Abstract
The objectives of the present study were to identify composition of fungal communities associated with healthy and declined oak trees and seedlings in Ilam Province, Iran, and to evaluate their role in occurrence of the oak decline. Fungal isolates were obtained from branches of healthy and declining Persian oak trees and seedlings in Ilam province during summer and autumn 2014–2015. Fungal species were identified according to both morphological and molecular characteristics obtained from ITS, 28S and 18S regions of ribosomal DNA. Some fungal species such as Neoscytalidium dimidiatum and Obolarina persica were obtained only from branches of Persian oak trees with decline symptoms. The Acremonium sp., Coniochaeta sp., Cytospora ribis, Fusarium tricinctum, Microsphaeriopsis olivacea and Neoetophoma samarorum species were found only in healthy trees as endophytic species. While, B. mediterrana, Didymella glomerata, Fusarium solani and Tricothecium roseum were isolated from both healthy and declined trees. The F. tricinctum and T. roseum species were found in healthy seedlings. However, D. glomerata was isolated from both healthy and dried seedlings. The species B. mediterrana, D. glomerata, N. dimidiatum and O. persica showed pathogenicity on the Persian oak seedlings in the greenhouse conditions. Finally, it could be concluded that for the first time two species, D. glomerata and N. dimidiatum, were recorded as pathogens associated with Persian oak. In addition, Acremonium sp., Coniochaeta sp., C. ribis, F. solani, F. tricinctum, N. samarorum and T. roseum were recorded for the first time as endophytic fungi on Persian oak trees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oaks (Quercus spp.) are the dominant tree species present in Zagros forest in Iran. Zagros forest is one of the most important forest area in Zagros mountains located in western parts of Iran and contains about 5.2 billion hectares of Iran’s forested area (Jazirehi and Ebrahimi Rostaghi 2013; Sagheb-Talebi et al. 2014). Persian oak (Quercus brantii) covers more than half of the Zagros forest area, and is more widespread and frequent than other species (Bordbar et al. 2010; Jazirehi and Ebrahimi Rostaghi 2013; Hassanzad Navroodi et al. 2015). Ilam province by itself comprises about 641,000 ha of Zagros forest, and the main tree species in these forests is Persian oak (Ahmadi et al. 2014). During the past three decades, occurrence of oak tree mortality has been frequently reported in many countries such as Austria, Hungary, Italy, United States, Turkey and other regions of the world (Hämmerli and Stadler 1989; Freer-Smith and Read 1995; Thomas and Büttner 1998; Pérez-Sierra et al. 2013; McConnell and Balci 2014; Linaldeddu et al. 2014; Frisullo et al. 2018). Also, the decline and mortality of oaks is one of the most important disease of oak trees in western regions of Iran. It has resulted in destruction of oak trees in Zagros forest ecosystems. The disease is widespread in all forests of Zagros from north to south especially on the southern side of the mountain in Ilam, Lorestan, Kohgilouyeh va Boyer-Ahmad, Fars and Kermanshah provinces (Fattahi 1994; Ahmadi et al. 2014; Mirabolfathi 2013). Symptoms on affected oak trees include significant mortality in all parts of the crown, brown-black discoloration of bark and dead branches. Therefore, production of Persian oak seedlings in nurseries and their transplantation to forests is a strategy for restoration of damaged areas in Ilam Province, though mostly transplanted seedlings die after a short time after moving to the forest.
Oak decline is a complex disease that is generally caused by interactions of several biotic and abiotic factors, and the symptoms and causal factors may not be the same in all regions (Akilli et al. 2013; Linaldeddu et al. 2011; Moreira and Martins 2005; Ragazzi et al. 1995). Undoubtedly, biotic stresses play an important role on occurrence of decline symptoms on their host plants (Akilli et al. 2013). The role of fungi, as an important factor causing decline, has been evaluated in different regions of the world, and a high numbers of studies have tried to determine the diversity of fungi associated with oak decline symptoms worldwide (Kowalski 1996; Bruhn et al. 2000; Luque et al. 2000; Thomas et al. 2002; Balci and Halmschlager 2003; Ragazzi et al. 2003; Kelley et al. 2009; Henriques et al. 2012; Akilli et al. 2013; Mirabolfathi 2013; Linaldeddu et al. 2014). For instance, the fungal species Botryosphaeria dothidea, Diplodia corticola, D. seriata and Neofusicoccum parvum were isolated by Linaldeddu et al. (2014) from trunk and branch cankers of declining holm oak (Q.ilex). Luque et al. (2000) reported Biscogniauxia mediterranea, Botryosphaeria stevensii and Ophiostoma quercus from stems of cork oak (Q. suber) with decline symptoms. Furthermore, six species including B. mediterranea, B. corticola, Cytospora sp., Discula quercina, Fusicoccum sp. and Pleurophoma cava have been reported as causal agents of canker and dieback in branches of cork oak in Tunisia (Linaldeddu et al. 2010). B. mediterranea and Obolarina persica have been also reported as important agents of Persian oak decline in Zagros forests (Mirabolfathi 2013; Mirabolfathi et al. 2013). In addition, Fungal endophytic communities are highly diverse group of fungi that inhabit in internal tissues of living plants without causing any immediate overt negative effects and present in many kinds of plants (Bacon and White 2000; Strobel and Daisy 2003; Hyde and Soytong 2008). Many studies have been performed to determine the diversity of endophytic fungi associated with oak trees worldwide (Faeth and Hammon 1997; Gennaro et al. 2003; Ghobad-Nejhad et al. 2017). In addition, the fungal species Deniquelata quercina, Cytospora sp., M. olivacea, Diatrype spp., Leptosphaerulina spp., Comoclathris sp. and Thyrostroma sp. have been reported as endophytic fungi from Persian oak in Iran (Ghobad-Nejhad et al. 2018; Alidadi et al. 2019). Nevertheless, endophytes usually survive in a latent phase in healthy tissues for the whole lifetime or for an extended period of time, but may turn pathogenic during a change in environmental conditions and can rapidly spread from several infection points during their pathogenic phase (Rodriguez et al. 2008). B. mediterranea can spend part of its life-cycle as an endophyte in all the aerial organs of the oak plants and then become opportunistic pathogen under water stress conditions. In those conditions, B. mediterraneais able to rapidly colonize the xylem and bark tissues, induces necrosis and canker formation (Anselmi et al. 2000; Mazzaglia et al. 2001; Linaldeddu et al. 2011; Safaee et al. 2016).
