al. 1999). DNA concentration was estimated with the microvolume spectrophotometer, Nanodrop (NanoDrop Lite, Thermo Scientific®, USA), while DNA integrity was evaluated through electrophoresis using 1.5% agarose gel.
PCR assays and sequencing of the fungal β-tubulin 2 (tub2) gene
PCR assays were carried out initially using a pair of primers targeting the β-tubulin 2 gene (tub2), viz. Bt2a (5′-GGT AAC CAA ATC GGT GCT GCT TTC-3′) and βt2b (5’-ACC CTC AGT GTA GTG ACC CTT GGC-3′) (Glass and Donaldson 1995). PCR mix was composed of 3 μL of fungal genomic DNA (10 ng/μL), 2 μL 10X Taq DNA Polymerase buffer (100 mM Tris-HCl, 500 mM KCl, pH 8.3), 0.5 μL MgCl2 (50 mM), 2 μL dNTPs (2.5 M each), 0.2 μL of recombinant Taq DNA polymerase (Invitrogen®, 5 units/μL), 2.5 μL of each primer (10 μM) and 7.3 μL of ultrapure Milli-Q water, with a final reaction volume adjusted to 20 μL. PCR assays were carried out employing the Verity PCR System (Applied Biosystems). In the PCR assays, the following steps were kept constant across all genomic regions under evaluation: initial denaturation at 94 °C and a final extension at 72 °C for 7 min. The number of haplotypes from the β-tubulin sequences for the Colletotrichum species were calculated using DnaSP v5 (Librado and Rozas 2009). A haplotype network to visualize the relationships among haplotypes representing six Brazilian states was reconstructed with PopART (Leigh and Bryant 2015) using the TCS algorithm (Clement et al. 2002) with gaps and missing data excluded.
PCR assays and sequencing of additional genomic regions of isolates representing the nine previously identified tub2 gene haplotypes
Altogether nine fungal tub2 gene haplotypes were separated by DnaSP to represent the total of fully-characterized isolates of our survey. Therefore, only genomic DNAs from these representative isolates obtained for each tub2 gene haplotype were employed as templates in subsequent PCR assays. Sequence information of following genes/genomic regions were also obtained for this subset of isolates: actin (act), the intergenic spacer (IGS) region between the DNA lyase (apn2) gene and the mating-type mat1–2-1 locus (APN2/MAT-IGS), calmodulin (cal) and glyceraldehyde-3-phosphate dehydrogenase (gapdh). The primer pair employed to amplify the act gene segment was: ACT-512F (5′-ATG TGC AAG GCC GGT TTC GC-3′) and ACT-783R (5’-TAC GAG TCC TTC TGG CCC AT-3′) (Carbone and Kohn 1999). The primers for amplification of the gapdh gene were: GDF1 (5’-GCC GTC AAC GAC CCC TTC ATT GA-3′) and GDR1 (5′-GGG TGG AGT CGT ACT TGA GCA TGT-3′) (Templeton et al. 1992). The primers for amplification of the APN2/MAT-IGS region were: CgDL_F6 (5′-AGT GGA GGT GCG GGA CGT T-3′) and CgMAT1_F2 (5′-TGA TGT ATC CCG ACT ACC G-3′) (Rojas et al. 2010). The primers for amplification of the cal gene were CL1C (5′-GAA TTC AAG GAG GCC TTC TC-3′) and CL2C (5’-CTT CTG CAT CAT GAG CTG GAC-3′) (O’Donnell et al. 2000). All temperatures of each cycle were used according to the original works, except for the cal and gapdh genes, where the annealing temperatures 59 °C and 60 °C were employed, respectively. For the APN2/MAT-IGS region the following PCR cycling program was used: initial step of 95 °C for 3 min, 10 cycles (Step Down function) of 95 °C for 45 s, 60 °C for 45 s (reducing 1 °C for each cycle), 72 °C for 1 min, 35 cycles of 95 °C for 45 s, 50 °C for 45 s, 72 °C for 1 min and final extension at 72 °C for 10 min. PCR reactions were performed with a final volume of 20 μL containing 11.1 μL of Milli-Q water, 1 μL of dNTP (Invitrogen®, Germany), 1.5 μL of each primer, 2 μL PCR buffer 10X, 0.6 μL of MgCl2, 0.3 μL of Taq DNA polymerase (Invitrogen®, Germany) and 2 μL of genomic DNA. The reactions were carried out in a MyCycler® Thermal Cycler (BioRad®, USA). PCR products were separated on electrophoresis using 1.5% agarose gel in 1X Tris-Acetate acid EDTA (TAE) and photographed in UV light after staining with ethidium bromide (0.5 μg mL−1) for 5 min. PCR products were purified using the Wizard® SV Gel and PCR Clean-Up System kit (Promega, USA) following the manufacturer’s protocol. Sequencing of the genomic regions previously amplified were performed by Macrogen Inc. (Seoul, South Korea).
