Abstract
Cochliobolus lunatus (teleomorph: Curvularia lunata) is an important plant pathogenic fungus that causes the maize foliar spot, resulting in serious yield losses. In ascomycetes, a single mating-type (MAT) locus with two idiomorphs controls sexual development. The structure and arrangement of the MAT genes were examined to understand the MAT locus of C. lunatus. MAT loci were MAT1–1-1 or MAT1–2-1, flanked upstream and downstream by regions encoding GTPase activating protein, pyridoxamine phosphate oxidase domain, and β-glucosidase. A MAT1–1 or MAT1–2 idiomorph was identified in single isolate, and sexual reproduction in vitro indicated that the species was heterothallic. In vitro crossing between isolates with opposite MATs produced perithecia, asci, and ascospores. A multiplex MAT-specific PCR method was developed and used to test mating-type genes in 177 C.lunatus isolates collected from China. The ratio of isolates of each mating-type in China was consistent with a 1:1 ratio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cochliobolus lunatus R.R.Nelson & F.A.Haasis [anamorph: Curvularia lunata (Wakker) Boedijn], the causal agent of Curvularia leaf spot of maize, is widely distributed worldwide (Macri and Lenna 1974). In China, Curvularia leaf spot of maize was firstly reported in 1984 and primarily found in the Northwest corn producing regions (Duan 1984). In 1996, loss of grain yield caused by Curvularia leaf spot of maize was estimated to be 2.5 × 105 tons in Liaoning Province (Lu et al. 1997). Curvularia leaf spot of maize have rapidly become one of the most important foliar diseases in corn-cultivating regions in the past two decades (Wang et al. 2006). The genus Cochliobolus is an important group of ascomycete fungi, including the maize pathogens C. heterostrophus, C. carbonum, and C. lunatus, the wheat/barley/cereal pathogen C. sativus, oat pathogen C. victoriae, and rice pathogen C. miyabeanus. Genomes of All these pathogens have been sequenced (JGI:http://genome.jgi.doe.gov/programs/fungi/indes.jsf) (Gao et al. 2014; Zhang et al. 2016).
Sexual compatibility is controlled by a single mating-type (MAT) locus with two idiomorphs in ascomycetes. In homothallic species, both idiomorphs are located within an individual often at the same locus, with few exceptions (Paoletti et al. 2007; Yun et al. 1999). By contrast, each individual in heterothallic species carries a single MAT locus. This locus is unique, because the two alternate forms (MAT1–1 and MAT1–2) have completely dissimilar sequences located in the same locus on the chromosome (Yun et al. 1999). MAT1–1 is identified by a gene MAT1–1-1 that encodes a protein containing alpha-box DNA binding domain, whereas MAT1–2 is identified by a gene MAT1–2-1 that encodes a protein with high mobility group (HMG) DNA binding domain (Brewer et al. 2011; Coppin et al. 1997; Pearce et al. 2016; Turgeon 1998).
The genus Cochliobolus employs various sexual reproductive strategies. C. luttrellii, C. homomorphus, C. cymbopogonis and C. kusanoi are homothallic species, while C. ellisii, C. heterostrophus, C .intermedius, C. miyabeanus, C. sativus, C. setariae, and C. victoriae are heterothallic species (Christiansen et al. 1998; Yun et al. 1999; Zhong and Steffenson 2001). C. lunatus is also heterothallic (Nelson and Haasis 1964). Although the genomes of strains m118 (sorghum) and CX-3 (maize) of C.lunatus had been sequenced,the MAT1–1 and MAT1–2 idiomorphs have not been identified yet. A thorough description of the MAT1–1 and MAT1–2 required comparison of sequences from both mating type to determine where idiomorphs end and homologous flanking regions begin. Identification of mating-type genes in C. lunatus would be useful for comparative studies on the structure and evolution of the MAT1 locus within the Cochliobolus, and among other ascomycetes.
Resurgence of Curvularia leaf spot of maize caused serious damages in several maize-producing regions in Liaoning, Anhui and Henan, China (Gao et al. 2014; Zhang et al. 2016). The increase in incidence of Curvularia leaf spot of maize has raised a question on the possible role of sexual reproduction in the incidence of Curvularia leaf spot of maize. The objectives of this study were to: (1) identify genes and sequences in idiomorphs at the MAT1–1 and MAT1–2 loci in C.lunatus, (2) pair MAT1–1 and MAT1–2 strains to produce asci and ascospore, and (3) determine if mating types are present in 1:1 ratio, which are expected under random mating, in C. lunatus populations from different regions in China.