The objectives of the present study were to: a) isolate and characterize the fungal species associated with healthy tree and dead branches of Persian oak trees with decline symptoms in the Zagros region, b) Isolate and characterize fungi from healthy and dead Persian oak seedlings (in nurseries and transplanted to the forest); and c) to determine the pathogenicity of the isolated fungal species on Persian oak seedling in greenhouse conditions.
Material and methods
Sampling and isolation of fungi
Sampling was done from healthy and dead branches of Persian oak trees with decline symptoms in different forest areas of Ilam province (Forest area including Arghavan, Tange Dalab, Chagha Sabz in Ilam County and Forest area in Malekshahi County) as well as healthy and dead seedlings in nurseries (located in Ilam province, Eyvan city) and transplanted seedlings in the forests during the summer and autumn of 2014–2015 (Fig. 1). The selected regions are the most affected area by oak decline (Forests, Range and Watershed Management Organization of Iran 2013). Several symptomatic samples were collected and placed in separate paper bags, recorded their information and then transferred to the mycology Lab. The samples were washed under running tap water in order to remove dusts or other surface contaminations, and subjected to air to be dried at room temperature for 2–3 h. In order to eliminate epiphytic contaminations, woody samples were disinfested by the ethanol 70% for 30 s and by the 1% sodium hypochlorite for 1 min, and rinsed twice by sterile water. To isolate the associated fungi, the samples were further cut into smaller segments (about 5 mm) in sterile conditions and paced on 2% Water Agar (2% WA) as well as Potato Dextrose Agar (PDA) and then incubated at 25 °C for 5–7 days. Fungi purification was performed by transferring single spores and/or single hyphal tips grown on 2% Water Agar (2% WA) onto PDA. The purified isolates were stored on PDA slants at 4 ̊ C for future studies.
Morphological and molecular identifications
Morphological identifications were conducted on general culture media, including Potato Dextrose Agar (PDA), Oat meal Agar (OA), Malt Extract Agar (MEA), Carnation Leaf Agar (CLA) and Synthetic Nutrient Agar (SNA). Macro- and micro-morphological characteristics, such as colonies morphology, color and diameter, and sexual and asexual states characteristics were recorded and compared with the literature. Total genomic DNA was extracted according to the protocol previously described by Zhong and Steffenson (2001). PCR amplifications were done with the primer pairs NS1/NS4 for 18S and ITS1/ITS4 for ITS-rDNA (White et al. 1990), and LROR/LR5 for 28S nrDNA (Rehner and Samuels 1995). Generated sequences were observed and edited in BioEdit V. 7.2.5 (Hall 1999) and were subjected to BLAST search tool in the GenBank nucleotide database. Required sequences for phylogenetic analysis were retrieved from GenBank and multiple sequence alignments were generated using the MAFFT version 7 web tool (https://mafft.cbrc.jp/alignment/server/) (Katoh and Standley 2013). Phylogenetic estimates were evaluated using the Maximum Parsimony Analyses (MP) in PAUP version 4.0b10 software (Swofford and Sullivan 2003). Ambiguously aligned regions were excluded and gaps were treated as missing data. The trees were inferred using the heuristic search option with TBR branch swapping and 1000 random sequence additions. Descriptive tree statistics for parsimony (Tree Length [TL], Consistency Index [CI], Retention Index [RI] and Homoplasy Index [HI]) were calculated for trees generated under different optimality criteria. Maximum parsimony bootstrap values (MP) equal or greater than 50% are given below or above each node. All identified species have been deposited in the Microbial of Agriculture Biotechnology Research Institute of Iran culture collections (ABRII CC).
Pathogenicity tests
To perform pathogenicity test, two-year-old cork oak seedlings obtained from the field-collected acorns (from healthy Persian oak trees in Ilam Province, Eyvan city). The acorns were sown in plastic bags containing peat vermiculite mixture in a 1:1 ratio. The plants were kept in a greenhouse with temperature 25–35 °C and irrigated twice a week to field capacity. The age of the seedlings for the experiment was 24 months and the mean plant size was 20–25 cm in height with a 4–6 mm stem diameter at the time of inoculation. The seedlings stems were surface-sterilized in 10% sodium hypochlorite for 10 min and artificially wounded (size of wound 4–5 mm). Inoculations were conducted by placing a 1-week-old 5-mm agar plug from each fungal culture and the wounded stems were wrapped with parafilm. Control plants were treated similarly with sterile PDA plug. All inoculated and non-inoculated seedlings were incubated at 25 °C. The inoculated plants examined after 1–2 months of incubation and inspected for symptoms development. Each plant was cut longitudinally through the point of inoculation and the extent of lesion and vascular discoloration was measured upward and downward from the point of inoculation. The statistical analysis of data was performed using a one-way analysis of variance (ANOVA), and the means were compared using Duncan’s test by SPSS for Windows version 17 (Chicago, SPSS Inc.) Small pieces of necrotic tissue from the edge of each lesion were cut and placed on PDA in an attempt to recover the inoculated fungus and complete Koch’s postulates.
Results
Sampling and isolation
Totally, 120 samples from branches of healthy/declining Persian oak trees in different forest areas and Persian oak seedlings in the nursery and forest were collected. Finally, 264 fungal isolates were obtained from branches of trees and seedlings. From that, 164 isolates were from trees branches (97 isolates from healthy and 67 isolates from declining trees) and 100 isolates associated with seedlings (70 isolates from healthy and 30 isolates from died seedlings) (Figs. 2, 3 and 4).