Phylogenetic analyses
Sequence assemblies were performed separately in Geneious R8 software (Kearse et al. 2012) and alignments with MAFFT plugin, also with Geneious R8. The tub2 gene sequences were initially analyzed for all 38 isolates. Ambiguities and other errors were verified in the corresponding electropherograms and then either removed, re-sequenced and/or corrected manually. The Bayesian inference method was chosen for the phylogenetic analysis using MrBayes (Huelsenbeck and Ronquist 2001) plugin in Geneious R8 (Kearse et al. 2012). The MEGA X (Kumar et al. 2018) program was used to select substitution model according to the Akaike Information Criterion for each analysis, resulting in HKY + I as the most appropriate for tub2. The sequences were compared with reference sequences obtained from GenBank (Table 1). Initial analyses, using the information derived from the tub2 genomic region, indicated that the isolates could be subdivided into two species complexes: C. acutatum and C. gloeosporioides. Because of this, a tree was generated with all the isolates used in this study along with a set of reference isolates from species of both the C. gloeosporioides complex and the C. acutatum complex (Fig. 2). The MEGA X (Kumar et al. 2018) program was again used to select substitution model according to the Akaike Information Criterion, resulting in HKY + G as the most appropriate for C. acutatum species complex and GTR + G + I for C. gloeosporioides species complex. A subset of isolates (corresponding to each of the nine initially identified tub2 gene haplotypes) were selected (Table 2) and used to generate two trees: one using sequences corresponding to the genomic regions tub2 (424 bp in length), act (248 bp) and gapdh (274 bp) and reference isolates of the C. acutatum complex (Fig. 4) and another tree using sequences corresponding to the genomic regions tub2 (413 bp), act (211 bp), cal (637), gapdh (269 bp) and APN2/MAT-GS (683 bp) and the reference isolates of the C. gloeosporioides complex (Fig. 5). In all phylogenetic analyses, the Maximum Likelihood method with RAxML (Stamatakis 2014) plugin in Geneious was used. All maximum likelihood analyses were performed with 1000 bootstrap repetitions.
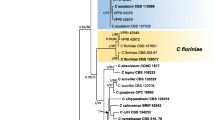
Phylogenetic tree of tub2 genomic region of 38 onion Colletotrichum (Coll) isolates along with GenBank sequences of reference isolates. Colletotrichum pseudoacutatum was employed as outgroup. Values on the branches are indicating the posterior probability of Bayesian inference (model HKY + I, 2 million generations and 25% burnin) followed by maximum likelihood bootstraps (model GTR + I, 1000 bootstraps). Values below 60 of either bootstrap or posterior probability were removed. The Colletotrichum isolates associated with onion foliar anthracnose employed in the present study are highlighted in blue
Morphological characterization of Colletotrichum isolates
Based upon the results of the phylogenetic analyses, isolates belonging to each of the nine Colletotrichum tub2 gene haplotypes were characterized for colony color and morphology as well as morphometric characteristics of the conidia. Disks of 4 mm in diameter were removed from the edges of the colonies (with five days of growth) and placed on plates containing PDA medium. Three replicates were made for each of the nine isolates in a completely randomized experimental design. Plates were incubated at 25 °C in photoperiod of 24 h. This assay was carried out twice. The colony growth (diameter increase) of each isolate was measured for 7 days. At the end of 7 days, the colony color and texture of each isolate were evaluated and according to these criteria they were classified into distinct morphological groups. The diameter of the colony was measured with the aid of a digital caliper (in millimeters) in two perpendicular directions. The mycelial growth of the colonies was expressed in mm day−1. After five days, the microscopic preparation on slides was performed using lactophenol and the images were produced through a Nikon® DS-Ri1 camera coupled to a Nikon® eclipse 80i microscope. The length and width of 50 conidia per isolate were measured with the aid of Motic Image Plus v. 2.0 (Motic Group Co., Beijing, China). In order to determine the differences of the conidial dimensions of the Colletotrichum species and the mycelial growth averages, the data were submitted to analysis of variance (ANOVA) and the means were grouped by the Scott-Knott test (Scott and Knott 1974), with significance of 5%, using the Sisvar V. 5.4 software (Ferreira 2014).
Pathogenicity assay of Colletotrichum isolates from onion via inoculation of the bulbs
The bulbs were disinfested according to a previously described protocol (Vila Nova et al. 2011). A subset of isolates representing each of tub2 gene haplotypes (Table 2) was grown in PDA medium at 25o C in continuous light for 24 h for five days. Afterwards, mycelial discs from each isolate were removed from the edges of the five days old colonies and placed in contact with onion bulbs with the help of autoclaved toothpicks. Each isolate was inoculated into five onion bulbs, each one being tested with two mycelial discs in symmetrical positions on each bulb. The mock-inoculated controls were treated in a similar manner; however, the discs were composed exclusively of PDA. The inoculated bulbs were then kept in moist chambers in trays covered with autoclaved paper towels and moistened with sterile distilled water. Trays remained enclosed by plastic bags for 7 days in the light under a temperature range of 20 ± 5 °C. Fourteen days after inoculation, the diameters of the two lesions of each bulb were measured in two opposite directions. The experimental design was completely randomized with five replications. The data obtained in the 11 treatments for all pathogenicity tests were submitted to analysis of variance (ANOVA) and the means were grouped by the Scott-Knott test (Scott and Knott 1974) with significance of 5%, using Sisvar V. 5.4 software (Ferreira 2014). This test was carried out twice and the results of the two assays were statistically similar (P > 0.05). Data were analyzed as a group, representing the average of the two assays.