Materials and methods
Maize sampling and fungal isolation
Maize plants exhibiting typical symptoms of Curvularia leaf spot of maize were collected in August between 2010 and 2016 in China, namely, Heilongjiang, Jilin, Liaoning, Hebei, Henan, Shandong, Hubei, Anhui, Sichuan, and Yunnan. These provinces had serious events of Curvularia leaf spot of maize in 2010–2016. The majority of samples were collected from Liaoning province (Table 1).
Symptomatic leaves were excised from the junction of diseased and healthy tissue to 0.5 cm2 pieces to isolate C. lunatus from maize tissue. The samples were washed in distilled water, disinfected for 30 s in 70% ethanol and 1 min in 0.1% mercuric chloride, and rinsed twice in sterile distilled water. A subsection was cut from each sterilized piece, placed on a plate of potato dextrose agar (PDA; Hangzhou Baisi Biotechnology Co., Ltd., Hangzhou, China), and incubated at 25 °C for 4–5 days to produce spores in the dark.
A spore suspension was filtered through four layers of lens tissue to remove hyphal fragments, and serially diluted and evenly plated out over the agar plate surface. After incubating for 3–5 h, individually germinated spores were picked from the plate using a sterile needle with dissecting microscope, and the spores were subcultured at 25 °C for 7 days on PDA. All isolates were stored at −80 °C in 20% glycerol. Finally, 177 C. lunatus isolates were obtained for genomic DNA extraction.
DNA extraction, PCR amplification, and sequencing
Mycelia from each single-spore isolate were scraped from the surface of cultures grown on PDA. The tissue was ground in liquid nitrogen with sterile pestle and mortar. Genomic DNA was extracted from the mycelia using TIANGEN DNAsecure Plant Kit [Tian Gen Biochemical Technology (Beijing) Co., Ltd., Beijing, China], following the manufacturer’s instruction. DNA was quantified with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, MA), and the concentration was adjusted to 10 ng/μL in sterilized water for PCR.
The PCR was performed in a volume of 20 μL. The reaction mixture contained 2.0 μL of 10× PCR buffer, 2.0 μL of dNTPs (2.5 mM each), 1.0 μL each of 10 μΜ forward and reverse primers, 0.5 U Taq polymerase, and 1.0 μL (10 ng) of genomic DNA template. All PCR reagents are from TaKaRa (Dalian, China). All amplifications were performed in a PTC-200 thermocycler (Bio-Rad Laboratories, Hercules, CA), and the cycling programs were as follows: initial denaturation at 94 °C for 2 min, followed by 35 cycles of 94 °C for 30 s, at annealing temperature for 30 s, and 72 °C for 0.5–3 min, followed by final extension at 72 °C for 5 min.
All PCR products were purified by gel electrophoresis in a 1.0% agarose gel. All the fragments of the predicted length were excised and purified with Axyprep DNA Gel extraction kit (Axygen Scientific Inc., Tewksbury, MA) and cloned into the pMD19-T vector (Takara Bio Inc., Dalian, China) following the manufacturer’s instructions. Plasmids were extracted using the TIANprep Mini Plasmid Kit [TianGen Biochemical Technology (Beijing) Co., Ltd., Beijing, China] and sequenced with forward (M13RV-M) and reverse primers (M13F-47) by Genewiz Co. Ltd. (Suzhou, China) using the dideoxy chain termination method on the ABI 3730 Genetic Analyzer (PerkinElmer, Inc., Foster City, CA).
Consensus sequences were obtained from pairwise alignment of forward and reverse sequences using DNAMAN 8 (Lynnon Corporation, San Ramon, CA) and Gene Runner (www.softpedia.com) software.