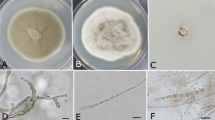
The morphological characteristics of six fungal species isolated in the present study, including a-b: the Acremonium sp. isolate 97SA, c-d: the B. mediterranea isolate 17SA, e-g: the Coniochaeta sp. isolate 92SA1, h-I: the C. ribis isolate 38SA1, j-n: the D. glomerata isolate N42 and o-p: the F. solani isolate 27RF
The morphological characteristics of six fungal species isolated in the present study, including a-b: the F. tricinctum isolate 60B1, c-e: the M. olivacea isolate 22SA, f-h: the N. samarorum isolate 88SA1, i-l: the N. dimidiatum isolate 112RA1, m-n: the O. persica isolate 66SA and o-p: the T. roseum isolate 115SA
Morphological identifications
All of the recovered isolates were investigated on the basis of morphological features of asexual or very rarely sexual reproduction stages, and finally were grouped in 12 morpho-types based on the similarity of their micro- and macro-morphological characteristics. Finally, ten fungal species, including B. mediterrana, C. ribis, D. glomerata, F. solani, F. tricinctum, M. olivacea, N. samarorum, N. dimidiatum, O. persica and T. roseum were identified. In addition, Acremonium sp., and Coniochaeta sp., were identified at the genus level. Table 1 reports a summarized information about morphological characteristic of the identified species in the study.
The information about relative abundance and origin of the isolates was included in the Table 3. The N. dimidiatum and O. persica were obtained only from Persian oak trees with decline symptoms, whereas Acremonium sp., C. ribis, F. tricinctum, M. olivacea and N. samarorum only from healthy Persian oak trees as endophytic species. However, the B. mediterrana, D. glomerata, F. solani and T. roseum were isolated from both healthy and declining trees. In addition, D. glomerata, F. tricinctum and T. roseum were isolated from healthy seedlings, while D. glomerat was isolated from all died seedlings in the nurseries and from the seedlings transported to the forest area (Table 2). Therefore, it could be concluded that D. glomerata was observed in all healthy/declining trees and seedlings (Fig. 5). B. mediterranea showed the highest frequency in healthy/declining Persian oak trees (20.61% and 37.31%, respectively) whereas the D. glomerata isolates was recovered with the highest frequency from healthy/died Persian oak seedlings (57.14% and 100%, respectively) (Fig. 5).
Phylogenetic analysis
The total number of 21 newly recovered isolates representing 12 genera, 10 species were selected for phylogenetic reconstruction using the ITS-rDNA sequences. The newly obtained sequences were deposited in the GenBank (Table 3). The Maximum parsimony trees consisted of two main clades including clad 1 (Dothideomycetes) and clade 2 (Sordariomycetes) with 53% and 93% bootstrap support, respectively (Fig. 6).
The parsimonious tree for the fungal species isolated in the present study based on the ITS rDNA sequences of 31 taxa belong to Dothideomycetes and Sordariomycetes. The bootstrap support values for maximum Parsimony values higher than 50 are given above each branch. The ITS sequence of Paecilomyces divaricatus was used as out group
The clade 1 included three groups A, B and C, which belonged to the taxonomic families of fungi including Botryosphaeriaceae, Didymellaceae, Leptosphaeriaceae, respectively. The isolates obtained in the present study including N. dimidiatum (isolate: 112RA1), D. glomerata (N43–1, N74–1, N42, N59–2, N29–2 and 66SA2), M. olivacea (isolate: 22SA) and N. samarorum (isolate: 88SA1) were located in these groups. However, ITS-rDNA sequences were not able to separate species of Neoscytalidium from each other (Fig. 6, clade 1-A). Therefore, phylogenetic analysis for Neoscytalidium spp. was performed based on the combined analysis of the ITS, 18S and 28S nrDNA dataset by MP method and the results showed that the isolate 112RA1 clustered with N. dimidiutum strains and is distinct from the isolates of N. novaehollandiae (Fig. 1, supplementary material).
Twelve isolates obtained in the present study were placed in the clade 2, which included seven groups D to J. The clade 2-D comprised of members of Nectriaceae family. F. solani species complex (isolates: 27RF and 113SA) and F. tricinctum (isolates: 60B11 and 56R3) were clustered to related taxa in this clade (Fig. 6, Clad 2-D). To determine the accurate taxonomic position of F. solani species complex, the phylogenetic analysis was performed by using TEF-1α gene for Fusarium spp. isolates. The isolates were identified as F. solani sensu stricto (Fig. 2, supplementary material). The O. persica (isolate: 66SA) and B. mediterranea (isolates: 17SA, 116SA1 and 60RP1) were located in the clade 2-E that consisted of members of Xylariaceae family (Fig. 6, Clade 2-E). The isolates of the clade 2-F and clade 2-G were related to incertae sedis family. The Acremonium sp. (isolate: 97SA) and T. roseum (isolate: 115SA) were placed in these clades, respectively (Fig. 6, Clade 2-F and G). The isolates belonging to Valsaceae and Coniochaetaceae families were located in the clade 2-H and I, respectively.