Pathogenicity assay of Colletotrichum isolates from onion via leaf inoculation
The same subset of selected isolates was transferred to Petri dishes containing oatmeal agar medium and incubated for two weeks at 25o C in continuous light for 24 h for 5 days. Twenty-five mL of 0.01% Tween 20 solution was added to each Petri dish. The mycelium was then dissolved with the aid of a soft bristle brush and the conidial suspension was then filtered in autoclaved double gauze. The suspension was quantified with the aid of a hemocytometer and dilutions were performed to obtain a final concentration of 1 × 105 conidia/mL. Onion plants cultivar BRS 367, were grown in Styrofoam trays filled with a commercial substrate (Rohrbacher®). Plants with three fully-expanded leaves were transplanted to 1.5-L pots (with four plants each) containing a substrate composed of a soil mixture, burned rice husk and bovine manure in the ratio 3:1:1, enriched with 8 g of a mixture of NPK (formulation 4–30-16) and 7.5 g of dolomitic limestone. The aereal organs of the plants, displaying at least five fully-expanded leaves were inoculated by using a suspension of 1 × 105 conidia/ml (containing 0.01% Tween) up to the point of drainage. Each plant was sprayed with about 2.5 mL of fungal suspension and then the pots were kept in moist chamber for 48 h. The experiment was carried out twice under greenhouse conditions in a completely randomized design, involving ten treatments (isolates) with three replicates, each one with four plants. Mock-inoculated plants were treated only with a Tween (0.01%) solution.
Results
Phylogenetic analyses
The 38 isolates were initially separated into nine tub2 gene haplotypes (Table 2). With this initial analysis using tub2 gene information, fungal species belonging to two Colletotrichum complexes (C. acutatum and C. gloeosporioides) were observed in both Bayesian and Maximum Likelihood inferences (Fig. 2). These two methods rendered phylogenetic trees with similar topologies. Eleven (28.9%) out of the 38 isolates grouped in the clade of the C. acutatum species complex and 27 isolates (71.1%) grouped in the C. gloeosporioides species complex. The tub 2 haplotype network (Fig. 3) revealed two distinct clades corresponding the clades already observed in the Fig. 2. The first clade is represented by isolates of the C. gloeosporioides species complex, originated from multiple geographic regions, including all sampled states. The second clade is represented by isolates of the C. acutatum species complex, also originated from multiple geographic regions, including all sampled states except Rio Grande do Sul. Six out of nine tub2 haplotypes were represented by only one isolate (Table 2 and Fig. 3).
TCS haplotype network generated for β-tubulin sequences representing six Brazilian states using PopArt. The sizes of the circles are proportional to the frequency of each haplotype. Black circles represent hypothetical haplotypes. Hatch marks along the network branches indicate the number of mutations. Each color represents a Brazilian state
In this preliminary phylogenetic analysis employing the tub2 information, three haplotypes (Hap 1, Hap 2, and Hap 6) grouped with the reference species of the C. acutatum complex and six haplotypes (Hap 3, Hap 4, Hap 5, Hap 7, Hap 8, and Hap 9) grouped with references of the C. gloeosporioides complex. Twenty-six out of the 27 isolates belonging to the C. gloeosporioides species complex grouped in a subclade together with the species C. theobromicola. The other isolate belonging to the C. gloeosporioides complex (Coll 269), grouped in a subclade with the species C. fructicola and related species of this complex. Among the eleven isolates that grouped with species of the C. acutatum species complex, seven grouped in a subclade with C. scovillei and C. guajavae, two species with close phylogenetic relationship. Another three isolates grouped in a subclade with C. tamarilloi, while the other isolate grouped in a clade with C. nymphaeae. Primers used for the APN2/MAT-IGS region failed to amplify in five representative isolates from the haplotypes that grouped with species of the C. acutatum complex species. However, amplicons corresponding to such region were observed in all the remaining isolates. The genomic regions act, cal, and gapdh were successfully amplified across all representative haplotype isolates. The concatenated trees corroborated the results of the tub2 phylogeny. The same set of species belonging to the C. acutatum and C. gloeosporioides complexes identified in the tub2 phylogeny were confirmed in this analysis. Three species were found in the C. acutatum complex and two species were detected in the C. gloeosporioides complex. The tub2, act, and gapdh genes tree of the four onion isolates of the C. acutatum complex displayed well-supported clades with high posterior probability values, indicating that all the isolates used in this tree are bona fide members of the C. acutatum complex (Fig. 4). With the maximum value of posterior probability, the representative isolate Coll 39 clustered with C. tamarilloi, while isolate Coll 734 grouped with C. nymphaeae. Both representative isolates Coll 20 and Coll 23 clustered with C. scovillei with posterior probability of 0.96 and separated from C. guajavae. The tub2, act, cal, gapdh and APN2/MAT-IGS genes tree displays the representative onion isolates and the references of the C. gloeosporioides complex (Fig. 5). The isolates Coll 26, Coll 35, Coll 70, Coll 374 and Coll 997 formed a clade associated with the reference isolate of C. theobromicola (syn. C. fragariae), with posterior probability of 1 (Fig. 5). The Coll 269 isolate grouped to C. fructicola with posterior probability of 0.98.