Identification of the MAT idiomorphs and flanking sequence
The nucleotide sequences of MAT1–1-1 and MAT1–2-1 of C. ellisii (GenBank: AF129746.1 and AF129747.1) were used as starting points to search for MAT1–2 or MAT1–1 in the genomic sequence of the C. lunatus strain CX-3 (GenBank:JFHG01001253.1). A search of scaffold 19 (GenBank:KN050451.1) in the identified strain CX-3 revealed sequences with 85% identity to a MAT1–2 idiomorph. Strain CX-3 with putative MAT1–2-1 gene was used in the PCR to amplify the entire MAT gene for sequencing. Based on this sequence, primers in the HMG domain were designed to amplify MAT1–2 by PCR from the genomic DNA of C. lunatus (Supplementary Fig. 1). The initial PCR used primers ClMat2-F and ClMat2-R to amplify a 698 bp fragment (Supplementary Table 1). The PCR was executed with annealing temperature at 62 °C and extension time of 1 min. The subsequent PCR with primer pairs ClMat2-UF/ClMat2-UR and ClMat2-DF/ClMat2-DR amplified MAT1–2-1 in two overlapping fragments. MAT1–2-1 gene was amplified by PCR with annealing temperature at 60/65 °C and extension time of 1/1.5 min. The three overlapping PCR products of MAT1–2-1 gene were sequenced and combined. The sequences were compared with sequences in the GenBank database using BLASTn to confirm the amplification of the gene homologous to MAT1–2-1. The HMG domain of MAT1–2 isolates was amplified and sequenced using specific primer ClMat2T-F/ClMat2T-R. Nucleotide sequences of MAT1–2-1 gene in C. lunatus had 86% similarity to C. ellisii.
The nucleotide sequences of MAT1–1-1 of C. ellisii (GenBank:AF129746.1), C. heterostrophus (GenBank:X68399.1), C. victoriae (GenBank:AF032369.1), C. sativus (GenBank:AF275373.1), C. carbonum (GenBank:AF032368.1), and C. miyabeanus (GenBank:HQ123463.1) were used as templates to design the primer ClMat1-F/ClMat1-R to screen candidate isolates containing MAT1–1-1 gene. Conditions for amplification included an extension time of 1 min at annealing temperature of 65 °C. Strain ZD958 with putative MAT1–1-1 gene was used in the PCR to amplify the entire MAT1–1 gene for sequencing (Supplementary Fig. 1). The initial PCR amplified a 796 bp fragment. The subsequent PCR with primer pairs ClMat1-UF/ClMat1-UR and ClMat1-DF/ClMat1-DR amplified MAT1–1-1 in two overlapping fragments (Supplementary Table 1). The PCR annealing temperature at 55 °C and extension time of 2.0/1.5 min was performed to amplify MAT1–1-1. The three overlapping PCR products of MAT1–1-1 were sequenced and concatenated. The sequences were compared with sequences in the GenBank database using BLASTn to confirm the amplification of gene homologous to MAT1–1-1. The alpha box domain of MAT1–1 isolates was amplified and sequenced using specific primer ClMat1T-F/ClMat1T-R.
To identify the putative genes upstream of the MAT genes, the GTPase activator-like protein (GAP) and protein with pyridoxamine 5′-phosphate oxidase domain (ORF1) genes of C. heterostrophus strain C5 (GenBank:AF029913.1) and C4 (GenBank:AF027687.1), which resided upstream of the MAT gene in C. heterostrophus (Turgeon et al. 1993), were used to search the database of C. lunatus strain CX-3 (GenBank:JFHG01001253.1) for homologous sequences. A search of the scaffold 19 (KN050451.1) in strain CX-3 showed 81% sequences homologous to each of the GAP and ORF1 genes. The entire GAP and ORF1 of MAT were amplified and sequenced with primer pairs ClMatg-F/ClMatg-R and ClMato-F/ClMato-R at annealing temperature of 55/60 °C.
To obtain the nucleotide sequence of two nucleotides spanning the partial sequences, three primer pairs, namely, ClMatgU-F/ClMatoD-R, ClMatoU-F/ClMat1I-R, and ClMat2I-R, were designed to target the partial sequences of GAP, ORF1, and MAT1–2-1/MAT1–1-1, respectively. The primers were used to amplify and sequence the nucleotides spanning the partial sequences of GAP and ORF1, as well as ORF1 and MAT1–2-1/MAT1–1-, in C. lunatus isolates CX-3 and ZD958. The PCR included 30 cycles with annealing temperature at 59/57/58 °C and extension time of 2 min. The two overlapping PCR products and amplicons of each gene were combined. The sequences were compared with sequences in the GenBank database using Seqman (DNASTAR) to confirm the amplification of genes homologous to GAP and ORF1.