Pathogenicity tests
Pathogenicity tests were conducted using various isolates of each species in six replicates. The D. glomerata isolates, including N43–1, N74–1, N42, N59–2, N29–2 and N-43 were tested on stems of Persian oak seedlings. Typical symptoms, including longitudinal lesion on the inoculation point with dark brown discoloration of wood were observed 45 days after inoculation. All the isolates of Didymella were able to grow in the xylem surrounding the inoculation point on stem of Persian oak (Fig. 7a-f). The length of necrosis in inoculated seedlings ranged from 12 to 26 mm in length, and all the strains produced lesions significantly (P < 0.05) different from the control (Fig. 8a). Also, 70% of inoculated seedlings were died two months after inoculation. These pathogenic fungi were re-isolated from the lesion areas, and the identity as D. glomerata species was confirmed by the morphological characterization. The pathogenicity tests using selected isolates of N. dimidiatum (112RA1, 38SA3, 41SA2 and 59SA3) resulted in sooty necrotic lesions that developed in both upwards and downwards directions from the inoculation points within 30 days after inoculation (18–35 mm long) and slight bark peeling was observed in the inoculated area and the black fungal spores appeared under the bark on the canker surface (Fig. 7g-k). All the strains of N. dimidiatum produced significantly (p < 0.05) different mean lesion lengths from the control (Fig. 8b). Inoculated seedlings were dead 30 days after inoculation. The morphological characteristics of the isolates obtained from the inoculated plants coincided with the inoculated strains, confirming the Koch’s postulates.
The results of pathogenicity tests on Persian oak seedlings using different D. glomerata and N. dimidiatum isolates. a-f: the symptoms caused by different D. glomerata,aD. glomerata isolate N43–1, bD. glomerata isolate N43, cD. glomerata isolate N29–2, dD. glomerata isolate N59–2, e- f. control, g-k: the symptoms caused by N. dimidiatum isolates, g inoculation of N. dimidiatum on oak seedling (right: inoculated seedling and left: control), hN. dimidiatum isolate 38SA3, iN. dimidiatum isolate 112RA1. j-k control
Average lesion length (in millimeters) resulting from inoculation of (a) Didymella glomerata, (b) Neoscytalidium dimidiatum strains. The vertical bars represent means standard error. The letters above bars indicate treatments with significant difference (P < 0.05). Note: the control showed only faint discoloration
The isolates 116SA and 17SA of B. mediterranea were used for the pathogenicity test experiments on Persian oak seedlings. After 45 days, a complete destruction of the wood texture was observed at the inoculation points and finally all the inoculated seedlings died. In addition, the O. persica pathogenicity tests showed visible brown to black necrotic lesions developed in both upwards and downwards directions from the inoculation point (17–30 mm) within 45 days after inoculation. No symptoms were observed on the control seedlings. Both pathogens were separately re-isolated from the lesions of all seedlings inoculated with B. mediterranea and O. persica, confirming the Koch’s postulates. Inoculation of different F. solani and T. roseum isolates on Persian oak seedlings did not show any symptoms after three months.
Discussion
The decline and mortality of oaks is one of the most important disease of oak trees worldwide. In Iran, it is especially serious in the West regions in the Zagros forest (Mirabolfathi 2013; Ahmadi et al. 2014). Production of Persian oak seedlings in nurseries and their transplantation into forests is taken in the account as an important strategy to restore the damaged areas in the West regions of Iran. Forest nurseries have an important role in keeping forest lands productive. Nurseries seedlings diseases are important from two points of view including a) Damage to seedlings in the nurseries and b) transmission of diseases from nurseries to forest areas. Therefore, occurrence of diseases on the nursery seedlings is of particular importance, and many studies have been focused on this subject (Peterson and Smith 1975; Sutherland et al. 1989; Lilja et al. 1992; Lilja 1994; Pathak et al. 2015; Pandey et al. 2018; Alidadi et al. 2018). The present study focused on isolation and characterization of fungal flora from healthy/declining Persian oak trees and healthy/dead Persian oak seedlings. N. dimidiatum with 10.44% frequency was isolated only from trees with declining symptoms and was proved a pathogen on Persian oak trees based on pathogenicity test results (Fig. 5; Fig. 7g-k). This species has been previously reported from various sources, including woody plants (Punithalingam and Waterston 1970; Sutton and Dyko 1989), soil, human skin and nails (Moore 1988; Punithalingam and Waterston 1970; Crous et al. 2006). N. dimidiatum is known as one of the most important pathogens of plants and has a wide host range. This species has been reported as causal agent of different plant diseases, such as leaf blight on Sansevieria trifasciata (Kee et al. 2017), shoot blight and fruit rot in almond trees (Nouri et al. 2018), wood canker on grapevine (Rolshausen et al. 2013), canker disease in Ficus nitida and F. benjamina (Al-Bedak et al. 2018), dieback in Mangifera indica (Ray et al. 2010), canker disease in Hylocereus spp. (Xu et al. 2018) in the world. The present study reported the fungus N. dimidiatum as a pathogen of Persian oak in Iran for the first time.
The D. glomerata species is a worldwide distributed soil fungus, which has been isolated from various kinds of plants as well as inorganic materials, and frequently found in association with symptoms of blight, leaf spots and fruit rot (Boerema 2004; Moral et al. 2018; Thomidis et al. 2011; Aghapour et al. 2009; Holz et al. 1989). In the present study, this species was isolated from branches of the healthy and declining Persian oak trees with frequency of 5.15% and 7.46%, respectively, and from healthy and died Persian oak seedlings with frequency of 57.14% and 100%, respectively (Fig. 5). In addition, by pathogenicity test, it was proved in the present study that the D. glomerata species has pathogenicity on Persian oak. So, for the first time, this species was introduced as new pathogen of oak trees. Moreover, it could be concluded that latent infection of oak seedlings by D. glomerata may cause the spread of the disease among forest areas as it was observed in both seedlings and trees. In addition, considering to the worldwide distribution and wide host range of this species, careful inspection of nursery products is essential to prevent introduction of the fungus to the forests.