Phylogenetic tree of concatenated sequences of tub2, act, and gapdh genes containing four representative isolates plus reference accessions of members from the Colletotrichum acutatum complex. Colletotrichum orchidophilum was employed as outgroup. Values on branches correspond to posterior probability of Bayesian inference (model HKY + G, 6 million generations and 25% burn-in) followed by maximum likelihood bootstraps (model GTR + G, 1000 bootstraps). Values below 60 of bootstrap or posterior probability were removed. The Colletotrichum isolates associated with onion foliar anthracnose employed in the present study are highlighted in blue
Phylogenetic tree of concatenated sequences of tub2, act, cal and APN2/MAT-IGS genomic regions, containing six representative isolates plus reference accessions from members of the Colletotrichum gloeosporioides complex. Colletotrichum alatae was employed as outgroup. Values on branches correspond to posterior probability of Bayesian inference (model HKY + I, 2 million generations and 25% burn-in) followed by maximum likelihood bootstraps (model GTR + I, 1000 bootstraps). Values below 60 of bootstrap or posterior probability were removed. The Colletotrichum isolates associated with onion foliar anthracnose employed in the present study are highlighted in blue
Morphological characterization
Based on colony morphology, the representative isolates of Colletotrichum were separated into seven different morphological groups. The morphological groups #1, #2, #3, #5, and #7 included the isolates Coll 35, Coll 23, Coll 39, Coll 269 and Coll 20, respectively. The morphological group #4 was represented by the isolate Coll 997, while the morphological group #6 was composed by the isolates Coll 26, Coll 70 and Coll 374. The representative isolate Coll 734 had its morphological analysis compromised due to successive contaminations. The morphological group #1 had a light gray colony with a dark green back and absence of conidia. The morphological group #2 displayed a slightly cottonous colony with pale orange color, with a few orange conidia, and a pale orange reverse. The morphological group #3 displayed colonies with olive color with absence of conidia, and reverse of coloration varying from white to olive. The morphological group #4 presented a colony with a color ranging from gray to white, the reverse of coloration ranging from white to pale orange and with no production of conidia. The morphological group #5 showed a colony with a gray and reverse coloration ranging from gray to white with absence of conidiomata. The morphological group #6 presented a colony with irregular borders and very cottonous of light gray color and the reverse ranging from gray to dark green and absence of conidiomata. The morphological group #7 presented a slightly cottonous colony of olive color with numerous conidiomata of orange color and the reverse of coloration varying from orange to olive.
The mycelial growth rates displayed significant statistical differences among the isolates. Four distinct groups were observed (Table 3). The isolates Coll 269 and Coll 997 displayed the highest average growth (mean of 10.4 mm.day-1), while the Coll 26 isolate had the lowest growth rate, 6.03 mm day−1. The isolates Coll 20, Coll 35, Coll 70 and Coll 23 were in the group with the second highest mycelial growth rate (mean of 9.6 mm day−1). The isolates Coll 374 and Coll 39 showed a mean of 8.55 mm day−1. Regarding the diameter of the colony measured at the end of seven days, all the isolates presented a distribution of means identical to the distributions of the average of the daily mycelial growth rate, grouping equally in four groups.
In relation to the morphological attributes, the isolates Coll 269, Coll 997, Coll 26, Coll 35 and Coll 374 displayed oblong conidia with both obtuse ends, ranging in size from 9.50 μm to 13.00 μm in length and from 3.50 μm to 4.50 μm in width. The isolates Coll 23, Coll 20 and Coll 39 displayed fusiform (sometimes oblong) conidia with one or in some cases, both acute ends, varying in dimensions of 12.50 μm to 15.00 μm in length and 3.50 μm to 4.50 μm in width. The Coll 70 isolate, in PDA medium, displayed an abundant production of a sterile cottonous mycelium, making it impossible to measure the conidia.
Pathogenicity assays in onion bulbs
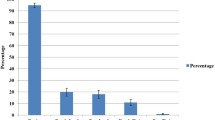
The representative subset of Colletotrichum isolates, except Coll 734, were pathogenic, causing typical anthracnose lesions on the onion bulbs seven days after inoculation. Two types of lesions were observed: one firm and other with an aqueous aspect, both with signs of the pathogen. Significant statistical difference was detected among the lesion size means (= aggressiveness) induced by the selected isolates. Isolates Coll 23 and Coll 20 were weakly aggressive and the isolates Coll 39, Coll 997, Coll 70, Coll 269, Coll 35, Coll 374, and Coll 26 were the most aggressive (Table 4). All isolates were re-isolated from the symptomatic bulbs.
Pathogenicity assays in onion leaves
All selected Colletotrichum isolates were able to induce foliar symptoms in onion plants. However, the means of severity did not display significant statistical difference (data not shown). The severity of the disease was classified as an overall “grade 1”, indicating a low rate of aggressiveness of isolates in onion leaves during the evaluation period of the experiment. The isolates were re-isolated in PDA media and they were morphologically similar to the original cultures.