To identify the putative gene downstream of the MAT genes, the β-glucosidase gene of C. heterostrophus, which resided downstream of the MAT gene in C. heterostrophus strains C5 (GenBank:AF029913.1) and C4 (GenBank:AF027687.1), was used to search the database of C. lunatus strain CX-3 (GenBank:JFHG01001253.1) for homologous sequences. A search of the scaffold 19 (KN050451.1) in strain CX-3 showed 85% sequences homologous to each of the β-glucosidase. To sequence the entire β-glucosidase of C. lunatus and confirm its location downstream from the MAT genes, primer (ClMatg1U–F/ClMatg1D-R) was designed in the mutual conserved region downstream of the MAT genes. The entire β-glucosidase of MAT1–2 and MAT1–1 isolates were amplified and sequenced at annealing temperature of 61 °C. To obtain the nucleotide sequence spanning the partial sequences, primers ClMat1–1-1 U-F and ClMat1–2-1 U-F/ClMatgD-R were designed to target the partial sequences of β-glucosidase and MAT1–2-1/MAT1–1-1 genes, respectively. The primers were used to amplify and sequence the nucleotides spanning the partial sequences of β-glucosidase and MAT1–2-1/MAT1–1-1 in C. lunatus isolates CX-3 and ZD958. The PCR included 30 cycles with annealing temperature at 58/61 °C and extension time of 2 min. The two overlapping PCR products and amplicons of each gene were combined. The sequences were compared with sequences in the GenBank database using Seqman (DNASTAR) to confirm the amplification of genes highly homologous to β-glucosidase.
To obtain the MAT idiomorphs and flanking genes, sequences were combined for C. lunatus isolates using de novo assembly to generate complete nucleotide sequence. The GAP, ORF, MAT, and β-glucosidase genes and the intergenic regions between these genes of the C. lunatus isolates were aligned using DNAMAN to identify sequence similarity within and between isolates of each MAT. In addition, the sequences were compared with other fungal species in the GenBank using BLASTn. Sequences were deposited in the GenBank.
Crosses to determine MAT phenotype in C. lunatus
The MAT phenotypes of isolates of C. lunatus in Liaoning were determined for comparison in the molecular genotyping by PCR. Each isolate was tested for MAT by crossing with a MAT1–1 or MAT1–2 isolate using techniques described by Nelson (1959). C. lunatus isolates of known MAT were plated onto PDA and incubated in the dark at 25 °C for 7 days. Dry sterile corn leaf was embedded in Sach’s agar. Crossing involved the transfer of plugs of PDA agar from the parental cultures of each isolate and either of the isolates designated as MAT1–1 or MAT1–2 to opposite sides of the corn leaf. All cultures were mated with both MATs. The plates were incubated at 25 °C for 21 days, and the presence of perithecia on the corn leaf was considered a compatible cross. Then, the number of perithecia from each mating was counted, and some mature perithecia were individually crushed in a drop of water and examined for asci and ascospores under a dissecting microscope. As a control, all isolates were also paired with themselves.
Analysis of MAT distributions in the populations of C. lunatus in Liaoning
A multiplex MAT-specific PCR assay, consisting of a single reverse primer and two forward primers, was designed to rapidly identify the MAT of C. lunatus isolates. The reverse primer ClMatspe-R was combined with the MAT-specific forward primers ClMat1T-F and ClMat2-DF. The forward primers were designed to anneal within the CDS of each MAT gene to differentiate PCR products of 1582 and 932 bp for MAT1–1 and MAT1–2 individuals, respectively. The primers were tested as pairs and as a multiplex against a subset of C. lunatus isolates of known MAT to confirm their ability to differentiate the MAT. The PCR contained 0.15 μΜ each primer and consisted of 30 cycles and an extension time of 75 s. The multiplex PCR was used to identify the MAT of isolates within the field population of C. lunatus. Amplified products were visualized as previously described, and the MATs of isolates were identified based on the PCR product length.
Results
Identification and arrangement of the MAT idiomorphus
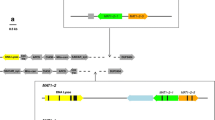
The predicted MAT1–1-1 (GenBank:KU749293) of C. lunatus was 1134 bp, possessed a single intron of 53 bp within the alpha domain at the nucleotide position from 218 bp to 270 bp, and encoded a protein of 378 amino acid (aa). Blastn analysis of MAT1–1-1 identified had the highest similarity to the MAT1–1-1 of heterothallic C. ellisii (GenBank:AF129746, 85% sequence homology) (Fig. 1A).