In both healthy and declining trees, B. mediterranea was the most abundant with 20.61% and 37.31%, respectively. This species reported as an agent of oak decline and charcoal disease in many regions of the world including Portugal, Spain, Italy, Slovenia (Henriques et al. 2012; Luque et al. 2000; Linaldeddu et al. 2014; Jurc and Ogris 2006). In addition, association of such fungi with charcoal disease in Quercus spp. trees have been previously reported in deferent region of Zagros forest including Lorestan, Ilam, Fars, Kohgiloye VA Boyer-Ahmad and Golestan Forests in Iran (Mirabolfathi 2013). In this study, B. mediterranea was isolated as an endophyte fungus from healthy oak trees as well as from declined trees in different sampling areas. O. persica (17.91%) was the second most abundant species observed in the declining trees, and isolated as a pathogen species only from branches of the Persian oak trees with decline symptoms (Fig. 5). This species previously have been reported as an important agent causing dying in Persian oak trees in Ilam province, Iran (Mirabolfathi et al. 2013).
F. solani was another species which was isolated from the healthy and declining trees with 7.21% and 11.94% frequency, respectively (Fig. 5). A brief overview on the literature reveals that this species was previously reported as the causing agent of canker and wilt symptoms on red and coast live oak, associated with branch and trunk cankers on citrus and stem canker on Tectona grandis (Vujanovic et al. 1999; Lynch et al. 2013; Nemec 1987; Huang et al. 2017). However, this species did not cause any disease and symptoms on Persian oak seedlings during three months. T. roseum was obtained from branches of healthy and declining trees with 9.27% and 14.92%, respectively as well as from healthy seedlings (17.14%) (Fig. 5). This species has shown high antagonistic activities against Diplodia corticola causal agent of cankers, vascular necrosis and dieback on various oak species (Campanile et al. 2007). The T. roseum has been risolated also from Maytenus hookeri as an endophyte and antagonist against other pathogenic fungi in vitro (Zhang et al. 2010). In the present study, the pathogenicity of this species was not observed on Persian oak seedlings and did not show any symptoms. As these species was isolated from both Persian oak trees and seedlings, it seems that it could be transferred by vertical transmission, and its antagonistic activity against Persian oak’s pathogens could be studied in the future. Therefore, this study may be taken in the account as a potential biocontrol agent of oak pathogens.
Finally, it could be concluded that four species, including B. mediterranea, D. glomerata, N. dimidiatum and O. persica, were pathogens on Persian oak. To the best of our knowledge, this is the first time that two species, N. dimidiatum and D. glomerata, are introduced as pathogens of Persian oak species. In addition, F. solani and T. roseum were isolated from trees with decline symptoms but were not pathogenic for Persian oak, and they are only associated with declined oak trees. In the previous studies, the fungal species Alternaria atra, A. infectoria, A. consortialis, A. molorum, Chaetomium globosum, Epicoccum nigrum, Immersidiscosia eucalypti, Kalmusia variispora, Petriella sordida, Neocamarosporium obiones and Sordaria fimicola have been also reported associated with declined Persian oak trees in Zagros forests(Alidadi et al. 2018).
In addition, the Acremonium sp., Coniochaeta sp., C. ribis, F. tricinctum and N. samarorum species were isolated from healthy Persian oaks and are new records of endophytic fungi on Persian oak throughout the world.
References
Aghapour, B., Fotouhifar, K. B., Ahmadpour, A., & Ghazanfari, K. (2009). First report of leaf spot disease on Ficus elastica caused by Phoma glomerata in Iran. Australasian Plant Disease Notes, 4(1), 82–83. https://doi.org/10.1071/DN09035.
Ahmadi, R., Kiadaliri, H., Mataji, A., & Kafaki, S. (2014). Oak forest decline zonation using AHP model and GIS technique in Zagros forests of Ilam Province. Biodiversity and Environmental Sciences, 4(3), 141–150.
Akilli, S., Ulubaş Serçe, Ç., Katırcıoğlu, Y. Z., & Maden, S. (2013). Phytophthora dieback on narrow leaved ash in the Black Sea region of Turkey. Forest Pathology, 43(3), 252–256. https://doi.org/10.1111/efp.12024.
Al-Bedak, O. A., Mohamed, R. A., & Seddek, N. H. (2018). First detection of Neoscytalidium dimidiatum associated with canker disease in Egyptian Ficus trees. Forest Pathology, 48(2), e12411. https://doi.org/10.1111/efp.12411.
Alidadi, A., Kowsari, M., Javan-Nikkhah, M., & Karami, S. (2018). First report of leaf spot caused by Truncatella angustata on Persian oak (Quercus brantii) in Iran. Plant Disease, 102(6), 1173–1173. https://doi.org/10.1094/PDIS-08-17-1258-PDN.
Alidadi, A., Kowsari, M., Javan-Nikkhah, M., Karami, S., Ariyawansa, H. A., & Jouzani, G. S. (2019). Deniquelata quercina sp. nov .; a new endophyte species from Persian oak in Iran. Phytotaxa, 405(4), 187–194. https://doi.org/10.11646/phytotaxa.405.4.2.
Anselmi, N., Mazzaglia, A. & Vannini, A. (2000) The role of endophytes in decline of oak species in Italy (A. Ragazzi & I. Dellavalle, eds): 131-144. Accademia Italiana di Scienze Forestali, Firenze.
Bacon, C. W., & White, J. F. (2000). Microbial endophytes (pp. 341–388). New York: Dekker.
Balci, Y., & Halmschlager, E. (2003). Incidence of Phytophthora species in oak forests in Austria and their possible involvement in oak decline. Forest Pathology, 33(3), 157–174. https://doi.org/10.1046/j.1439-0329.2003.00318.x.
Boerema, G. H. (Ed.). (2004). Phoma identification manual: differentiation of specific and infra-specific taxa in culture. CABI Publishing, Wallingford, U.K, pp 497.
Bordbar, K., Sagheb-Talebi, K., Hamzehpour, M., Joukar, L., Pakparvar, M., & Abbasi, A. R. (2010). Impact of environmental factors on distribution and some quantitative characteristics of manna oak (Quercus brantii Lindl.) in Fars province. Iranian Journal of Forest and Poplar Research, 18(3), 390–404. https://doi.org/10.22092/ijfpr.2015.105659.