Discussion
Extensive characterization studies of the putative complex of Colletotrichum species associated to foliar anthracnose of onion via a combination of morphological and molecular tools are scarce in the literature. These analyses were carried out with a representative collection of 38 isolates obtained from diseased onion plants across major onion-producing areas in four macro geographic regions of Brazil. The results indicated that there is at least five Colletotrichum species as members of this complex. Distinct Colletotrichum species have been reported causing anthracnose in onions in the several countries such as C. coccodes in USA (Rodriguez-Salamanca et al. 2012; Baysal-Gurel et al. 2014; Hay et al. 2016) and C. siamense and C. truncatum in India (Chowdappa et al. 2015). However, in our study, despite the diversity of sampled fields in the four main onion producing regions of Brazil, these species were not found associated with the foliar anthracnose of onion. In fact, novel information about the fungal diversity associated with this important tropical and subtropical diseases was obtained. Species belonging to the C. gloeosporioides complex are often reported in association with onion anthracnose, while species of the C. acutatum complex have not yet been reported in the literature. Therefore, our work is the first formal report of the association of three novel species of the C. acutatum complex (C. scovillei, C. nymphaeae and C. tamarilloi) with the foliar anthracnose of onions.
Thus far, only C. gloeosporioides (sensu lato) has been reported as the causal agent of this disease in Brazil. However, we found here that two distinct species of the C. gloeosporioides complex (C. theobromicola and C. fructicola) are associated with this disease in the country. Twenty-six out of 38 isolates were identified as C. theobromicola, indicating this fungus as the prevalent anthracnose pathogen under Brazilian conditions. This fungal species was recently reported as causal agent of foliar anthracnose on welsh onion (Allium fistulosum L.) in Brazil (Matos et al. 2017). Other species of Colletotrichum, as C. spaethianum (Santana et al. 2016) and C. truncatum (Matos et al. 2017) were also reported as causal agent of foliar anthracnose on welsh onion in Brazil. However, none of these fungal species was detected in association with onion in our work, which may indicate the occurrence of host species-specific Colletotrichum diversity. Additional surveys will confirm this hypothesis.
The species C. fructicola, C. nymphaeae, C. scovillei, C. tamarilloi and C. theobromicola have already been reported in Brazil causing anthracnose in distinct crops (Alexandre et al. 2014; Silva et al. 2017; Veloso et al. 2018; Moreira et al. 2019). The species C. tamarilloi was reported as the causal agent of anthracnose in scarlet eggplant fruits (Alexandre et al. 2014). The species C. theobromicola (syn. C. fragariae) and C. fructicola were reported as causal agents of cashew anthracnose (Veloso et al. 2018). Colletotrichum nymphaeae and C. scovillei were reported causing anthracnose on Capscium species (Silva et al. 2017) and apple fruits (Moreira et al. 2019), respectively.
Unlike the incongruities that may occur between morphology and phylogeny, two isolates showed similarity across these two analyzes. For example, the isolates Coll 70 and Coll 374 were grouped in the same clade and in the same morphological group (#7). These isolates are phylogenetically close to each other, and similar to C. theobromicola, although they were obtained from two geographically very distant regions, São José do Norte-RS and Cristalina-GO. The fact that Colletotrichum spp. can be disseminated by contaminated onion seeds (Massola Jr et al. 2016) can explain the presence of the same species causing anthracnose in onions in these two far apart geographic regions of Brazil.
The information derived from only three genes (tub2, act, and gapdh) has been sufficient to obtain well-supported clades for studies involving the genetic diversity of the species from the C. acutatum complex (Dean et al. 2012; Vieira et al. 2020). For the C. gloeosporioides complex, it is well-known that the APN2/MAT-IGS region in association with other genomic regions such as act, cal, chs (chitin synthetase), gs (glutamine synthetase) and his (histone) provides crucial information to obtain a precise characterization of isolates (Sharma et al. 2013; Vieira et al. 2020). Therefore, the genomic regions used here were suitable to identify and characterize our sample of isolates.
In the morphological analysis, it was observed that the color and texture of the morphological groups #1, #5, and #6 are congruent with reference isolates of the species employed by Weir et al. (2012) to define the C. gloeosporioides species complex. The development of mycelium with white, gray, and olive colors have also been reported in other studies as being a major characteristic associated with the members of the C. gloeosporioides species complex (Bernstein et al. 1995; Gunnell and Gubler 1992). The occurrence, however, of colonies with rosy to orange colorations and with the reverse color ranging from white to pink are characteristic of C. acutatum species complex (Bernstein et al. 1995; Damm et al. 2012). The morphological groups #2, #3, #4, and #7 were composed of species belonging to this complex and displayed these peculiar morphological characteristics. In addition, the differences in the morphology of the conidia produced in these colonies are in agreement with colony morphology data for most isolates. The isolates included in each morphological group, with presence of colony characteristic of species included in the C. gloeosporioides complex, also displayed typical conidia of the members of this complex, without shape variations. However, for the isolates included in the morphological groups #2, #3, #4, and #7 there was no consistence in the conidial shape. Damm et al. (2012) also observed a conspicuous variation of the conidial shape within species of the C. acutatum complex, and this fact can be due several reasons. One is that fungi preserved over a long period and subcultured multiple times, may be prone to morphophysiological variations when compared to wild/fresh isolates (Budiie et al. 1999). A subgroup of our Colletotrichum isolates from onion has been maintained for over a decade (data not shown). Besides influencing the shape of the conidia, the successive subculture and the preservation during long periods of Colletotrichum isolates promote changes in the colony texture. This type of fungal manipulation can result in denser and cottony colonies, which may display considerable loss in the capacity of conidial production (Damm et al. 2012). This condition may explain the fact that the isolates such as Coll 70 and Coll 734 displayed only sterile mycelium. It is important to highlight that these changes in colony morphology are not necessary associated with loss of pathogenicity, as observed here for the isolate Coll 70, which was among the most aggressive in the bioassay using onion bulbs (Table 4). The abnormalities in conidial size and mycelial growth of all isolates found in this study were similar to the overall means described for Colletotrichum species. Weir et al. (2012) obtained conidial dimension values quite similar to those obtained in this study after analyzing colonies with 10 days of growth. This observation indicates that the cultivation period ranging from seven to 10 days may not have strong influence in the overall Colletotrichum conidial dimensions.