Alignments of putative amino acid sequences in Cochliobolus lunatus to mating-type (MAT) genes in other species of Cochliobolus: a MAT1–1 alpha HMG domain of C. lunatus (GenBank accession No. AML61199), C. ellisii (Q9Y8C7), C. heterostrophus (CAA06843), C. sativus (CAD62166), C. kusanoi (Q9Y8D3), C. cymbopogonis (Q9Y8D1), C. luttrellii (AAD33439), C. homomorphus (AAD33441), and C. carbonum (O13402). b MAT1–2 HMG domain of C. lunata (AML61200), C. ellisii (AAD33451), C. heterostrophus (Q02991), C. sativus (Q9P445), C. kusanoi (AAD33442), C. cymbopogonis (AAD33447), C. luttrellii (AAD33439), and C. homomorphus (AAD33441)
The predicted MAT1–2-1 (GenBank:KU749294.1) of C. lunatus was 1087 bp, possessed one single intron (55 bp long) within HMG domain at nucleotide positions 488–542 bp, and encoded a protein of 343 aa. Blastn analysis of MAT1–1-1 identified had the highest similarity to the MAT1–1-1 of heterothallic C. ellisii (GenBank:AF129747, 86% sequence homology) (Fig. 1B).
The highly conserved mutual regions were found upstream of the start codon of MAT1–1-1 and MAT1–2-1. ORF1 encoding a protein homologous to the domain of pyridoxamine phosphate oxidase-like protein of C. ellisii (AAD33450.1, 95% sequence homology) and ORF1 proteins of C. heterostrophus (GenBank:AAB82944.1, 85% sequence homology) were identified. In C. lunatus, the ORF1 resided further upstream from the MAT gene start codon in MAT1–1 (1095 bp) and MAT1–2 (1106 bp) isolates. In addition, the ORF1 nucleotide sequences of C. lunatus MAT1–1 and MAT1–2 strains had 99% homology. The highly conserved mutual regions were found between GAP and ORF1, as well as ORF1 and MAT1–1-1/MAT1–1-2. The GAP gene encodes a protein homologous to the GTPase activating protein (GAP) of C. heterostrophus (GenBank:AAB82943.1, 96% aa sequence homology at 88% coverage). The predicted GAP of MAT1–1 and MAT1–2 was 2388 bp, with six introns of 49,79, 47, 55, 95, and 97 bp (corresponding nucleotide positions 113–161 bp, 442–520 bp,1286–1332 bp, 1942–1996 bp, 2203–2297 bp, and 2302–2388 bp), and encoded a protein of 649 aa. A highly conserved mutual region located between MAT1–1-1/MAT1–2-1 and beta glucosidases (BGL1) was found downstream of the two MAT idiomorphs. The conserved region varied in length from 2386 bp to 2401 bp for MAT1–1 and MAT1–2 isolates, respectively. The region between MAT1–1 and MAT1–2 isolates was more highly conserved (98%). The BGL1 gene encoded a protein homologous to the beta glucosidases of BGL1 of C. heterostrophus (GenBank:AAB82946.1; 93% aa sequence homology). The BGL1 constituted 2852 bp, with 2 introns of 51 and 27 bp (corresponding nucleotide positions of 541–592 bp and 813–940 bp), and encoded a protein of 849 aa. The isolates of MAT1–1 and MAT1–2 had identical encoded BGL1 protein sequences.
Alignment of the MAT idiomorphs and flanking genes resulted in sequences of 10.89 kb for MAT1–1 (GenBank:KY471562) isolates and 10.81 kb for MAT1–2 (GenBank:KY471563) isolates (Fig. 2). The sizes of the idiomorph, which was determined by the length of dissimilar nucleotides, were 1.13 and 1.08 kb for MAT1–1 and MAT1–2 isolates, respectively. The MAT genes were successfully amplified in C. lunatus isolates tested with the primer. All isolates contained a MAT gene with a structure homologous to either the MAT1–2-1 of C. lunatus strain CX-3 or MAT1–1-1 of C. lunatus strain ZD958.