Bruhn, J. N., Wetteroff, J. J., Jr., Mihail, J. D., Kabrick, J. M., & Pickens, J. B. (2000). Distribution of Armillaria species in upland Ozark Mountain forests with respect to site, overstory species composition and oak decline. Forest Pathology, 30(1), 43–60. https://doi.org/10.1046/j.1439-0329.2000.00185.x.
Campanile, G., Ruscelli, A., & Luisi, N. (2007). Antagonistic activity of endophytic fungi towards Diplodia corticola assessed by in vitro and in planta tests. European Journal of Plant Pathology, 117(3), 237–246. https://doi.org/10.1007/s10658-006-9089-1.
Crous, P. W., Slippers, B., Wingfield, M. J., Rheeder, J., Marasas, W. F., Philips, A. J., Alves, A., Burgess, T., Barber, P., & Groenewald, J. Z. (2006). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology, 55, 235–253.
Faeth, S. H., & Hammon, K. E. (1997). Fungal endophytes in oak trees: Long-term patterns of abundance and associations with leafminers. Ecology, 78(3), 810–819. https://doi.org/10.1890/0012-9658(1997)078[0810:FEIOTL]2.0.CO;2.
Fattahi, M. (1994). Study on Zagros oak forests and the most important their destruction causes. Institute of Forests and Rangelands Research press. Iran: Sanandaj.
Freer-Smith, P. H., & Read, D. B. (1995). The relationship between crown condition and soil solution chemistry in oak and Sitka spruce in England and Wales. Forest Ecology and Management, 79(3), 185–196. https://doi.org/10.1016/0378-1127(95)03614-8.
Frisullo, S., Lima, G., Magnano di San Lio, G., Camele, I., Melissano, L., Puglisi, I., Pane, A., Agosteo, G. E., Prudente, L., & Cacciola, S. O. (2018). Phytophthora cinnamomi involved in the decline of holm oak (Quercus ilex) stands in southern Italy. Forest Science, 64(3), 290–298.
Gennaro, M., Gonthier, P., & Nicolotti, G. (2003). Fungal endophytic communities in healthy and declining Quercus robur L. and Q. cerris L. trees in northern Italy. Journal of Phytopathology, 151(10), 529–534. https://doi.org/10.1046/j.1439-0434.2003.00763.x.
Ghobad-Nejhad, M., Asgari, B., & Chaharmiri Dokhaharani, S. (2017). Notes on some endophytic fungi isolated from Quercus brantii in Dena region of Kohgiluyeh and Boyer-Ahmad province. Mycologia Iranica, 4(1), 1–12. https://doi.org/10.22043/mi.2018.115893.
Ghobad-Nejhad, M., Meyn, R., & Langer, E. (2018). Endophytic fungi isolated from healthy and declining Persian oak (Quercus brantii) in western Iran. Nova Hedwigia, 106(1–2), 1–2. https://doi.org/10.1127/nova_hedwigia/2018/0470.
Hall, T. A. (1999). BioEdit: A user–friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series, 41(41), 95–98.
Hämmerli, F., & Stadler, B. (1989). Eichenschäden - Eine Übersicht zur Situation in Europa und in der Schweiz. Schweit. Z. Forstwes., 140, 357–374.
Hassanzad Navroodi, I., Zarkami, R., Basati, M., & Mohammadi Limaei, S. (2015). Quantitative and qualitative characteristics of Persian oak along altitudinal gradation and gradient (Case study: Ilam province, Iran). Journal of Forest Science, 61(7), 297–305. https://doi.org/10.17221/13/2015-JFS.
Henriques, J., Inácio, M. L., Lima, A., & Sousa, E. (2012). New outbreaks of charcoal canker on young cork oak trees in Portugal. IOBC/wprs Bulletin, 76, 85–88.
Holz, G., Morley, M., & Schreuder, W. (1989). Pathogenicity of Phoma glomerata, P. cava, P. eupyrena and Cytospora chrysosperma on blackthorn (Acacia mellifera subsp. detinens). Agricola, 7, 37–42.
Huang, S. P., Li, Z. L., Wei, J. G., Mo, J. Y., Li, Q. L., Guo, T. X., Luo, J. T., Yang, X. H., Tan, X. F., & Yang, X. B. (2017). First report of stem canker caused by Fusarium solani on Tectona grandis in China. Plant Disease, 101(12), 2148–2148. https://doi.org/10.1094/PDIS-04-17-0514-PDN.
Hyde, K. D., & Soytong, K. (2008). The fungal endophyte dilemma. Fungal Diversity, 33(163), e173.
Jazirehi, M. H., & Ebrahimi Rostaghi, M. (2013). Silviculture in Zagros. University of Tehran Press. 2nd edition, pp 560.
Jurc, D., & Ogris, N. (2006). First reported outbreak of charcoal disease caused by Biscogniauxia mediterranea on Turkey oak in Slovenia. Plant Pathology, 55(2), 299–299. https://doi.org/10.1111/j.1365-3059.2005.01297.x.
Katoh, K., & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution, 30(4), 772–780. https://doi.org/10.1093/molbev/mst010.
Kee, Y. J., Suhaimi, N. N., Zakaria, L., & Mohd, M. H. (2017). Characterisation of Neoscytalidium dimidiatum causing leaf blight on Sansevieria trifasciata in Malaysia. Australasian Plant Disease Notes, 12(1), 60. https://doi.org/10.1007/s13314-017-0284-z.
Kelley, M. B., Fierke, M. K., & Stephen, F. M. (2009). Identification and distribution of Armillaria species associated with an oak decline event in the Arkansas Ozarks. Forest Pathology, 39(6), 397–404. https://doi.org/10.1111/j.1439-0329.2009.00600.x.
Kowalski, T. (1996). Oak decline II. Fungi associated with various types of lesions on stems and branches of young oaks (Quercus robur). Österreichische Zeitschrift für Pilzkunde, 5, 51–63.