The differences in symptom expression observed in our bioassay with onion bulbs employing representative isolates of the C. acutatum and C. gloeosporioides complexes were also observed by Vila Nova et al. (2011) with a distinct collection of C. gloeosporioides isolates from Pernambuco State, Brazil. The aqueous type of lesions, observed in both works, indicates activity of extracellular pectinolytic enzymes produced by members of this group of pathogens. According to Bailey et al. (1992), tissue-degrading enzymes can act in the establishment of Colletotrichum species infections and are, therefore, important components associated with the divergences observed in aggressiveness across isolates. Vila Nova et al. (2011) also found differences in the aggressiveness of Colletotrichum isolates in onion leaves, contrasting with results of our study. The variation in the levels of aggressiveness is a useful information from the onion breeding standpoint, guiding the identification of most suitable isolates for anthracnose resistance selection programs (Reis and Oliveira 2009).
For a long time, the taxonomy of the genus Colletotrichum has been problematic due to the absence of uniform criteria to characterize species (Hibbett and Taylor 2013). In addition, morphological characterizations are not always accurate because they deal with variable and plastic characteristics. Changes in the composition of the medium, storage time and the number of times that a given isolate has been subcultured are major factors that alter the results of morphological analyses, leading to imprecise characterization (Cai et al. 2009; Weir et al. 2012).
The results of our work demonstrated the association of at least five Colletotrichum species causing onion anthracnose in Brazil. This diversity of Colletotrichum species in onions was not identified in previous diversity studies. Thus far, only Colletotrichum gloeosporioides has been reported as the causal agent of this disease in Brazil (Bajungu 1979; Wordell Filho et al. 2006; Vila Nova et al. 2011). However, our work is clearly indicating that anthracnose disease is, in fact, caused by a complex of Colletotrichum species, with the prevalence of C. theobromicola isolates. In this scenario, the identification of a fungal complex in this pathossystem is a relevant contribution since the correct identification of the causal agents is essential for the establishment of more effective anthracnose management strategies, including epidemiological aspects, chemical and cultural control as well as the identification and deployment of onion cultivars with species-specific and/or wide-spectrum tolerance/resistance.
References
Alexandre, E. R., Herculano, L. M., Silva, J. M., & Oliveira, S. M. A. (2014). Fosfitos no manejo da antracnose do jiló. Pesquisa Agropecuária Brasileira, 49(12), 930–938.
Bailey, J. A., O’Connell, R. J., Pring, R. J., & Nash, C. (1992). Infection strategies of Colletotrichum species. In A. J. Bailey & J. M. Jeger (Eds.), Colletotrichum: Biology, pathology and control (pp. 88–120). Oxford: British Society for Plant Pathology.
Bajungu, H. E. (1979). Caracterização patogênica, serológica e fisiológica de Colletotrichum gloeosporioides Penz. (Sensu Arx, 1957) f. sp. cepae n.c. Piracicaba: USP-ESALQ. 35p (Dissertação de mestrado).
Baysal-Gurel, F., Subedi, N., Mamiro, D.P, & Miller, S.A. (2014). First report of anthracnose of onion caused by Colletotrichum coccodes in Ohio. Plant Disease,98(9), 1271.
Bernstein, B., Zehr, E. I., & Dean, E. A. N. (1995). Characteristics of Colletotrichum from peach, apple, pecan, and other hosts. Plant Disease, 79(5), 478–482.
Boiteux, L. S., Fonseca, M. E. N., & Simon, P. W. (1999). Effects of plant tissue and DNA purification method on RAPD-based genetic fingerprinting analysis in carrot (Daucus carota L.). Journal of American Society for Horticultural Science, 124(1), 32–38.
Budiie, A., Martinez-Culebras, P., Bridge, P., García, M., Querol, A., Cannon, P., & Monte, E. (1999). Molecular characterization of Colletotrichum strains derived from strawberry. Mycological Research, 103(4), 385–394.
Cai, L., Hyde, K. D., Taylor, P. W. J., Weir, B. S., Waller, J., Abang, M. M., Zhang, J. Z., Yang, Y. L., Phoulivong, S., Liu, Z. Y., Prihastuti, H., Shivas, R. G., McKenzie, E. H. C., & Johnston, P. R. (2009). A polyphasic approach for studying Colletotrichum. Fungal Diversity, 39, 183–204.
Cannon, P. F., Damm, U., Johnston, P. R., & Weir, B. S. (2012). Colletotrichum - current status and future directions. Studies in Mycology, 73, 181–213.