Structural analysis and comparison of major open reading frames (ORFs) identified in the MAT region in C. lunatus (Accession numbers MAT1–1 = KY471562 and MAT1–2 = KY471563). The flanks are nearly identical between MATs, in sharp contrast to the idiomorphs. 5′ of MAT: GAP, GTPase activating protein; ORF1, pyridoxamine 5′-phosphate oxidase domain. 3′ of MAT: a β-glucosidase. Numbers are in bases or kilobases. Arrows represent ORFs, and the direction represents the directions of transcription
In vitro production of sexual morphology
MAT1–1 and MAT1–2 isolates of C. lunatus were co-inoculated and paired on detached corn leaves to produce perithecia. Perithecia were produced on the detached leaves between 21 days and 25 days. The amount of perithecia produced was highly variable and ranged from approximately 4–15 perithecia/cm2 of leaf tissue for a prolific cross between ZD958 and CX-3 to approximately 85–317 ascospores/cm2 of leaf tissue.
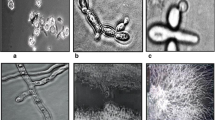
Mature perithecia were black and from ellipsoidal to globose. A well-defined ostiolate break, from subconical to paraboloid, was produced. Asci were cylindrical or clavate and straight or slightly curved with a short stipe. Mature asci contained 1–8 ascospores. Ascospores arrangement varied from complete coiling in a close helix to a nearly straight and parallel condition. Coiling was frequently evident in the apical portion of the ascus, with little or no coiling in the basal portion. Ascospores were discharged by the splitting of the ascus wall. Ascospores were filiform or flagelliform and somewhat tapered at the extremities. Mature ascospores were typically hyaline, with 6–15 septa (Fig. 3).
C. lunatus strains ZD958 × CX-3 form perithecia, asci, and ascospore. A: Small agar blocks of mycelium of compatible isolates on opposite sides of dry corn leaf on Sach’s medium; B: Black and ellipsoidal perithecia with suconical and ostiolate beak; C: Cylindrical and straight curved asci with coiling in a close helix. D: Flagelliform ascospores. B, C, and D: Microscopic images of perithecia, asci, and ascospore taken at 600 × magnification; Red arrow indicates mature perithecium, ascus, and ascospore in B, C, and D
MAT distribution in the C. lunatus population in China
The multiplex MAT-specific PCR produced single-amplification band in the C. lunatus isolates, with PCR products of 1582 and 932 bp amplified in the known MAT1–1 and MAT1–2 individuals, respectively (Fig. 4). No PCR products were produced when a random isolate (CX-3 or ZD958) was screened with PCR primer specific for the opposite MAT to detect heterothallic individuals. Thus, 48.02% of isolates were identified as MAT1–1 and 51.98% as MAT1–2 (Table 2). The frequency varied at a region level from 36.36% to 75.0% and 25.0% to 63.64% for MAT1–1 and MAT1–2, respectively. However, within China, the ratio of MAT1–1 to MAT1–2 isolates was consistent with 1:1 ratio (χ2 < 6.895, P > 0.05) (Table 2).
Discussion
To our knowledge, this study was the first to examine the MAT locus structure and distribution of C. lunatus in China. The identified MAT loci exhibited the typical characteristics and organization of a heterothallic Ascomycete species (Wirsel et al. 1998). The characteristics included a single gene per idiomorph, the location of the conserved introns in the HMG and alpha domain motifs, and ORFs flanking the idiomorphs encoding GAP, ORF1, and beta glucosidase. The occurrence of homologous gene sequences flanking both the MAT idiomorphs indicated that the MAT genes of C. lunatus resided in the same chromosome location.
The sequence length and identity upstream of the MAT1–1-1 and MAT1–2-1 genes identified in C. lunatus had also been reported in the MAT idiomorphs of many heterothallic Ascomycete species (Coppin et al. 1997). In addition, between the idiomorphs in the length, amino acid sequence, and protein stop codons of GAP and ORF1, which resided upstream of the MAT idiomorphs, beta glucosidase located downstream of the MAT idiomorphs were common for C. heterostrophus. The structural organization of the MAT loci of nine heterothallic species (C. heterostrophus, C. carbonum, C. ellisii, C. intermedius, C. miyabeanus, C. sativus, C. setariae, C. victoriae and C. lunatus) is highly conserved, with each strain carrying a single MAT gene. MAT loci genes sequence and arrangement in C. lunatus further support the hypothesis that heterothallism is ancestral in Cochliobolus species (Yun et al. 1999).