Lilja, A. (1994). The occurrence and pathogenicity of uni-and binucleate Rhizoctonia and Pythiaceae fungi among conifer seedlings in Finnish forest nurseries. European Journal of Forest Pathology, 24(3), 181–192. https://doi.org/10.1111/j.1439-0329.1994.tb00984.x.
Lilja, A., Lilja, S., Poteri, M., & Ziren, L. (1992). Conifer seedling root fungi and root dieback in Finnish nurseries. Scandinavian Journal of Forest Research, 7(1–4), 547–556. https://doi.org/10.1080/02827589209382746.
Linaldeddu, B. T., Hasnaoui, F., & Franceschini, A. (2010). Fungi associated with canker and dieback diseases of Quercus suber in Tunisia. Fungi associated with canker and dieback diseases of Quercus suber in Tunisia. IOBC wprs Bull, 57, 69–71.
Linaldeddu, B. T., Sirca, C., Spano, D., & Franceschini, A. (2011). Variation of endophytic cork oak-associated fungal communities in relation to plant health and water stress. Forest Pathology, 41(3), 193–201. https://doi.org/10.1111/j.1439-0329.2010.00652.x.
Linaldeddu, B. T., Scanu, B., Maddau, L., & Franceschini, A. (2014). Diplodia corticola and Phytophthora cinnamomi: the main pathogens involved in holm oak decline on Caprera Island (Italy). Forest Pathology, 44(3), 191–200 http://www.iobc-wprs.org/pub/bulletins/bulletin_2010_57_table_of_contents_abstracts.pdf.
Luque, J., Parladé, J., & Pera, J. (2000). Pathogenicity of fungi isolated from Quercus suber in Catalonia (NE Spain). Forest Pathology, 30(5), 247–263. https://doi.org/10.1046/j.1439-0329.2000.00208.x.
Lynch, S. C., Zambino, P. J., Mayorquin, J. S., Wang, D. H., & Eskalen, A. (2013). Identification of new fungal pathogens of coast live oak in California. Plant Disease, 97(8), 1025–1036. https://doi.org/10.1094/PDIS-11-12-1055-RE.
Mazzaglia, A., Anselmi, N., Gasbarri, A., & Vannini, A. (2001). Development of a polymerase chain reaction (PCR) assay for the specific detection of Biscogniauxia mediterranea living as an endophyte in oak tissues. Mycological Research, 105(8), 952–956. https://doi.org/10.1016/S0953-7562(08)61951-6.
McConnell, M. E., & Balci, Y. (2014). Phytophthora cinnamomi as a contributor to white oak decline in mid-Atlantic United States forests. Plant Disease, 98(3), 319–327. https://doi.org/10.1094/PDIS-06-13-0649-RE.
Mirabolfathi, M. (2013). Outbreak of charcoal disease on Quercus spp. and Zelkova carpinifolia trees in forests of Zagros and Alborz Mountaıns in Iran. Iranian Journal of Plant Pathology, 49(2), 77–79.
Mirabolfathi, M., Ju, Y. M., Hsieh, H. M., & Rogers, J. D. (2013). Obolarina persica sp. nov., associated with dying Quercus in Iran. Mycoscience, 54(5), 315–320. https://doi.org/10.1016/j.myc.2012.11.003.
Moore, M. K. (1988). Morphological and physiological studies of isolates of Hendersonula toruloidea Nattrass cultured from human skin and nail samples. Journal of Medical and Veterinary Mycology, 26(1), 25–39. https://doi.org/10.1080/02681218880000041.
Moral, J., Lichtemberg, P. S. F., Papagelis, A., Sherman, J., & Michailides, T. J. (2018). Didymella glomerata causing leaf blight on pistachio. European Journal of Plant Pathology, 151, 1–5. https://doi.org/10.1007/s10658-018-1422-y.
Moreira, A. C., & Martins, J. M. S. (2005). Influence of site factors on the impact of Phytophthora cinnamomi in cork oak stands in Portugal. Forest Pathology, 35(3), 145–162. https://doi.org/10.1111/j.1439-0329.2005.00397.x.
Nemec, S. (1987). Fusarium solani association with branch and trunk cankers on citrus weakened by cold weather in Florida. Mycopathologia, 97(3), 143–150. https://doi.org/10.1007/BF00437237.
Nouri, M. T., Lawrence, D. P., Yaghmour, M. A., Michailides, T. J., & Trouillas, F. P. (2018). Neoscytalidium dimidiatum Causing Canker, Shoot Blight and Fruit Rot of Almond in California. Plant Disease, 102(8), 1638–1647. https://doi.org/10.1094/PDIS-12-17-1967-RE.
Pandey, A., Juwantha, R., Chandra, S., Kumar, A., Kannojia, P., Khanna, D., Arora, S., Dwivedi, V. D., & Pandey, S. (2018). First report of Fusarium solani causing wilt of Melia dubia. Forest pathology, 48(1), p.e12398. https://doi.org/10.1111/efp.12398.
Pathak, H., Maru, S., & HN, S. (2015). Fungal diseases of trees in forest nurseries of Indore, India. Plant Pathology & Microbiology, 6, 297. https://doi.org/10.4172/2157-7471.1000297.
Pérez-Sierra, A., López-García, C., León, M., García-Jiménez, J., Abad-Campos, P., & Jung, T. (2013). Previously unrecorded low-temperature Phytophthora species associated with Quercus decline in a Mediterranean forest in eastern Spain. Forest Pathology, 43(4), 331–339. https://doi.org/10.1111/efp.12037.
Peterson, G. W., & Smith, R. S. (1975). Forest nursery diseases in the United States. USDA-Forest Service. Agricultural Handbook No, 470.
Punithalingam, E., & Waterston, J. M. (1970). Hendersonula toruloidea. CMI descriptions of pathogenic fungi and bacteria, (274).