Carbone, I., & Kohn, L. M. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia, 91(3), 553–556.
Castellani, A. (1939). Viability of some pathogenic fungi in distilled water. Journal of Tropical Medicine and Hygiene, 24, 270–276.
Chowdappa, P., Chethana, C.S., & Pavani, K.V. (2015). Colletotrichum siamense and C. truncatum are responsible for severe outbreaks of anthracnose on onion in Southwest India. Journal of Plant Pathology, 97(1), 77-86.
Clement, M., Snell, Q., Walke, P., Posada, D., & Crandall, K. (2002). TCS: Estimating gene genealogies. Proc. 16th Int. Parallel Distributed Process Symposium, 2:184.
Damm, U., Cannon, P. F., Woudenberg, J. H. C., & Crouss, P. W. (2012). The Colletotrichum acutatum species complex. Studies in Mycology, 73, 37–113.
Dean, R., Van Kan, J. A. L., Pretorius, Z. A., Hammond-Kosack, K. E., Di Pietro, A., Pietro, D. S., Rudd, J. J., Dickman, M., Kahmann, R., Ellis, J., & Foster, G. D. (2012). The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology, 13(4), 414–430.
Doyle, V. P., Oudemans, P. V., Rehner, S. A., & Litt, A. (2013). Habitat and host indicate lineage identity in Colletotrichum gloeosporioides s. l. from wild and agricultural landscapes in North America. PLoS One, 8, e62394.
Ferreira, D.F. (2014). Sisvar: A guide for its bootstrap procedures in multiple comparisons. Ciência e Agrotecnologia, 38(2), 109-112. Avaliable at: https://doi.org/10.1590/S1413-70542014000200001.
Glass, N. L., & Donaldson, G. (1995). Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology, 61, 1323–1330.
Gunnell, P. S., & Gubler, W. D. (1992). Taxonomy and morphology of Colletotrichum species pathogenic to strawberrry. Mycologia, 84(2), 157–165.
Hay, F. S., Strickland, D., & Maloney, E. (2016). Anthracnose of onion caused by Colletotrichum coccodes in New York. Plant Disease, 100(10), 2117.
Hibbett, D. S., & Taylor, J. W. (2013). Fungal systematics: is a new age of enlightenment at hand? Nature Reviews Microbiology, 11(2), 129–133.
Hill, J. P. (1996). Twister. In H. F. Schwartz & S. K. Mohan (Eds.), Compendium of onion and garlic diseases (p. 26). St. Paul: APS Press.
Huelsenbeck, J. P., & Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17(8), 754–755.
Hyde, K. D., Nilsson, R. H., Alias, S. A., Ariyawansa, H. A., Blair, J. E., Cai, L., Cock, A., Wa, M., Dissanayake, A. J., Glockling, S. L., Goonasekara, I. D., Gorzak, M., Hahn, M., Jayawardena, R. S., Jal, V. K., Laurence, M. H., Lévesque, C., Li, X., Liu, J. K., Maharachchikumbura, S. S. N., Manamgoda, D. S., Martin, F. N., McKenzie, E. H. C., McTaggart, A. R., Mortimer, P. E., Nair, P. V. R., Pawlowska, J., Rintoul, T. L., Shivas, R. G., Spies, C. F., Summerell, B. A., Taylor, P. W. J., Terhem, R. B., Udayanga, D., Vaghefi, N., Walther, G., Wilk, M., Wrzosek, M., Xu, J. C., Yan, J., & Zhoun, N. (2014). One stop shop: backbones trees for important phytopathogenic genera. Fungal Diversity, 67, 21–125.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P., & Drummond, A. (2012). Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28(12), 1647–1649.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547–1549.
Leigh, J. W., & Bryant, D. (2015). POPART: Full-feature software for haplotype network construction. Methods in Ecology and Evolution, 6, 1110–1116.
Librado, P., & Rozas, J. (2009). DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics, 25(11), 1451–1452.
Maranhão, E. H. A., Cavalcante, V. A. L. B., Candeia, J. A., Lyra Filho, H. P., & Rodrigues, V. J. L. B. (1997). Avaliação do controle químico do "mal-de-sete-voltas" em cebola, causado pelo fungo Colletotrichum gloeosporioides Pens, no estado de Pernambuco. Horticultura Brasileira, 15, 230.
Massola Jr, N., Jesus Jr, W. C., Krause-Sakate, R., Pavan, M. A., Frare, V. C., & Mituti, T. (2016). Doenças do alho e da cebola. In: Amorin, L.; Rezende, J. A. M., Bergamin Filho, A., & Camargo, L. E. A. Manual de Fitopatologia: Doenças das Plantas Cultivadas. V.2, 5ª. Ed., Piracicaba, CERES, p. 63-73
Matos, K. S., Santana, K. F. A., Catarino, A. M., & Hanada, R. E. (2017). First report of anthracnose on welsh onion (Allium fistulosum) in Brazil caused by Colletotrichum theobromicola and C. truncatum. Plant Disease, 101(6), 1055.
Moreira, R. R., Peres, N. A., & May De Mio, L. L. (2019). Colletotrichum acutatum and C. gloeosporioides species complexes associated with apple in Brazil. Plant Disease, 103(2), 268–275.