In this study, we determined the distribution of MAT1–1 and MAT1–2 C. lunatus isolates form corn planting regions in china which ever and presently severe occurred of this disease. The occurrence of both mating types was at a 1:1 ratio overall and in northeast region of China. C. lunatus is heterothallic, and the presence of MAT 1–1 and MAT 1–2 mating types and production of ascospore are the evidence for the possible existence of a sexually reproducing population. Although this study was not able to confirm the occurrence of a sexual morph in the field, it indicated that a cryptic reproductive cycle may occur within field population. A further consequence of sexually reproducing heterothallic species is genetic recombination. Fungal populations that reproduce sexually are likely to be genetically more diverse and have a higher adaptive potential than asexually reproducing population. It may be reason for resurgence of corn curvularia leaf spot disease in recently years.
The function of MAT genes has been investigated extensively in many pathogenic fungi, such as Cochliobolus spp., Sordaria spp., Bipolaris sacchari, Penicillium chrysogenum, Aspergillus fumigatus, and Schizosaccharomyces spp. (Turgeon et al. 1995; Zheng et al. 2013; Sharon et al. 1996; O’Gorman et al. 2008; Dahlmann et al. 2015). Studies have shown that conversion often occurs in the MATs between homothallism and heterothallism, and they even exist simultaneously in certain environmental pressure and genetic conditions (Lin and Heitman 2007; Yun et al. 1999; Hastings 1992; O’Gorman et al. 2008).The roles of the MAT1–1-1 and MAT1–2-1 genes on the sexual development in C. lunatus are still unknown. Studies proved that the MAT genes have certain universality and encode a transcription factor (Becker et al. 2015). The genes not only played important roles in regulating the sexual reproduction, cell recognition of the mating process, cell fusion, heterokaryon formation, and nuclear fusion and meiosis but also in regulating the hyphal morphology, asexual development, amino acid synthesis, iron absorption, secondary metabolism, and genome-wide transcription regulatory networks (Böhm et al. 2013).
References
Becker, K., Beer, C., Freitag, M., & Kück, U. (2015). Genome-wide identification of target genes of a MAT α-domain transcription factor reveals functions beyond sexual development. Molecular Microbiology, 96(5), 1002–1022.
Böhm, J., Hoff, B., O'Gorman, C. M., Wolfers, S., Klix, V., & Binger, D. (2013). Sexual production and MAT-mediated strain development in the penicillin-producing fungus penicillium chrysogenum. Proceedings of the National Academy of Sciences, 110, 1476–1481.
Brewer, M. T., Cadle-Davidson, L., Cortesi, P., Spanu, P. D., & Milgroom, M. G. (2011). Identification and structure of the MAT locus and development of PCR-based markers for MAT in powdery mildew fungi. Fungal Genetics and Biology, 48, 704–713.
Christiansen, S. K., Wirsel, S., Yun, S. H., Yoder, O. C., & Turgeon, G. B. (1998). The two Cochliobolus MAT genes are conserved among species but one of them is missing in C. victoriae. Mycological Research, 102(8), 919–929.
Coppin, E., Debuchy, R., Arnaise, S., & Picard, M. (1997). MATs and sexual development in filamentous ascomycetes. Microbiology and Molecular Biology Reviews, 61, 411–428.
Dahlmann, T. A., Böhm, J., Becker, K., & Kück, U. (2015). Sexual recombination as a tool for engineering industrial Penicillium chrysogenum strains. Current Genetics, 61(4), 1–5.
Duan, S. K. (1984). Survey on yellow spot of Huangzaosi. Plant Protection, 6, 12.
Gao, S. G., LI, Y. Q., Gao, J. X., Suo, Y. J., Fu, K. H., Li, Y. Y., & Chen, J. (2014). Genome sequence and virulence variation-related transcriptome profiles of Curvularia lunata, an important maize pathogenic fungus. BMC Genomics, 15, 627.
Hastings, I. M. (1992). Population genetic aspects of deleterious cytoplasmic genomes and their effect on the evolution of sexual reproduction. Genetical Research, 59, 215–225.
Lin, X., & Heitman, J. (2007). Mechanisms of homothallism in fungi and transitions between heterothallism and homothallism. Sex in fungi: Molecular Determination and Evolutionary Implications (pp. 283–300).