Ragazzi, A., Vagniluca, S., Moricca, S., & Vigniluca, S. (1995). European expansion of oak decline: Involved microrganisms and methodological approaches. Phytopathologia Mediterranea, 207–226.
Ragazzi, A., Moricca, S., Capretti, P., Dellavalle, I., & Turco, E. (2003). Differences in composition of endophytic mycobiota in twigs and leaves of healthy and declining Quercus species in Italy. Forest Pathology, 33(1), 31–38. https://doi.org/10.1046/j.1439-0329.2003.3062003.x.
Ray, J. D., Burgess, T., & Lanoiselet, V. M. (2010). First record of Neoscytalidium dimidiatum and N. novaehollandiae on Mangifera indica and N. dimidiatum on Ficus carica in Australia. Australasian Plant Disease Notes, 5(1), 48–50. https://doi.org/10.1071/DN10018.
Rehner, S. A., & Samuels, G. J. (1995). Molecular systematics of the Hypocreales: A teleomorph gene phylogeny and the status of their anamorphs. Canadian Journal of Botany, 73(S1), 816–823. https://doi.org/10.1139/b95-327.
Rodriguez, R. J., Henson, J., Van Volkenburgh, E., Hoy, M., Wright, L., Beckwith, F., Kim, Y. O., & Redman, R. S. (2008). Stress tolerance in plants via habitat-adapted symbiosis. The ISME Journal, 2(4), 404–416. https://doi.org/10.1038/ismej.2007.106.
Rolshausen, P. E., Akgül, D. S., Perez, R., Eskalen, A., & Gispert, C. (2013). First report of wood canker caused by Neoscytalidium dimidiatum on grapevine in California. Plant Disease, 97(11), 1511–1511. https://doi.org/10.1094/PDIS-04-13-0451-PDN.
Safaee, D., Khodaparast, S. A., Mirabolfathi, M., & Mousanejad, S. (2016). Relationship between dieback of Persian oak (Quercus brantii) and apparent and latent infection of Biscogniauxia mediterranea in Zagros forests. Iranian Journal of Plant Pathology, 52(4).
Sagheb-Talebi, K., Sajedi, T., & Pourhashemi, M. (2014). Forests of Iran- a treasure from the past, a hope for the future. Dordrecht Heidelberg New York London: Springer.
Strobel, G., & Daisy, B. (2003). Bioprospecting for microbial endophytes and their natural products. Microbiology and Molecular Biology Reviews, 67(4), 491–502. https://doi.org/10.1128/MMBR.67.4.491-502.2003.
Sutherland, J. R., Shrimpton, G. M., & Sturrock, R. N. (1989). Diseases and insects in British Columbia forest seedling nurseries FRDA Report 065, 27–28.
Sutton, B. C., & Dyko, B. J. (1989). Revision of Hendersonula. Mycological Research, 93(4), 466–488. https://doi.org/10.1016/S0953-7562(89)80040-1.
Swofford, D. L., & Sullivan, J. (2003). Phylogeny inference based on parsimony and other methods using PAUP*. The Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny, cáp, 7, 160–206.
Thomas, F. M., & Büttner, G. (1998). Nutrient relations in healthy and damaged stands of mature oaks on clayey soils: Two case studies in northwestern Germany. Forest Ecology and Management, 108(3), 301–319. https://doi.org/10.1016/S0378-1127(98)00239-4.
Thomas, F. M., Blank, R., & Hartmann, G. (2002). Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. Forest Pathology, 32(4–5), 277–307. https://doi.org/10.1046/j.1439-0329.2002.00291.x.
Thomidis, T., Michailides, T. J., & Exadaktylou, E. (2011). Phoma glomerata (Corda) Wollenw. & Hochapfel a new threat causing cankers on shoots of peach trees in Greece. European journal of plant pathology, 131(2), 171. https://doi.org/10.1007/s10658-011-9796-0.
Vujanovic, V., Cogliastro, A., St-Arnaud, M., Neumann, P., & Gagnon, D. (1999). First report of Fusarium solani canker and wilt symptoms on red oak (Quercus rubra) in Quebec, Canada. Plant Disease, 83(1), 78–78. https://doi.org/10.1094/PDIS.1999.83.1.78B.
White, T. J., Bruns, T., Lee, S. J. W. T., & Taylor, J. L. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications, 18(1), 315–322.
Xu, M., Peng, Y., Qi, Z., Yan, Z., Yang, L., He, M.D., Li, Q.X., Liu, C.L., Ruan, Y.Z., Wei, S.S. and Xie, J., 2018. Identification of Neoscytalidium dimidiatum causing canker disease of pitaya in Hainan, China. Australasian Plant Pathology, pp1–7.
Zhang, X. M., Li, G. H., Ma, J., Zeng, Y., Ma, W., & Zhao, P. (2010). Endophytic Fungus Trichothecium roseum LZ93 Antagonizing Pathogenic Fungi In Vitro and Its Secondary Metabolites. The Journal of Microbiology, 48(6), 784–790. https://doi.org/10.1007/s12275-010-0173-z.
Zhong, S., & Steffenson, B. J. (2001). Virulence and molecular diversity in Cochliobolus sativus. Phytopathology, 91(5), 469–476. https://doi.org/10.1094/PHYTO.2001.91.5.469.
Acknowledgments
The authors would like to thank of Saadi Karami and Ebrahim Karimi for their help in sampling and the Agriculture Biotechnology Research Institute of Iran (ABRII) and University of Tehran for their financial supports of this projects.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 456 kb)
Rights and permissions
About this article
Cite this article
Alidadi, A., Kowsari, M., Javan-Nikkhah, M. et al. New pathogenic and endophytic fungal species associated with Persian oak in Iran. Eur J Plant Pathol 155, 1017–1032 (2019). https://doi.org/10.1007/s10658-019-01830-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01830-y