O’Donnell, K., Nirenberg, H. I., Aoki, T., & Cigelnik, E. (2000). A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience, 41, 61–78.
Phoulivong, S. (2011). Colletotrichum, naming, control, resistance, biocontrol of weeds and current challenges. Current Research in Environmental and Applied Mycology, 1(1), 53–73.
Reis, A., & Oliveira, V. R. (2009). Agressividade de Isolados de Colletotrichum gloeosporioides em Cebola. Brasília: EMBRAPA-CNPH, Boletim de Pesquisa e Desenvolvimento, 53. 17 p.
Rodriguez-Salamanca, L.M., Enzenbacher, T.B., Derie, M.L., du Toit, L.J., Feng, C., Correll, J.C., and Hausbeck, M.K. (2012). First report of Colletotrichum coccodes causing leaf and neck anthracnose on onions (Allium cepa) in Michigan and the United States. Plant Diseae, 96(4), 769.
Rojas, E. I., Rehner, S. A., Samuels, G. J., Van Bael, S. A., Herre, E. A., Cannon, A., Chen, R., Pang, J., Wang, R., Zhang, Y., Peng, Y., & Sha, T. (2010). Colletotrichum gloeosporioides s.l. associated with Theobroma cacao and other plants in Panamá: Multilocus phylogenies distinguish host associated pathogens from asymptomatic endophytes. Mycologia, 102(6), 1318–1338.
Santana, K. F. A., Garcia, C. B., Matos, K. S., & Hanada, R. E. (2016). First report of anthracnose caused by Colletotrichum spaethianum on Allium fistulosum in Brazil. Plant Disease, 100(1), 224.
Scott, A. J., & Knott, M. (1974). A cluster analysis method for grouping means in the analysis of variance. Biometrics, 30(3), 507–512.
Sharma, G., Kumar, N., Weir, B. S., Hyde, K. D., & Shenoy, B. D. (2013). The ApMat marker can resolve Colletotrichum species: a case study with Mangifera indica. Fungal Diversity, 61, 117–138.
Silva, J. R. A., Chaves, T. P., Silva, A. R. G., Barbosa, L. F., Costa, J. F. O., Ramos-Sobrinho, R., Teixeira, R. R. O., Silva, S. J. C., Lima, G. S. A., & Assunção, I. P. (2017). Molecular and morpho-cultural characterization of Colletotrichum spp. associated with anthracnose on Capsicum spp. in northeastern Brazil. Tropical Plant Pathology, 42, 315–319. https://doi.org/10.1007/s40858-017-0151-7.
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30(9), 1312–1313.
Sutton, B. C. (1980). The Coelomycetes. Commonwealth Mycological Institute: 696p.
Templeton, M. D., Rikkerink, E. H. A., Solon, S. L., & Crowhurst, R. N. (1992). Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene, 122(1), 225–230.
Veloso, J. S., Camara, M. P. S., Lima, W. G., Michereff, S. J., & Doyle, V. P. (2018). Why species delimitation matters for fungal ecology: Colletotrichum diversity on wild and cultivated cashew in Brazil. Fungal Biology, 122, 677–691.
Vieira, W. A. S., Michereff, S. J., Morais Jr., M. A., Hyde, K. D., & Câmara, M. P. S. (2014). Endophytic species of Colletotrichum associated with mango in northeastern Brazil. Fungal Diversity, 67, 181–202.
Vieira, W. A. S., Bezerra, P. A., Silva, A. C., Veloso, J. S., Câmara, M. P. S., & Doyle, V. P. (2020) Optimal markers for the identification of Colletotrichum species. Molecular Phylogenetics and Evolution 14: online. https://doi.org/10.1016/j.ympev.2019.106694.
Vila Nova, M. X., Borges, L. R., Sousa, A. C. B., Brasileiro, B. T. R. V., Lima, E. A. L., Costa, A. F., & Oliveira, N. T. (2011). Pathogenicity for onion and genetic diversity of isolates of the pathogenic fungus Colletotrichum gloeosporioides (Phyllachoraceae) from the state of Pernambuco, Brazil. Genetics and Molecular Research, 10(1), 311–320.
Weir, B., Damm, U., & Johnston, P. R. (2012). The Colletotrichum gloeosporioides species complex. Studies in Mycology, 73, 115–180.
Wordell Filho, J. A., Rowe, E., Gonçalves, P. A. S., Debarba, J. F., Boff, P., & Thomazelli, L. F. (2006). Manejo Fitossanitário na Cultura da Cebola (226p). Florianópolis: Epagri.
Acknowledgements
To “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) for the scholarship of the first author during his MSc course. Leonardo S. Boiteux, Maria E. N. Fonseca and Ailton Reis are recipients of CNPq Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflict of interest
The authors do not have any conflict of interest.
Research involving human participants and/or animals
Not applicable.
Informed consent
All authors have reviewed the manuscript and approved its submission to Journal of Plant Pathology.
Rights and permissions
About this article
Cite this article
Lopes, L.H.R., Boiteux, L.S., Rossato, M. et al. Diversity of Colletotrichum species causing onion anthracnose in Brazil. Eur J Plant Pathol 159, 339–357 (2021). https://doi.org/10.1007/s10658-020-02166-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02166-8