Lu, G. Z., Chen, J., Bai, J. K., & Wang, C. P. (1997). Occurrence situation and control measures of corn disease in China. Plant Protection, 23(4), 20–21.
Macri, F., & Lenna, P. (1974). Leaf corn blight incited by Curvularia lunata (Wakker) Boed. Journal of Plant Pathology, 10, 27–35.
Nelson, R. R. (1959). Genetics of Cochliobolus heterostrophus. I. Variability in Degree of Compatibility. Mycologia, 51(1), 18–23.
Nelson, R. R., & Haasis, F. A. (1964). The perfect stage of Curvularia lunata. Mycologia, 56(2), 316–317.
O’Gorman, C. M., Fuller, H. T., & Dyer, P. S. (2008). Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature, 457, 471–474.
Paoletti, M., Seymour, F. A., Alcocer, M. J. C., Kaur, N., Calvo, A. M., Archer, D. B., & Dyer, P. S. (2007). MAT and the genetic basis of self-fertility in the model fungus Aspergillus nidulans. Current Biology, 17, 1384–1389.
Pearce, T. L., Scott, J. B., Hay, F. S., & Pethybridge, S. J. (2016). MAT gene structure and spatial distribution of Didymella tanaceti in pyrethrum fields. Phytopathology, 106(12), 1521–1529.
Sharon, A., Yamaguchi, K., Christiansen, S., Horwitz, B. A., Yoder, O. C., & Turgeon, B. G. (1996). An asexual fungus has the potential for sexual development. Molecular & General Genetics, 251(1), 60–68.
Turgeon, B. G. (1998). Application of MAT gene technology to problems in fungal biology. Phytopathology, 36, 115–137.
Turgeon, B. G., Bohlmann, H., Ciuffetti, L. M., Christiansen, S. K., & Yang, G. (1993). Cloning and analysis of the MAT genes from Cochliobolus heterostrophus. Molecular & General Genetics, 238, 270–284.
Turgeon, G. B., Sharon, A., Wirsel, S., Christiansen, S. K., Yamaguchi, K., & Yoder, O. C. (1995). Structure and function of mating type genes in Cochliobolus spp. and asexual fungi. Canadian Journal of Botany, 73, S788–S783.
Wang, X. M., Jin, Q. M., Shi, J., Wang, Z. Y., & Li, X. (2006). The status of maize diseases and the possible effect of variety resistance on disease occurrence in the future. Acta Phytopathologica Sinica, 36, 1–11.
Wirsel, S., Horwitz, B., Yamaguchi, K., Yoder, O. C., & Turgeon, B. G. (1998). Single MAT-specific genes and their 3′ UTRs control mating and fertility in Cochliobolus heterostrophus. Molecular & General Genetics, 259, 272–281.
Yun, S. H., Berbee, M. L., Yoder, O. C., & Turgeon, B. G. (1999). Evolution of the fungal self-fertile reproductive life style from self-sterile ancestors. Proceedings of the National Academy of Sciences, 96, 5592–5597.
Zhang, L., Li, H., Xiao, S. Q., Lu, Y. Y., Li, G. F., Xue, C. S., & Chen, J. (2016). Efficient Agrobacterium tumefaciens-mediated target gene disruption in the maize pathogen Curvularia lunata. European Journal of Plant Pathology, 145(1), 155–165.
Zheng, Q., Hou, R., Zhang, J. Y., Ma, J. W., Wu, Z. S., Wang, G. H., Wang, C. F., & Xu, J. R. (2013). The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. PLoS One, 8(6), e66980.
Zhong, S., & Steffenson, B. J. (2001). Genetic and molecular characterization of MAT genes in Cochliobolus sativus. Mycologia, 93(5), 852–863.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant number 31271992) and China Agriculture Research System (Grant number CARS-02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Conflict of interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Human and animal studies
Research did not involving Human Participants and/or Animals.
Electronic supplementary material
Supplementary Table 1
(DOCX 17 kb)
Supplementary Fig 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Lu, Y.Y., Liu, K.X., Li, G.F. et al. Identification mating-type locus structure and distribution of Cochliobolus lunatus in China. Eur J Plant Pathol 151, 487–500 (2018). https://doi.org/10.1007/s10658-017-1393-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1393-4